Abstract
Hormone therapy is still used by millions of women for menopausal symptoms. Concerns regarding hormone therapy and breast cancer were initially based on case reports and retrospective case–control studies. However, recent results from large prospective cohort studies and the Women’s Health Initiative (WHI) randomized placebo-controlled hormone therapy trials have substantially changed concepts regarding how estrogen alone and estrogen plus progestin influence breast cancer. The preponderance of observational studies suggested that estrogen alone and estrogen plus progestin both increased the risk of breast cancer, with cancers commonly diagnosed at an early stage. However, substantially different results emerged from the WHI randomized hormone therapy trials. In the WHI trial evaluating estrogen plus progestin in postmenopausal women with an intact uterus, combined hormone therapy statistically significantly increased the risk of breast cancer and hindered breast cancer detection, leading to delayed diagnosis and a statistically significant increase in breast cancer mortality. By contrast, estrogen alone use by postmenopausal women with prior hysterectomy in the WHI trial did not substantially interfere with breast cancer detection and statistically significantly decreased the risk of breast cancer. Differential mammography usage patterns may explain differences between observational study and randomized trial results. In clinical practice, hormone therapy users have mammograms more frequently than nonusers, leading to more and earlier stage cancer detection. By contrast, in the WHI randomized trials, mammogram frequency was protocol mandated and balanced between comparison groups. Currently, the different effects of estrogen plus progestin vs estrogen alone on breast cancer are not completely understood.
Concepts regarding menopausal hormone therapy and breast cancer have been evolving for decades. When exogenous estrogen was introduced into clinical practice for menopausal symptom management in the early 1940s, there were theoretical concerns regarding potential adverse effects on breast cancer, a cancer believed to be under the influence of reproductive hormones. Information linking use of exogenous estrogen to breast cancer risk initially came from case reports and retrospective case–control studies. Given the known limitations of such reports, perceptions about the nature of the relationship between hormone therapy and breast cancer developed slowly and controversy began. Subsequently, large-scale prospective observational studies that were designed to provide more reliable evidence were initiated in the 1980s and began reporting results in the 1990s (Table 1).
Table 1.
Estrogen and breast cancer: findings from selected studies
| Year | First author (reference) | Study design | Finding |
| 1896 | Beatson (1) | Case report | Oophorectomy associated with breast cancer regressions |
| 1968 | Feinleib (2) | Cohort analysis | Oophorectomy associated with lower breast cancer risk |
| 1970 | MacMahon (3) | International collaborative study | Age at first birth related to breast cancer risk |
| 1973 | McGuire (4) | Summary, findings from clinical correlative studies | Estrogen receptor quantitative status correlated with clinical breast cancer response to hormone-directed therapy |
| 1976 | Hoover (5) | Incidence rate in cohort vs rate in general population | Exogenous estrogen alone associated with higher breast cancer risk |
| 1980 | Ross (6) | Case–control analysis | Exogenous estrogen associated with higher breast cancer risk |
| 1983 | Pike (7) | Analysis | Model of endogenous hormonal risk factors with breast cancer |
| 1989 | Bergkvist (8) | Cohort analysis | Exogenous estrogen alone and exogenous estrogen plus progestin both associated with higher breast cancer risk |
| 1995 | Colditz (9) | Cohort analysis | Exogenous estrogen alone and exogenous estrogen plus progestin both associated with higher breast cancer risk |
| 1997 | Collaborative Group on Hormonal Factors in Breast Cancer (10) | Collaborative reanalysis of 51 case–control studies | Hormone therapy (80% exogenous estrogen alone) associated with higher breast cancer risk |
| 2003 | Beral (11) | Cohort analysis with mammography at entry | Exogenous estrogen alone and exogenous estrogen plus progestin both associated with higher breast cancer risk. Trend for higher breast cancer mortality in estrogen plus progestin users |
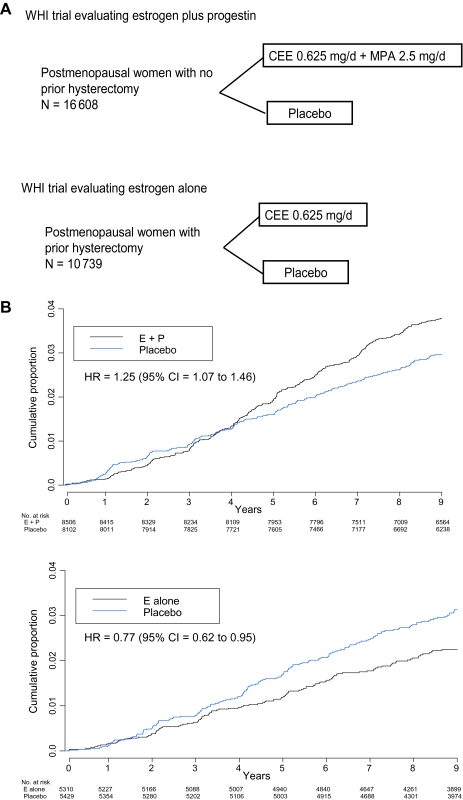
During this period, observational studies and biomarker studies of lipid profiles suggested that exogenous estrogen, alone or in combination with progestin, was beneficial for the prevention and treatment of osteoporosis (12), cardiovascular disease (13), and dementia (14) and had net favorable effects on diseases of aging (15), but this concept lacked clinical trial evidence. After more than 50 years of exogenous estrogen use in clinical practice, a strategy for the definitive assessment of this approach was proposed under the direct impetus of the late Dr Bernadine Healy, the first female Director of the US National Institutes of Health. Consequently, in 1993, the Women’s Health Initiative (WHI) began two full-scale randomized placebo-controlled clinical trials that separately evaluated estrogen plus progestin (in women with an intact uterus) as well as estrogen alone (in women with a previous hysterectomy) (Figure 1, A) (16). Results that incorporate longer post-intervention follow-up from both trials are now available to inform the current understanding of the influence of hormone therapy on breast cancer and other chronic diseases in postmenopausal women.
Figure 1.
Women's Health Initiative (WHI) Hormone Therapy Clinical Trials. A) Program design. The depicted study implementation details have been described previously (16–18). Entry criteria for both trials were similar, except that previous hysterectomy was required for estrogen-alone trial entry. Eligible were postmenopausal women aged 50–79 years with anticipated 3 years or greater survival. Exclusions included prior breast or endometrial cancer at any time or other cancer (except nonmelanoma skin cancer) within the previous 10 years. Hormone therapy users at screening were eligible after a 3-month washout. Despite similar eligibility, participant characteristics in the two trials differed, primarily with respect to factors that are associated with hysterectomy. Women in the estrogen-alone trial were heavier and more likely to have had bilateral oophorectomy and to have used hormone therapy in the past (18). Between 1993 and 1998, 10 739 women with prior hysterectomy were randomly assigned to estrogen alone (conjugated equine estrogen [CEE], 0.625 mg/d) or matching placebo and 16 808 women with an intact uterus were randomly assigned to estrogen plus progestin (conjugated equine estrogen, 0.625 mg/d plus medroxyprogesterone acetate [MPA], 2.5 mg/d) or a matching placebo. A mammogram and a clinical breast examination with no evidence of cancer were required for entry. Annual mammography and clinical breast examinations with negative findings were a prerequisite to dispensing ongoing study medication. Breast biopsies were clinically directed. Colorectal cancer screening was not protocol defined, but information on sigmoidoscopy and/or colonoscopy and fecal occult blood testing was collected semiannually. All cancer outcomes were verified by blinded medical record review. Depicted are the study designs of the WHI placebo-controlled clinical trials evaluating estrogen plus progestin and estrogen alone. B) Invasive breast cancer incidence by hormone therapy group. Hazard ratios (HRs) are from Cox proportional hazards regression models, stratified by age and randomization group in the WHI dietary modification trial and prior disease if applicable. CI = confidence interval; E + P = estrogen plus progestin; E alone = estrogen alone.
