A signaling module known to organize the actin cytoskeleton during neuronal guidance also instructs synaptic vesicle clustering during synapse assembly.
Abstract
Netrin is a chemotrophic factor known to regulate a number of neurodevelopmental processes, including cell migration, axon guidance, and synaptogenesis. Although the role of Netrin in synaptogenesis is conserved throughout evolution, the mechanisms by which it instructs synapse assembly are not understood. Here we identify a mechanism by which the Netrin receptor UNC-40/DCC instructs synaptic vesicle clustering in vivo. UNC-40 localized to presynaptic regions in response to Netrin. We show that UNC-40 interacted with CED-5/DOCK180 and instructed CED-5 presynaptic localization. CED-5 in turn signaled through CED-10/Rac1 and MIG-10/Lamellipodin to organize the actin cytoskeleton in presynaptic regions. Localization of this signaling pathway to presynaptic regions was necessary for synaptic vesicle clustering during synapse assembly but not for the subcellular localization of active zone proteins. Thus, vesicle clustering and localization of active zone proteins are instructed by separate pathways downstream of Netrin. Our data indicate that signaling modules known to organize the actin cytoskeleton during guidance can be co-opted to instruct synaptic vesicle clustering.
Introduction
Netrin is a chemotrophic factor originally discovered for its requirement in cell migration and axon guidance (Hedgecock et al., 1990; Ishii et al., 1992; Serafini et al., 1994). Genetic studies in Drosophila later indicated that the Netrin receptor Frazzled (homologous to Caenorhabditis elegans UNC-40 and vertebrate deleted in colorectal cancer [DCC]) is also required for short-range synaptic targeting (Winberg et al., 1998). Recent studies in C. elegans have also demonstrated that Netrin plays instructive roles in directing the formation of en passant presynaptic specializations (Colón-Ramos et al., 2007; Poon et al., 2008; Park et al., 2011). The capacity of Netrin to instruct synapse formation is also conserved in vertebrates (Manitt et al., 2009; Xu et al., 2010). How Netrin instructs these conserved roles in synapse formation and how these mechanisms relate to Netrin’s canonical guidance role are not understood.
The capacity of guidance molecules to instruct synapse formation is not unique to Netrin. Many of the molecules originally identified for their roles in axon guidance, such as Ephrins, Semaphorins, and Slits, are now known to play important roles during synapse formation (Chen and Cheng, 2009; Shen and Cowan, 2010). Morphogenic signals such as Wnts can also spatially regulate the patterning of synaptic connections (Inaki et al., 2007; Klassen and Shen, 2007; Davis et al., 2008; Miech et al., 2008; Poon et al., 2008; Salinas and Zou, 2008). Although examples of guidance molecules controlling synapse formation abound, we understand less about how these molecules regulate synapse biology.
Here we use the C. elegans AIY interneuron to identify the molecular mechanisms that act downstream of the UNC-40 receptor to regulate synaptic vesicle clustering in vivo (Fig. 1 A). We show that core components of the canonical Netrin guidance pathway, such as Rac GTPases, have a novel role in synaptic vesicle clustering. We also demonstrate that CED-5 (a Rac guanine nucleotide exchange factor [GEF]) and MIG-10 (homologous to vertebrate proteins RIAM and Lamellipodin) localize to presynaptic regions in a Netrin-dependent manner. Our data indicate that Netrin instructs synaptic vesicle clustering by directing the asymmetric localization of downstream signaling molecules that organize the actin cytoskeleton at synaptic sites.
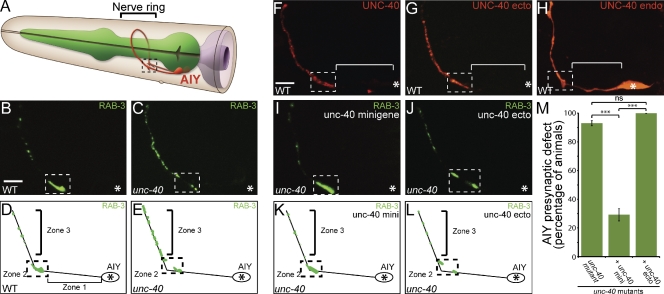
Figure 1.
The UNC-40 intracellular domain is required for instructing synaptic vesicle clustering in AIY. (A) Schematic diagram of AIY morphology in the nerve ring (bracket). Dashed box represents Zone 2 of AIY where AIY:RIA synapses form (White et al., 1986). Modified image adapted from www.wormatlas.org with permission from Zeynep Altun. (B–E) Distribution of synaptic vesicles in AIY in wild-type (B and D) or unc-40(e271) (C and E) animals as visualized with GFP::RAB-3 and represented in cartoon diagrams (D and E). RAB-3::GFP marker used here has been shown to associate with vesicles during the late stage of the secretory pathway (Matteoli et al., 1991). Bar (B and C), 5 µm. (F and G) Localization of UNC-40::GFP (F) and UNC-40 ecto::mCh (G) expressed cell-specifically in AIY using the ttx-3 promoter. Note the enrichment of UNC-40::GFP (F) and UNC-40 ecto::mCh (G) in Zones 2 and 3, but absent from asynaptic Zone 1 (bracket). Bar (F–J), 5 µm. (H) Localization of UNC-40 intracellular domain::mCherry expressed cell-specifically in AIY. Note diffuse localization throughout neurite, including Zone 1 (bracket, compare with F and G). Although expression of the endodomain in other neurons has been shown to cause gain-of-function outgrowth and guidance phenotypes (Gitai et al., 2003), we did not observe gain-of-function phenotypes in AIY resulting from expression of this construct (see Fig. S3, H and I). (I–L) unc-40(e271) mutant animals expressing an unc-40 minigene (I and K) or an unc-40 minigene lacking the intracellular domain (called unc-40 ecto; J and L). Presynaptic pattern was assayed with the GFP::RAB-3 probe in AIY. (M) Quantification of the AIY presynaptic phenotype in unc-40(e271) animals with the unc-40 minigene or the unc-40 ecto minigene. Error bars represent 95% confidence interval. In all images, asterisk represents location of the cell body and dashed box encloses AIY Zone 2; ***, P < 0.0001 between indicated groups by Fisher’s exact test.
Results
The intracellular domain of UNC-40 is required for synaptic vesicle clustering
The Netrin receptor UNC-40 has the conserved capacity to instruct synapse formation (Colón-Ramos et al., 2007; Poon et al., 2008; Manitt et al., 2009; Xu et al., 2010; Park et al., 2011). For example, in C. elegans, UNC-40 is cell-autonomously required for presynaptic assembly in the AIY interneurons (Fig. 1, B–E; Fig. S1, G–I; Fig. S2, G–I; Colón-Ramos et al., 2007). The role of UNC-40 in presynaptic assembly is not the result of a guidance role, as antero-dorsal guidance in AIY is not dependent on UNC-40 (Altun-Gultekin et al., 2001; Colón-Ramos et al., 2007). Furthermore, UNC-40 is localized to synaptic regions in response to Netrin (Fig. 1 F; Fig. S3 C; Colón-Ramos et al., 2007). These data suggest that local UNC-40 activity mediates presynaptic assembly.
To determine which domain of UNC-40 is required for synaptic vesicle clustering, we conducted structure-function studies. We generated “UNC-40 ectodomain::mCh”, a transgene containing the intact transmembrane and ectodomains of UNC-40 with mCherry in lieu of the cytoplasmic domain (Fig. S3 A). We expressed this transgene cell-specifically in AIY and observed UNC-40 ectodomain::mCh enrichment in Zones 2 and 3, regions where presynaptic sites assemble (Fig. 1 G). The observed subcellular localization for the UNC-40 ectodomain is indistinguishable from that of full-length UNC-40 (Fig. 1 F). In addition, as observed for the full-length receptor, the localization of the ectodomain to Zones 2 and 3 is dependent on its ligand, UNC-6 (Fig. S3, C and G). These data suggest that the ectodomain is sufficient for UNC-40 localization to presynaptic sites in response to UNC-6.
To determine if the ectodomain is necessary for localization, we generated “UNC-40 endodomain::mCh” and expressed it cell-specifically in AIY. This transgene consists of the intact transmembrane and endodomains of UNC-40 fused to mCherry (Fig. S3 A). Consistent with the importance of the ectodomain in UNC-40 localization, we observed that the UNC-40 endodomain was not enriched in presynaptic regions. Instead, it displayed a diffuse localization pattern throughout the entire AIY neurite (Fig. 1 H). Overexpression of the endodomain in AIY did not alter AIY synaptic vesicle patterning (Fig. S3 H). Together, our data indicate that the UNC-40 ectodomain is both necessary and sufficient to instruct the localization of UNC-40 to presynaptic regions.
Given the capacity of the ectodomain to recapitulate the localization of the full-length receptor, we tested whether it was sufficient to rescue the synaptic vesicle clustering phenotype observed in unc-40(e271) mutants in AIY. To achieve this, we expressed the UNC-40 ectodomain under the endogenous unc-40 promoter in unc-40(e271) mutant animals. We then visualized the presynaptic specializations in Zone 2 and Zone 3 of AIY by cell-specifically expressing the presynaptic vesicle marker GFP::RAB-3. As previously reported, unc-40(e271) mutant animals display a highly penetrant presynaptic patterning defect in AIY (Fig. 1, C, E, and M; Colón-Ramos et al., 2007). Expression of full-length UNC-40 rescues the observed presynaptic patterning defect (Fig. 1, I, K, and M; Colón-Ramos et al., 2007). In contrast, we observed that expression of the UNC-40 ectodomain alone does not rescue the synaptic vesicle clustering defect in unc-40(e271) mutant animals (Fig. 1, J, L, and M). These data indicate that: (1) the ectodomain localizes to presynaptic sites in response to Netrin, but is not sufficient for rescue; and (2) the cytoplasmic domain of UNC-40 is required for instructing synaptic vesicle clustering in AIY, but is not sufficient for rescue.
