Abstract
Flavonoids are common components of the human diet and appear to be of interest in cancer prevention or therapy, but their structure-activity relationships (SAR) remain poorly defined. In this study, were compared 24 flavonoids for their cytotoxicity on cancer cells (B16 and Lewis lung), and their morphological effect on endothelial cells (EC) that could predict antiangiogenic activity. Ten flavonoids presented inhibitory concentrations for 50% of cancer cells (IC50, 48 h) below 50 μM: rhamnetin, 3′,4′-dihydroxyflavone, luteolin, 3-hydroxyflavone, acacetin, apigenin, quercetin, baicalein, fisetin, and galangin. Important SAR for cytotoxicity included the C2-C3 double bond and 3′,4′-dihydroxylation. Concerning the morphological effects on EC, only fisetin, quercetin, kaempferol, apigenin, and morin could induce the formation of cell extensions and filopodias at non cytotoxic concentrations. The SAR for morphologic activity differed from cytotoxicity and involved hydroxylation at C-7 and C-4′. Fisetin, the most active agent, presented cell morphology that was distinct compared to colchicine, combretastatin A-4, docetaxel, and cytochalasin D. Resistance to cold depolymerization and a 2.4-fold increase in acetylated α-tubulin demonstrated that fisetin was a microtubule stabilizer. In conclusion, this study disclosed several SAR that could guide the choice or the rational synthesis of improved flavonoids for cancer prevention or therapy.
Keywords: Acetylation; Animals; Carcinoma, Lewis Lung; drug therapy; pathology; Cytoskeleton; drug effects; Endothelial Cells; cytology; drug effects; Flavonoids; pharmacology; Melanoma, Experimental; drug therapy; pathology; Mice; Microtubules; chemistry; drug effects; Structure-Activity Relationship; Tubulin; metabolism
Keywords: flavonoid, fisetin, endothelial cells, morphology
Introduction
Tumor vasculature has become an attractive target for cancer therapy because a single vessel provides oxygen and nutrients to numerous tumor cells and also because it is the main route for metastatic spread of cancer cells (reviewed in (1)). Tumor angiogenesis is the result of an imbalance between pro-angiogenic factors, e.g., vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and endogenous antiangiogenic factors such as angiostatin and endostatin (2,3). Tumor vasculature can be targeted at the angiogenesis level to prevent the formation of new vessels with antiangiogenic agents, or at the vascular level when the vessels are already formed with vascular disrupting agents (4,5). The antiangiogenesis approach has already proven its clinical effectiveness in colon, breast, and non small cell lung cancer by the combination of VEGF antibody with cytotoxic drugs (6–8).
Several small molecules, e.g., colchicine and combretastatin analogues, have demonstrated antitumoral antivascular properties by binding to tubulin and inhibiting its polymerization. Several tumor vascular disrupting agents are currently being tested in the clinic, e.g., combretastatin analogues (CA4P, Oxi 4503, AVE8062), a dolastatin analogue (TZT1027), and an analogue of N-acetylcolchinol (ZD6126) (5,9). The mechanism of action of these vascular targeting agents in vivo is not completely elucidated, but the endothelial cells of the tumor blood vessels appear to be morphologically modified (rounding up) by the disorganization of the cytoskeleton consecutive of the inhibition of tubulin polymerization. This rounding up of endothelial cells induces a vascular collapse which causes a blood flow shut down with consequent tumor cells death (10).
Flavonoids belong to a large family of natural polyphenolic compounds that are commonly found in the human diet especially in fruits, vegetables, and beverages such as tea and red wine (reviewed in (11)). These compounds display a variety of pharmacological properties of interest in the therapy of several diseases including cancer, as cytotoxic, antiangiogenic or antivascular agents (12–16). Although some flavonoid anticancer effects are recognized, their structure-activity relationships (SAR) remain poorly defined for their cytotoxic activity on cancer cells (12,13) and for their effect on the tumor vasculature.
Because flavonoids can be considered as good candidates for antiangiogenic or antivascular agents useful in cancer prevention or therapy, the purpose of this study was to examine the SAR that could be predictive of their in vivo cytotoxic and/or antivascular effects. We report here on a series of 24 flavonoids bearing minimally substituted groups with regard to their cytotoxic activity, their morphological effects on endothelial cells, and their effects on cytoskeleton organization. Our results indicate that the SAR found for the morphological effects of flavonoids on endothelial cells are markedly different from the SAR required for their cytotoxic activity. In addition, the rapid morphological effects induced by some flavonoids on endothelial cells was clearly distinct compared to morphologically active standard agents known to interfere with the cytoskeleton integrity (e.g., colchicine, docetaxel, combretastatin A4 and cytochalasin D). Our data also show that some flavonoids may have an effect on cytoskeleton organization independently of a significant effect on in vitro tubulin assembly. We show that fisetin, the most active compound of our series, can induce a rapid morphological modification of endothelial cells which is correlated with an increase in microtubule stability and the acetylation of α-tubulin, a marker of tubulin stabilization.