To understand how concepts about hormone therapy and breast cancer have evolved over time, we compare and contrast findings from selected early case–control studies and case reports with results of randomized clinical trials and prospective cohort analyses. We address areas of agreement and controversies against a background of potential mediating mechanisms of action. Findings with estrogen alone are addressed separately from those with estrogen plus progestin.
Breast Cancer Hormonal Associations: Observational Study Findings
The concept that reproductive hormones influence breast cancer risk has a sound basis. Although it is beyond the scope of this review to provide a comprehensive assessment of all clinical findings from the past decades that informed the development of concepts regarding exogenous estrogen use and breast cancer, we provide a timeline of selected studies to outline the overall pace of discovery (Table 1). In 1896, Beatson (1) reported that oophorectomy could result in the regression of breast cancer. Subsequent reports (3,7,19–21) described associations between reproductive events (such as a woman's age at birth of first child, an earlier menarche, and a later menopause) and increased risks of breast cancer, suggesting a role for endogenous estrogen in breast cancer development. The finding that a previous oophorectomy was associated with lower breast cancer incidence solidified this relationship (2). An extensive body of evidence now supports the concept that, in postmenopausal women, higher endogenous estrogen levels are associated with an increased risk of breast cancer (22,23).
The higher incidence of breast cancer among women who used exogenous estrogen was initially reported in 1976 (5) (Table 1). In 1980, a case–control study with 138 breast cancers reported that the breast cancer risk ratio for cumulative estrogen doses greater than 1500 mg vs no hormone therapy was 2.5 (6). In 1989, in a prospective cohort of Swedish women, Bergkvist et al. (8) found a modestly increased risk of breast cancer associated with hormone therapy, particularly longer durations of use, compared with no hormone therapy use (after 9 years of use, relative risk = 1.7, 95% confidence interval [CI] = 1.1 to 2.7). However, an editorial in The Lancet (24) outlined the study limitations and, reflecting the then prevalent perspective, concluded “the Bergkvist data should not cause us to change our advice to patients.”
In the 1990s, new evidence emerged from two influential sources: a meta-analysis that consolidated data from previous case–control studies (10) and mature data from a large cohort study (9). The comprehensive collaborative reanalysis of 51 epidemiological studies of 52 705 women with breast cancer and 108 411 control subjects (80% of whom used estrogen alone) concluded that women who used hormone therapy had a statistically significant increase in the risk of breast cancer and that the risk increased with increasing duration of use (10). Parallel analyses in the Nurses’ Health Study cohort found that estrogen alone and estrogen plus progestin were both associated with an increased risk of breast cancer (9). In 1998, tamoxifen, a selective estrogen receptor modulator that competes with estrogen for binding to the estrogen receptor, was shown to reduce the incidence of hormone receptor–positive breast cancer in a primary prevention trial (25).
Regarding potential mechanisms mediating hormone action on breast cancer, tumor expression of the estrogen receptor was found to correlate with clinical response to hormonal interventions such as tamoxifen (4,17,26). These findings suggested a relatively linear relationship among endogenous estrogen level, exogenous estrogen use, estrogen receptor activation, and breast cancer incidence.
When the WHI hormone therapy trials began in 1993, results of previous observational studies suggested that both estrogen alone and estrogen plus progestin increase the risk of breast cancer, with the increased risk with estrogen alone perhaps requiring longer exposures (Box 1). The preponderance of recent reviews and reports from large cohorts (27–29) has reached similar conclusions.
Box 1. Changing concepts regarding hormone therapy and breast cancer.
Concepts in 2002
Combined estrogen plus progestin use
Estrogen plus progestin increases breast cancer risk
Estrogen-alone use
Estrogen alone increases breast cancer risk but may require longer duration exposure than combined estrogen plus progestin for an effect
Hormone therapy*
Breast cancer associated with hormone therapy are mainly hormone receptor–positive cancers
Breast cancers associated with hormone therapy are diagnosed at earlier stage
Breast cancers associated with hormone therapy have a favorable prognosis
Current Concepts
Combined estrogen plus progestin use
Estrogen plus progestin increases breast cancer risk and the effect on risk may be greater in women who initiate therapy closer to menopause†
Estrogen plus progestin broadly increases breast cancer risk with the increase in risk not limited to hormone receptor–positive cancers†
Estrogen plus progestin interferes with breast cancer mammographic detection resulting in cancers diagnosed at more advanced stage†
Estrogen plus progestin increases breast cancer mortality†
Estrogen-alone use
Estrogen alone reduces breast cancer risk†
Estrogen alone does not substantially interfere with breast cancer detection by mammography†
*Hormone therapy concepts refer to findings that are similar for estrogen-alone and estrogen plus progestin use or findings where estrogen alone and estrogen plus progestin were combined in analyses.
†Findings that differ from concepts in 2002.
WHI: The Estrogen Plus Progestin Trial
The WHI began two randomized placebo-controlled clinical trials to provide definitive assessment of the effects of estrogen alone (in women with prior hysterectomy) and of estrogen plus progestin (in women with an intact uterus). In the WHI clinical trial evaluating estrogen plus progestin in postmenopausal women with no previous hysterectomy, intervention was terminated early after mean follow-up of 5.6 years, when substantial evidence emerged of an adverse effect on breast cancer risk in the context of an assessment that overall harms exceeded benefits for the combined hormone therapy group. By that time, approximately 40% of the participants had stopped taking study medication. Women who took estrogen plus progestin were at higher risk for heart disease, blood clots, stroke, and breast cancer, but at lower risk for fracture and colon cancer. Since the initial report (30), detailed analyses by cancer site and with post-intervention follow-up have been published (31–35) (Table 2).
Table 2.
