Abstract
Background
Considerable effort has been expended on tobacco control strategies in the United States since the mid-1950s. However, we have little quantitative information on how changes in smoking behaviors have impacted lung cancer mortality. We quantified the cumulative impact of changes in smoking behaviors that started in the mid-1950s on lung cancer mortality in the United States over the period 1975–2000.
Methods
A consortium of six groups of investigators used common inputs consisting of simulated cohort-wise smoking histories for the birth cohorts of 1890 through 1970 and independent models to estimate the number of US lung cancer deaths averted during 1975–2000 as a result of changes in smoking behavior that began in the mid-1950s. We also estimated the number of deaths that could have been averted had tobacco control been completely effective in eliminating smoking after the Surgeon General’s first report on Smoking and Health in 1964.
Results
Approximately 795 851 US lung cancer deaths were averted during the period 1975–2000: 552 574 among men and 243 277 among women. In the year 2000 alone, approximately 70 218 lung cancer deaths were averted: 44 135 among men and 26 083 among women. However, these numbers are estimated to represent approximately 32% of lung cancer deaths that could have potentially been averted during the period 1975–2000, 38% of the lung cancer deaths that could have been averted in 1991–2000, and 44% of lung cancer deaths that could have been averted in 2000.
Conclusions
Our results reflect the cumulative impact of changes in smoking behavior since the 1950s. Despite a large impact of changing smoking behaviors on lung cancer deaths, lung cancer remains a major public health problem. Continued efforts at tobacco control are critical to further reduce the burden of this disease.
CONTEXT AND CAVEATS
Prior knowledge
The proportion of smokers among the US population has gradually declined since the mid-1950s, as the dangers of tobacco use became apparent and tobacco control laws were enacted. However, there are few estimates of how many lung cancer deaths were spared by the decline in cigarette smoking.
Study design
Six groups of investigators built independent models based on cohort, case–control, or registry data and calibrated to mortality or other data to estimate the number of lung cancer deaths averted in 1975–2000. The data were stratified by sex and birth decade (1890–1970), and the prevalence of smoking and lung cancer deaths under three scenarios were considered: actual tobacco control (ATC), based on historical changes in smoking rates; no tobacco control (NTC), based on predicted smoking rates if tobacco control had not been enacted; and complete tobacco control (CTC), which considers what might have happened if all smoking ceased in 1965.
Contribution
In the United States in 1975–2000, there were 2 067 775 lung cancer deaths among men and 1 051 978 lung cancer deaths among women. The models predict that over 550 000 lung cancer deaths among men and over 240 000 lung cancer deaths among women were averted by tobacco control efforts. If all smokers had quit in response to the Surgeon General's first report in 1964, over 1.6 million lung cancer deaths might have been averted among men, and over 880 000 among women.
Implication
Tobacco control efforts do appear to have reduced smoking behavior and lung cancer deaths.
Limitations
There is some variation in the estimates among models, depending on data source and calibration. Data were not available for other exposures related to lung cancer incidence and non-cigarette forms of tobacco use were not considered. The relative contributions of decreased smoking initiation and increased smoking cessation were not addressed.
From the Editors
After the US Surgeon General's first report on Smoking and Health was issued in 1964, several initiatives, collectively known as “tobacco control,” included restrictions on smoking in public places, large increases in cigarette excise taxes, reduced access to cigarettes, and increased public awareness of the hazards of smoking. These smoking regulations have been cited as the principal contributors to the observed decline in US adult tobacco use from 1975 to 2003 (1,2) and to subsequent declines in smoking-related mortality (3). In 2004, the Surgeon General (4) estimated that during 1965–1999, approximately 3 million lung cancer deaths (2.2 million among men and 0.8 million among women) in the United States were attributable to smoking. Using a straightforward demographic projection, Thun and Jemal (5) estimated that reductions in tobacco smoking averted approximately 146 000 lung cancer deaths among US men during 1991–2003.
In this article, we analyzed the direct influence that changes in smoking behaviors that began in the mid-1950s had on lung cancer mortality rates among men and women aged 30-84 years in the United States during 1975–2000. We also estimated the total number of lung cancer deaths averted among men and women during the same period as a direct result of changes in smoking behavior. Finally, we estimated the numbers of avoidable deaths, that is, the number of lung cancer deaths that could have been averted had smoking been completely eliminated as of 1965.
