Abstract
The synthetic utility of 3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis (3-oxopropanenitrile) (1) in the synthesis of some novel bis-[1,3,4-thiadiazole] 6a–g and bis-thiazole 10 and 13 derivatives with thieno[2,3-b]thiophene moiety is reported. Antimicrobial evaluation of some selected examples from the synthesized products was carried out and showed promising results.
Keywords: thieno[2,3-b]thiophene; nucleophilic addition; hydrazonoyl halides; bis-thiadiazoles; bis-thiazoles; antimicrobial activity
1. Introduction
Thiophene compounds are well known to exhibit various biological and medicinal activities such as BACE1 inhibitors [1], antitubercular [2], anti-depressant [3], anti-inflammatory [4], anti-HIV PR inhibitors [5], and anti-breast cancer activities [6]. In addition, thienothiophenes have potential applications in a wide variety of optical and electronic systems [7–9]. Furthermore, 1,3,4-thiadiazoles were recently reported as highly anti-inflammatory [10,11], and anticonvulsant agents [10,12]. Also, thiazoles and their derivatives found application in drug development for the treatment of allergies [13], hypertension [14], inflammation [15], schizophrenia [16], bacterial [17] and HIV infections [18]. Encouraged by all these findings and in continuation of our ongoing research program investigating the utilization of compound 1 as versatile and useful building blocks for the synthesis of a wide variety of bis-heterocycles systems [19,20], we report in the present work an efficient and rapid method for the synthesis of a series of thienothiophene pendant to thiadiazole or thiazole moieties.
2. Results and Discussion
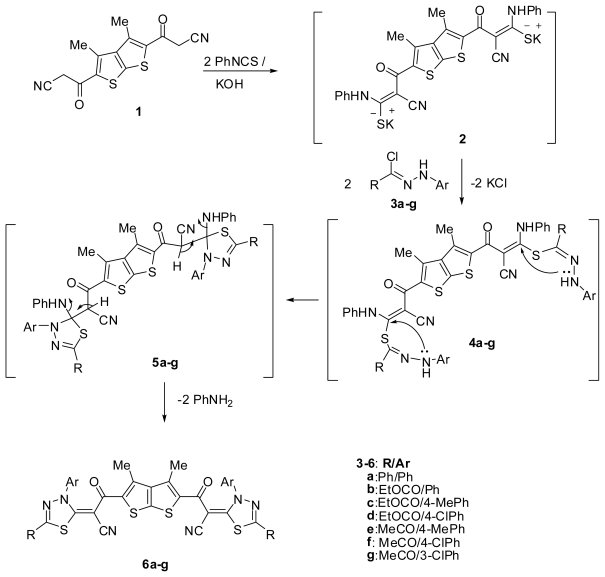
The nucleophilic addition of thieno[2,3-b]thiophene 1 [19] to phenyl isothiocyanate in DMF, in the presence of potassium hydroxide, afforded the corresponding potassium salt 2. Heterocyclisation of the intermediate 2 with hydrazonoyl chlorides 3a [21] or 3b–d [22] or 3e–g [23] furnished in each case, one isolable product (as tested by TLC). The reaction products were identified as bis-[1,3,4]-thiadiazole structures 6a–g (Scheme 1).
Scheme 1.
The structure of the products 6a–g was determined from spectroscopic as well as elemental analytical data. Thus, compound 6a, taken as a typical example, showed absorption bands at 1674 and 2199 cm−1 corresponding to C=O and C ≡ N groups, respectively. Its 1H NMR spectrum revealed the absence of CH2 protons of compound 1 and showed signals at δ 2.49 due to CH3 protons, in addition to an aromatic multiplet in the region δ 7.57–7.97. The aforementioned results indicate that the reaction proceeds via S-alkylation [24] to give S-alkylated intermediate 4 which cyclized in situ under the employed reaction conditions to give intermediate 5. Elimination of two aniline molecules from 5 gave the desired product 6 (Scheme 1).