Collectively, our data disclosed several interesting SAR for natural flavonoids present in the human diet that are acting on both cancer and endothelial cells. These data can be useful in the choice of food containing the most active natural flavonoids acting on cancer and/or endothelial cells, and could also be of value in the rational synthesis of improved flavonoids.
Materials and Methods
Chemicals
Naringenin, hesperidin, isoquercitrin, cynaroside, and combretastatin A4 were kindly donated by Pr. Michel Koch (Faculty of Pharmacy, University Paris Descartes, Paris, France); flavone-8-acetic acid was a gift from Dr. Jean-Jacques Berthelon (Merck Lipha Santé, Lyon, France); rhamnetin, quercetagetin, and 3′,4′-dihydroxyflavone were purchased from ExtraSynthese (Lyon, France); docetaxel was obtained from Sanofi-Aventis (Vitry sur Seine, France). All other flavonoids and chemicals were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). Flavonoids stock solutions were prepared in dimethylsulfoxide (DMSO) and kept at 4°C in the dark.
Cytotoxicity
Murine B16 melanoma and Lewis lung carcinoma cells were grown in Dulbecco’s modified essential medium (DMEM) containing 2 mM L-glutamine, 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin (37°C, 5% CO2). Exponentially growing cancer cells were plated onto 96-well plates at 5000 cells per well in 200 μl DMEM, and 24 h later the cells were exposed for 48 h to the solvent alone or to the flavonoid at the indicated concentrations. Viability was assessed using the MTT (1-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium) test and absorbance was read at 562 nm in a microplate reader (BioKinetics Reader EL340, Fisher Bioblock Scientific, Illkirch, France). Control cells were exposed to 1% DMSO. Experiments were run in triplicate and repeated 3–6 times. Results are presented as the inhibitory concentrations for 50% of cells (IC50) for a 48 h exposure time.
Morphologic effects on EA·hy 926 endothelial cells
To assess the effect of flavonoids on the morphology of endothelial cells, normal HUVEC (human umbilical vein endothelial cells) and EA·hy 926 endothelial cells were used (17,18). EA·hy 926 cells were grown in DMEM containing 2 mM L-glutamine, 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin (37°C, 5% CO2). Exponentially growing EA·hy 926 cells were plated onto 96-well plates at 5000 cells/200 μl/well. Twenty-four h after plating, the medium was aspirated, and 100 μl of medium containing the test compound was added to the well (triplicate) at the indicated final concentrations (37°C, 5% CO2), and 2 h later digital photographs were recorded of representative center areas of each well at a magnification of X320. Morphological evaluation was assessed by drawing the cell contours on a computer screen and morphometric parameter values were obtained using the ImageJ software (19). The form factor was defined as (1-circularity), where circularity is calculated by the following formula: 4π × area × perimeter −2 (20). The form factor is therefore zero for a circle, and increases as the shape elongates, or with the formation of cellular processes. The mean form factor of control EA·hy 926 endothelial cells was approximately 0.5. The results were expressed as a percent of the controls using the following formula: 100 × [1-(circularity of treated cells)/(circularity of control cells)]. Experiments were performed in triplicate for each concentration and repeated 3 times. Results are presented as the efficacious concentration for the change in cell shape for 50% of cells for a 2 h exposure time (EC50) relative to controls. Percent cell viability of EA·hy 926 cells was assessed by the MTT test. Because the concentrations at which morphological activity was observed was not cytotoxic for the 2 h exposure time, we determined the “therapeutic index” as the ratio of the IC50 (cytotoxicity at 48 h)/EC50 (morphology at 2 h) on endothelial cells.
Immunofluorescence microscopy
EA·hy 926 endothelial cells were fixed (4% paraformaldehyde, 0.5% glutaraldehyde), permeabilized and saturated with a 3% BSA solution containing 0.1% Triton X100. Cells were processed for indirect immunofluorescence as follows: cells were incubated overnight at 4°C with the mouse anti-α-tubulin monoclonal antibody (Sigma) at 1/2000 dilution, and further incubated with an anti-mouse fluorescein isothiocyanate (FITC) secondary antibody (Sigma) at 1/400 dilution for 45 min in the dark at room temperature, in the presence of phalloidin coupled to tetramethylrhodamine isothiocyanate (TRITC) (1 μg/ml). Photographs were taken on a Zeiss fluorescence microscope and a Zeiss LSM-510 confocal microscope (Cal Zeiss France, Le Pecq, France) (FITC, excitation 488 nm, emission 530 nm; TRITC, excitation 541 nm, emission 572 nm).