The Rac-GEF CED-5 mediates synaptic vesicle clustering downstream of UNC-40
UNC-40 instructs synaptic vesicle clustering via its cytoplasmic domain, suggesting that molecules known to associate with this domain may be required for synaptic vesicle clustering. Genetic studies in C. elegans and biochemical studies in vertebrates show that Rac GTPases are activated in response to Netrin signaling via the UNC-40 cytoplasmic domain (Li et al., 2002a,b; Shekarabi and Kennedy, 2002; Gitai et al., 2003; Shekarabi et al., 2005; Quinn et al., 2008; Ziel et al., 2009). In vertebrates, Rac activation is mediated through the association of the UNC-40 homologue, DCC, with one of two GEFs, Trio (homologous to UNC-73) or DOCK180 (homologous to CED-5; Honigberg and Kenyon, 2000; Forsthoefel et al., 2005; Briançon-Marjollet et al., 2008; Li et al., 2008). We examined the genetic requirement of these two GEFs in AIY synaptic vesicle clustering. Mutation of unc-73 resulted in severe axon outgrowth defects in AIY. We observed that 97.3% of the unc-73(e936) mutants failed to extend dorsally into the nerve ring (n = 148; Fig. S4, F and H). Our observations are consistent with other studies of the role of unc-73 in AIY neurodevelopment (Altun-Gultekin et al., 2001). This phenotype for unc-73(e936) mutants is different from that observed for unc-40(e271) mutants, in which 93% of animals display correct axon guidance (Fig. S4 B; Altun-Gultekin et al., 2001; Colón-Ramos et al., 2007). The requirement of UNC-73 in axon outgrowth prevented us from assessing its role in synaptic vesicle clustering. Although we cannot discard a potential role for this GEF in synaptic vesicle clustering events, the observed outgrowth phenotype indicates that UNC-73 is involved in UNC-40–independent outgrowth events in AIY.
We next examined whether the DOCK180 homologue, CED-5, is required for correct AIY neurodevelopment. We examined three putative null alleles of ced-5 (n1812, tm1949, and tm1950) and observed that unlike unc-73(e936) animals, ced-5 mutants do not display axon guidance defects (Fig. S4, C, F, and I). We then examined synaptic vesicle markers in ced-5 mutants. Like unc-40(e271) mutants, ced-5 mutant animals displayed a highly penetrant presynaptic patterning defect as visualized using synaptic vesicle markers GFP::RAB-3 and SNB-1::YFP (Fig. 2, C and D; Fig. S1, J–L; Nonet, 1999). We found that 94.4% of ced-5(n1812) mutant animals have reduced synaptic vesicle clustering in Zone 2. Quantification of average fluorescence of GFP::RAB-3 revealed a twofold reduction as compared with wild-type animals in all synaptic regions (n = 108; Fig. 2, G and H; unpublished data). Interestingly, unlike unc-40 mutants, ced-5 mutant animals do not display guidance or postsynaptic receptor distribution defects in postsynaptic partner RIA (Fig. S5, B and D–J). Thus, our data indicate that CED-5 is required for synaptic vesicle clustering in AIY. Our data also indicate that the UNC-40–mediated guidance mechanisms in RIA are genetically separable from the UNC-40–mediated synaptic vesicle clustering mechanisms in AIY.
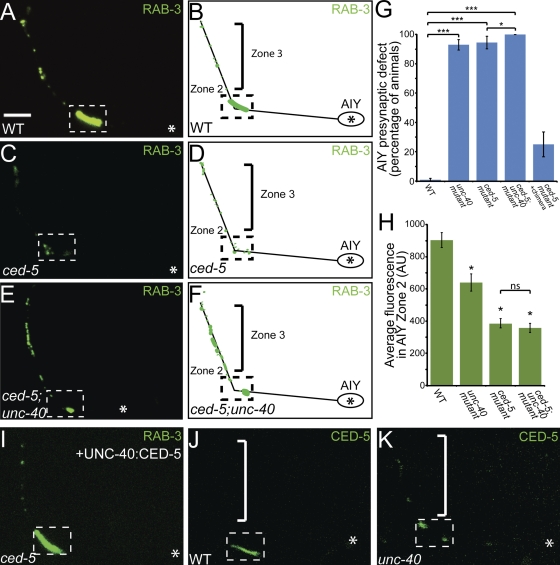
Figure 2.
The Rac GEF CED-5 is required downstream of UNC-40 for AIY synaptic vesicle clustering. (A–F) Distribution of synaptic vesicles in AIY in wild type (A and B), ced-5(n1812) (C and D), and ced-5(n1812);unc-40(e271) (E and F) mutants. Note that the distribution of synaptic vesicles is disrupted in ced-5(n1812) and ced-5(n1812);unc-40(e271) double mutants compared with wild type. (G) Quantification of the percentage of animals displaying an AIY presynaptic defect in wild type (n = 318), unc-40(e271) (n = 299), ced-5(n1812) (n = 108), ced-5(n1812);unc-40(e271) (n = 105), and ced-5(n1812) + chimeric rescue construct UNC-40::CED-5 (n = 100). ***, P < 0.001 between indicated groups by Fisher’s exact test. Error bars represent 95% confidence interval. (H) Quantification of the relative distribution of GFP::RAB-3 at the presynaptic Zone 2 (dashed box) in wild type (n = 25), unc-40(e271) (n = 37), ced-5(n1812) (n = 37), and ced-5(n1812);unc-40(e271) (n = 53) mutants (AU, arbitrary units). *, P < 0.05 between mutants and wild type by Fischer individual and Tukey simultaneous confidence intervals. Error bars represent SEM. (I) Distribution of synaptic vesicles in AIY in ced-5(n1812) mutants expressing the UNC-40:CED-5 chimera protein. Note that the synaptic vesicle clustering defect in ced-5 mutants is significantly rescued with the chimera UNC-40::CED-5 construct (compare with C). (J and K) Confocal micrographs of animals expressing CED-5::GFP cell-specifically in AIY in wild-type (J) and unc-40(e271) mutant animals (K). Note that there is decreased CED-5::GFP signal in the unc-40(e271) mutants in Zone 2 (dashed box) relative to that in wild-type animals. In all images, asterisk represents location of the cell body and dashed box encloses AIY Zone 2. Bar, 5 µm.
ced-5 mutants phenocopy unc-40 mutants, consistent with the hypothesis that these two molecules act in the same pathway to instruct synaptic vesicle clustering in AIY. To further test this hypothesis, we constructed a ced-5(n1812);unc-40(e271) double-mutant strain and visualized axon guidance and synaptic vesicle clustering in AIY. The ced-5(n1812);unc-40(e271) double mutant had enhanced guidance defects. For instance, 26.7% of the ced-5(n1812);unc-40(e271) double mutants exhibit AIY neurite truncations (n = 107). We analyzed the presynaptic pattern of ced-5(n1812);unc-40(e271) mutant animals that displayed normal guidance (Fig. 2 G). We observed that animals displaying normal guidance in the double mutants still displayed a presynaptic patterning defect that was similar to the presynaptic phenotypes seen in the single mutants; all displayed a reduction of synaptic vesicle clusters in the Zone 2 region and disorganized presynaptic sites in the Zone 3 region (Fig. 2, E and F). To further assay the phenotype, we quantified the average fluorescence in Zone 2 and observed that the reduction of synaptic vesicle clusters in the double-mutant animals is similar to that observed in ced-5(n1812) single mutants (Fig. 2 H). We also observed that the reduction of average AIY total fluorescence in unc-40(e271);ced-5(n1812) mutants was similar to that of ced-5 mutants (unpublished data).
The fact that the expressivity of the presynaptic patterning phenotype in the double mutant is qualitatively and quantitatively similar to that seen for the ced-5 single mutants is consistent with our hypothesis that CED-5 acts downstream of UNC-40 to instruct synaptic vesicle clustering in AIY. To further examine the genetic interaction of CED-5 and UNC-40 in synaptic vesicle clustering, we built transheterozygotes of these alleles. ced-5(n1812) and unc-40(e271) are recessive alleles that, in heterozygote animals, predominantly display a wild-type phenotype in AIY. However, when we generated ced-5/+;unc-40/+ transheterozygote animals, we observed that while AIY guidance was wild type, there was a significant enhancement of the percentage of animals displaying a synaptic vesicle clustering defect in AIY (Fig. 3 P). Together, our findings indicate that CED-5 and UNC-40 genetically interact to instruct synaptic vesicle clustering in AIY.