Methods
A consortium of six universities and research centers (Erasmus Medical Center [Erasmus MC], Rotterdam, the Netherlands; Fred Hutchinson Cancer Research Center [FHCRC], Seattle, WA; Pacific Institute for Research and Evaluation [PIRE], Calverton, MD; Rice University and M.D. Anderson Cancer Center [Rice-MDA], Houston, TX; Massachusetts General Hospital and Harvard Medical School [MGH-HMS], Cambridge, MA; and Yale University, New Haven, CT) developed independent models to estimate the impact of tobacco control policies on lung cancer mortality. Although the models shared common inputs, each group developed its own model, based on mathematical descriptions of lung carcinogenesis as it relates to smoking behaviors. The models explicitly consider factors associated with the risk of smoking, including the number of cigarettes smoked per day, the age of initiation, and the number of years quit.
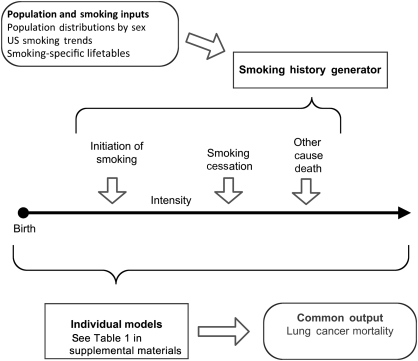
All models shared the same overall structure (Figure 1). The central component of each model was a dose–response module that provided a quantitative description of the age-specific lung cancer mortality among never smokers and age-specific lung cancer mortality among continuing smokers and former smokers by detailed history of smoking. This module was used to predict age-specific lung cancer mortality rates associated with three specific smoking scenarios. With the exception of the MGH-HMS group, which used a set of logistic regression models and tumor progression functions (6,7), the other groups used multistage models (8–11) for the underlying dose–response module. Multistage models, based on mathematical formalisms representing the biological paradigm of initiation, promotion, and progression, recognize that carcinogenesis includes accumulation of mutations and clonal expansion of partially altered cells on the pathway to malignancy (8,12–17). These models may be used to explore biological hypotheses regarding the mechanism of tobacco-induced lung cancer. They have generally shown clonal expansion (promotion) of partially altered (initiated) cells by cigarette smoke to be the dominant mechanism and have confirmed the disproportionate importance of smoking duration on lung cancer risk (10,11,18–21). Both the multistage models and the probabilistic model of MGH-HMS are capable of accommodating detailed individual-level smoking histories, including temporal factors, such as age at start, age at cessation, and temporal changes in the level of smoking. The parameters of these models were estimated as described in the supplementary material (available online) by fitting the model to specific epidemiological cohort data (Erasmus MC, FHCRC, PIRE, Yale), case–control data (Rice-MDA), or registry data (MGH-HMS). The Erasmus, FHCRC, and Yale model parameter estimates were obtained by fits of the Two-Stage Clonal Expansion (TSCE) model to the Nurses Health Study (NHS) and Health Professionals Follow-up Study (HPFS) cohort data (11). The model parameters for the PIRE model were derived by fitting the TSCE model to the Cancer Prevention Study II (CPS II) data (10). The parameters for the Rice-MDA model were derived from fitting the TSCE model to data from a case–control study conducted to evaluate the interaction of smoking and genetics on lung cancer risk (21). The parameters for the MGH-HMS model were estimated by fitting a nested set of logistic regression models for cancer development and spread along with a tumor growth function to lung cancer incidence in the Surveillance, Epidemiology, and End Results (SEER) registry during the period 1990–2000 (6,7).
Figure 1.
Process shared by all models. Population and smoking inputs were used to develop the smoking history generator, which, in turn, simulates detailed individual-level smoking and other-cause mortality histories. These individual histories were used by each of the modeling groups to estimate lung cancer mortality rates in the population.
Three specific smoking scenarios, the common inputs for the models, were simulated using a smoking history generator as briefly described below. Each involved a detailed description of smoking behaviors by sex and birth cohort starting with the birth cohort of 1890 and ending with the birth cohort of 1970. The actual tobacco control (ATC) scenario is a quantitative description of the actual smoking behaviors of men and women in the United States. The no tobacco control (NTC) scenario is a quantitative description of the predicted smoking behaviors of men and women in the United States under the assumption that tobacco control efforts starting mid-century had never been implemented. Initiation rates in the NTC scenario are from models fitted to survey data after age and cohort effects had stabilized. For men, these are cohorts born after 1904. The history for women is more complex because age effects stabilized after 1919 but cohort effects continued to increase; therefore, a linear cohort trend for 1930–1955 was used in the model for log rate, and the estimated parameter was held constant after 1955. For smoking cessation rates, we used data for individuals born in 1900–1904 because they would have had little knowledge of the health effects of smoking for most of their lives while at the same time being sufficiently well represented in surveys to provide accurate estimates of these rates. The complete tobacco control (CTC) scenario is a quantitative description of the predicted smoking behaviors of men and women in the United States under the assumption that all smoking ceased abruptly in 1965, that is, all smokers quit permanently at that time and there was no initiation of smoking after 1964. Clearly, the CTC scenario represents the best imaginable outcome for smoking behavior. Our justification for using this scenario is that it is transparent and unambiguous. More details about the construction of these scenarios are on the Cancer Intervention and Surveillance Modeling Network (CISNET) web site (http://cisnet.cancer.gov last accessed Feb 19, 2012) and in a forthcoming group of articles (22).