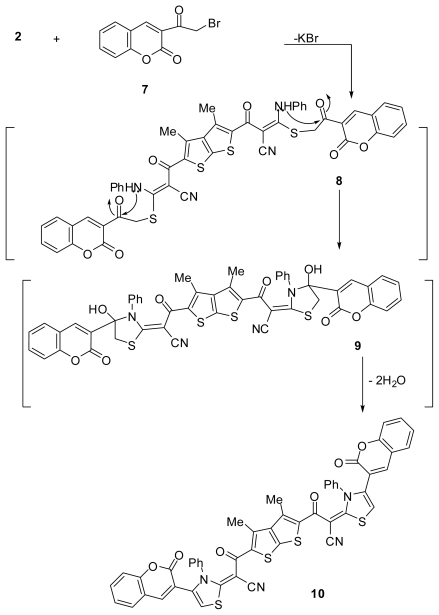
Next, the reactivity of the potassium salt 2 towards 3-(2-bromoacetyl)-2H-chromen-2-one (7) [25,26] was also investigated. Thus, treatment of potassium salt 2 with compound 7 gave one product that was identified as 3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(3-oxo-2-(4-(2-oxo-2H-chromen-3-yl)-3-phenylthiazol-2(3H)-ylidene)propanenitrile) (10) as shown in Scheme 2. The reaction proceeds via nucleophilic displacement of bromide to give S-alkylated intermediate 8, followed by nucleophilic addition of (PhNH) group to carbonyl group of chromen-2-one ring to give the respective intermediate 9. Dehydration of the latter intermediate gave bis-thiazole derivative 10 as the final product. The IR spectrum of the isolated product showed absorption bands at 2195, 1647 and 1724 cm−1 due to nitrile function and carbonyl groups, respectively. Its 1H NMR spectrum showed singlet signal at δ 2.49 ppm due to methyl protons, in addition to aromatic multiplets in the region δ 7.02–8.6 ppm.
Scheme 2.
Synthesis of 3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(3-oxo-2-(4- (2-oxo-2H-chromen-3-yl)-3-phenylthiazol-2(3H)-ylidene)propanenitrile (10).
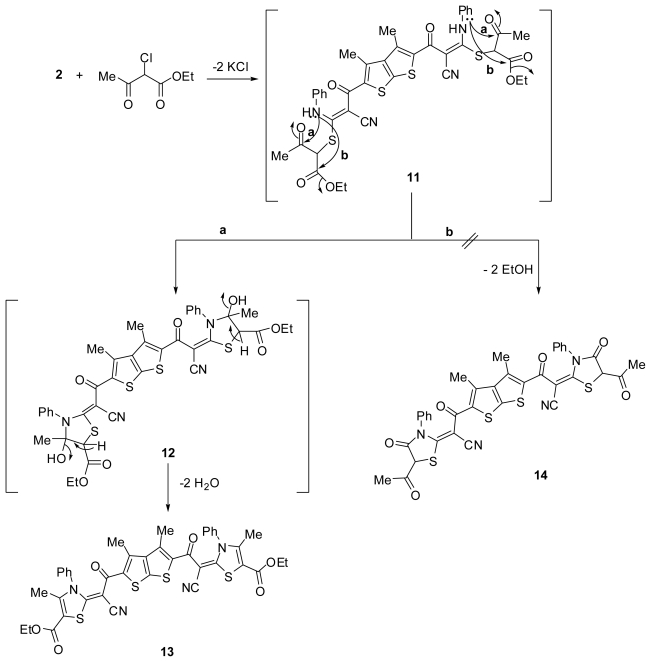
Similarly, treatment of the potassium salt 2 with ethyl 2-chloro-3-oxobutanoate afforded diethyl 2,2′-(2,2′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(1-cyano-2-oxoethan-2-yl-1-ylidene))bis(4- methyl-3-phenyl-2,3-dihydrothiazole-5-carboxylate) (13) as outlined in Scheme 3. The bis-thiazole structure 13 was confirmed from its elemental analyses and spectral data. The IR spectrum of compound 13 revealed absorption bands at 2206, 1713 and 1643 cm−1 due to nitrile function and two carbonyl groups, respectively. Its 1H-NMR spectrum showed a triplet signal at δ 1.30 (J = 7.2 Hz) due to CH3 protons, two singlet signal at δ 2.24 and 2.49 characteristics for two methyl protons, a quartet signal at δ 4.32 (J = 7.2 Hz) due to CH2 protons, in addition to an aromatic multiplet in the region δ 7.62. A proposed mechanism for the formation of the bis-thiazole structure 13 is depicted in Scheme 3. The foregoing spectral data supported the proposed structure 13 and ruled out the other bis-thiazole structure 14 (Scheme 3).