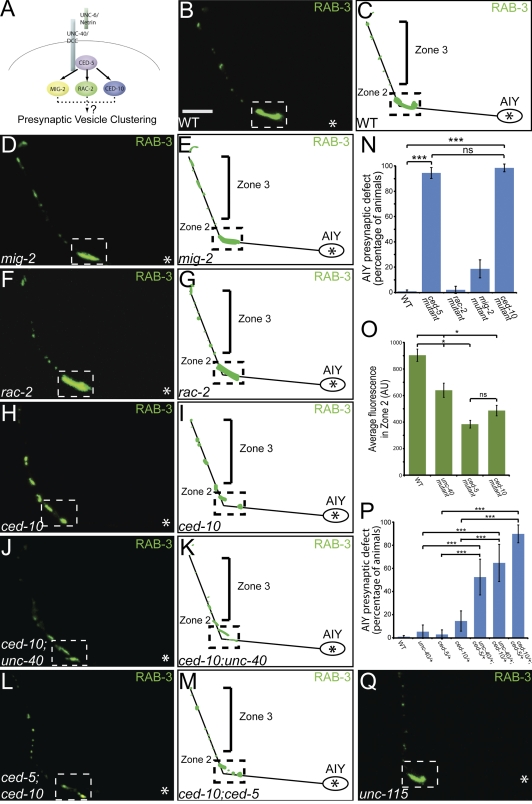
Figure 3.
The RacGTPase CED-10 is required for AIY synaptic vesicle clustering. (A) Schematic of examined Rac GTPases downstream of CED-5. (B–M) Distribution of AIY synaptic vesicles in wild type (B and C), mig-2(mu28) (D and E), rac-2(ok326) (F and G), ced-10(n3246) (H and I), ced-10(n3246);unc-40(e271) (J and K), and ced-10(n1993);ced-5(n1812) (L and M) mutants. Note that distribution of synaptic vesicles is disrupted in ced-10(n3246), ced-10(n3246);unc-40(e271), and ced-10(n1993);ced-5(n1812) mutants compared with wild type. (N) Quantification of the percentage of animals displaying a disrupted AIY presynaptic pattern in wild type (n = 318), ced-5(n1812) (n = 109), rac-2(ok326) (n = 96), mig-2(mu28) (n = 113), and ced-10(n3246) (n = 63) mutants. ***, P < 0.001 between indicated groups by Fisher’s exact test. Error bars represent 95% confidence interval. (O) Quantification of the relative distribution of GFP::RAB-3 at the presynaptic Zone 2 in wild type (n = 25), unc-40 (n = 37), ced-5 (n = 37), and ced-10 (n = 39) mutants (AU, arbitrary units). *, P < 0.05 between indicated groups by Fischer individual confidence intervals. Error bars represent SEM. (P) Transheterozygote analysis. Quantification of the percentage of animals displaying a disrupted presynaptic pattern in AIY in wild type (n = 318), unc-40(e271)/+ heterozygotes (n = 57), ced-5(n1812)/+ heterozygotes (n = 69), ced-10(n3246)/+ heterozygotes (n = 62), unc-40(e271)/+; ced-5(n1812)/+ transheterozygotes (n = 40), unc-40(e271)/+;ced-10(n3246)/+ transheterozygotes (n = 34), and ced-5(n1812)/+;ced-10(n3246)/+ transheterozygotes (n = 59). Note that a significant percentage of transheterozygote animals display synaptic vesicle clustering defects in AIY as compared with wild-type or heterozygote animals. ***, P < 0.0001 between indicated groups by Fisher’s exact test. Error bars represent 95% confidence interval. (Q) Distribution of synaptic vesicles in unc-115(ky275) mutants. Note that the presynaptic pattern resembles that of wild-type animals (B). In all images, asterisk represents the location of the cell body; dashed box encloses Zone 2. Bar, 5 µm.
We had previously shown that local signaling by Netrin induces UNC-40 clustering at presynaptic regions and results in presynaptic assembly (Colón-Ramos et al., 2007). The source of Netrin in this region is a neuroglial cell (Wadsworth et al., 1996). Altering the position or morphology of these neuroglia affects UNC-40 localization and presynaptic assembly in AIY (Colón-Ramos et al., 2007). To understand how CED-5 interacts with UNC-40 in synaptic vesicle clustering, we first investigated whether the reported mutant backgrounds altered the morphology or position of the UNC-6–secreting neuroglia. When we visualized the UNC-6–secreting cell in the examined mutant backgrounds, we did not observe a change in the position or morphology of the neuroglia with respect to AIY, suggesting that the observed effects in synaptic vesicle clustering were not due to disruption of the morphology of the UNC-6–secreting cell (Fig. S4, A–E).
To understand the synaptic role of CED-5, we then examined its subcellular localization in AIY. To achieve this, we generated transgenes expressing CED-5::GFP cell-specifically in AIY. We observed that in wild-type animals, CED-5 is enriched in presynaptic Zone 2 (Fig. 2 J). We then examined if CED-5 enrichment in Zone 2 was dependent on UNC-40. Interestingly, we observed a decrease of CED-5::GFP localization to presynaptic regions in unc-40(e271) mutants, indicating that UNC-40 is required for CED-5 clustering in Zone 2 (Fig. 2 K). We did not detect any disruption in UNC-40::GFP localization in ced-5(n1812) mutants (Fig. S3 D). These cell biological data are consistent with our genetic analysis and further suggest that CED-5 acts downstream of UNC-40 in synaptic vesicle clustering.
In vertebrates, the CED-5 homologue, DOCK180, directly interacts with the endodomain of the UNC-40 homologue, DCC, to direct guidance (Li et al., 2008). Based on our genetic and cell biological data, we hypothesized that CED-5 directly interacts with the UNC-40 endodomain in AIY to instruct synaptic vesicle clustering. To test this hypothesis in vivo, we generated an UNC-40::CED-5 chimera, in which the CED-5 cDNA was fused in-frame with the full-length UNC-40 receptor at the C terminus. To determine if the direct interaction between UNC-40 and CED-5 was sufficient for instructing synaptic vesicle clustering, we examined the ability of this chimeric protein to rescue the ced-5(n1812) mutant phenotype in AIY. Indeed, we observed that expression of the chimeric transgene significantly rescued the AIY presynaptic patterning defect in ced-5(n1812) mutants (Fig. 2, G and I). Together, our data indicate that CED-5 localizes to presynaptic sites in response to UNC-40, where it instructs synaptic vesicle clustering.
CED-10 is required for synaptic vesicle clustering in AIY
In both vertebrates and invertebrates, the major targets of CED-5 are Rac GTPases (Kiyokawa et al., 1998; Nolan et al., 1998; Reddien and Horvitz, 2000; Lundquist et al., 2001). Therefore, we hypothesized that UNC-40 and CED-5 might regulate synaptic vesicle clustering through Rac GTPases. C. elegans has three Rac genes that interact with the CED-5 GEF during development to control neuronal cell migration and axon pathfinding: ced-10, mig-2, and rac-2 (Fig. 3 A; Lundquist et al., 2001). In AIY, mig-2(mu28) and rac-2(ok326) mutant animals did not display cell migration or axon guidance defects (unpublished data). ced-10(n3246) mutants exhibited mildly penetrant outgrowth defects (16.2% of mutant animals had AIY truncations; n = 148), but in most animals, cell migration and axon guidance were wild type (Fig. S4, D, F, and J).
To examine the role of these Racs in AIY synaptic vesicle clustering, we visualized GFP::RAB-3 in loss-of-function mutants for each of these three Racs. mig-2(mu28) and rac-2(ok326) mutants did not display major defects in synaptic vesicle clustering (Fig. 3, D–G and N). However, ced-10(n3246) loss-of-function mutants displayed a highly penetrant presynaptic patterning defect as visualized using synaptic vesicle markers GFP::RAB-3, SNB-1::YFP, and SNG-1::YFP (98.4% of ced-10(n3246) animals, n = 63; Fig. 3, H, I, and N; Fig. S1, M–O; Fig. S2, J–L; Nonet, 1999; Zhao and Nonet, 2001). A weaker loss-of-function ced-10 allele, ced-10(n1993), exhibited a similar phenotype (unpublished data). Our data suggest that MIG-2 and RAC-2 are not required for AIY development, but that CED-10 is required for synaptic vesicle clustering in AIY.
We then examined if CED-10 acts downstream of CED-5 and UNC-40 in synaptic vesicle clustering by assaying the presynaptic patterning defect in ced-10(n1993);ced-5(n1812) and ced-10(n3246);unc-40(e271) double mutants. Consistent with CED-10 acting in the same pathway as UNC-40 and CED-5, we observed that ced-10(n1993);ced-5(n1812) and ced-10(n3246);unc-40(e271) double mutants displayed phenotypes that were qualitatively similar to that seen for the ced-10(n3246) single mutants (Fig. 3, J–M).
To further examine the potential genetic interaction between UNC-40, CED-5, and CED-10 in synaptic vesicle clustering, we built transheterozygotes of these genes. ced-10(n3246) is a recessive allele and heterozygote animals predominantly display a wild-type phenotype. When we generated ced-10/+;unc-40/+ and ced-10/+;ced-5/+ transheterozygote animals, we observed a significant enhancement of the percentage of animals displaying synaptic vesicle clustering defects in AIY (Fig. 3 P). Together, our findings indicate that UNC-40, CED-5, and CED-10 interact to instruct synaptic vesicle clustering in AIY.