The models used in CISNET did not incorporate other known or suspected risk factors, such as environmental tobacco smoke, radon exposure, diet, and air pollution (23–25), which could have influenced trends in lung cancer mortality in the United States. Moreover, the datasets from which the parameters of the individual dose–response modules were estimated may not be representative of the US population. For these reasons, the outputs of the models under the ATC scenario cannot be expected to reproduce the observed lung cancer rates in the US population. Rather, without further calibration, these models estimate lung cancer rates in hypothetical populations with the same smoking behaviors and the same age structure as the US population in 1975–2000. To compensate for these limitations, some groups (FHCRC, MGH-HMS, Yale, PIRE) chose to calibrate their models further to describe actual deaths in the US population under the ATC scenario during the period 1975–2000. In the models of the FHCRC, MGH-HMS, and Yale groups, this calibration was achieved by embedding the dose–response module in an age–period–cohort model. The exception was the PIRE model, which used only a period calibration. Other groups (Erasmus MC, Rice-MDA) chose not to perform this additional calibration. The overall model structure and the specific models used by each group are described in greater detail in the supplementary materials (available online).
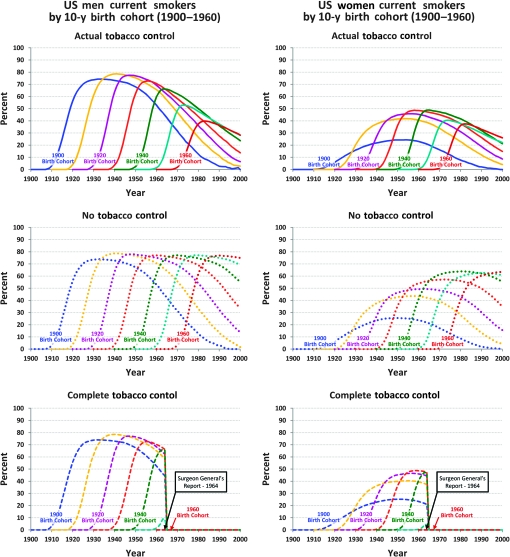
For each smoking scenario (ATC, NTC, CTC), the smoking history generator provided, as its output, detailed smoking histories during the period 1975–2000 for individuals born between 1890 and 1970. As an example, we show one output of the smoking history generator, the proportion of current smokers in the three smoking scenarios (Figure 2). For the ATC scenario, the initial increase in smoking prevalence for each cohort results primarily from initiation and the subsequent decline reflects both smoking cessation and increased mortality among smokers. Deaths from causes other than lung cancer were also simulated by the level of smoking; each individual history was terminated at 84 years or at the age of death from a cause other than lung cancer if the death occurred before 84 years. Details of the construction of the smoking history generator can be found on the CISNET website (http://cisnet.cancer.gov and supplementary material, available online). Using the outputs of the smoking history generator, each group estimated the number of lung cancer deaths during the period 1975–2000 while adjusting for other-cause mortality in each smoking scenario.
Figure 2.
Percentage of current smokers in the US population by sex and birth cohort, assuming three different tobacco control scenarios. This is one of the outputs that can be generated from the smoking history generator. The output from the actual tobacco control scenario describes the observed data well (not shown).