Scheme 3.
Synthesis of diethyl 2,2′-(2,2′-(3,4-dimethylthieno[2,3-b]thiophene-2,5- diyl)bis(1-cyano-2-oxoethan-2-yl-1-ylidene))bis(4-methyl-3-phenyl-2,3-dihydrothiazole-5- carboxylate) (13).
3. Experimental Section
All melting points were measured on a Gallenkamp melting point apparatus (Weiss-Gallenkamp, London, UK). The infrared spectra were recorded in potassium bromide disks on a pye Unicam SP 3300 and Shimadzu FT IR 8101 PC infrared spectrophotometers (Pye Unicam Ltd. Cambridge, England and Shimadzu, Tokyo, Japan, respectively). The NMR spectra were recorded on a BRUKER VX-500 NMR spectrometer (Varian, Palo Alto, CA, USA). 1H spectra were run at 500 MHz in deuterated dimethyl sulfoxide (DMSO-d6). Chemical shifts were related to that of the solvent. Elemental analyses were carried out at the Micro-analytical Center of Cairo University, Giza, Egypt. The biological evaluation of the products 6a–g and 10 were carried out in the Medical Mycology Laboratory of the Regional Center for Mycology and Biotechnology of Al-Azhar University, Cairo, Egypt. Thieno[2,3-b]thiophene 1 [19], and hydrazonoyl chlorides 3a [21], 3b–d [22], 3e–g [23], and 3-(2-bromoacetyl)-2H-chromen-2-one (7) [25,26] were prepared following the literature procedure.
Reactions of Compound 1 with Hydrazonoyl Halides 3a or 3b-d or 3e-g or 3-(2-bromoacetyl)-2Hchromen- 2-one (7)
General Procedure
To a stirred solution of potassium hydroxide (0.11 g, 2 mmol) in 20 mL DMF was added compound 1 (0.302 g, 1 mmol). After stirring for 30 min, phenyl isothiocyanate (0.27 g, 2 mmol) was added to the resulting mixture. Stirring was continued for 6 h, and then the appropriate hydrazonoyl chlorides 3a–g (2 mmol) or 3-(2-bromoacetyl)-2H-chromen-2-one (7) (0.534 g, 2 mmol) or ethyl 2-chloro-3-oxobutanoate (0.329 g, 2 mmol) was added portion-wise over a period of 30 min. After the addition was complete, the reaction mixture was stirred for additional 12 h, during which the hydrazonoyl chloride or 3-(2-bromoacetyl)-2H-chromen-2-one went into solution and a yellow product precipitated. The solid product was filtered off, washed with EtOH and dried, Recrystallization from DMF/EtOH (3:1) afforded the corresponding bis-[1,3,4]thiadiazole derivatives 6a–g or bis-thiazole derivatives 10 or 13, respectively.
3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(2-(3,5-diphenyl-1,3,4-thiadiazol-2(3H)- ylidene)-3-oxopropanenitrile) (6a)
Yield (61%), m.p. 276 °C; IR (KBr) νmax: 2905 (aliphatic CH), 2199 (C ≡ N), 1674 (C=O) cm−1; 1H-NMR (DMSO-d6): δ2.49 (s, 6H, 2CH3), 7.57–7.97 (m, 20H, ArH). MS m/z (%): 775 (M+, 0.16), 774 (0.14), 471 (46.73), 304 (4.13), 77 (70.79). Anal. Calcd for C42H26N6O2S4 (774.95): C, 65.09; H, 3.38; N, 10.84. Found: C, 65.01; H, 3.45; N, 10.90%.