Microtubule polymerization assay
Rat brain microtubules were purified according to Shelanski et al. (21) as modified by Fellous et al. (22) and resuspended at a final concentration of 1 mg/ml in the assembly buffer (pH 6.4) containing 0.1 M MES (2-morpholino-ethanesulfonic acid monohydrate), 0.5 mM MgCl2, 1 mM EGTA, and 1 mM GTP. Microtubule assembly was monitored and recorded continuously by turbidimetry at 400 nm in a UV spectrophotometer equipped with a thermostated cell at 37°C (23).
Cold-induced microtubule depolymerization
EA·hy 926 cells (12500 cells) were seeded on microscope glass slides in a 24 well plate and cultured overnight. Fisetin (87.5 μM) was applied to cell cultures for a 2 h exposure time, and control cells were treated with 1% DMSO. After the 2 h exposure time, the plates were put on ice for 0, 10, 15, 30, 45, or 60 min. Cells were fixed with a 4% solution of paraformaldehyde and 0.5% glutaraldehyde for 10 min at ambient temperature, and then permeabilized and saturated with a solution of 0.1% Triton X100 in phosphate buffered saline (PBS)-3% bovine serum albumin (BSA) for 1 h at room temperature. Cells were incubated overnight at 4°C with a primary monoclonal antibody anti-α-tubulin (Sigma), and then incubated for 1 h at room temperature with a mouse secondary FITC labeled antibody (Sigma). Nucleus were stained with 4′,6-diamidino-2-phenyindole (DAPI) for 30 min at room temperature in the dark. Glasses were mounted and cells were photographed on a Zeiss fluorescence microscope (FITC, excitation 488 nm, emission 530 nm; DAPI, excitation 365 nm, emission 420 nm).
α-Tubulin acetylation evaluation by Western blotting
After a 2 h exposure time to 1% DMSO (control) or fisetin (87.5 μM), cells were trypsinized, centrifuged, washed with PBS, and solubilized in a RIPA buffer for 30 min on ice. After vortexing, the sample was ultracentrifuged (100000 × g) for 40 min at 4°C. The supernatant was harvested and an aliquot was saved for protein concentration determination using the bicinchoninic acid assay, and the other aliquot was diluted with a Laemmli buffer at a final concentration of 1X and boiled for 5 min. The samples were applied on a Bis-Tris 10% gel (InVitrogen, R&D Systems Europe, Abingdon, Oxon, U.K.) and migration was accomplished during 90 min with an application of 150 V. Proteins were transferred on a nitrocellulose membrane in 2 h at 25 V. The membrane was saturated with 3% BSA and 0.5% Tween 20 in PBS (30 min) and exposed overnight at 4°C to the primary mouse antibody against α-tubulin or against acetylated α-tubulin. The secondary mouse antibody coupled with peroxidase was applied for 1 h at room temperature, and ECL revelation was used for detection. Quantification was accomplished using the ImageJ software (19). The level of acetylated α-tubulin was normalized against the level of total α-tubulin in each sample.
Statistical analysis
Results are expressed as the mean ± SEM from at least 3 independent experiments. Comparisons between means were made using the Student t test for unpaired data. If unequal variance was observed, the Welch’s correction was applied. A P value ≤ 0.05, was considered significant.
Results
Flavonoids selection
To define potential flavonoid structure-activity relationships (SAR) for cytotoxicity on cancer cells and morphologic activity on endothelial cells (as potential antiangiogenic or antivascular agents), a series of flavonoids bearing minimal substituents were selected (Table 1). Most flavonoids were mono- or poly-hydroxylated on rings A, B, or C, some compounds were methoxylated, one flavanone was included to determine the influence of the C2-C3 double bond, and the presence of sugar substituents was also investigated.
Table 1.
Selected flavonoids cytotoxicity on cancer cell lines and morphological activity on endothelial cells (EA.hy 926).