MIG-10 acts cell-autonomously in AIY to mediate synaptic vesicle clustering downstream of UNC-40
CED-10 acts through a number of effectors that regulate the actin cytoskeleton (Quinn and Wadsworth, 2008). One of them, UNC-115 (homologous to abLIM), interacts with Racs in axon pathfinding (Lundquist et al., 1998; Struckhoff and Lundquist, 2003). However, unc-115(ky275) mutants did not display major defects in synaptic vesicle clustering (Fig. 3 Q).
Another effector, MIG-10 (homologous to RIAM and Lamellipodin), is an evolutionarily conserved molecule that regulates actin polymerization and can directly bind to CED-10 (Fig. 4 A; Quinn et al., 2008). Interestingly, MIG-10 has been shown to asymmetrically localize in response to Netrin to mediate directional axon outgrowth and cell migration (Manser and Wood, 1990; Manser et al., 1997; Adler et al., 2006; Chang et al., 2006; Quinn et al., 2008).
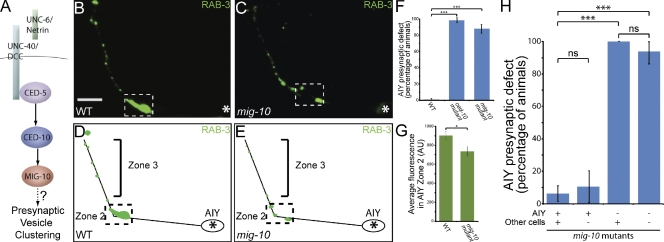
Figure 4.
MIG-10 is cell-autonomously required in AIY for synaptic vesicle clustering in Zone 2. (A) Schematic of the pathway downstream of UNC-40. (B–E) Distribution of synaptic vesicles in AIY in wild type (B and D) and mig-10(ct41) (C and E) mutants. Note that the distribution of synaptic vesicles is disrupted in mig-10(ct41) mutants. Bar (B and C) 5 µm. (F) Quantification of the percentage of animals displaying a disrupted presynaptic pattern in AIY in wild type (n = 318), ced-10(n3246) (n = 63) and mig-10(ct41) (n = 141) mutants. ***, P < 0.001 between indicated groups by Fisher’s exact test. Error bars represent 95% confidence interval. (G) Quantification of the relative distribution of GFP::RAB-3 at the presynaptic Zone 2 (dashed box) in wild type (n = 25) and mig-10(ct41) (n = 37) mutants (AU, arbitrary units). *, P < 0.05 between indicated groups by unpaired t test. Error bars represent SEM. (H) mig-10(ct41) mutant animals expressing an unstable transgene containing a mig-10 rescuing fragment and cytoplasmic cell-specific markers in AIY and RIA were scored for retention of the transgene and rescue of the AIY presynaptic phenotype. Note that retention of the rescuing transgene in AIY, but not other cells (as determined by retention in RIA), results in rescue of the presynaptic defect. ***, P < 0.001 between indicated groups by Fisher’s exact test. Error bars represent 95% confidence interval. In all images, asterisk represents the location of the cell body; dashed box encloses Zone 2.
We did not detect significant guidance or cell migration defects in mig-10(ct41) mutants in AIY (Fig. S4, E, F, and K). Instead, mig-10(ct41) mutants displayed a highly penetrant presynaptic patterning defect in AIY Zone 2 as visualized using synaptic vesicle markers GFP::RAB-3, SNB-1::YFP and SNG-1::YFP (87.9% of animals, n = 141; Fig. 4, C, E, and F; Fig. S1, P–R; Fig. S2, M–O). Additionally, quantification of the average GFP::RAB-3 fluorescence revealed that there was a statistically significant reduction of synaptic vesicle clusters when compared with wild-type animals (Fig. 4 G; unpublished data). Together, our data are consistent with a model in which UNC-40 instructs synaptic vesicle clustering in AIY through CED-5, CED-10, and MIG-10.
Because UNC-40 acts cell-autonomously in AIY to instruct presynaptic assembly (Colón-Ramos et al., 2007), we examined whether MIG-10 acts cell-autonomously in AIY to instruct synaptic vesicle clustering. To achieve this, we used a fosmid containing the mig-10 gene. Expression of this rescuing fosmid in mig-10(ct41) mutant animals resulted in robust rescue of the AIY synaptic vesicle clustering defect (Fig. 4 H). To examine where MIG-10 functions to direct AIY synaptic vesicle clustering, we took advantage of the mitotic instability of the transgene arrays and analyzed mig-10(ct41) mosaic animals retaining the rescuing fosmid array in subsets of cells (Fig. 4 H; Yochem and Herman, 2003). We observed that mosaic animals retaining the array in AIY were rescued for the AIY presynaptic defect, whereas animals that did not retain the array in AIY, but retained it in other cells, such as postsynaptic partner RIA, did not show any detectable rescue of the AIY presynaptic defect (Fig. 4 H). Consistent with this result, we also observed that MIG-10 is expressed in AIY (unpublished data). Additionally, similar to ced-5 mutants, we did not observe guidance or postsynaptic patterning defects in the postsynaptic partner, RIA, in mig-10(ct41) mutants (Fig. S5, B, G, and L). Thus, our data indicate that mig-10 acts cell-autonomously in AIY to instruct synaptic vesicle clustering.
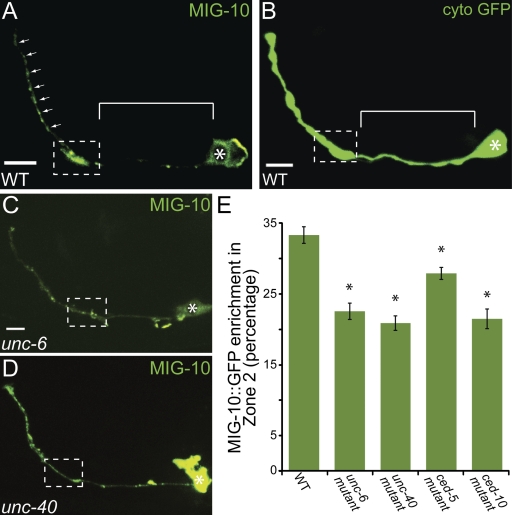
To better understand how local signaling by Netrin results in synaptic vesicle clustering, we examined the subcellular localization of MIG-10 by generating a transgene expressing MIG-10 cDNA cell-specifically in AIY. We observed that MIG-10::GFP is enriched in presynaptic regions (Fig. 5 A). This localization is different from that seen for cytoplasmic expression of GFP (Fig. 5 B), but is reminiscent of CED-5::GFP localization (Fig. 2 J). Interestingly, MIG-10::GFP has previously been shown to localize asymmetrically during axon outgrowth in other neurons in response to Netrin (Adler et al., 2006; Quinn et al., 2008).
Figure 5.
MIG-10 localizes to presynaptic regions in response to Netrin. (A) Confocal micrograph showing MIG-10::GFP subcellular localization cell-specifically in AIY. Bar, 5 µm. (B) Confocal micrograph showing cytoplasmic GFP expressed cell-specifically in AIY. Note that cytoplasmic signal is diffuse throughout the neurite, including Zone 1 (B, bracket), whereas MIG-10 is enriched in presynaptic regions (A, dashed box and arrows). Bar, 5 µm. (C and D) Confocal micrographs showing MIG-10::GFP subcellular localization expressed cell-specifically in AIY in unc-6(ev400) (C) and unc-40(e271) (D) mutant animals. Note that MIG-10::GFP is less enriched in Zone 2 in the two mutant backgrounds, as compared with wild-type animals (A). Bar (C and D), 5 µm. (E) Quantification of the relative MIG-10::GFP distribution in AIY presynaptic Zone 2 region in wild type (n = 49), unc-6(ev400) (n = 31), unc-40(e271) (n = 18), ced-5(n1812) (n = 43), and ced-10(n3246) (n = 17) mutant animals. *, P < 0.05 between mutants and wild type by Fischer Individual and Tukey simultaneous confidence intervals. Error bars represent SEM. Dashed box encloses AIY Zone 2 and asterisk represents location of the cell body.
We next tested whether MIG-10 localization to presynaptic regions was dependent on UNC-6 and UNC-40 by visualizing MIG-10::GFP in unc-6 (ev400) and unc-40(e271) mutant backgrounds (Fig. 5, C and D). We observed a statistically significant decrease of MIG-10::GFP enrichment in presynaptic regions in unc-6(ev400) and unc-40(e271) mutants, suggesting that UNC-6 and UNC-40 are required for MIG-10 localization to presynaptic regions (Fig. 5 E). Conversely, there was no noticeable disruption in UNC-40::GFP localization in mig-10(ct41) mutants (Fig. S3 F). We also observed a statistically significant decrease of MIG-10::GFP enrichment in presynaptic regions in ced-5(n1812) and ced-10(n3246) mutant animals (Fig. 5 E). These cell biological data are consistent with our genetic analyses and reaffirm that MIG-10 acts downstream of UNC-6, UNC-40, CED-5, and CED-10 in synaptic vesicle clustering.