Diethyl 5,5′-(2,2′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(1-cyano-2-oxoethan-2-yl-1- ylidene))bis(4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate)(6b)
Yield (52%), m.p. > 300 °C; IR (KBr) νmax: 2982 (aliphatic CH), 2199 (C≡N), 1744 and 1674 (2C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.33 (s, 6H, 2CH3, J = 6.9 Hz), 2.49 (s, 6H, 2CH3), 4.44 (q, 4H, 2CH2, J = 6.9 Hz),7.53–7.92 (m, 10H, ArH). MS m/z (%): 767 (M+, 1.57), 167 (19.92), 149 (36.71), 77 (7.77). Anal. Calcd for C36H26N6O6S4 (766.89): C, 56.38; H, 3.42; N, 10.96. Found: C, 56.30; H, 3.36; N, 10.88%.
Diethyl 5,5′-(2,2′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(1-cyano-2-oxoethan-2-yl-1- ylidene))bis(4-p-tolyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate) (6c)
Yield (66%), m.p. > 300 °C; IR (KBr) νmax: 2986 (aliphatic CH), 2203 (C≡N), 1747 and 1674 (2C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.35 (s, 6H, 2CH3, J = 7.0 Hz), 2.42 (s, 6H, 2CH3), 2.52 (s, 6H, 2CH3),4.46 (q, 4H, 2CH2, J = 7.0 Hz),7.41 (d, 4H, J = 8.0 Hz), 7.62 (d, 4H, J= 8.0 Hz). MS m/z (%): 793 (3.44), 222 (4.85), 221 (4.55), 167 (9.11), 91 (50.33), 77 (51.22). Anal. Calcd for C38H30N6O6S4 (794.94): C, 57.41; H, 3.80; N, 10.57. Found: C, 57.52; H, 3.88; N, 10.66 %.
Diethyl 5,5′-(2,2′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(1-cyano-2-oxoethan-2-yl-1- ylidene))bis(4-(4-chlorophenyl)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate)(6d)
Yield (53%), m.p. > 300 °C; IR (KBr) νmax: 2986 (aliphatic CH), 2206 (C≡N), 1744 and 1674 (2C=O) cm−1; 1H-NMR (DMSO-d6): δ1.37 (s, 6H, 2CH3, J = 7.0 Hz), 2.52 (s, 6H, 2CH3), 4.47 (q, 4H, 2CH2, J = 7.0 Hz),7.73 (d, 4H, J= 10.0 Hz), 7.84 (d, 4H, J = 10.0 Hz). MS m/z (%): 835 (M+, 2.81), 334 (6.05), 168 (8.37), 112 (6.37), 111 (23.38), 77 (39.48). Anal. Calcd for C36H24Cl2N6O6S (835.78): C, 51.73; H, 2.89; N, 10.06. Found: C, 51.67; H, 2.79; N, 10.12%.
3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(2-(5-acetyl-3-p-tolyl-1,3,4-thiadiazol-2(3H)- ylidene)-3-oxopropanenitrile)(6e)
Yield (52%), m.p. 240 °C; IR (KBr) νmax: 2199 (C≡N), 1690 and 1674 (2C=O) cm−1; 1H-NMR (DMSO-d6): δ 2.29 (s, 6H, 2CH3), 2.45 (s, 6H, 2CH3), 2.50 (s, 6H, 2CH3), 7.22 (d, 4H, J = 8.5 Hz), 7.33 (d, 4H, J = 8.5 Hz). MS m/z (%): 732 (0.04), 647 (0.06), 221 (2.03), 166 (1.33), 106 (100.0), 91, (58.18), 77 (84.54). Anal. Calcd for C36H26N6O4S4 (734.89): C, 58.84; H, 3.57; N, 11.44. Found: C, 58.77; H, 3.49; N, 11.38%.