 |
B16 melanoma cells | Lewis lung cells | EAhy 926 endothelial cells | ||
|---|---|---|---|---|---|
| Cytotoxicity IC50 (μM) 1 | Morphology EC50 (μM)3 2 h | Cytotoxicity IC50 (μM)4 48 h | Therapeutic window5 | ||
| Flavonoid aglycones | |||||
| Rhamnetin (3,3′,4′,5-tetrahydroxy-7-methoxyflavone) | 10 ± 0.3 | - | |||
| 3′,4′-Dihydroxyflavone | 15 ± 2 | - | |||
| Luteolin (3′,4′,5,7-tetrahydroxyflavone) | 15 ± 2 | - | |||
| 3-Hydroxyflavone | 20 ± 2 | - | |||
| Acacetin (5,7-dihydroxy-4′-methoxyflavone) | 22 ± 7 | - | |||
| Apigenin (4′,5,7-trihydroxyflavone) | 22 ± 4 | 16 ± 2 | 52 ± 7 | 16 ± 5 | 0.31 |
| Quercetin (3,3′,4′,5,7-pentahydroxyflavone) | 26 ± 4 | 35 ± 7 | 39 ± 3 | 36 ± 2 | 0.91 |
| Baicalein (5,6,7-trihydroxyflavone) | 28 ± 3 | - | |||
| Fisetin (3,3′,4′,7-tetrahydroxyflavone) | 29 ± 5 | 37 ± 2 | 16 ± 4 | 36 ± 5 | 2.26 |
| Galangin (3,5,7-trihydroxyflavone) | 48 ± 4 | - | |||
| Chrysin (5,7-dihydroxyflavone) | 51 ± 3 | - | |||
| Kaempferol (3,4′,5,7-tetrahydroxyflavone) | 51 ± 5 | 32 ± 4 | 41 ± 3 | 31 ± 8 | 0.76 |
| Flavone | 93 ± 14 | - | |||
| Quercetagetin (3,3′,4′,5,6,7-hexahydroxyflavone) | 110 ± 21 | - | |||
| 5-Hydroxyflavone (primuletin) | 123 ± 37 | - | |||
| 7-Hydroxyflavone | 179 ± 5 | - | |||
| Myricetin (3,3′,4′,5,5′,7-hexahydroxyflavone) | 283 ± 35 | - | |||
| Morin (2′,3,4′,5,7-pentahydroxyflavone) | 298 ± 51 | 258 ± 46 | 166 ± 6 | 257 ± 37 | 1.55 |
| Naringenin (4′,5,7,-trihydroxyflavanone) | 361 ± 32 | - | |||
| Flavone-8-acetic acid | 364 ± 32 | - | |||
| Flavonoid glycosides | |||||
| Rutin (quercetin-3-O-rutinoside) | no effect2 | - | |||
| Isoquercitrin (quercetin-3-O-glucoside) | no effect2 | - | |||
| Cynaroside (luteolin-7-O-glucoside) | no effect2 | - | |||
| Naringin (naringenin-7-O-rutinoside) | no effect2 | - | |||
B16 melanoma cells or Lewis lung carcinoma cells were exposed to the flavonoid for 48 h and viability was assessed by the MTT test. Results are expressed as the inhibitory concentration for 50% of the cells (IC50) compared to controls (1% DMSO). Mean ± SEM of 3 to 6 independent experiments each performed in triplicate.
No effect: indicates no cytotoxicity observed at 400 μM, the maximum concentration tested.
The EC50 is the effective concentration to obtain a change in the morphology for 50% of EA.hy 926 cells after a 2 h exposure time at non cytotoxic concentrations as described in Materials and Methods.
IC50, inhibitory concentration for 50% of cells relative to controls for a 48 h exposure time.
The “therapeutic window” for the EA.hy 926 endothelial cells represents the ratio of the IC50 value at 48 h (cytotoxicity) divided by the EC50 value at 2 h (morphology).
Flavonoids cytotoxicity on B16 melanoma cells
Table 1 presents the flavonoids inhibitory concentrations for 50% (IC50) of B16 melanoma cells for a 48 h exposure time. The most cytotoxic compounds in this series were rhamnetin, 3′,4′-dihydroxyflavone, luteolin and 3-hydroxyflavone with IC50 values below 20 μM. In this series, we did not observe obvious SAR, except that the 3′,4′-dihydroxylation on the B ring was present for the first three compounds but not for the 3-hydroxyflavone.
Intermediate cytotoxicity (21 to 100 μM) was observed with the following compounds: acacetin, apigenin, quercetin, baicalein, fisetin, galangin, chrysin, kaempferol, and flavone (Table 1). In this series, some substituents were identified to increase the cytotoxicity potential. An increased in cytotoxicity was observed with a hydroxyl group at position 6 of ring A, as seen in baicalein (28 μM) versus chrysin (51 μM), and a also with a hydroxyl group at the 4′-position as seen with apigenin (22 μM) versus chrysin (51 μM). The 5-OH substituent did not appear to markedly influence the cytotoxicity as seen with quercetin (26 μM) and fisetin (29 μM) which displayed similar IC50 values. A similar observation was made with the 3-OH position which did not significantly change cytotoxicity, as seen with galangin which bears a 3-OH (48 μM) and chrysin with no 3-OH (51 μM).
Concerning the flavonoids with rather high IC50 values (>100 to 400 μM), i.e., quercetagetin, 5-hydroxyflavone, 7-hydroxyflavone, myricetin, morin, naringenin, and flavone-8-acetic acid, no apparent SAR could be deduced. The least cytotoxic compounds ( > 400 μM) of this series were the flavonoid glycosides.