Results
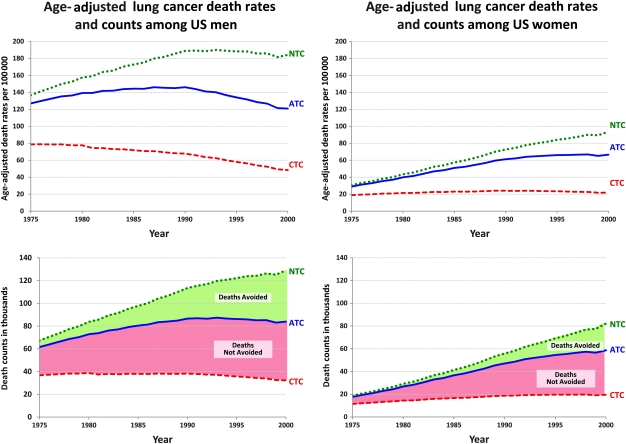
The models yielded a range of results for the numbers of lung cancer deaths among the three smoking scenarios, but the estimates of the fraction of lung cancer deaths averted were reasonably consistent across models. For purposes of illustration, we chose one of the models calibrated against the US population data (the Yale model) as an exemplar model to present our results (Figure 3). Based on this model, we could estimate the actual age-adjusted rates and the actual number of lung cancer deaths among men and women aged 30-84 years in the United States during the period 1975–2000 and the numbers that would have been expected assuming the NTC and CTC scenarios. During the period 1975–2000, there were 2 067 775 lung cancer deaths among men and 1 051 978 lung cancer deaths among women in the United States. Assuming NTC conditions, the Yale model estimated 2 670 897 lung cancer deaths among men and 1 273 151 lung cancer deaths among women, whereas assuming CTC conditions, the deaths numbered 958 862 and 438 858, respectively, among men and women. The difference between the NTC and observed numbers provides an estimate of the numbers of lung cancer deaths averted (A), which for the Yale model are 603 122 and 221 173 for men and women, respectively. The difference between NTC and CTC (B) is an estimate of the total number of lung cancer deaths that could have been averted if tobacco control efforts had been immediately and completely successful with all smoking ending in 1965, which were approximately 1 712 035 and 834 293 for men and women, respectively, in the Yale model. The averages for these figures across all models are 1 620 686 and 883 356.
Figure 3.
Lung cancer death rates and counts for men and women aged 30-84 years as observed and for modeled tobacco control scenarios. ATC = Actual Tobacco Control; CTC = Complete Tobacco Control; NTC = No Tobacco Control.
We also examined the difference between the NTC and observed numbers to obtain an estimate of the numbers of lung cancer deaths averted in all models. Approximately 795 851 US lung cancer deaths were averted. The high estimate for men was 658 529, the low estimate was 454 517, and the average was 552 574 (Table 1). For women, the high estimate was 333 976, the low estimate was 201 788, and the average was 243 277. When we looked at the difference between NTC and CTC, again, as an estimate of the total number of lung cancer deaths that could have been averted if tobacco control efforts had been immediately and completely successful, we estimated that approximately 2 504 042 lung cancer deaths could have been averted in men and women combined. This estimate represents the average of the estimates for the models shown in Table 1. In the year 2000 alone, approximately 70 218 lung cancer deaths were averted: 44 135 among men and 26 083 among women. These numbers are estimated to represent approximately 32% of lung cancer deaths that could have potentially been averted during the period 1975–2000, 38% of the lung cancer deaths that could have been averted in 1991–2000, and 44% of lung cancer deaths that could have been averted in 2000.
Table 1.
Realized and potential reductions in lung cancer mortality from changes in smoking behavior among men and women aged 30-84 years.*
| Realized proportion of potential benefit from tobacco control, by year(s) | Women | Men | Overall | ||||||
| Realized (NTC–ATC) | Potential (NTC–CTC) | Proportion realized | Realized (NTC–ATC) | Potential (NTC–CTC) | Proportion realized | Realized (NTC–ATC) | Potential (NTC–CTC) | Proportion realized | |
| 1975–2000 | |||||||||
| Erasmus MC | 201 788 | 806 ,320 | 0.25 | 658 529 | 1 757 857 | 0.37 | 860 317 | 2 564 177 | 0.34 |
| FHCRC | 202 817 | 862 ,610 | 0.24 | 508 777 | 1 680 867 | 0.30 | 711 594 | 2 543 477 | 0.28 |
| MGH-HMS | 214 830 | 854 112 | 0.25 | 487 263 | 1 597 733 | 0.30 | 702 092 | 2 451 845 | 0.29 |
| PIRE | 333 976 | 1 064 443 | 0.31 | 454 517 | 1 329 972 | 0.34 | 788 493 | 2 394 415 | 0.33 |
| Rice-MDA | 285 079 | 878 359 | 0.32 | 603 236 | 1 645 651 | 0.37 | 888 316 | 2 524 010 | 0.35 |
| Yale | 221 173 | 834 293 | 0.27 | 603 122 | 1 712 035 | 0.35 | 824 294 | 2 546 328 | 0.32 |
| Mean | 243 277 | 883 356 | 0.28 | 552 574 | 1 620 686 | 0.34 | 795 851 | 2 504 042 | 0.32 |
| 1991–2000 | |||||||||
| Erasmus MC | 143 273 | 462 528 | 0.31 | 384 882 | 834 310 | 0.46 | 528 155 | 1 296 837 | 0.41 |
| FHCRC | 152 574 | 521 040 | 0.29 | 318 279 | 842 602 | 0.38 | 470 853 | 1 363 642 | 0.35 |
| MGH-HMS | 153 549 | 511 509 | 0.30 | 310 210 | 846 300 | 0.37 | 463 759 | 1 357 809 | 0.34 |