CED-5 and MIG-10 are not required for the localization of active zone proteins SYD-1 and SYD-2
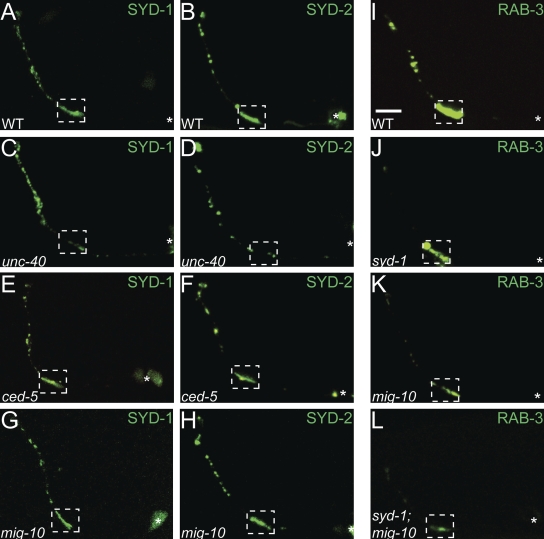
UNC-40 is required for presynaptic assembly in the AIY interneurons, and unc-40 mutant animals display a significant reduction of both synaptic vesicle clusters and active zone proteins at presynaptic sites (Fig. S1, G–I; Fig. S2, G–1; Fig. 6, A–D; Colón-Ramos et al., 2007). To determine if the examined downstream pathway was also required for the localization of active zone proteins to presynaptic sites, we visualized the subcellular localization of SYD-1::GFP and SYD-2::GFP (Fig. 6, A–H). SYD-1 and SYD-2 are evolutionarily conserved proteins that localize to active zones and are required for active zone assembly in a number of neurons, including AIY (Fig. 6, A, B, and J; unpublished data; Zhen and Jin, 1999; Hallam et al., 2002; Yeh et al., 2005; Dai et al., 2006; Patel et al., 2006; Jin and Garner, 2008). Interestingly, although UNC-40 is required for the subcellular localization of SYD-1::GFP and SYD-2::GFP to presynaptic sites, we could not detect defects in the subcellular localization of these active zone proteins in ced-5(n1812) or mig-10(ct41) mutants (Fig. 6, A–H). In addition, we observed that although syd-1(ju82) and mig-10(ct41) single mutants both display highly penetrant synaptic vesicle clustering defects, the expressivity of the defects is qualitatively different. When we created a syd-1(ju82); mig-10(ct41) double mutant, we observed an enhancement of the vesicle clustering defects as compared with the single mutants (Fig. 6, J–L). Together, our data indicate the existence of a parallel pathway downstream of UNC-40 required for localization of active zone proteins to presynaptic sites. Our data also suggest that the mechanisms that instruct active zone formation and synaptic vesicle clustering downstream of UNC-40 are genetically separable.
Figure 6.
CED-5 and MIG-10 are not required for the presynaptic localization of active zone proteins. (A–H) Localization of active zone proteins, SYD-1::GFP (A, C, E, and G) and SYD-2::GFP (B, D, F, and H) in wild type (A and B), unc-40(e271) (C and D), ced-5(n1812) (E and F), and mig-10(ct41) (G and H) mutant animals. Note that in unc-40 mutants SYD-1::GFP and SYD-2::GFP display disrupted localization in presynaptic zones compared with wild type. However, mig-10 and ced-5 mutants do not display altered localization of these active zone proteins as compared with wild type. (I–L) Distribution of synaptic vesicle clusters (visualized with GFP:RAB-3) in wild type (I), syd-1(ju82) (J), mig-10(ct41) (K), and syd-1(ju82);mig-10(ct41) (L) mutants. Although both syd-1 and mig-10 mutants display a high penetrance defect in synaptic vesicle distribution, syd-1;mig-10 double mutants display an enhanced expressivity of the mutant phenotype. In all images, the dashed box encloses Zone 2 and asterisk represents location of the cell body. Bar, 5 µm.
Netrin instructs F-actin accumulation at presynaptic sites
Our data indicate that Netrin instructs synaptic vesicle clustering through a Rac GTPase and MIG-10. These molecules have the shared capacity to induce local changes in cytoskeletal dynamics by regulating actin polymerization (de Curtis, 2008; Quinn and Wadsworth, 2008; Quinn et al., 2008). A number of studies have shown that disruption of F-actin specifically during synaptogenesis results in fewer presynaptic assemblies, indicating a role for F-actin during synaptogenesis (Colicos et al., 2001; Zhang and Benson, 2001, 2002; Sankaranarayanan et al., 2003; Lucido et al., 2009). Therefore, we hypothesized that CED-5, CED-10, and MIG-10 are required downstream of Netrin to organize the actin cytoskeleton in presynaptic regions (Fig. 7 A).
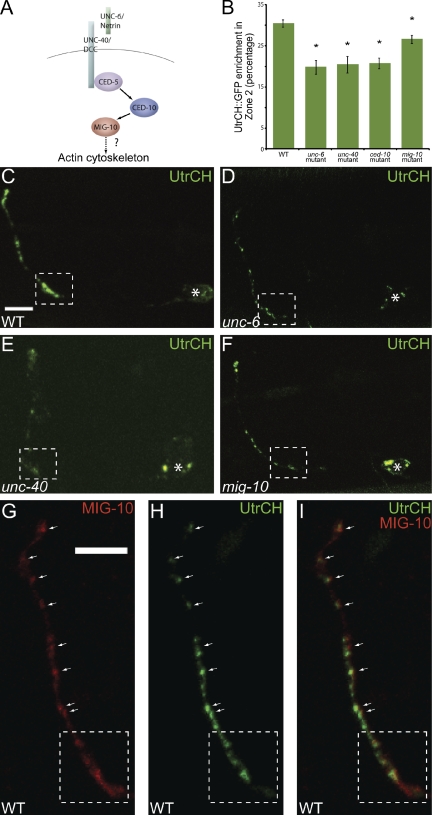
Figure 7.
MIG-10 is required for F-actin organization in presynaptic regions. (A) Schematic of the examined pathway. (B) Quantification of the distribution of F-actin, as visualized by the UtrCH::GFP probe, in AIY Zone 2 region in wild type (n = 66), unc-6(ev400) (n = 11), unc-40(e271) (n = 17), ced-10(n3246) (n = 25), and mig-10(ct41) (n = 45) mutants. *, P < 0.05 between mutants and wild type by Fischer individual and Tukey simultaneous confidence intervals. Error bars represent SEM. (C–F) Confocal micrographs of animals expressing the F-actin probe, UtrCH::GFP, cell-specifically in AIY in wild type (C), unc-6(ev400) (D), unc-40(e271) (E), and mig-10(ct41) (F) mutant animals. Note that F-actin is disorganized in unc-6(ev400), unc-40(e271), mig-10(ct41) mutants and that there is decreased F-actin accumulation in Zone 2 compared with wild type. Bar (C–F), 5 µm. (G–I) Confocal micrographs of Zones 2 and 3 of a transgenic animal expressing MIG-10::mCh (G) and UtrCH::GFP (H) cell-specifically in AIY. Merge in panel I. Synaptic regions are expanded in these images to allow evaluation of partial colocalization of the two proteins (arrows). In all images, the dashed box encloses Zone 2 and asterisk represents location of the cell body. Bar (G–I), 5 µm.
To examine this hypothesis, we visualized F-actin in AIY by cell-specifically expressing the in vivo F-actin probe UtrCH::GFP (Burkel et al., 2007). In wild-type animals, we observed that F-actin was enriched in the presynaptic regions of AIY (n = 66; Fig. 7, B and C). We then examined the requirement of UNC-6 and its receptor, UNC-40, in presynaptic F-actin accumulation by visualizing the UtrCH::GFP marker in unc-6(ev400) and unc-40(e271) mutants (Fig. 7, D and E). We observed a statistically significant reduction of F-actin accumulation in presynaptic regions in unc-6(ev400) and unc-40(e271) mutants, consistent with a role for Netrin in regulating the organization of actin in presynaptic regions (n = 11, n = 17, respectively; Fig. 7 B).
To examine if Rac GTPases and MIG-10 were also required for F-actin accumulation at presynaptic sites, we visualized the UtrCH::GFP marker in ced-10(n3246) and mig-10(ct41) mutants (unpublished data and Fig. 7 F, respectively). Similar to unc-6(ev400) and unc-40(e271) mutants, ced-10(n3246) and mig-10(ct41) mutants displayed statistically significant reductions of F-actin accumulation at presynaptic sites (n = 25, n = 45, respectively; Fig. 7 B). These data indicate that Netrin, Rac GTPases, and MIG-10 are required for F-actin accumulation in presynaptic regions. In addition, we observed that MIG-10 partially colocalized with F-actin at presynaptic regions (Fig. 7, G–I). Therefore, our data indicate that CED-10 and MIG-10 are required downstream of Netrin to organize the actin cytoskeleton and instruct synaptic vesicle clustering in AIY presynaptic regions.
Discussion
Studies in C. elegans, Drosophila, and vertebrates have demonstrated that Netrin plays a conserved role in instructing synapse formation (Winberg et al., 1998; Colón-Ramos et al., 2007; Poon et al., 2008; Manitt et al., 2009; Xu et al., 2010; Park et al., 2011). How Netrin instructs synapse formation and how these mechanisms relate to Netrin’s canonical guidance role are not understood. Here we identify the molecular mechanisms by which Netrin instructs synaptic vesicle clustering in the C. elegans AIY interneuron. Our data indicate that Netrin instructs synaptic vesicle clustering through the GEF CED-5, the GTPase CED-10, and cytoplasmic adapter molecule MIG-10. These three molecules are required for Netrin-mediated cell migration and axon guidance in other neurons (Manser and Wood, 1990; Lundquist et al., 2001; Gitai et al., 2003; Adler et al., 2006; Chang et al., 2006; Quinn et al., 2008). In AIY, these molecules are instead required for synaptic vesicle clustering downstream of Netrin. Although our cell biological study was focused on the AIY interneuron, the identified pathways are well conserved throughout evolution. Therefore, we hypothesize the mechanisms reported here will also instruct vesicle clustering in other cells and organisms where Netrin and DCC direct synapse formation.