Women's Health Initiative hormone therapy trials: monitored clinical outcomes during active intervention and overall, by study, comparing hormone therapy with placebo*
| Outcome | Estrogen plus progestin vs placebo |
Estrogen alone vs placebo |
||
| Events during intervention phase | Events, overall | Events during intervention phase | Events, overall | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Invasive breast cancer | 1.24 (1.01 to 1.54) | 1.25 (1.07 to 1.46) | 0.80 (0.62 to 1.04) | 0.77 (0.62 to 0.95) |
| Coronary heart disease | 1.29 (1.02 to 1.63) | 1.11 (0.94 to 1.11) | 0.91 (0.75 to 1.12) | 0.95 (0.82 to 1.11) |
| Stroke | 1.41 (1.07 to 1.85) | 1.28 (1.05 to 1.56) | 1.39 (0.62 to 1.04) | 1.19 (0.98 to 1.43) |
| Pulmonary embolus | 2.13 (1.39 to 3.25) | 1.66 (1.22 to 2.25) | 1.34 (0.87 to 2.06) | 1.18 (0.87 to 1.60) |
| Endometrial cancer | 0.83 (0.47 to 1.47) | 0.78 (0.52 to 1.16) | Not applicable | Not applicable |
| Colorectal cancer | 0.56 (0.38 to 0.81) | 0.75 (0.57 to 1.00) | 1.12 (0.77 to 1.63) | 1.15 (0.81 to 1.64) |
| Hip fracture | 0.66 (0.45 to 0.98) | 0.78 (0.60 to 1.00) | 0.61 (0.41 to 0.91) | 0.92 (0.71 to 1.18) |
| Global index† | 1.15 (1.03 to 1.28) | 1.12 (1.03 to 1.21) | 1.01 (0.91 to 1.12) | 1.03 (0.95 to 1.11) |
| Dementia | 2.05 (1.21 to 3.48) | Not reported | 1.49 (0.83 to 2.66) | Not reported |
| Deaths (all causes) | 0.97 (0.81 to 1.16) | 1.04 (0.91 to 1.18) | 1.04 (0.89 to 1.22) | 1.02 (0.91 to 1.15) |
Results from (18,30,35–37). Active intervention phase: 5.6 years (mean) in estrogen plus progestin trial and 7.1 years (mean) in estrogen-alone trial. Results are reported for outcomes occurring during active intervention with study medications and overall including total follow-up for the estrogen plus progestin trial of 11.0 years (mean) for breast cancer and 7.9 years (mean) for other outcomes. For the estrogen-alone trial, total follow-up was 10.7 years (mean). HRs for hormone therapy vs placebo are from Cox proportional regression models stratified by age, randomization assignment in the dietary modification trial, and prior disease when applicable. CI = confidence interval; HR = hazard ratio.
The global index measured time-to-first event for clinical conditions likely under hormone influence that were associated with increased risk for death (coronary heart disease, stroke, pulmonary embolus, hip fracture, invasive breast cancer, colorectal cancer, endometrial cancer [only in the combined hormone therapy trial], and death due to other causes) (4,16,38,39).
In the WHI randomized clinical trial, estrogen plus progestin was compared with placebo in postmenopausal women with an intact uterus, and compared with placebo, estrogen plus progestin increased the risk of invasive breast cancer by 24% (P = .003). In sensitivity analyses that excluded nonadherent participants, the risk of invasive breast cancer was increased by 49% (P < .001) (31). During the first 4 years of the trial (follow-up), fewer breast cancers were diagnosed in the estrogen plus progestin group than in the placebo group; thereafter, more breast cancers were diagnosed in the estrogen plus progestin group (Figure 1, B) (31). Estrogen plus progestin resulted in a higher cumulative frequency of abnormal mammograms vs placebo (35% vs 23%, P < .001) while reducing the mammographic sensitivity for breast cancer detection (32). Similarly, there were more clinically indicated breast biopsies in the estrogen plus progestin group vs placebo, but those biopsies less frequently diagnosed breast cancer (31,32). The statistically significant increase in mammographic breast density associated with estrogen plus progestin (33) is one likely factor contributing to the poor diagnostic performance of mammograms and breast biopsies.
The relatively rapid influence of estrogen plus progestin use on breast cancer incidence is consistent with it having a predominant influence on preclinical already established cancers. In addition, the fact that longer duration of the combined hormone therapy had a greater effect on breast cancer risk (31,34) suggested an influence on breast cancer development as well.
After a mean cumulative follow-up of 11 years with 678 invasive breast cancers, a 25% increase in the risk of invasive breast cancer was seen with estrogen plus progestin compared with placebo (P = .004) (Table 3, Figure 1, B) (35). Proportionally more lymph node–positive cancers were observed in the combined hormone therapy group vs placebo (P = .03). There were numerically more estrogen receptor–positive, estrogen receptor–negative, HER2-overexpressing, and triple-negative (ie, hormone receptor–negative and HER2-nonoverexpressing) breast cancers in the estrogen plus progestin group vs the placebo group (Table 3) (35). Estrogen plus progestin had a greater influence on breast cancer if initiated closer to menopause (incidence of invasive breast cancer in the hormone therapy group compared with the placebo group: for therapy initiated <5 vs ≥5 years from menopause, hazard ratio [HR] = 1.41, 95% CI = 1.14 to 1.74 vs HR = 1.15, 95% CI = 0.96 to 1.37; Pinteraction = .08).
Table 3.
Distribution of invasive breast cancer characteristics in the WHI estrogen plus progestin clinical trial by randomization group*
| Characteristic | Estrogen plus progestin | Placebo | HR of breast cancer (95% CI) |
| No. (%) | No. (%) | ||
| Invasive breast cancer (all) | 385 (100) | 293 (100) | 1.25 (1.07 to 1.46) |
| Histology | |||
| Ductal | 238 (62.1) | 195 (66.6) | 1.16 (0.96 to 1.41) |
| Lobular | 36 (9.4) | 20 (6.8) | 1.63 (0.94 to 2.81) |
| Ductal and lobular | 57 (14.9) | 35 (11.9) | 1.55 (1.02 to 2.37) |
| Tubular | 13 (3.4) | 9 (3.1) | 1.39 (0.59 to 3.25) |
| Other | 39 (10.2) | 34 (11.6) | 1.10 (0.70 to 1.75) |
| Estrogen receptor† | |||
| Positive | 308 (86.5) | 230 (87.5) | 1.27 (1.07 to 1.51) |
| Negative | 48 (13.5) | 33 (12.5) | 1.40 (0.90 to 2.18) |
| Progesterone receptor† | |||
| Positive | 262 (75.2) | 194 (75.7) | 1.29 (1.07 to 1.55) |
| Negative | 86 (24.8) | 62 (24.3) | 1.31 (0.95 to 1.82) |
| ERBB2 (HER2) overexpression† | |||
| Yes | 54 (18.8) | 26 (13.9) | 2.00 (1.25 to 3.19) |
| No | 233 (81.2) | 161 (86.1) | 1.37 (1.12 to 1.68) |
| Triple negative‡ | |||
| Yes | 26 (9.1) | 14 (7.4) | 1.78 (0.93 to 3.41) |
| No | 259 (90.9) | 173 (92.6) | 1.42 (1.17 to 1.72) |
Mean follow-up for analysis was 11.0 years, including 5.6 years of study medication intervention. HRs are from Cox proportional hazards regression models, stratified by age and randomization group in the Women's Health Initiative dietary modification trial. CI = confidence interval; HR = hazard ratio.
The percentage for each category reflects findings from tumors with available information. For example, of tumors with information on estrogen receptor status in the estrogen plus progestin group, 86.5% were positive and 13.5% were negative. Estrogen receptor status missing: 7.5% (estrogen plus progestin) vs 9.9% (placebo); Progesterone receptor status missing: 8.3% (estrogen plus progestin) vs 11.6% (placebo); HER2 status missing: 24.7% (estrogen plus progestin) vs 35.8% (placebo).
Triple negative = estrogen receptor–negative, progesterone receptor–negative, and ERBB2 (HER2) not overexpressed.