3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(2-(5-acetyl-3-(4-chlorophenyl)-1,3,4- thiadiazol-2(3H)-ylidene)-3-oxopropanenitrile)(6f)
Yield (49%), m.p. 295 °C; IR (KBr) νmax: 2199 (C≡N), 1693 and 1655 (2C=O) cm−1; 1H-NMR (DMSO-d6): δ 2.41 (s, 6H, 2CH3), 2.52 (s, 6H, 2CH3), 7.72 (d, 4H, J = 8.8 Hz), 7.84 (d, 4H, J = 8.8 Hz). MS m/z (%): 776 (3.02), 500 (3.36), 471 (9.6), 304 (3.99), 276 (6.27), 166 (10.71), 112 (6.32), 111 (16.73). Anal. Calcd for C34H20Cl2N6O4S4 (775.73): C, 52.64; H, 2.60; N, 10.83. Found: C, 52.58; H, 2.54; N, 10.77%.
3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(2-(5-acetyl-3-(3-chlorophenyl)-1,3,4- thiadiazol-2(3H)-ylidene)-3-oxopropanenitrile)(6g)
Yield (49%), m.p. > 300 °C; IR (KBr) νmax: 2199 (C≡N), 1690 and 1647 (2C=O) cm−1; 1H-NMR (DMSO-d6): δ1.89 (s, 6H, 2CH3), 2.49 (s, 6H, 2CH3), 6.97–8.00 (m, 8H, ArH). MS m/z (%): 771 (3.28), 304 (6.34), 166 (22.08), 112 (13.36), 111 (18.98). Anal. Calcd for C34H20Cl2N6O4S4 (775.73): C, 52.64; H, 2.60; N, 10.83. Found: C, 52.55; H, 2.52; N, 10.74%.
3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(3-oxo-2-(4-(2-oxo-2H-chromen-3-yl)-3- phenylthiazol-2(3H)-ylidene)propanenitrile) (10)
Yield (68%), m.p. > 300 °C; IR (KBr) νmax: 2195 ((C≡N)), 1724 and 1647 (2C=O) cm−1; 1H-NMR (DMSO-d6): δ 2.49 (s, 6H, 2CH3), 7.02–8.6 (m, 22H, ArH). MS m/z (%): 909 (2.45), 166 (2.75), 145 (4.05), 77 (15.41). Anal. Calcd for C50H28N4O6S4 (909.04): C, 66.06; H, 3.10; N, 6.16. Found: C, 66.15; H, 3.21; N, 6.25%.
Diethyl 2,2′-(2,2′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(1-cyano-2-oxoethan-2-yl-1- ylidene))bis(4-methyl-3-phenyl-2,3-dihydrothiazole-5-carboxylate) (13)
Yield (44%), m.p. 278–280 °C; IR (KBr) νmax: 2986 (aliphatic CH), 2206 ((C≡N)), 1713 and 1643 (2C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.30 (t, 6H, 2CH3, J = 7.2 Hz), 2.24 (s, 6H, 2CH3), 2.49 (s, 6H, 2CH3), 4.32 (q, 4H, 2CH2, J = 7.2 Hz),7.62 (s, 10H, ArH). Anal. Calcd for C40H32N4O6S4 (792.97): C, 60.59; H, 4.07; N, 7.07. Found: C, 60.48; H, 4.16; N, 7.15%.
3.1. Antimicrobial Evaluation
The newly synthesized target compounds (6a–g and 10) were evaluated for their in vitro antibacterial activity against Staphylococcus aureus (SA) and Bacillis subtilis (BS) as examples of Gram-positive bacteria and Pseudomonas aeruginosa (PA) and Escherichia coli (EC) as examples of Gram-negative bacteria. They were also evaluated for their in vitro antifungal potential against Aspergillus fumigatus (AF), Geotrichum candidum (GC), Candida albicans (CA) and Syncephalastrum racemosum (SR) fungal strains. The organisms were tested against the activity of solutions of concentrations (5 μg/mL) and using inhibition zone diameter (IZD) in mm as criterion for the antimicrobial activity (agar diffusion method). The fungicides Itraconazole, Clotrimazole and the bactericides Penicillin G, Streptomycin were used as references to evaluate the potency of the tested compounds under the same conditions. The results are depicted in Table 1.