Interestingly, we find that these molecules are dispensable for instructing the localization of active zone proteins, which suggests a genetic separation of these signaling pathways downstream of UNC-40. Our data are consistent with observations from other genetic studies. For instance, a genetic study in C. elegans GABAergic neurons resulted in the identification of mutants required for the localization of active zone protein SYD-2, but not for the localization of synaptic vesicle protein SNB-1 (Yeh et al., 2005). Moreover, in Drosophila, ghost boutons, which are not in direct contact with postsynaptic partners, lack active zone proteins yet contain synaptic vesicles (Ataman et al., 2006). Therefore, in some developmental contexts, including AIY, active zone assembly and synaptic vesicle localization are genetically separable events. It should be noted, however, that we do observe that active zone proteins are required for the clustering of synaptic vesicles in AIY. Double-mutant analyses between syd-1(ju82);mig-10(ct41) suggest that these two molecules act in parallel in vesicle clustering. Together, our findings indicate that a single receptor, UNC-40, can instruct vesicle clustering by simultaneously activating a Rac GTPase pathway and instructing the localization of active zone proteins.
We show that synaptic vesicle clustering at the synaptogenic site results from the asymmetric localization of a signaling module in response to Netrin. We find that the asymmetric organization of the signaling molecules starts with the localization of the Netrin receptor UNC-40. Glia-derived Netrin modulates the asymmetric accumulation of UNC-40 through an interaction with the UNC-40 ectodomain. UNC-40 then directs synaptic vesicle clustering at the Netrin-specified coordinate through the UNC-40 intracellular domain. We found that CED-5 localizes to presynaptic sites in response to Netrin and is required for synaptic vesicle clustering in AIY. Our data also suggest that CED-5 signals through the Rac GTPase CED-10 to instruct synaptic vesicle clustering. In many disparate neurodevelopmental events, Racs modulate the asymmetric reorganization of the actin cytoskeleton in response to extracellular cues (de Curtis, 2008; Miyamoto and Yamauchi, 2010). In synapse formation, the role of Racs is best understood at postsynaptic spines (de Curtis, 2008). We now find that at presynaptic sites, Racs can also instruct asymmetric organization of the actin cytoskeleton and direct synaptogenesis in response to Netrin.
The pathway we report here is required for the correct organization of the actin cytoskeleton in response to Netrin. Thus, Netrin-mediated cytoskeletal reorganization underlies both axon guidance and synaptogenesis. Surprisingly little is known regarding the role of actin during presynaptic assembly (Dillon and Goda, 2005; Cingolani and Goda, 2008; Geraldo and Gordon-Weeks, 2009). Tissue culture studies have demonstrated that F-actin is essential for the early development of presynaptic sites (Zhang and Benson, 2001; Dillon and Goda, 2005; Cingolani and Goda, 2008). Given our data and these findings, we propose that Netrin instructs synaptic vesicle clustering by organizing F-actin in presynaptic regions. Therefore, a signaling module used in other cells during axon guidance is synaptically localized in AIY to instruct synaptic vesicle clustering.
The development of the nervous system is orchestrated with a limited number of molecular cues. As we learn more about these molecular factors, we are discovering that distinct neurodevelopmental events share common signaling molecules. Our findings indicate that Netrin activates similar pathways while instructing guidance and synaptic vesicle clustering. We find that while directing synaptic vesicle clustering, UNC-40 and its downstream signaling module are subcellularly localized to presynaptic regions of AIY. Our work provides evidence that in vivo neurodevelopmental outcomes may be differentially instructed between cells, not only through novel intracellular signaling pathways, but also through the directed asymmetric localization of known signaling complexes.
Materials and methods
Strains and genetics
Worms were raised on NGM plates at 20°C using OP50 Escherichia coli as a food source. N2 Bristol was used as the wild-type reference strain. The following mutant strains were obtained through the Caenorhabditis Genetics Center: ced-10(n3246)IV, unc-40(e271)I, unc-6(ev400)X, mig-10(ct41)III, ced-5(n1812)IV, unc-73((e936)I, mig-2(mu28)X, rac-2(ok326)IV, ced-10(n1993)IV, syd-1(ju82)II, and unc-115(ky275)X. The alleles ced-5(tm1949)IV and ced-5(tm1950) were kindly provided by the Mitani laboratory of the Tokyo Women’s Medical University School of Medicine (Tokyo, Japan). The ced-5(n1812);ced-10(n1993) parent strain for the double mutant was kindly provided by the Horvitz laboratory of the Massachusetts Institute of Technology (Cambridge, MA).
The unc-40(e271) mutation is a null allele. The nucleotide polymorphism is c7968t and results in a R824* (Arg to opal stop) in the ectodomain (Roy, P., personal communication).
Molecular biology and transgenic lines
Expression clones were made in the pSM vector (Shen and Bargmann, 2003). The plasmids and transgenic strains (0.5–30 ng/µl) were generated using standard techniques and coinjected with markers Punc122::gfp or Punc122::dsRed (15–30 ng/µl): wyIs45 [Pttx3::gfp::rab3], wyEx1149 [Pttx3::unc40::gfp], wyEx523 [Pttx3::unc40 ectodomain::mch], wyEx540 [Pttx3::unc40 endodomain::mch], wyEx1697 [Punc40::unc40 ectodomain], wyEx650 [Punc40::unc40], olaEx586 [Pttx3::gfp, Phlh17::mch], olaEx738 [Pttx3::gfp, Phlh17::mch], olaEx745 [Punc40::unc40minigene::ced5], olaEx581 [Pttx3::ced5::gfp], olaEx135 [Pttx3::rfp, Pglr3::rfp, Fosmid WRM 066dB08], olaEx324 [Pttx3::mig10b::gfp], olaEx294 [Pttx3::UtrCH::gfp], olaEx410 [Pttx3::mig10b::mCh, Pttx3::UtrCh::gfp], olaEx602 [Pmig10b::gfp, Pttx3::rfp], olaEx866 [Pttx3::snb-1::yfp, Pttx3::mCh::rab3], olaEx915 [Pttx3::sng1::yfp, Pttx3::mCh::rab3], wyIs93 [Pglr3::mCh::rab3, Pglr3::glr1::gfp], wyEx1826 [Pttx3::syd2::gfp], and olaEx297 [Pttx3::syd1::gfp].
Detailed subcloning information will be provided upon request. MIG-10B cDNA was isolated by PCR from cDNA collected from a mixed stage population. CED-5, MIG-2, and RAC-2 cDNA were obtained by the same methods. The UNC-40:CED-5 chimera fusion protein was constructed with CED-5 cDNA fused in-frame to the C terminus of the UNC-40 minigene (Colón-Ramos et al., 2007), driven by the endogenous UNC-40 promoter.
Fluorescence microscopy and confocal imaging
Images of fluorescently tagged fusion proteins (eGFP, GFP, YFP, and mCherry with excitation wavelengths of 488 and 561 nm) were captured at room temperature in live C. elegans using a 60x CFI Plan Apochromat VC, NA 1.4, oil objective (Nikon) on an UltraView VoX spinning-disc confocal microscope (PerkinElmer) on a stand (Ti-E; Nikon) with an EM-CCD camera (model C9100-50; Hamamatsu Photonics). Images were acquired and processed with Volocity software (PerkinElmer). All images are maximal projections (“Extended Focus” in Volocity). Final processing of images, including rotation and cropping, was performed with Adobe Photoshop CS4 (Adobe Systems, Inc.). Any quantifications were performed on maximal projections of raw data. Worms were immobilized using 10 mM levamisole (Sigma-Aldrich) and oriented anterior to the left and dorsal up.
Mosaic analysis
Mosaic analysis was conducted on mig-10(ct41) animals by expressing unstable transgenes with a mig-10 rescuing construct and cytoplasmic markers in RIA and AIY (olaEx135). Animals were inspected for retention of the transgene and rescue of AIY presynaptic patterning using a microscope (model DM5000 B; Leica). We conducted mosaic analysis for AIY presynaptic patterning by scoring mig-10(ct41) animals for rescue of synaptic vesicle distribution in AIY, as defined by an enrichment of synaptic vesicles in Zone 2 and discrete synaptic vesicle clusters in Zone 3. Array retention was tracked in AIY and RIA with cytoplasmic RFP (Yochem and Herman, 2003; Colón-Ramos et al., 2007; Herman, 2007).
Quantification
Quantification of the axon guidance or cell migration phenotype in AIY was conducted as described previously (Colón-Ramos et al., 2007). In brief, 3D reconstructions of confocal micrographs were closely examined and scored for the cell migration of the AIY cell body to the retrovesicular ganglion and the three navigational decisions AIY makes as it traverses the ventral nerve cord and the nerve ring: (1) anterior projection through the ventral nerve cord, (2) dorsal turn at the nerve ring, and (3) circumnavigation of the nerve ring until the AIY neurite contacts the sister AIY at the dorsal midline. Significance was determined using a two-tailed Fisher’s exact test.