The more advanced stage of breast cancers diagnosed in the estrogen plus progestin group was reflected in the mortality results. In the estrogen plus progestin group, the risk of death attributed to breast cancer was increased by a factor of 1.96 (P = .049), and the risk of deaths from all causes after a breast cancer diagnosis was increased by a factor of 1.57 (P = .045) (35).
The WHI findings for estrogen plus progestin and breast cancer are similar to those from observational studies with respect to incidence but differ from most reports with respect to breast cancer characteristics and clinical outcome (27,40). As in most observational studies (41), estrogen plus progestin use had a somewhat greater influence on cancers with some lobular histology in the WHI trial. Most, but not all (42), observational studies suggest that estrogen plus progestin use is mainly associated with estrogen receptor–positive early-stage breast cancers (43–45), and some report either no increase (46) or a decrease (47,48) in death rates following a breast cancer diagnosis while on hormone therapy. A review published in 2002 (49) cited five recent cohort studies that showed either no difference in mortality or decreased mortality after a breast cancer diagnosis in hormone therapy users compared with nonusers (relative risks of death ranged from 0.5 to 1.0). By contrast, in the WHI randomized trial, the increase in breast cancer incidence was not limited to favorable prognosis estrogen receptor–positive tumors: the cancers that were diagnosed had more lymph node involvement. In addition, breast cancer mortality, measured from initial randomization, was statistically significantly increased (35).
There has been interest in the potential modulating influence of body weight, as measured by body mass index [BMI], on the effects of menopausal hormone therapy on the risk of breast cancer. In several observational studies (10,41,50,51), the risk of breast cancer associated with hormone therapy use was substantially higher in women of normal weight (ie, BMI < 25 kg/m2) compared with those who were overweight (BMI = 25–30 kg/m2) or obese (BMI ≥ 30 kg/m2). By contrast, in the WHI randomized trial, no interaction was observed between BMI and the influence of estrogen plus progestin on the risk of breast cancer (31). Even after additional follow-up and 678 invasive breast cancer cases, the increased risk of breast cancer associated with estrogen plus progestin use did not differ by BMI (BMI < 25 kg/m2: 29% increase; BMI = 25–30 kg/m2: 34% increase; BMI ≥ 30 kg/m2: 14% increase) (35). No compelling hypothesis has been offered to explain this difference between observational study results and the WHI randomized clinical trial. It has been suggested (52) that the proportional increase in circulating estrogen level among postmenopausal women taking exogenous estrogens might be smaller in obese women compared with normal weight women. However, a 2009 study (53) reported that obese postmenopausal women had a greater increase in circulating free estradiol in response to oral estrogen compared with normal weight women.
Differences between the WHI randomized trial findings and those of observational studies may arise from methodological issues. The limited number of breast cancers in the randomized trial yield somewhat imprecise effect estimates, particularly in subgroups of interest such as HER2-overexpressing tumors and lobular carcinomas. In the WHI trial, mammogram frequency was protocol defined and comparable between groups. In other non-trial settings, hormone therapy users have mammograms more frequently compared with nonusers (34,54), likely because of concerns about breast tenderness and/or breast cancer. In one report from the Nurses’ Health Study (54), 69% of hormone users reported having undergone screening mammography in the previous 2 years compared with only 44% of nonusers. In addition, the frequency of mammography is not reliably determined retrospectively by query (55). Consequently, confounding can occur in observational studies that are unable to accurately control for frequency of mammography because screened populations have more cancers detected than non-screened populations (54,56) and cancers in the screened population are diagnosed at earlier stage (57,58). Also, observational studies of breast cancer mortality that begin analyses at the time of cancer diagnosis (47,48) or control for stage (43,45) make analysis adjustments that mitigate adverse effects of combined hormone use on breast cancer incidence and characteristics. In this regard, the Million Women Study, which began analysis at cohort entry and controlled for previous mammography, also reported higher breast cancer mortality for women who were diagnosed with breast while using estrogen plus progestin compared with non–hormone therapy users (HR of death = 1.22; 95% CI = 1.00 to 1.48, P = .05) (11).
Public Health Consequences of the WHI Hormone Therapy Trial
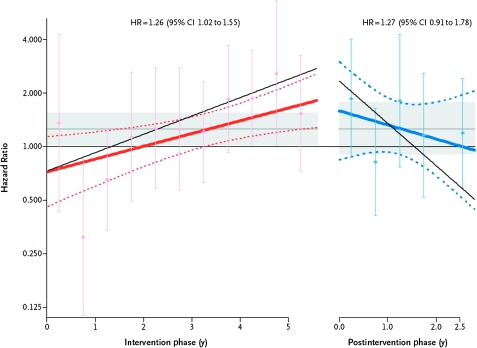
After the WHI reported the net adverse effects of estrogen plus progestin (30,59), hormone therapy use among postmenopausal women in the United States rapidly decreased over 1 year from approximately 40%–20% (60). A corresponding and substantial population-based decrease in breast cancer incidence was observed, particularly for estrogen receptor–positive breast cancers in women aged 50–69 years, which was attributed to the decreased use of hormone therapy (61,62). Although a relationship between a decrease in combined hormone therapy use and a decrease in breast cancer incidence has been generally supported in other populations in the United States (63), Germany (64), Australia (65), France (66), and Canada (67), the potential influence of changes in mammography use on the rapid decrease in breast cancer raised concerns. These concerns have been largely addressed by analyses of the WHI hormone trial, in which almost all participants discontinued study medication as instructed (68). In that randomized trial setting, the risk of breast cancer rapidly decreased after intervention ended while annual mammography utilization was comparable in the two randomization groups (34) (Figure 2).
Figure 2.
Breast cancer risk during intervention and after intervention in the Women's Health Initiative Estrogen Plus Progestin Trial. Depicted are time-varying linear hazard ratios (solid red and blue lines) and 95% confidence intervals (95% CIs) (dashed lines) for the effect of estrogen plus progestin vs placebo on breast cancer incidence during the intervention (left) and post-intervention (right) phases of the Women's Health Initiative randomized clinical trial. Circles and error bars represent hazard ratios of breast cancer and 95% CIs, respectively, for analyses based on events during each 6-month period. Results for a sensitivity analyses, with events censored 6 months after participants became nonadherent (defined as using <80% of study pills or starting non-protocol hormone therapy) are depicted by the slanting thin black solid lines in the intervention and post-intervention phases. The shaded areas indicate the overall mean and 95% CIs for the hazard ratios in the intervention and post-intervention phases. Modified from and reproduced with permissions from New England Journal of Medicine (34).
The rapid decrease in largely estrogen receptor–positive breast cancers seen in the United States and other countries is biologically reasonable given that a sudden change in hormonal environment likely represents a therapeutic intervention for subclinical breast cancers. In the WHI randomized trial, the influence of combined hormone therapy on breast cancer incidence does not appear to be limited to hormone receptor–positive cancers. However, in the clinic, extensive clinical trial and practical experience indicates that, for patients with established breast cancer, only hormone receptor–positive breast cancers respond to hormone-targeted therapies (38,69).