| PIRE | 253 711 | 687 156 | 0.37 | 342 558 | 865 306 | 0.40 | 596 269 | 1 552 462 | 0.38 |
| Rice-MDA | 185 782 | 461 559 | 0.40 | 346 266 | 785 168 | 0.44 | 532 048 | 1 246 727 | 0.43 |
| Yale | 157 388 | 507 085 | 0.31 | 366 815 | 871 273 | 0.42 | 524 203 | 1 378 358 | 0.38 |
| Mean | 174 380 | 525 146 | 0.33 | 344 835 | 840 827 | 0.41 | 519 214 | 1 365 972 | 0.38 |
| 2000 | |||||||||
| Erasmus MC | 20 277 | 55 337 | 0.37 | 48 897 | 94 979 | 0.51 | 69 173 | 150 316 | 0.46 |
| FHCRC | 22 271 | 63 373 | 0.35 | 39 076 | 92 434 | 0.42 | 61 347 | 155 807 | 0.39 |
| MGH-HMS | 21 532 | 60 774 | 0.35 | 38 375 | 92 187 | 0.42 | 59 907 | 152 961 | 0.39 |
| PIRE | 40 496 | 90 001 | 0.45 | 50 943 | 110 800 | 0.46 | 91 439 | 200 802 | 0.46 |
| Rice-MDA | 28 365 | 55 988 | 0.51 | 42 351 | 86 863 | 0.49 | 70 716 | 142 851 | 0.50 |
| Yale | 23 559 | 62 628 | 0.38 | 45 165 | 96 794 | 0.47 | 68 723 | 159 422 | 0.43 |
| Mean | 26 083 | 64 684 | 0.40 | 44 135 | 95 676 | 0.46 | 70 218 | 160 360 | 0.44 |
ATC = Actual Tobacco Control; CTC = Complete Tobacco Control; NTC = No Tobacco Control. The realized benefits of ATC are estimated by the difference (NTC–ATC); the potential total benefits are estimated by the difference (NTC–CTC); the proportion realized is given by the quotient of realized benefits and total potential benefits: (NTC–ATC)/(NTC–CTC). The six study groups that produced models are as follows: Erasmus MC = Erasmus Medical Center, Rotterdam, the Netherlands; FHCRC = Fred Hutchinson Cancer Research Center, Seattle, WA; MGH-HMS = Massachusetts General Hospital and Harvard Medical School, Cambridge, MA; PIRE = Pacific Institute for Research and Evaluation, Calverton, MD; Rice-MDA = Rice University and M.D. Anderson Cancer Center, Houston, TX; and Yale = Yale University, New Haven, CT.
The other models calibrated to US mortality yielded similar estimates of the number of lung cancer deaths among the three scenarios (data not shown). Counts of the differences in the number of deaths between scenarios are shown for all models in Table 1 and in Supplementary Figure 1 (available online). The ratio of deaths averted to total deaths that could potentially have been avoided (ie, A/B) is also presented in Table 1. The models estimate that of all avoidable deaths from smoking-related lung cancer, between 24% and 32% among women and between 30% and 37% among men were actually averted as a result of the changes in smoking behaviors that actually began in the mid-1950s some years before the first Surgeon General's Report. For both sexes combined, approximately 32% (28%–35% across models) of all avoidable deaths were averted. Table 1 also shows the impact of tobacco control efforts on lung cancer mortality for the decade 1991–2000 and for the year 2000. In the decade 1991–2000, the fraction of lung cancer deaths averted in men and women combined increased to about 38% (34%–43% across models). In the year 2000, this fraction increased to roughly 44% (39%–50% across models). The increasing trend in the fraction of lung cancer deaths averted reflects both changes in smoking behaviors and a continuing decrease in risk among former smokers.
Discussion
A consortium of six research groups used data from common sources to recreate detailed cigarette smoking histories under three distinct tobacco control scenarios as inputs for mathematical models to quantify the impact of changing smoking behavior on lung cancer mortality rates in the United States during 1975–2000. We used a comparative modeling approach to address this complex problem; comparative modeling produces a range of results across models but, when these are reasonably consistent, enhances their credibility. During the period 1975–2000, approximately 2 504 042 lung cancer deaths among men and women combined could have been averted had tobacco control efforts been completely effective in eliminating smoking as of 1965; of these, we estimate that approximately 795 851 lung cancer deaths were averted or about one-third of what was possible. During the period 1991–2000, we estimate that approximately 345 000 lung cancer deaths among US men and 175 000 deaths among US women were averted due to changes in smoking behaviors starting in the mid-1950s. These estimates of reduced lung cancer mortality associated with reduced tobacco use are much larger than an estimate from demographic projections that 146 000 lung cancer deaths among men were averted in 1991–2003 (5). We estimate that in the year 2000 alone, approximately 44 000 deaths were averted among US men and 26 000 deaths among US women.