Table 1.
Antibacterial and antifungal activities of the synthesized compounds (6a–g) and 10.
| Sample/Tested Organism | 6a | 6b | 6c | 6d | 6e | 6f | 6g | 10 | Standard | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fungi | Itraconazole | Clotrimazole | ||||||||
| Aspergillus fumigatus(AF) | 11.7 ± 0.2 | 15.4 ± 0.09 | 13.3 ± 0.2 | 16.4 ± 0.3 | 9.3 ± 0.2 | 17.4 ± 0.08 | 12.2 ± 0.09 | 14.3 ± 0.2 | 28.5 ± 0.05 | 26 ± 0.1 |
| Geotrichum candidum(GC) | 13.5 ± 0.1 | 14.9 ± 0.05 | 14.4 ± 0.1 | 18.1 ± 0.08 | 11.4 ± 0.1 | 18.3 ± 0.3 | 14.4 ± 0.03 | 16.7 ± 0.08 | 27.1 ± 0.06 | 23.1 ± 0.03 |
| Candida albicans(CA) | 10.4 ± 0.08 | NA | 10.2 ± 0.09 | 13.7 ± 0.05 | NA | NA | NA | 11.9 ± 0.1 | 26.1 ± 0.02 | 18.3 ± 0.01 |
| Syncephalastrum racemosum(SR) | NA | 12.1 ± 0.08 | NA | NA | 8.2 ± 0.09 | 14.2 ± 0.08 | 9.2 ± 0.08 | NA | 22.3 ± 0.09 | 20.5 ± 0.02 |
| Gram Positive Bacteria | Penicillin G | Streptomycin | ||||||||
| Staphylococcus aureus(SA) | 11.2 ± 0.1 | 17.9 ± 0.05 | 11.3 ± 0.05 | 15.4 ± 0.5 | 9.4 ± 0.05 | 18.9 ± 0.01 | 13.8 ± 0.1 | 13.4 ± 0.3 | 29.4 ± 0.08 | 25.1 ± 0.08 |
| Bacillis subtilis(BS) | 13.7 ± 0.07 | 16.1 ± 0.01 | 9.0 ± 0.08 | 18.4 ± 0.1 | 10.6 ± 0.08 | 20.9 ± 0.03 | 16.6 ± 0.03 | 14.7 ± 0.09 | 32.5 ± 0.06 | 29.1 ± 0.04 |
| Gram Negative Bacteria | Penicillin G | Streptomycin | ||||||||
| Pseudomonas aeruginosa(PA) | NA | 10.1 ± 0.01 | NA | NA | NA | 12.1 ± 0.01 | NA | NA | 28.3 ± 0.05 | 24.3 ± 0.08 |
| Escherichia coli(EC) | 8.3 ± 0.09 | 14.5 ± 0.2 | 10.1 ± 0.07 | 13.7 ± 0.05 | 7.4 ± 0.07 | 15.2 ± 0.5 | 9.5 ± 0.2 | 10.9 ± 0.2 | 33.5 ± 0.7 | 25.6 ± 0.04 |
NA: No activity, data are expressed in the form of mean ± SD. Mean zone of inhibition in mm ± Standard deviation beyond well diameter (6 mm) produced on a range of environmental and clinically pathogenic microorganisms using (5 mg/mL) concentration of tested samples.
The results depicted in Table 1 revealed that most of the tested compounds displayed variable inhibitory effects on the growth of the tested Gram-positive bacteria and Gram-negative bacteria strains and also against fungal strains. In general, most of the tested compounds revealed better activity against the Gram-positive bacteria rather than the Gram-negative bacteria: Compounds 6a, 6c–d and 10 exhibited almost no activity against Syncephalastrum racemosum and Pseudomonas aeruginosa; Compounds 6b and 6e–g exhibited almost no activity against Candida albicans. Compounds 6e and 6g exhibited almost no activity against Pseudomonas aeruginosa; Compounds 6d, 6f and 10 showed comparatively good activity against all the bacterial and fungal strains. The good activity of 6d and 6f is attributed to the presence of pharmacologically active 4-chlorophenyl at position 4 of the thiadiazole ring.