Such a treatment effect would largely be a one-time phenomenon because such a large-scale decrease in hormone therapy use in a population would not be duplicated year to year. Although a lower incidence of hormone receptor–positive breast cancer has been maintained in the US population through at least 2005 (63), studies of subsequent trends in annual breast cancer incidence have provided mixed results with reports of continued decline (66), stabilization (63), and subsequent increase (70). The complex interplay among withdrawal of estrogen plus progestin, subsequent breast cancer incidence, and associated societal and medical factors, such as increasing obesity and use of medications that have a potential influence on breast cancer such as bisphosphonates (39) and metformin (71), will influence subsequent breast cancer incidence trends.
WHI: The Estrogen-Alone Trial
In the WHI randomized placebo-controlled clinical trial evaluating estrogen alone in postmenopausal women with a previous hysterectomy, intervention ended at a mean follow-up of 7.1 years because of an increased risk of stroke and the absence of overall clinical benefit, despite a decrease in the risk of fracture (18). By that time, 54% of participants had stopped taking study medications. Other monitored outcomes, including coronary heart disease, pulmonary emboli, colorectal cancer, and hip fracture, were not influenced by the use of estrogen alone; however, there were fewer breast cancers in the estrogen-alone group compared with the placebo group (Table 2), but the difference was not statistically significant. In addition, subgroup analyses suggested that estrogen alone may be associated with more favorable outcomes in younger women compared with older women (18,36).
Breast Cancer: Randomized Trial Findings, Estrogen Alone
In the estrogen-alone trial, as in the WHI estrogen plus progestin trial, the designated primary outcome for benefit was a reduction in coronary heart disease and the designated primary adverse outcome was an increase in incidence of invasive breast cancer (72). There was a lower breast cancer incidence seen with estrogen-alone use compared with placebo during the 5.9 years (median) of intervention, but the difference was not statistically significant (73). In adherence-adjusted analyses at that time based on events during the intervention, use of estrogen alone was associated with a statistically significant 33% lower incidence of breast cancer (P = .03). After longer follow-up (10.7 years [mean]), use of estrogen alone was associated with a statistically significant 23% lower incidence of invasive breast cancer (P = .02) (Figure 1, B and Table 2) (36). Similar to the WHI findings with combined hormone therapy, estrogen alone increased the frequency of breast biopsies compared with placebo (74). In contrast to estrogen plus progestin, use of estrogen alone did not substantially interfere with breast cancer detection by mammography (74) and increased mammographic density only by a modest 1% compared with placebo (75).
In the WHI trial, women who started using estrogen alone less than 5 years after menopause (ie, women who had a short gap time) did not demonstrate a reduction in breast cancer incidence compared with the reduction seen in women who started using estrogen alone 5 or more years after menopause (76,77). However, this interaction with gap time was not statistically significant. The concept that breast cancer risk associated with hormone therapy use could vary based on gap time was reported initially in the WHI (108) and later in the French E3N cohort (78). In the Million Women Study, an increased risk of breast cancer with estrogen alone was seen only in participants who initiated hormone use within 5 years of menopause (28). Because women in these cohorts more commonly began using estrogen within 5 years of menopause, gap time considerations can perhaps explain why cohort analyses associated estrogen use with higher breast cancer incidence but not why a statistically significant lower breast cancer incidence with estrogen alone use was seen in the WHI randomized trial (36,77).
As outlined above, an imbalance in the use of mammography with greater screening for hormone users could explain some of the increase in breast cancer incidence with estrogen alone seen in cohort studies because screened populations have more cancers detected than unscreened populations (56). In this regard, in a large US cohort with reliable information on serial mammography use, a somewhat lower breast cancer incidence was reported for estrogen-alone use (relative risk = 0.92, 95% CI = 0.84 to 1.00) (42), and a recent update of the Nurses’ Health Study cohort found estrogen exposures for more than 20 years were required before an increase in breast cancer incidence was seen (79).
The long-term effect of estrogen use on the risk of breast cancer should be considered still an open question. Nonetheless, some of the substantial differences between findings from the preponderance of observational studies and those from the WHI randomized trial regarding the use of 5 years of estrogen alone and the risk of breast cancer remain unexplained. Future observational studies should include only populations for which reliable information on serial mammography use is available. In summary, in the WHI randomized trial, estrogen-alone use was associated with a statistically significant decrease in breast cancer incidence.
Current Concepts: Potential Mediating Mechanisms of Action of Hormone Therapy on Breast Cancer
Recently, investigators from the randomized placebo-controlled primary prevention MAP.3 trial reported that the aromatase inhibitor exemestane, which substantially reduces estrogen levels in postmenopausal women, reduced breast cancer incidence by 65% (P = .002) (80). Consequently, a biological understanding of the relationship between estrogen and breast cancer must account for clinical trial results showing that both estrogen addition, by conjugated equine estrogen use, as well as estrogen reduction, by aromatase inhibitor use, lower breast cancer incidence. These apparently paradoxical findings are nonetheless consistent with preclinical studies over the past decade indicating there are complex time- and condition-dependent changes in the influence of estrogen on breast cancers based on evidence from mammary tumor preclinical models (81,82).
Typically, estrogen stimulates breast cell proliferation and inhibits apoptosis (82). However, preclinical studies have found changes in breast tumor gene expression profile after a period of estrogen deprivation (82–84), and in this environment, estrogen administration induces apoptosis (85,86). Two pathways have been identified as regulating estrogen-induced apoptosis: the extrinsic hormone receptor–mediated death regulatory pathway, which involves an increase in Fas ligand (87), and the receptor-independent intrinsic mitochondrial pathway, which involved the release of cytochrome C (88).
These preclinical findings are reflected in breast cancer outcomes in the clinic. For example, high-dose estrogen as diethylstilbesterol is a recognized breast cancer therapy, albeit one with high level of toxicity (89,90). Moreover, Ellis et al. (91) found that some tumors in patients with metastatic breast cancer that progressed after a period of aromatase inhibitor use (ie, estrogen deprivation) subsequently regressed in response to relatively low-dose estradiol use (91).
A period of estrogen deprivation also can result in enhanced estrogen receptor expression whereby even low levels of endogenous estrogen can stimulate breast tumor growth. This action may be mediated not only by increased expression of estrogen receptor alpha but also increased expression of MAP kinase and mTOR pathways (92). As a result, the substantial 90%–95% reduction in estradiol levels associated with aromatase inhibitor use can result in tumor regression (93).
The findings in the clinic, taken together with preclinical evidence, suggest that many breast cancers that present in postmenopausal women can survive only a limited range of estrogen exposures. As a result, a substantial change in the estrogen environment—either by increasing estrogen with exogenous estrogen use or decreasing estrogen with an aromatase inhibitor—can inhibit breast tumor growth.
The influence of estrogen on the full range of estrogen receptor–mediated signaling pathways has not yet been integrated into a comprehensive model regarding estrogen and breast cancer risk. In addition to estrogen-dependent action on nuclear DNA via binding to estrogen receptors, there are estrogen-dependent actions mediated without involvement of estrogen receptors through other transcription factors. Activation of estrogen receptors can also be mediated through other pathways not involving estrogen (86). Interactions between estrogen and mitochondrial DNA (94) as well as membrane estrogen receptor–mediated activation of second messenger and protein kinase signaling have also been described (21). In the future, a better understanding of interactions among these pathways and how they influence breast cell proliferation and apoptosis will likely lead to more accurate breast cancer risk assessments and new therapeutic strategies.