It is not surprising that the various models used in this article yielded a range of estimates of the fraction of lung cancer deaths averted by the tobacco control efforts in the United States. This range of results represents the uncertainty associated with model choice. First, some of these models were calibrated against US mortality data, and, as a consequence, these models describe the lung cancer mortality trends in the United States very well under the ATC scenario. Second, although five of the six groups used the TSCE version of multistage models (8,10,11) as the dose–response module, the estimated parameters were different because they were estimated by their fit to different cohorts. In addition to the TSCE model, the Yale group also used the models developed by Knoke et al. (9) and Flanders et al. (26) and obtained similar estimates of the relative effect of tobacco control. It is well known that the risks of tobacco smoking have changed over time; moreover, they could be modified by other factors such as diet that are not accounted for in any of the models. Despite these limitations, the estimated numbers of deaths averted and deaths that could have been averted under the assumption of CTC were reasonably consistent across models (Table 1). The main message of these analyses is clear. Tobacco control strategies implemented mid-century have averted hundreds of thousands of lung cancer deaths in the United States during the period 1975–2000, but these are only approximately 30% of the lung cancer deaths that could have been averted had all cigarette smoking ended in 1965.
The FHCRC, MGH-HMS, and Yale groups calibrated their models to US mortality during 1975–2000 using birth cohort and period effects. These calibrations are necessary to describe lung cancer mortality rates and trends in the United States and indicate that the lung cancer mortality experience of the entire population cannot be adequately described by extrapolating from the SEER registry in one decade, or from various cohort and case–control studies of smoking and lung cancer (please see the supplementary material, available online, for the datasets used by each of the groups for parameter estimation). Particularly among men, US lung cancer mortality is considerably higher than would be expected from the cohort studies against which the dose–response modules were calibrated. In addition, models from cohort studies and available population smoking histories cannot adequately describe temporal components of trend, that is, the effects of age, period, and birth cohort.
There could be several reasons why the models were poor at predicting population lung cancer rates without additional calibrations. First, the datasets used for estimating the parameters of the dose–response modules were almost certainly not representative of the US population. Second, the smoking history generator was based on smoking histories for birth cohorts in the general population that were inferred from simulations using cross-sectional histories that often relied on subjects’ recall of events that occurred several years earlier. Third, potentially important covariates (eg, diet, air pollution, and radon exposure) and occupational exposures (including asbestos and ionizing radiation) were not available for the overall population, and different exposure distributions could contribute to rate discrepancies. Fourth, although the models discussed assume a consistent effect of exposure on lung cancer mortality, temporal changes in the manufacture of cigarettes and smoking behaviors could explain some of the discrepancies in trend, and data on changes in cigarette manufacturing and composition are not readily available. Changes in tobacco or cigarette composition, which were not explicitly addressed in these analyses, could be important contributors to population trends in lung cancer mortality. However, one would expect changes in tobacco or cigarette composition to manifest themselves as period effects, whereas models that used age–period–cohort calibrations find that trends are dominated by birth cohort effects. Finally, uncertainty remains with respect to the models themselves.
In particular, our estimates of the lung cancer rates in the US population under the CTC scenario appear to be higher than would have been expected on the basis of recent work on lung cancer rates among never smokers (27). However, for the reasons given above (cohorts not representative of the general population, omission of important covariates), the never-smoker rates reported by Thun et al. (27) may not reflect the never-smoker rates in the general population. Some confidence in the lung cancer rates under the CTC scenario estimated from the models in this article can be derived from the fact that the dose–response modules describe lung cancer rates among former smokers well (9,10,11).
One limitation of the calibrations is that the same period and cohort parameters are applied to current smokers, former smokers, and never smokers. Factors, such as diet, that could affect trends in lung cancer rates might be expected to have different effects among current smokers, former smokers, and never smokers. However, different cohort and period effects could not be estimated in these subgroups because of identifiability issues. The FHCRC group did fit period and cohort effects to never smokers alone and to current smokers and former smokers separately, but the original model in which these effects are applied equally to all groups described the data better as judged by the Akaike Information Criterion Statistics (28).
Overall, our study shows that changes in smoking behaviors led to a substantial reduction in the lung cancer mortality that would have been expected had the smoking trends in the 1950s continued into the future. Our analysis was conducted through to the year 2000, the latest year for which we were able to obtain sufficiently detailed data when this project was initiated. Consistent with trends for continued gains due to past tobacco control policies, smoking prevalence continued to fall from 23.2% in 2000 to 20.6% in 2008. Much of this decrease can be attributed to tobacco control policies, especially the cigarette price increases in 1998–1999 (29).