4. Conclusions
In conclusion, the reactivity of diethyl 3,3′-(3,4-dimethylthieno[2,3-b]thiophene-2,5-diyl)bis (3-oxopropanenitrile) (1) was investigated as a versatile and readily accessible building block for the synthesis of new bis-heterocycles incorporating thieno[2,3-b]thiophene moiety of biological and pharmaceutical importance.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP- VPP- 007.
References
- 1.Giordanetto F., Karlsson O., Lindberg J., Larsson L.O., Linusson A., Evertsson E., Morgan D.G.A., Inghardt T. Discovery of cyclopentane- and cyclohexane-trans-1,3-diamines as potent melanin-concentrating hormone receptor 1 antagonists. Bioorg. Med. Chem. Lett. 2007;17:5222–5231. doi: 10.1016/j.bmcl.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Parai M.K., Panda G., Chaturvedi V., Manju Y.K., Sinha S. Thiophene containing triarylmethanes as antitubercular agents. Bioorg. Med. Chem. Lett. 2008;18:289–292. doi: 10.1016/j.bmcl.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 3.Wardakhan W.W., Abdel-Salam O.M.E., Elmegeed G.A. Screening for antidepressant, Sedative and analgesic activities of novel fused thiophene derivatives. Acta Pharm. 2008;58:1–14. doi: 10.2478/v10007-007-0041-5. [DOI] [PubMed] [Google Scholar]
- 4.Kumar P.R., Raju S., Goud P.S., Sailaja M., Sarma M.R., Reddy G.O., Kumar M.P., Reddy V.V., Suresh T., Hegde P. Synthesis and biological evaluation of thiophene [3,2-b] pyrrole derivatives as potential anti-inflammatory agents. Bioorg. Med. Chem. 2004;12:1221–1230. doi: 10.1016/j.bmc.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Bonini C., Chiummiento L., Bonis M.D., Funicello M., Lupattelli P., Suanno G., Berti F., Campaner P. Synthesis, biological activity and modelling studies of two novel anti HIV PR inhibitors with a thiophene containing hydroxyethylamino core. Tetrahedron. 2005;61:6580–6583. [Google Scholar]
- 6.Brault L., Migianu E., Néguesque A., Battaglia E., Bagrel D., Kirsch G. New thiophene analogues of kenpaullone: Synthesis and biological evaluation in breast cancer cells. Eur. J. Med. Chem. 2005;40:757–763. doi: 10.1016/j.ejmech.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Litvinov V.P. The latest achievements in thienothiophene chemistry. Russ. Chem. Rev. 2005;74:217–248. [Google Scholar]
- 8.Gather M.C., Heeny M., Zhang W., Whitehead K.S., Bradley D.D.C., McCulloch I., Campbell A.J. An alignable fluorene thienothiophene copolymer with deep-blue electrolumenescent emission at 410 nm. Chem. Commun. 2008;9:1079–1081. doi: 10.1039/b716510b. [DOI] [PubMed] [Google Scholar]
- 9.He M., Li J., Sorensen M.L., Zhang F., Hancock R.R., Fong H.H., Pozdin V.A., Smilgies D., Malliaras G.G. Alkylsubstituted thienothiophene semiconducting materials: Structure property relationships. J. Am. Chem. Soc. 2009;131:11930–11938. doi: 10.1021/ja903895s. [DOI] [PubMed] [Google Scholar]
- 10.Dawood K.M., Abdel-Gawad H., Ragab E.A., Ellithey M., Mohamed H.A. Synthesis, anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. Bioorg. Med. Chem. 2006;14:3672–3680. doi: 10.1016/j.bmc.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Schenone S., Bruno O., Ranise A., Bondavalli F., Filippelli W., Falcone G., Giordano L., Vitelli M.R. 3-Arylsulphonyl-5-arylamino-1,3,4-thiadiazol-2(3H)ones as anti-inflammatory and analgesic agents. Bioorg. Med. Chem. 2001;9:2149–2153. doi: 10.1016/s0968-0896(01)00121-3. [DOI] [PubMed] [Google Scholar]