Whereas estrogen alone and combined estrogen plus progestin have different influences on breast cancer risk, the mechanisms that mediate these differential effects on the breast of adding progestin to estrogen are not established (95,96). A mechanistic explanation of the breast cancer findings from the WHI must also address the completely opposite effects of estrogen plus progestin and estrogen alone on the risk of endometrial cancer in women with a uterus, that is, estrogen alone increases risk and progestin addition mitigates the estrogen-associated risk (97). Preclinical studies suggest that progestin stimulation of breast cell proliferation (98,99) and angiogenesis (100), which leads to growth and metastatic spread of established cancers, as well as progestin stimulation of stem cells by a paracrine receptor–associated mechanism involving nuclear factor-κB ligand (RANKL), represent two potential mediators of the adverse influence of adding progestin to estrogen on breast cancer risk (98,101). Given the complex, condition-dependent, and conflicting findings from experimental studies, further clinical studies are needed to clarify the influence of progestin on human breast cancer.
Preparation, Dose, and Schedule Considerations
The WHI hormone therapy trials evaluated conjugated equine estrogen with or without medroxyprogesterone acetate, hormone preparations that represent the vast majority of menopausal hormone therapy still being used in US clinical practice (102). However, large observational studies that have incorporated other estrogen plus progestin regimens, including those with estradiol and progesterone-like progestins that are more frequently used in Europe, have generally reported similar breast cancer findings, namely an increase in breast cancer risk associated with estrogen plus progestin use (11,29,78,103). In contrast to most progestins, micronized progesterone has less influence on breast cancer cell proliferation in preclinical studies (104) and on breast epithelial proliferation in a small randomized trial in primates (105). Although estrogen regimens that include micronized progesterone (which is used mainly in France) are less strongly associated with an increased risk of breast cancer compared with other progestins (29,106,107), they also provide limited protection against endometrial cancer (HR of endometrial cancer for current users vs never users = 2.42; 95% CI = 1.53 to 3.83) (97). These results, which require confirmation, suggest that micronized progesterone may provide a trade-off between an increased risk of endometrial cancer and a decreased risk of breast cancer. On balance, however, the current results provide no compelling evidence of the relative overall safety of micronized progesterone combinations with regard to cancer.
Summary
In Box 1, we present current concepts regarding breast cancer and hormone therapy, giving substantial weight to findings from the WHI randomized clinical trials, and concepts that were prevalent in 2002, before the initial reports of the WHI hormone therapy trials, the maturation of large prospective cohorts, and expanding preclinical evidence regarding mediating mechanisms of action. In the WHI trials, fewer breast cancers were seen in postmenopausal women with a previous hysterectomy who were receiving estrogen alone (36). In contrast, estrogen plus progestin statistically significantly increased breast cancer incidence (31,35) and breast cancer mortality (35).
Although the absolute risk of death due to breast cancer associated with estrogen plus progestin use is relatively modest, from a public health perspective, a near doubling of breast cancer deaths with estrogen plus progestin (35) represents a considerable concern. Finally, unlike coronary heart disease, where risk was increased only in the first few years of use (108,109) and was lower in women who initiated hormone use closer to menopause (108), the risk of developing breast cancer continues to increase with longer duration of use (35,37) and appears to be somewhat greater in women who start hormone therapy near menopause (77,110). In addition, because estrogen plus progestin interferes with breast cancer detection and delays diagnosis (31,32), it is not possible to define a safe interval for estrogen plus progestin use with respect to breast cancer risk.
There are caveats regarding concepts that have emerged from the WHI hormone therapy trials. The findings are limited to the duration of intervention and adherence achieved in the trials; cancer effects of longer duration hormone therapy cannot be inferred from these data. Furthermore, nonadherence likely attenuated the effect estimates.
In summary, concepts regarding menopausal hormone therapy and breast cancer have undergone considerable change in the last decade (Table 1). Randomized clinical trial data suggest that estrogen plus progestin is associated with increases in breast cancer incidence and death from breast cancer, whereas approximately 5 years of estrogen alone use in postmenopausal women with a previous hysterectomy is associated with reduced breast cancer incidence. Although the underlying complex biology provides a framework for understanding the mechanisms mediating these hormone effects, currently they are not completely understood.
Footnotes
R. T. Chlebowski has received consulting fees from AstraZeneca and Novartis and Pfizer, lecture fees from AstraZeneca and Novartis, and grant funding from Amgen. G. L. Anderson has no industry relations. The authors are solely responsible for the study design, data collection, analysis and interpretation of the data, writing the article, and decision to submit the article for publication.
Funding
The WHI program described in this report is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
References
- 1.Beatson CT. On treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet. 1896;148(3803):162–165. [PMC free article] [PubMed] [Google Scholar]
- 2.Feinlieb M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41(2):315–329. [PubMed] [Google Scholar]
- 3.MacMahon B, Cole P, Lin M, et al. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43(2):209–221. [PMC free article] [PubMed] [Google Scholar]
- 4.McGuire WL, Chamness GC. Studies on the estrogen receptor in breast cancer. Adv Exp Med Biol. 1973;36(0):113–136. doi: 10.1007/978-1-4684-3237-4_7. [DOI] [PubMed] [Google Scholar]
- 5.Hoover R, LA Gray, Sr, Cole P, MacMahon B. Menopausal estrogens and breast cancer. N Engl J Med. 1976;295(8):401–405. doi: 10.1056/NEJM197608192950801. [DOI] [PubMed] [Google Scholar]
- 6.Ross RK, Paganini-Hill A, Gerkins VR, et al. A case-control study of menopausal estrogen therapy and breast cancer. JAMA. 1980;243(16):1635–1639. [PubMed] [Google Scholar]
- 7.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303(5920):767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 8.Bergkvist L, Adami HO, Persson I, Hoover R, Schairer C. The risk of breast cancer after estrogen and estrogen-progestin replacement. N Engl J Med. 1989;321(5):293–297. doi: 10.1056/NEJM198908033210505. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;322(24):1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 10.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 11.Beral V. Breast cancer and hormone replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 12.Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy I: a 10-year prospective study in the relationship of osteoporosis. Obstet Gynecol. 1979;53(3):277–281. [PubMed] [Google Scholar]
- 13.Stampfer MJ, Colditz GA, Willet WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurse's Health Study. N Engl J Med. 1991;325(11):756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 14.McBee WL, Dailey ME, Dugan E, Shumaker SA. Hormone replacement therapy and other potential treatments for dementias. Endocrinol Metab Clin North Am. 1997;26(2):329–345. doi: 10.1016/s0889-8529(05)70250-4. [DOI] [PubMed] [Google Scholar]
- 15.Greendale GA, Judd HL. The menopause: health implications and clinical management. J Am Geriatr Soc. 1993;41(4):426–436. doi: 10.1111/j.1532-5415.1993.tb06953.x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13(9 suppl):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 17.Leung BS, Fletcher WS, Lindell TD, Wood DC, Krippaechne WW. Predictability of response to endocrine ablation in advanced breast carcinoma. A correlation to estrogen receptor and steroid sulfurylation. Arch Surg. 1973;106(4):515–519. doi: 10.1001/archsurg.1973.01350160129021. [DOI] [PubMed] [Google Scholar]