There are also other limitations to our study. We did not quantitatively assess the relative contributions made by changing patterns of smoking initiation and cessation to decreases in lung cancer mortality. It is clear, however, that most of the benefits of tobacco control policies during the period 1975–2000 have accrued from smoking cessation because changing patterns of smoking initiation would have impacted only individuals who were aged 55 or younger in 2000 and thus younger than the age at which lung cancer mortality begins to increase rapidly. Also, our numbers are likely to greatly underestimate the overall health impact of tobacco control efforts because they neither consider the substantial impact of non-cigarette forms of tobacco use (eg, cigars and pipes) nor the impact of tobacco smoking behaviors on diseases other than lung cancer. Smoking-associated diseases other than lung cancer, such as cardiovascular disease, were outside the scope of this work.
The results of this article show the dramatic impact of the reduction in smoking associated with tobacco control efforts in the second half of the 20th century on lung cancer mortality during the period 1975–2000. Even though other factors, including genetic polymorphisms (27), contribute to lung cancer risk, the vast majority of lung cancer cases could be eliminated by eliminating smoking. Our results indicate that only approximately 30% of the total lung cancer deaths that could have been averted had tobacco control been complete were actually averted. This is because smoking rates took time to decline after the first Surgeon General's Report in 1965; smokers’ risk of lung cancer remains elevated for many years after smoking cessation and a sizable fraction of the population continued to smoke.
Clearly, further reductions in smoking rates will be required to reduce lung cancer incidence and mortality rates substantially. The recently reported 20% reduction in lung cancer mortality (30) as a result of early detection using low-dose spiral CT suggests that screening of high-risk individuals may play a role in reducing mortality from this disease. Because risk of lung cancer remains elevated for a long time among smokers who quit, effective screening techniques may have a role in reducing lung cancer mortality among ex-smokers. However, continued implementation of evidence-based tobacco control policies, programs, and services remains the most promising approach to reducing the burden of lung cancer.
Supplementary Material
Footnotes
The sponsoring agencies had no role in designing and conducting the research and in the collection and analyses of the data. The sponsoring agencies did not participate in the writing of the article and had no role in the decision to publish.
Dr R. Boer is affiliated with RAND and is a consultant at Verner LifeSciences. Dr G. S. Gazelle is a consultant for GE Healthcare. However, the authors declare no conflicts of interest.
Funding
The research reported in this article was funded by the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET; http://cisnet.cancer.gov/). Specifically, this work was supported by grants from the National Cancer Institute at the National Institutes of Health (5U01CA097415-04 to SHM, 2U01CA097432-04 to TRH, 5U01CA097450-04 to DTL, 5U01CA097416-04 to RB and HJK, 2U01CA097431-04 to MK, 1U01CA152956-01 to PMM HJK, DTL, SHM, TRH). GSG acknowledges support from the National Cancer Institute at the National Institutes of Health (5R01CA097337-02) and the American Cancer Society (ACS RSG 2008A060554), PMM from the National Cancer Institute at the National Institutes of Health (R00CA126147), and CYK from the National Cancer Institute at the National Institutes of Health (K25CA133141).
References
- 1.Cokkinides V, Bandi P, McMahon C, Jemal A, Glynn T, Ward E. Tobacco control in the United States—recent progress and opportunities. CA Cancer J Clin. 2009;59(6):352–365. doi: 10.3322/caac.20037. [DOI] [PubMed] [Google Scholar]
- 2.Reducing Tobacco Use. Rockville, MD: Office of the Surgeon General, U.S. Department of Health and Human Services; 2000. . http://www.cdc.gov/tobacco/data_statistics/sgr/2000. Accessed January 18, 2011. [Google Scholar]
- 3.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Health Consequences of Smoking: A Report of the Surgeon General. [Atlanta, GA] Washington, DC: Dept of Health and Human Services, Centers for Disease Control and Prevention, Office of Smoking and Health; 2004. [PubMed] [Google Scholar]
- 5.Thun MJ, Jemal A. How much of the decrease in cancer death rates in the United States is attributable to reductions in tobacco smoking? Tob Control. 2006;15(5):345–347. doi: 10.1136/tc.2006.017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon PM, Kong CY, Weinstein MC, et al. Adopting helical CT screening for lung cancer: potential health consequences during a 15-year period. Cancer. 2008;113(12):3440–3449. doi: 10.1002/cncr.23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMahon PM, Kong CY, Johnson BE, et al. Estimating long-term effectiveness of lung cancer screening in the Mayo CT screening study. Radiology. 2008;248(1):278–287. doi: 10.1148/radiol.2481071446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moolgavkar SH, Knudson AG. Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst. 1981;66(6):1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- 9.Knoke JD, Shanks TG, Vaughn JW, Thun MJ, Burns DM. Lung cancer mortality is related to age in addition to duration and intensity of cigarette smoking: an analysis of CPS-I data. Cancer Epidemiol Biomarkers Prev. 2004;13(6):949–957. [PubMed] [Google Scholar]