An overall analysis of the SAR responsible for an increase in cytotoxic potency yielded the following observations: methoxylation of the 7-OH can increase the cytotoxicity as in rhamnetin (10 μM) compared to quercetin with no methoxyl at the 7 position (26 μM). The 3′,4′ dihydroxylation on cycle B led to a significant increase in cytotoxicity of several flavonoids, e.g., as in 3′,4′-dihydroxyflavone, in luteolin, fisetin and quercetin, which were potent cytotoxic compounds in this series. The importance of the 3′,4′-dihydroxylation for cytotoxicity is also underlines by morin which lacks a 3′-OH, and bears a 2′-OH instead, presented a dramatic decrease in cytotoxicity (298 μM) compared to quercetin (26 μM).
The C2-C3 double bond of cycle C was found to be essential for the cytotoxicity. For example, naringenin which lacks this double bond, presents an IC50 of 361 μM, whereas apigenin with a C2-C3 double bond presented an IC50 of 22 μM.
The presence of a sugar moiety was always correlated with a decrease of cytotoxic potential. For example, quercetin, luteolin and naringenin lost their cytotoxic activity when combined with a sugar (e.g., rutin, isoquercitrin, cynaroside and naringin).
Selected flavonoids cytotoxicity on Lewis lung carcinoma cells
For comparison purposes, Table 1 also presents some flavonoids inhibitory concentrations for 50% (IC50) for another cancer cell line, i.e., the Lewis lung carcinoma cells. It can be observed that the IC50 for this cell line are similar to the IC50 noted above for the B16 melanoma cells.
Flavonoid effect on the morphology of endothelial cells
We next assessed the effect of these flavonoids on the morphology of endothelial cells (EA·hy 926) which could be predictive of antiangiogenic or antitumor activity. Of the 24 flavonoids tested, only 5 compounds were morphologically active after a short 2 h exposure time at non cytotoxic concentrations, i.e., fisetin, apigenin, kaempferol, quercetin and morin. The exposure time was deliberately chosen to be short to simulate more closely the acute effects of these flavonoids upon ingestion, because most of these compounds have a short half-life in vivo and endothelial cells are therefore exposed to high concentrations for a short exposure time. Figure 1 depicts the characteristic morphology of endothelial cells after exposure to these morphologically active flavonoids compared to controls. Similar morphological modifications were also observed with HUVEC (data not shown). The morphological changes induced by active flavonoids were characterized by the rapid (< 2 h) formation of numerous large cell extensions prolonged by thin and dense filopodias. These morphological changes were detected as early as 15 min after the application of fisetin, as determined by microcinematography over a 2 h observation time (data not shown). To better quantitate these morphological changes, a morphometric analysis based on the form factor (1-circularity) was applied. The form factor, as defined in Materials and Methods, increases as a function of cell contour irregularities, as observed with the formation of cellular processes. In our study, the form factor increased as a dose-dependent manner, when exposed to the morphologically active flavonoids (Figure 2). In addition, it is noteworthy that these morphological effects were observed at non cytotoxic concentrations for this brief 2 h exposure time.
Figure 1. Morphological effects of flavonoids on endothelial cells.
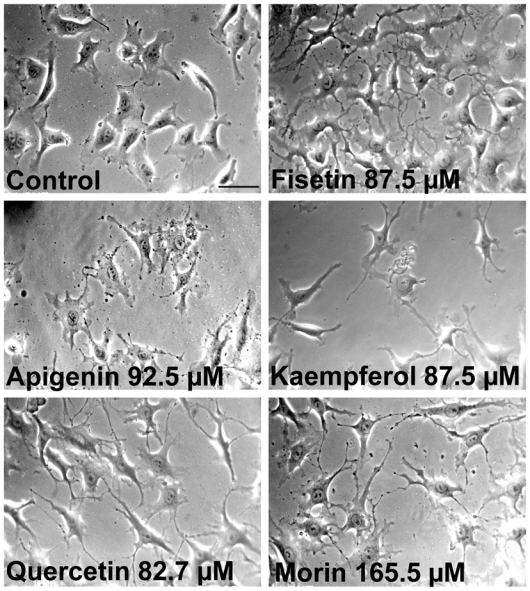
Endothelial cells (EA·hy 926) were exposed to the indicated flavonoid for 2 h at the indicated non cytotoxic concentration. Control cells were exposed to 1% DMSO (solvent). Magnification X 320. Scale bar, 50 μm.
Figure 2. Effects of morphologically active flavonoids on endothelial cells at non cytotoxic concentrations.