Quantification of the distribution of GFP::RAB-3 in AIY was completed by measuring average fluorescence intensity over the Zone 2 region in confocal micrographs. Fluorescence intensity was measured using the line scan function of ImageJ (National Institutes of Health, Bethesda, MD) and tracing the AIY process. To measure the average fluorescence intensity in Zone 2, Zone 2 was morphologically defined in adult animals as the region of the AIY process which encompasses the dorsal turn as the process exits the anterior ventral nerve cord and enters the nerve ring (∼5 µm; boxed in all figures; Colón-Ramos et al., 2007). This anatomical definition of Zone 2 was informed by the reconstructions of EM micrographs in White et al. (1986). In wild-type animals AIY forms synapses with RIA only in the Zone 2 region, as determined by reconstructions of EM micrographs. In addition, in wild-type animals the AIY axon width is roughly four times larger in Zone 2 than in Zone 3 (White et al., 1986; Colón-Ramos et al., 2007). Total fluorescence intensity in this region was divided by the exact length of the measured Zone 2, yielding the average fluorescence intensity over the Zone 2 region. To determine average total AIY fluorescence intensity of GFP::RAB-3, Zone 2 average fluorescence was added to Zone 3 average fluorescence. To determine the average fluorescence intensity of GFP::RAB-3 in Zone 3, Zone 3 was morphologically defined as the region of the AIY neurite immediately dorsal to the Zone 2 region extending to the end of the dorsal midline. Total fluorescence intensity in this region was divided by the exact length of the measured Zone 3, yielding the average fluorescence intensity over Zone 3.
To quantify the enrichment of MIG-10::GFP and UtrCH::GFP in AIY, we first identified extrachromosomal transgenic lines that displayed stable expression of MIG-10::GFP or UtrCH::GFP (olaEx324 and olaEx294, respectively). To ensure consistency across experiments, the same extrachromosomal line for each construct was then crossed into the specified mutant backgrounds. Quantification was completed by using the line scan function of ImageJ and tracing the AIY process. Quantifications were performed blindly, without knowledge of the genotype. The fluorescence intensity in Zones 2 and 3 were determined as described above. Total fluorescence intensity of the Zone 2 region was divided by total fluorescence intensity of both Zones 2 and 3, yielding MIG-10::GFP or UtrCH::GFP enrichment in Zone 2.
Statistical analyses
Statistical significance for categorical data was determined using Fischer’s exact test. Error bars for categorical data were calculated using 95% confidence intervals. For Figs. 2 H, 3 O, 4 G, 5 E, and 7 B, statistical significance for continuous data was determined using one-way ANOVA with post-hoc analysis by Fischer individual and Tukey simultaneous confidence intervals using Minitab16 software. Error bars for continuous data were calculated using standard errors of the mean.
Online supplemental material
Fig. S1 and Fig. S2 display colocalization of mCh::RAB-3 and SNB-1::YFP or SNG-1::YFP, respectively, in relevant mutant backgrounds. Fig. S3 demonstrates that UNC-40 localization to presynaptic regions is dependent on UNC-6. Fig. S4 depicts AIY cell migration and axon guidance in relevant mutant backgrounds. Fig. S5 shows RIA cell migration, guidance, and pre- and postsynaptic localization in relevant mutant backgrounds. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201110127/DC1.
Acknowledgments
We thank D. Chao, K. Spilker, M. Margeta, W. Bement, the Caenorhabditis Genetic Center, and the Japanese National BioResource Project (NBPR) for strains and reagents. We thank Z. Altun (www.wormatlas.org) for diagrams used in the figures. We thank in particular K. Shen and J. Nelson for helpful discussions and generous sharing of advice and reagents. We also thank C. Gao and G. Chatterjee for technical assistance and M. Hammarlund, S. Margolis, C. Smith, M. Hurwitz, and members of the Colón-Ramos laboratory for thoughtful comments on the manuscript.
This work was funded by the following grants to D. Colón-Ramos: R00 NS057931 from the National Institutes of Health (NIH), a fellowship from the Klingenstein Foundation, the Alfred P. Sloan Foundation, and a March of Dimes research grant. A. Stavoe was supported by Cellular and Molecular Biology Training grant T32-GM007223 from the NIH.
Footnotes
Abbreviations used in this paper:
- DCC
- deleted in colorectal cancer
- GEF
- guanine nucleotide exchange factor
References
- Adler C.E., Fetter R.D., Bargmann C.I. 2006. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat. Neurosci. 9:511–518 10.1038/nn1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun-Gultekin Z., Andachi Y., Tsalik E.L., Pilgrim D., Kohara Y., Hobert O. 2001. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 128:1951–1969 [DOI] [PubMed] [Google Scholar]
- Ataman B., Ashley J., Gorczyca D., Gorczyca M., Mathew D., Wichmann C., Sigrist S.J., Budnik V. 2006. Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc. Natl. Acad. Sci. USA. 103:7841–7846 10.1073/pnas.0600387103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briançon-Marjollet A., Ghogha A., Nawabi H., Triki I., Auziol C., Fromont S., Piché C., Enslen H., Chebli K., Cloutier J.F., et al. 2008. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol. Cell. Biol. 28:2314–2323 10.1128/MCB.00998-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel B.M., von Dassow G., Bement W.M. 2007. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton. 64:822–832 10.1002/cm.20226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Adler C.E., Krause M., Clark S.G., Gertler F.B., Tessier-Lavigne M., Bargmann C.I. 2006. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr. Biol. 16:854–862 10.1016/j.cub.2006.03.083 [DOI] [PubMed] [Google Scholar]
- Chen S.Y., Cheng H.J. 2009. Functions of axon guidance molecules in synapse formation. Curr. Opin. Neurobiol. 19:471–478 10.1016/j.conb.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani L.A., Goda Y. 2008. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9:344–356 10.1038/nrn2373 [DOI] [PubMed] [Google Scholar]
- Colicos M.A., Collins B.E., Sailor M.J., Goda Y. 2001. Remodeling of synaptic actin induced by photoconductive stimulation. Cell. 107:605–616 10.1016/S0092-8674(01)00579-7 [DOI] [PubMed] [Google Scholar]
- Colón-Ramos D.A., Margeta M.A., Shen K. 2007. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 318:103–106 10.1126/science.1143762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Taru H., Deken S.L., Grill B., Ackley B., Nonet M.L., Jin Y.S. 2006. SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat. Neurosci. 9:1479–1487 10.1038/nn1808 [DOI] [PubMed] [Google Scholar]
- Davis E.K., Zou Y., Ghosh A. 2008. Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Dev. 3:32 10.1186/1749-8104-3-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I. 2008. Functions of Rac GTPases during neuronal development. Dev. Neurosci. 30:47–58 10.1159/000109851 [DOI] [PubMed] [Google Scholar]
- Dillon C., Goda Y. 2005. The actin cytoskeleton: integrating form and function at the synapse. Annu. Rev. Neurosci. 28:25–55 10.1146/annurev.neuro.28.061604.135757 [DOI] [PubMed] [Google Scholar]
- Forsthoefel D.J., Liebl E.C., Kolodziej P.A., Seeger M.A. 2005. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development. 132:1983–1994 10.1242/dev.01736 [DOI] [PubMed] [Google Scholar]
- Geraldo S., Gordon-Weeks P.R. 2009. Cytoskeletal dynamics in growth-cone steering. J. Cell Sci. 122:3595–3604 10.1242/jcs.042309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitai Z., Yu T.W., Lundquist E.A., Tessier-Lavigne M., Bargmann C.I. 2003. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 37:53–65 10.1016/S0896-6273(02)01149-2 [DOI] [PubMed] [Google Scholar]
- Hallam S.J., Goncharov A., McEwen J., Baran R., Jin Y. 2002. SYD-1, a presynaptic protein with PDZ, C2 and rhoGAP-like domains, specifies axon identity in C. elegans. Nat. Neurosci. 5:1137–1146 10.1038/nn959 [DOI] [PubMed] [Google Scholar]
- Hedgecock E.M., Culotti J.G., Hall D.H. 1990. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 4:61–85 10.1016/0896-6273(90)90444-K [DOI] [PubMed] [Google Scholar]
- Herman R.K. 2007. Mosaic analysis in the nematode Caenorhabditis elegans. J. Neurogenet. 21:219–242 10.1080/01677060701693446 [DOI] [PubMed] [Google Scholar]
- Honigberg L., Kenyon C. 2000. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development. 127:4655–4668 [DOI] [PubMed] [Google Scholar]
- Inaki M., Yoshikawa S., Thomas J.B., Aburatani H., Nose A. 2007. Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr. Biol. 17:1574–1579 10.1016/j.cub.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Ishii N., Wadsworth W.G., Stern B.D., Culotti J.G., Hedgecock E.M. 1992. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 9:873–881 10.1016/0896-6273(92)90240-E [DOI] [PubMed] [Google Scholar]
- Jin Y., Garner C.C. 2008. Molecular mechanisms of presynaptic differentiation. Annu. Rev. Cell Dev. Biol. 24:237–262 10.1146/annurev.cellbio.23.090506.123417 [DOI] [PubMed] [Google Scholar]