- 10.Hazelton WD, Clements MS, Moolgavkar SH. Multistage carcinogenesis and lung cancer mortality in three cohorts. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1171–1181. doi: 10.1158/1055-9965.EPI-04-0756. [DOI] [PubMed] [Google Scholar]
- 11.Meza R, Hazelton WD, Colditz GA, Moolgavkar SH. Analysis of lung cancer incidence in the Nurses’ Health and the Health Professionals’ Follow-Up studies using a multistage carcinogenesis model. Cancer Causes Control. 2008;19(3):317–328. doi: 10.1007/s10552-007-9094-5. [DOI] [PubMed] [Google Scholar]
- 12.Little MP. Are two mutations sufficient to cause cancer? Some generalizations of the two-mutation model of carcinogenesis of Moolgavkar, Venzon, and Knudson, and of the multistage model of Armitage and Doll. Biometrics. 1995;51(4):1278–1291. [PubMed] [Google Scholar]
- 13.Kopp-Schneider A. Carcinogenesis models for risk assessment. Stat Methods Med Res. 1997;6(4):317–340. doi: 10.1177/096228029700600403. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich WF, Wellmann J, Jacob P, Wichmann HE. Mechanistic modelling in large case-control studies of lung cancer risk from smoking. Stat Med. 2002;21(20):3055–3070. doi: 10.1002/sim.1246. [DOI] [PubMed] [Google Scholar]
- 15.Luebeck EG, Moolgavkar SH. Multistage carcinogenesis and the incidence of colorectal cancer. Proc Natl Acad Sci U S A. 2002;99(23):15095–15100. doi: 10.1073/pnas.222118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meza R, Jeon J, Moolgavkar SH, Luebeck EG. Age-specific incidence of cancer: phases, transitions, and biological implications. Proc Natl Acad Sci U S A. 2008;105(42):16284–16289. doi: 10.1073/pnas.0801151105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meza R, Jeon J, Renehan AG, Luebeck EG. Colorectal cancer incidence trends in the united states and united kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res. 2010;70(13):5419–5429. doi: 10.1158/0008-5472.CAN-09-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health. 1978;32(4):303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachet B, Siemiatycki J, Abrahamowicz M, Leffondre K. A flexible modeling approach to estimating the component effects of smoking behavior on lung cancer. J Clin Epidemiol. 2004;57(10):1076–1085. doi: 10.1016/j.jclinepi.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Schollnberger H, Manuguerra M, Bijwaard H, et al. Analysis of epidemiological cohort data on smoking effects and lung cancer with a multi-stage cancer model. Carcinogenesis. 2006;27(7):1432–1444. doi: 10.1093/carcin/bgi345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng L, Kimmel M, Foy M, Spitz M, Wei Q, Gorlova O. Estimation of the effects of smoking and DNA repair capacity on coefficients of a carcinogenesis model for lung cancer. Int J Cancer. 2009;124(9):2152–2158. doi: 10.1002/ijc.24149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moolgavkar SH, Feuer EJ, Levy DT, Marek K. The impact of tobacco control efforts on US lung cancer mortality: 1975-2000. Risk Analysis. 2012;32(suppl) doi: 10.1111/j.1539-6924.2012.01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krewski D, Burnett RT, Goldberg MS, et al. Reanalysis of the Harvard Six-Cities study and the American Cancer Society Study of Particulate Air Pollution and Mortality, Part II: Sensitivity Analysis. A Special Report of the Institute's Particle Epidemiology Reanalysis Project. Cambridge, Massachusetts: Health Effects Institute; 2000. [Google Scholar]
- 24.Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330(7485):223. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Rockville, MD: Office of the Surgeon General, U.S. Department of Health and Human Services; 2006. http://www.cdc.gov/tobacco/data_statistics/sgr/2006. Accessed January 18, 2011. [Google Scholar]
- 26.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63(19):6556–6562. [PubMed] [Google Scholar]
- 27.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto Y, Ishiguro M, Kitagawa G. Akaike Information Criterion Statistics. Tokyo, Japan: KTK Scientific Publishers; 1986. [Google Scholar]
- 29.Levy DT, Nikolayev L, Mumford E. Recent trends in smoking and the role of public policies: Results from the SimSmoke tobacco control policy simulation model. Addiction. 2005;100(10):1526–1536. doi: 10.1111/j.1360-0443.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 30. http://www.cancer.gov/newscenter/pressreleases/NLSTresultsRelease. Accessed January 18, 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.