- 18.Anderson GL, Limacher M, Assaf R, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(13):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski RT. Reducing the risk of breast cancer. N Engl J Med. 2000;343(3):191–198. doi: 10.1056/NEJM200007203430307. [DOI] [PubMed] [Google Scholar]
- 20.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 21.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone, and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106(1–5):24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Key TJ. Endogenous oestrogens and breast cancer risk in premenopausal and postmenopausal women. Steroids. 2011;76(8):812–815. doi: 10.1016/j.steroids.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Hormone replacement therapy and cancer: is there cause for concern. Lancet. 1989;2(8659):368–369. doi: 10.1016/s0140-6736(89)90545-x. [DOI] [PubMed] [Google Scholar]
- 25.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast Cancer and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 26.Jensen EV, Block GE, Smith S, Kyser K, DeSombre ER. Estrogen receptors and breast cancer response to adrenalectomy. Natl Cancer Inst Monogr. 1971;34:55–70. [PubMed] [Google Scholar]
- 27.Salagame U, Canfell K, Banks E. An epidemiological overview of the relationship between hormone replacement therapy and breast cancer. Expert Rev Endocrinol Metab. 2011;6(3):397–409. doi: 10.1586/eem.11.31. [DOI] [PubMed] [Google Scholar]
- 28.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakken K, Fournier A, Lund E, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128(1):144–156. doi: 10.1002/ijc.25314. [DOI] [PubMed] [Google Scholar]
- 30.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Anderson GL, Pettinger M, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168(4):370–377. doi: 10.1001/archinternmed.2007.123. [DOI] [PubMed] [Google Scholar]
- 33.McTiernan A, Martin CF, Peck JD, et al. Estrogen plus progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst. 2005;97(18):1366–1376. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 34.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after estrogen plus progestin use in postmenopausal women. New Eng J Med. 2009;360(6):573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacroix AZ, Chlebowski RT, Manson JE, et al. Health risks and benefits 5 years after stopping randomized treatment with conjugated equine estrogens in postmenopausal women with prior hysterectomy. JAMA. 2011;305(13):1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen plus progestin. JAMA. 2008;299(9):1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 38.Davies C, Godwin J, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomized trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chlebowski RT, McTiernan A, Aragaki AK, et al. Metformin and breast cancer incidence in postmenopausal diabetic women in the Women’s Health Initiative (WHI) J Clin Oncol. 2011;29(suppl) Abstract 1503. [Google Scholar]
- 40.Chen WY. Exogenous and endogenous hormones and breast cancer. Breast Pract Res Clin Endocrinol Metab. 2008;22(4):573–585. doi: 10.1016/j.beem.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reeves GK, Beral V, Green J, Gathani T, Bull D. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort and meta-analysis. Lancet Oncol. 2006;7(11):910–918. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 42.Kerlikowske K, Miglioretti DL, Ballard-Barbash R, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. J Clin Oncol. 2003;21(23):4314–4321. doi: 10.1200/JCO.2003.05.151. [DOI] [PubMed] [Google Scholar]
- 43.Holli K, Isola J, Cuzick J. Low biologic aggressiveness in breast cancer in women using hormone replacement therapy. J Clin Oncol. 1998;16(9):3115–3120. doi: 10.1200/JCO.1998.16.9.3115. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg LU, Granath F, Dickman PW, et al. Menopausal hormone therapy in relation to breast cancer characteristics and prognosis: a cohort study. Breast Cancer Res. 2008;10(5):R78. doi: 10.1186/bcr2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sener SF, Winchester DJ, Winchester DP, et al. The effects of hormone replacement therapy on postmenopausal breast cancer biology and survival. Am J Surg. 2009;197(3):403–407. doi: 10.1016/j.amjsurg.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Norman SA, Weber AL, Localio AR, et al. Hormone therapy and fatal breast cancer. Pharmacoepidemiol Drug Saf. 2010;19(5):440–447. doi: 10.1002/pds.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newcomb PA, Egan KM, Trentham Dietz A, et al. Prediagnostic use of hormone therapy and mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(4):864–871. doi: 10.1158/1055-9965.EPI-07-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reding KW, Doody DR, McTiernan A, et al. Age-related variation in the relationship between menopausal hormone therapy and the risk of dying from breast cancer. Breast Cancer Res Treat. 2001;126(3):749–761. doi: 10.1007/s10549-010-1174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 50.Schairer C, Lubin, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283(4):485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 51.Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92(4):328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 52.Marsdon J. Hormone-replacement therapy and breast cancer. Lancet Oncol. 2002;3(5):303–311. doi: 10.1016/s1470-2045(02)00732-5. [DOI] [PubMed] [Google Scholar]
- 53.Karim R, Mack WJ, Hodis HN, Roy S, Stanczyk FZ. Influence of age and obesity on serum estradiol, estrone, and sex hormone binding globulin concentrations following oral estrogen administration in postmenopausal women. J Clin Endocrinol Metab. 2009;94(11):4136–4143. doi: 10.1210/jc.2009-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joffe MM, Byrne C, Colditz GA. Postmenopausal hormone use, screening and breast cancer characterization and control of a bias. Epidemiology. 2001;12(4):429–438. doi: 10.1097/00001648-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Gordon NP, Hiatt RA, Lampert DI. Correspondence of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. 1993;85(7):566–570. doi: 10.1093/jnci/85.7.566. [DOI] [PubMed] [Google Scholar]
- 56.Hofvind S, Sorum R, Haldorsen T, Langmark F. Incidence of breast cancer before and after implementation of mammography screening. Tidsskr Nor Laegeforen. 2006;126(22):2935–2938. [PubMed] [Google Scholar]
- 57.Sihto H, Lundin J, Lehtimaki T, et al. Molecular subtypes of breast cancers detected in mammography screening and outside of screening. Clin Cancer Res. 2008;14(13):4103–4110. doi: 10.1158/1078-0432.CCR-07-5003. [DOI] [PubMed] [Google Scholar]
- 58.Dong W, Berry DA, Bevers TB, et al. Prognostic role of detection method and its relationship with tumor biomarkers in breast cancer: the University of Texas M.D. Anderson Cancer Center experience. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1096–1103. doi: 10.1158/1055-9965.EPI-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;28(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 60.Hersh AL, Stefanick ML, Stafford RS. National use of menopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 61.Clarke CA, Glaser SL, Uratsu CS, Selby JV, Kushi LH, Herinton LJ. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and popilation-based evidence. J Clin Oncol. 2006;24(33):e49–e50. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 62.Ravdin PM, Cronin KA, Howlander N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 63.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980-2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99(15):1152–1161. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 64.Katalinic A, Rawal R. Decline in breast cancer incidence after decrease in utilisation of hormone replacement therapy. Breast Cancer Res Treat. 2008;107(3):427–430. doi: 10.1007/s10549-007-9566-z. [DOI] [PubMed] [Google Scholar]
- 65.Canfell K, Banks E, Moa AM, Beral V. Decrease in breast cancer incidence following a rapid fall in use of hormone replacement therapy in Australia. Med J Aust. 2008;188(11):641–644. doi: 10.5694/j.1326-5377.2008.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 66.Seradour B, Allemand H, Weill A, Ricordeau P. Changes by age in breast cancer incidence, mammography screening and hormone therapy use in France from 2000 to 2006. Bull Cancer. 2009;96(4):E1–E6. doi: 10.1684/bdc.2009.0869. [DOI] [PubMed] [Google Scholar]