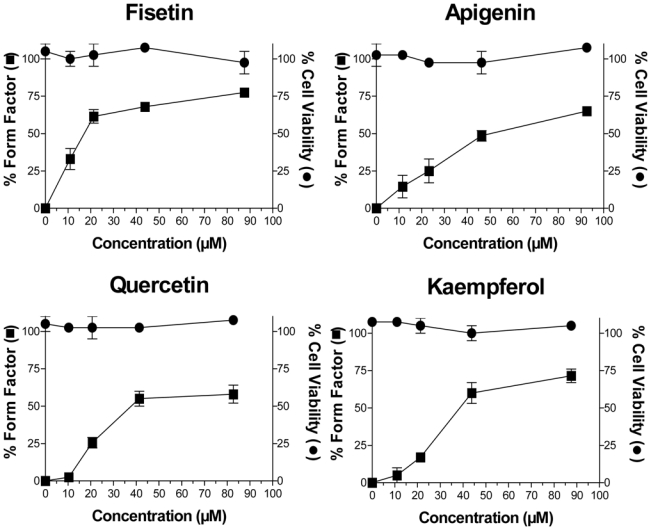
EA·hy 926 cells were exposed for 2 h to increasing concentrations of the indicated flavonoids. The percent form factor is an indicator of the increase in perimeter and cell prolongation, and was defined by the following formula: 100 x [1-(circularity of treated cells)/(circularity of control cells)]. Percent viability was evaluated by the MTT test relative to controls (1% DMSO). Each data point is the mean (+SEM) of 3 independent experiments, each performed in triplicate.
Table 1 also presents the concentration for 50% change in EA.hy 926 endothelial cell shape (EC50) of the morphologically active flavonoids relative to control cells for a 2 h exposure time. Fisetin was the most potent compound with an effective concentration for 50% change in cell shape (EC50) of 16 ± 4 μM, followed by quercetin (39 ± 3 μM), kaempferol (41 ± 3 μM), apigenin (52 ± 7 μM), and morin (166 ± 6 μM). It was of interest to note that these morphological changes observed at non cytotoxic concentrations were completely reversible overnight after flavonoid removal.
In addition, Table 1 presents a “therapeutic window” for the 5 morphologically active flavonoids, calculated as the ratio of the cytotoxicity value for a 48 h exposure time (IC50) over the effective concentration to obtain a change in the morphology for 50% of the cells (EC50). It can be noted that fisetin was the compound with the largest therapeutic window with a value of 2.26.
The SAR deduced from these 5 morphologically active flavonoids disclosed that hydroxylation on ring A at carbon 7, and hydroxylation on ring B at the 4′ position appeared necessary for morphological activity on endothelial cells (Table 1).
Endothelial cell cytoskeleton structure after fisetin exposure
To better understand the morphological changes induced by flavonoids on endothelial cells at non cytotoxic concentrations, we next examined the cytoskeleton structure after a 2 h exposure time to fisetin, which was the most morphologically active flavonoid of our series. A comparison of control and fisetin-treated cells using confocal microscopy is presented in Figures 3a and 3b. Microtubule and actin filaments distribution in control EA·hy 926 cells exposed to 1% DMSO presented a regular cell contour with few and short extensions, a loose hair-like structure for microtubules, and a large distribution of actin filaments (red) at the cell periphery (see arrows in Fig. 3-a). Fisetin-treated cells (Figure 3-b) showed several long extensions and filopodias with the loss of the loose hair-like structures observed in controls. In addition, the microtubule network in fisetin-treated cells presented dense and parallel microtubule organization in the numerous extensions and also a narrow peripheral distribution of actin structures (red).
Figure 3. Effect of fisetin and standard agents on the morphology of endothelial cells.
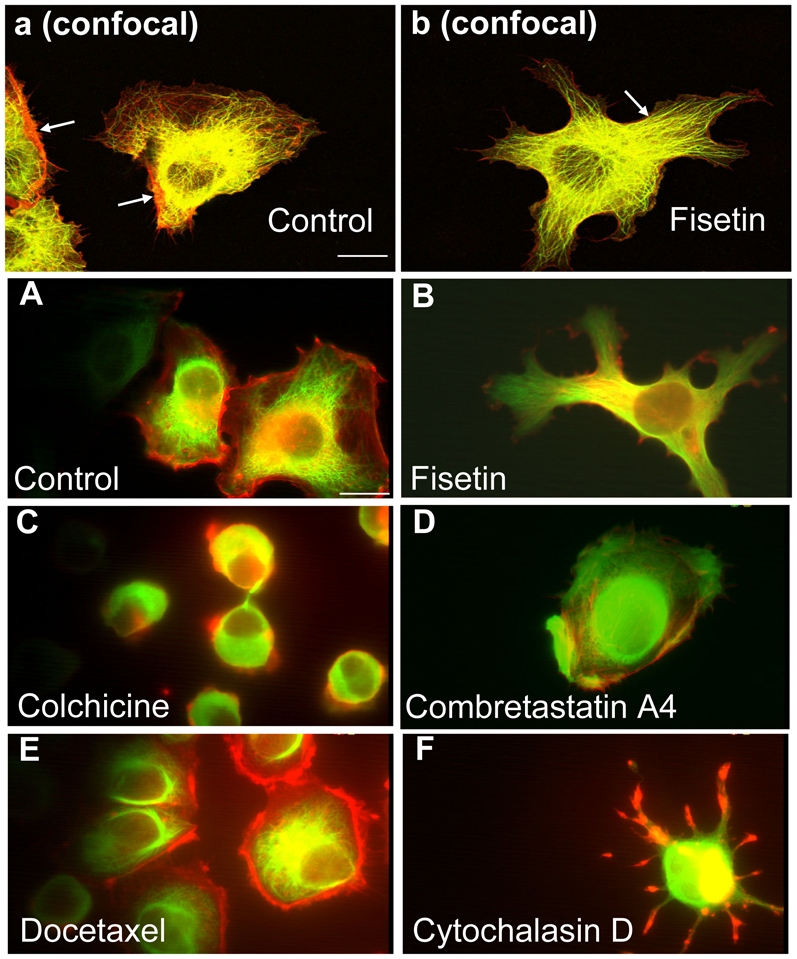
Panels a and b(Confocal microscopy) analysis of microtubules and actin filament distribution in EA·hy 926 endothelial cells exposed to DMSO 1% (control, panel a), or fisetin at 87.5 μM for 2 h (panel b), prepared for indirect immunofluorescence using a dual staining for α-tubulin (green) and actin (red) as described in Materials and Methods. The 2 arrows in panel “a” indicate the actin filament large peripheral distribution, and the arrow in panel b indicates parallel structures of microtubules in fisetin treated cells. Photographs were recorded at a magnification X 630. Scale bar, 16 μm.