- Kiyokawa E., Hashimoto Y., Kobayashi S., Sugimura H., Kurata T., Matsuda M. 1998. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Gene Dev. 12:3331–3336 10.1101/gad.12.21.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen M.P., Shen K. 2007. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 130:704–716 10.1016/j.cell.2007.06.046 [DOI] [PubMed] [Google Scholar]
- Li X., Meriane M., Triki I., Shekarabi M., Kennedy T.E., Larose L., Lamarche-Vane N. 2002a. The adaptor protein Nck-1 couples the netrin-1 receptor DCC (deleted in colorectal cancer) to the activation of the small GTPase Rac1 through an atypical mechanism. J. Biol. Chem. 277:37788–37797 10.1074/jbc.M205428200 [DOI] [PubMed] [Google Scholar]
- Li X., Saint-Cyr-Proulx E., Aktories K., Lamarche-Vane N. 2002b. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J. Biol. Chem. 277:15207–15214 10.1074/jbc.M109913200 [DOI] [PubMed] [Google Scholar]
- Li X., Gao X., Liu G., Xiong W., Wu J., Rao Y. 2008. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat. Neurosci. 11:28–35 10.1038/nn2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey F.J. 1999. Netrins and netrin receptors. Cell. Mol. Life Sci. 56:62–68 10.1007/s000180050006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucido A.L., Suarez Sanchez F., Thostrup P., Kwiatkowski A.V., Leal-Ortiz S., Gopalakrishnan G., Liazoghli D., Belkaid W., Lennox R.B., Grutter P., et al. 2009. Rapid assembly of functional presynaptic boutons triggered by adhesive contacts. J. Neurosci. 29:12449–12466 10.1523/JNEUROSCI.1381-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist E.A., Herman R.K., Shaw J.E., Bargmann C.I. 1998. UNC-115, a conserved protein with predicted LIM and actin-binding domains, mediates axon guidance in C. elegans. Neuron. 21:385–392 10.1016/S0896-6273(00)80547-4 [DOI] [PubMed] [Google Scholar]
- Lundquist E.A., Reddien P.W., Hartwieg E., Horvitz H.R., Bargmann C.I. 2001. Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development. 128:4475–4488 [DOI] [PubMed] [Google Scholar]
- Manitt C., Nikolakopoulou A.M., Almario D.R., Nguyen S.A., Cohen-Cory S. 2009. Netrin participates in the development of retinotectal synaptic connectivity by modulating axon arborization and synapse formation in the developing brain. J. Neurosci. 29:11065–11077 10.1523/JNEUROSCI.0947-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser J., Wood W.B. 1990. Mutations affecting embryonic cell migrations in Caenorhabditis elegans. Dev. Genet. 11:49–64 10.1002/dvg.1020110107 [DOI] [PubMed] [Google Scholar]
- Manser J., Roonprapunt C., Margolis B. 1997. C. elegans cell migration gene mig-10 shares similarities with a family of SH2 domain proteins and acts cell nonautonomously in excretory canal development. Dev. Biol. 184:150–164 10.1006/dbio.1997.8516 [DOI] [PubMed] [Google Scholar]
- Matteoli M., Takei K., Cameron R., Hurlbut P., Johnston P.A., Südhof T.C., Jahn R., De Camilli P. 1991. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J. Cell Biol. 115:625–633 10.1083/jcb.115.3.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech C., Pauer H.U., He X., Schwarz T.L. 2008. Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J. Neurosci. 28:10875–10884 10.1523/JNEUROSCI.0164-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Yamauchi J. 2010. Cellular signaling of Dock family proteins in neural function. Cell. Signal. 22:175–182 10.1016/j.cellsig.2009.09.036 [DOI] [PubMed] [Google Scholar]
- Nolan K.M., Barrett K., Lu Y., Hu K.Q., Vincent S., Settleman J. 1998. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 12:3337–3342 10.1101/gad.12.21.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M.L. 1999. Visualization of synaptic specializations in live C. elegans with synaptic vesicle protein-GFP fusions. J. Neurosci. Methods. 89:33–40 10.1016/S0165-0270(99)00031-X [DOI] [PubMed] [Google Scholar]
- Park J., Knezevich P.L., Wung W., O’Hanlon S.N., Goyal A., Benedetti K.L., Barsi-Rhyne B.J., Raman M., Mock N., Bremer M., Vanhoven M.K. 2011. A conserved juxtacrine signal regulates synaptic partner recognition in Caenorhabditis elegans. Neural Dev. 6:28 10.1186/1749-8104-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M.R., Lehrman E.K., Poon V.Y., Crump J.G., Zhen M., Bargmann C.I., Shen K. 2006. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat. Neurosci. 9:1488–1498 10.1038/nn1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon V.Y., Klassen M.P., Shen K. 2008. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 455:669–673 10.1038/nature07291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C.C., Wadsworth W.G. 2008. Axon guidance: asymmetric signaling orients polarized outgrowth. Trends Cell Biol. 18:597–603 10.1016/j.tcb.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C.C., Pfeil D.S., Wadsworth W.G. 2008. CED-10/Rac1 mediates axon guidance by regulating the asymmetric distribution of MIG-10/lamellipodin. Curr. Biol. 18:808–813 10.1016/j.cub.2008.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P.W., Horvitz H.R. 2000. CED-2/Crkll and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2:131–136 10.1038/35004000 [DOI] [PubMed] [Google Scholar]
- Round J., Stein E. 2007. Netrin signaling leading to directed growth cone steering. Curr. Opin. Neurobiol. 17:15–21 10.1016/j.conb.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Salinas P.C., Zou Y. 2008. Wnt signaling in neural circuit assembly. Annu. Rev. Neurosci. 31:339–358 10.1146/annurev.neuro.31.060407.125649 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S., Atluri P.P., Ryan T.A. 2003. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci. 6:127–135 10.1038/nn1002 [DOI] [PubMed] [Google Scholar]
- Serafini T., Kennedy T.E., Galko M.J., Mirzayan C., Jessell T.M., Tessier-Lavigne M. 1994. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 78:409–424 10.1016/0092-8674(94)90420-0 [DOI] [PubMed] [Google Scholar]
- Shekarabi M., Kennedy T.E. 2002. The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell. Neurosci. 19:1–17 10.1006/mcne.2001.1075 [DOI] [PubMed] [Google Scholar]
- Shekarabi M., Moore S.W., Tritsch N.X., Morris S.J., Bouchard J.F., Kennedy T.E. 2005. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 25:3132–3141 10.1523/JNEUROSCI.1920-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K., Bargmann C.I. 2003. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 112:619–630 10.1016/S0092-8674(03)00113-2 [DOI] [PubMed] [Google Scholar]
- Shen K., Cowan C.W. 2010. Guidance molecules in synapse formation and plasticity. Cold Spring Harb. Perspect. Biol. 2:a001842 10.1101/cshperspect.a001842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struckhoff E.C., Lundquist E.A. 2003. The actin-binding protein UNC-115 is an effector of Rac signaling during axon pathfinding in C. elegans. Development. 130:693–704 10.1242/dev.00300 [DOI] [PubMed] [Google Scholar]
- Wadsworth W.G., Bhatt H., Hedgecock E.M. 1996. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 16:35–46 10.1016/S0896-6273(00)80021-5 [DOI] [PubMed] [Google Scholar]
- White J.G., Southgate E., Thomson J.N., Brenner S. 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. 314:1–340 10.1098/rstb.1986.0056 [DOI] [PubMed] [Google Scholar]
- Winberg M.L., Mitchell K.J., Goodman C.S. 1998. Genetic analysis of the mechanisms controlling target selection: complementary and combinatorial functions of netrins, semaphorins, and IgCAMs. Cell. 93:581–591 10.1016/S0092-8674(00)81187-3 [DOI] [PubMed] [Google Scholar]
- Xu B., Goldman J.S., Rymar V.V., Forget C., Lo P.S., Bull S.J., Vereker E., Barker P.A., Trudeau L.E., Sadikot A.F., Kennedy T.E. 2010. Critical roles for the netrin receptor deleted in colorectal cancer in dopaminergic neuronal precursor migration, axon guidance, and axon arborization. Neuroscience. 169:932–949 10.1016/j.neuroscience.2010.05.025 [DOI] [PubMed] [Google Scholar]
- Yeh E., Kawano T., Weimer R.M., Bessereau J.L., Zhen M. 2005. Identification of genes involved in synaptogenesis using a fluorescent active zone marker in Caenorhabditis elegans. J. Neurosci. 25:3833–3841 10.1523/JNEUROSCI.4978-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J., Herman R.K. 2003. Investigating C. elegans development through mosaic analysis. Development. 130:4761–4768 10.1242/dev.00701 [DOI] [PubMed] [Google Scholar]
- Zhang W., Benson D.L. 2001. Stages of synapse development defined by dependence on F-actin. J. Neurosci. 21:5169–5181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Benson D.L. 2002. Developmentally regulated changes in cellular compartmentation and synaptic distribution of actin in hippocampal neurons. J. Neurosci. Res. 69:427–436 10.1002/jnr.10313 [DOI] [PubMed] [Google Scholar]
- Zhao H.J., Nonet M.L. 2001. A conserved mechanism of synaptogyrin localization. Mol. Biol. Cell. 12:2275–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M., Jin Y. 1999. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 401:371–375 [DOI] [PubMed] [Google Scholar]
- Ziel J.W., Hagedorn E.J., Audhya A., Sherwood D.R. 2009. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat. Cell Biol. 11:183–189 10.1038/ncb1825 [DOI] [PMC free article] [PubMed] [Google Scholar]