- 67.Neutel CI, Morrison H. Could recent decreases in breast cancer incidence really be due to lower HRT use? Trends in attributable risk for modifiable breast cancer risk factors in Canadian women. Can J Public Health. 2010;101(5):405–409. doi: 10.1007/BF03404862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294(2):183–193. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 69.International Breast Cancer Study Group (IBCSG) Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2003;95(24):1833–1846. doi: 10.1093/jnci/djg119. [DOI] [PubMed] [Google Scholar]
- 70.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2001;103(18):1–6. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chlebowski RT, Chen Z, Cauley JA, et al. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J Clin Oncol. 2010;28(22):3582–3590. doi: 10.1200/JCO.2010.28.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prentice RL, Pettinger M, Anderson GL. Statistical issues arising in the Women's Health Initiative. Biometrics. 2005;61(4):899–911. doi: 10.1111/j.0006-341X.2005.454_1.x. [DOI] [PubMed] [Google Scholar]
- 73.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295(14):647–657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 74.Chlebowski RT, Anderson GL, Manson JE, et al. Estrogen alone in postmenopausal women and breast cancer detection by means by mammography and breast biopsy. J Clin Oncol. 2010;28(16):690–697. doi: 10.1200/JCO.2009.24.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McTiernan A, Chlebowski RT, Martin C, et al. Conjugated equine estrogen influence on mammographic density in postmenopausal women in a sub-study of the Women's Health Initiative randomized trial. J Clin Oncol. 2009;27(36):6135–6143. doi: 10.1200/JCO.2008.21.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167(12):1407–1415. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chlebowski RT, Anderson GL. The influence of time from menopause and mammography on hormone therapy-related breast cancer risk assessment. J Natl Cancer Inst. 2011;103(4):284–285. doi: 10.1093/jnci/djq561. [DOI] [PubMed] [Google Scholar]
- 78.Fournier A, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Estrogen-progestagen menopausal hormone therapy and breast cancer: does delay from menopause onset to treatment initiation influence risks? J Clin Oncol. 2009;27(31):5138–5143. doi: 10.1200/JCO.2008.21.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166(9):1027–1032. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 80.Goss PE, Ingle JN, Ales-Martinez J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 81.Jordan VC, Ford LG. Paradoxical clinical effects of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosis. Cancer Prev Res. 2011;4(5):633–637. doi: 10.1158/1940-6207.CAPR-11-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin LA, Ghazoui Z, Weigel MT, et al. An in vitro model showing adaptation to long-term oestrogen deprivation highlights the clinical potential for targeting kinase pathways in combination with aromatase inhibition. Steroids. 2011;76(8):772–776. doi: 10.1016/j.steroids.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 83.Lewis JS, Keeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97(23):1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 84.Yao K, Lee ED, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6(5):2028–2036. [PubMed] [Google Scholar]
- 85.Jordan VC. The 38th David A Karnofsky lecture: the paradoxical actions of estrogen in breast cancer – survival or death? J Clin Oncol. 2008;26(18):3073–3082. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 86.Dunbier AK, Martin LA, Dowsett M. New and translational perspective of oestrogen deprivation in breast cancer. Mol Cell Endocrinol. 2011;340(2):137–141. doi: 10.1016/j.mce.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 87.Song RXD, Mor G, Naftolin F, et al. Effect of long term estrogen deprivation on apoptotic responses of breast cancer cells to 17B-estradiol. J Natl Cancer Inst. 2001;93(22):1414–1423. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 88.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11(3):206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ingle JN, Ahmann DL, Green SJ, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304(1):16–21. doi: 10.1056/NEJM198101013040104. [DOI] [PubMed] [Google Scholar]
- 90.Lonning PE, Taylor PD, Anker G, et al. High dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67(2):111–116. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- 91.Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs. high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer. JAMA. 2009;302(2):774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santen RJ, Song RX, Zhang Z, et al. Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. J Steroid Biochem Mol Biol. 2005;95(1–5):155–165. doi: 10.1016/j.jsbmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 93.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348(24):2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 94.Chen JQ, Brown TR, Yager JD. Mechanisms of hormone carcinogenesis: evolution of views, role of mitochondria. Adv Exp Med Biol. 2008;630:1–18. [PubMed] [Google Scholar]
- 95.Santen RJ. Risk of breast cancer with progestins: critical assessment of current data. Steroids. 2003;68(10–13):953–964. doi: 10.1016/s0039-128x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- 96.Gadducci A, Biglia N, Cosio S, Sismondi P, Genazzani AR. Progestagen component in combined hormone replacement therapy in postmenopausal women and breast cancer risk: a debated clinical issue. Gynecological Endocrin. 2009;25(12):807–815. doi: 10.3109/09513590903056878. [DOI] [PubMed] [Google Scholar]
- 97.Allen NE, Tsilidis KK, Key TJ, et al. Menopausal hormone therapy and risk of endometrial carcinoma among postmenopausal women in the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172(12):1394–1403. doi: 10.1093/aje/kwq300. [DOI] [PubMed] [Google Scholar]
- 98.Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84(12):4559–4568. doi: 10.1210/jcem.84.12.6194. [DOI] [PubMed] [Google Scholar]
- 99.Joshi PA, Jackson HW, Beristain AG, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 100.Liang Y, Besch-Williford C, Brekken RA, Hyder SM. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating anti-tumor therapeutics. Cancer Res. 2007;67(20):9929–9936. doi: 10.1158/0008-5472.CAN-07-1103. [DOI] [PubMed] [Google Scholar]
- 101.Beleut M, Rajaram RD, Caikovski M, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci U S A. 2010;107(7):2989–2994. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000-2009. Menopause. 2011;18(4):385–392. doi: 10.1097/gme.0b013e3181f43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stahlberg C, Pedersen AT, Lynge E, et al. Increased risk of breast cancer following different regimens of hormone replacement therapy frequently used in Europe. Int J Cancer. 2004;109(5):721–727. doi: 10.1002/ijc.20016. [DOI] [PubMed] [Google Scholar]
- 104.Seeger H, Wallwiener D, Mueck AO. The effect of progesterone and synthetic progestins on serum and estradiol-stimulated proliferation of human breast cancer cells. Horm Metab Res. 2003;35(2):76–80. doi: 10.1055/s-2003-39061. [DOI] [PubMed] [Google Scholar]
- 105.Wood CE, Register TC, Lees CJ, Chen H, Kimrey S, Cline JM. Effects of estradiol with micronized progesterone or medroxyprogesterone acetate on risk markers for breast cancer in postmenopausal monkeys. Breast Cancer Res Treat. 2007;101(2):125–134. doi: 10.1007/s10549-006-9276-y. [DOI] [PubMed] [Google Scholar]
- 106.Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448–454. doi: 10.1002/ijc.20710. [DOI] [PubMed] [Google Scholar]
- 107.Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N-EPIC cohort study. Breast Cancer Res Treat. 2008;107(1):103–111. doi: 10.1007/s10549-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 109.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 110.Prentice RL, Chlebowski RT, Stefanick ML, et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol. 2008;167(10):1207–1216. doi: 10.1093/aje/kwn044. [DOI] [PMC free article] [PubMed] [Google Scholar]