Panels A to F(Phase-contrast microscopy). Morphology and cytoskeleton structure of endothelial cells exposed to fisetin and standard agents. EA·hy 926 cells were exposed for 2 h to either DMSO 1% (control, panel A) or to the indicated compounds at the following non cytotoxic concentrations: fisetin, 87.5 μM (panel B); colchicine, 0.15 μM (panel C); combretastatin A4, 1.0 μM (panel D); docetaxel, 2.5 μM (panel E); cytochalasin D, 5.0 μM (panel E). Cells were fixed, permeabilized and processed for indirect immunofluorescence as described in the Materials and Methods using the anti-α-tubulin monoclonal antibody and a secondary antibody coupled to FITC (green). Actin was visualized using phalloidin coupled to TRITC (red). Photographs were recorded at a magnification X 1000. Scale bar, 16 μm.
Endothelial cells morphology and cytoskeleton structure after exposure to standard agents
To further explore the mechanisms involved in these rapid (< 2 h) and unusual fisetin-induced changes in the morphology of endothelial cells, we also compared these fisetin induced effects (Figure 3-B) to standard agents known to rapidly induce cytoskeleton reorganization and/or alteration. The morphological effects of colchicine, combretastatin A4, docetaxel, and cytochalasin D are presented in Figures 3-C to 3-F (phase-contrast microscopy). As expected with colchicine, we observed a depolymerization of the microtubules which led to a rounding up of endothelial cells (Figure 3-C) and a loss of the hair-like microtubule structure as seen in controls (Figure 3-A). Combretatastin A4 also induced a cell rounding up (Figure 3-D) due to microtubule depolymerization, and also led to the loss of the hair-like microtubule structure. Docetaxel also presented a cell rounding up with a retraction of intact microtubules and actin filaments (Figure 3-E). Exposure of endothelial cells to cytochalasin D induced a characteristic “star-like” shape with actin filopodias in red (Figure 3-F) and the loss of the hair-like microtubule organization seen in control cells. Considering the above results, it can be concluded that none of the standard agents used could induce similar morphological changes on endothelial cells as the ones observed with fisetin exposure (compare to Figures 3-b and 3-B).
Effect of morphologically active flavonoids on microtubule polymerization
To better understand the mechanism of action of morphologically active flavonoids, we next assessed their effects on the in vitro polymerization of microtubules. No correlation was found with the morphological effects observed on endothelial cells by the 5 active flavonoids and their in vitro effect on microtubule polymerization. Fisetin, kaempferol, quercetin, and apigenin were found inactive at their maximum solubility in the incubation buffer, which were 349 μM for fisetin and kaempferol, 83 μM for quercetin, and 46 μM for apigenin (data not shown). However, morin which was the least morphologically active flavonoid of our series, was found to be a weak inhibitor of microtubule polymerization (30% inhibition at a concentration of 83 μM).