- 12.Ilies M.A., Masereel B., Rolin S., Scozzafava A., Câmpeanu G., Cîmpeanu V., Supuran C.T. Carbonic anhydrase inhibitors: Aromatic and heterocyclic sulfonamides incorporating adamantyl moieties with strong anticonvulsant activity. Bioorg. Med. Chem. 2004;12:2717–2726. doi: 10.1016/j.bmc.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Hargrave K.D., Hess F.K., Oliver J.T. N-(4-Substituted-thiazolyl)oxamic acid derivatives, new series of potent, orally active antiallergy agents. J. Med. Chem. 1983;26:1158–1163. doi: 10.1021/jm00362a014. [DOI] [PubMed] [Google Scholar]
- 14.Patt W.C., Hamilton H.W., Taylor M.D., Ryan M.J., Taylor D.G., Jr, Connolly C.J.C., Doherty A.M., Klutchko S.R., Sircar I., Steinbaugh B.A., et al. Structure-activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J. Med. Chem. 1992;35:2562–2572. doi: 10.1021/jm00092a006. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P.K., Sawnhney S.N., Gupta A., Singh G.B., Bani S. Synthesis and antiinflammatory activity of some 3-(2-thiazolyl)-1,2-benzisothiazoles. Indian J. Chem. 1998;37B:376–381. [Google Scholar]
- 16.Jean J.C., Wise L.D., Caprathe B.W., Tecle H., Bergmeier S., Humblet C.C., Heffner T.G., Meltzner L.T., Pugsley T.A. 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. J. Med. Chem. 1990;33:311–317. doi: 10.1021/jm00163a051. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji K., Ishikawa H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg. Med. Chem. Lett. 1994;4:1601–1606. [Google Scholar]
- 18.Bell F.W., Cantrell A.S., Hogberg M., Jaskunas S.R., Johansson N.G., Jordon C.L., Kinnick M.D., Lind P., Morin J.M., Jr, Noreen R., et al. Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem. 1995;38:4929–4936. doi: 10.1021/jm00025a010. [DOI] [PubMed] [Google Scholar]
- 19.Mabkhoot Y.N. Synthesis and analysis of some bis-heterocyclic compounds containing sulphur. Molecules. 2009;14:1904–1914. doi: 10.3390/molecules14051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mabkhoot Y.N, Kheder N.A., Al-Majid A.M. Facile and convenient synthesis of new thieno[2,3-b]thiophene derivatives. Molecules. 2010;15:9418–9426. doi: 10.3390/molecules15129418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolkoff P. A new method of preparing hydrazonyl halides. Can. J. Chem. 1975;53:1333–1335. [Google Scholar]
- 22.Shawali A.S., Eweiss N.F., Hassaneen H.M., Al-gharib M.S. Synthesis and rearrangement of ethyl aryloxyglyoxalate arylhydrazones. Bull. Chem. Soc. Jpn. 1975;48:365–366. [Google Scholar]
- 23.Eweiss N.F., Osman A. Synthesis of heterocycles. Part II. New routes to acetylthiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980;17:1713–1717. [Google Scholar]
- 24.Geies A.A., Kamal-Eldeen A.M., Abdelhafez A.A., Gaber A.M. Synthesis of some thiazolo(3,2-a) pyrimidines. Phosphor. Sulfur Silicon Relat. Elem. 1991;56:87–93. [Google Scholar]
- 25.Koelsch C.F. Bromination of acetocoumarin. J. Am. Chem. Soc. 1950;72:2993–2995. [Google Scholar]
- 26.Czerney P., Hartman H. 3-α-bromacetyl-coumarines as synthones for heterocyclic substituted coumarines. J. Prakt. Chem. 1983;325:551–560. [Google Scholar]