Evaluation of microtubule stability
Because the appearance of dense microtubules in fisetin-treated cells (cf. Figures 3b and 3B) could be due to an increased stabilization of the microtubule network, we next evaluated their resistance to cold-induced depolymerization. We submitted control and fisetin-treated cells to cold for various periods of time and processed for indirect α-tubulin immunofluorescence. Figures 4A to E present control cells submitted to cold for 0 to 45 min: microtubule depolymerization was clearly observed as early as 10 min in control cells (Figure 4-B) and was almost complete by 15 min (Figure 4-C). In fisetin-treated cells (Figures 4-F to J), no microtubule depolymerization was noticeable before 15 min, indicating an increase of microtubule network stability. At 30 min some microtubule disorganization was observed, and at 45 min, the microtubule network was completely disorganized and tubulin appeared to form aggregates (Figure 4-J). It can also be seen that the fisetin-induced cell extensions and filopodias remained intact for 45 min (Figure 4-J) and beyond (up to 90 min, data not shown).
Figure 4. Increased microtubule stability of fisetin-treated endothelial cells.
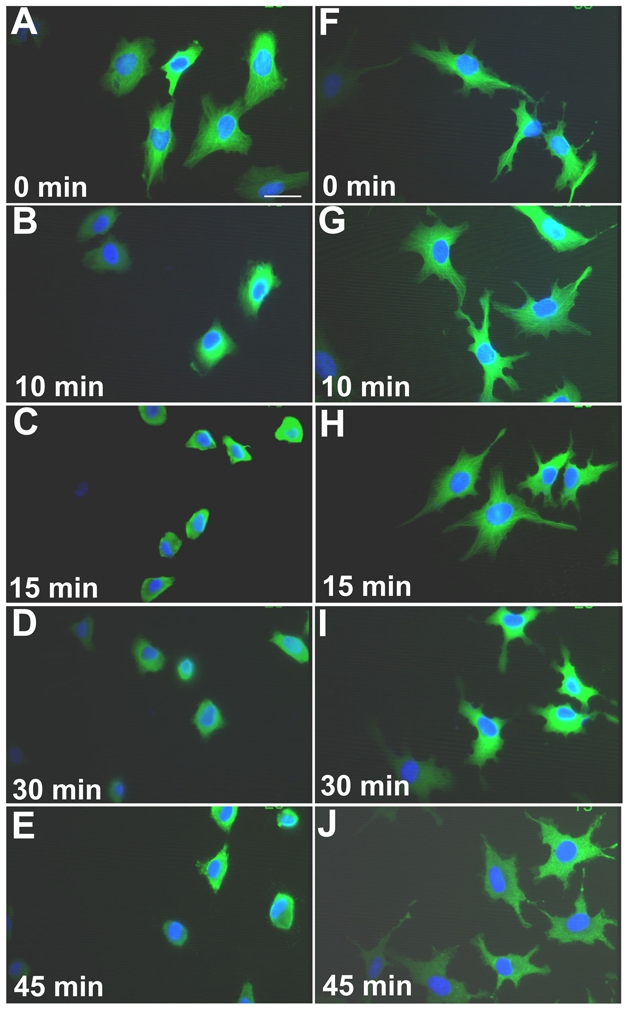
EA·hy 926 endothelial cells were exposed to either DMSO 1% (controls) or to fisetin at 87.5 μM for a 2 h exposure time, and then exposed to cold (0°C) for the indicated time to induce microtubules depolymerization. After fixing, cells were stained using a monoclonal antibody anti-α-tubulin coupled with an FITC secondary antibody (green), and nucleus were stained using DAPI (blue). Panels A to E present control cells submitted to cold for 0 to 45 min. Panels F to J, fisetin-treated cells exposed to cold for 0 to 45 min. Scale bar, 50 μm.
Evaluation of α-tubulin acetylation
Because acetylation of α-tubulin is considered as a marker of stable microtubule structures (reviewed in (24)), we next tested whether increased acetylated α-tubulin correlated with increased microtubule stability in fisetin-treated cells. Figure 5-A shows a representative Western blot of acetylated α-tubulin and total α-tubulin in control and fisetin-treated cells after a 2 h exposure time. It can be observed in Figure 5-B that fisetin could induce a rapid (within 2 h) and important 2.4-fold increase in expression of acetylated α-tubulin relative to total α-tubulin.
Figure 5. Increased acetylated α-tubulin in fisetin-exposed endothelial cells.
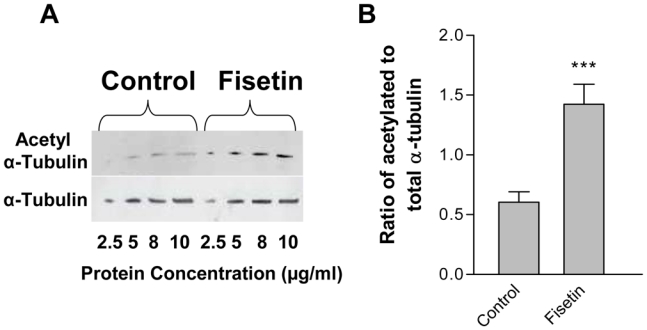
EA·hy 926 endothelial cells were exposed to 1% DMSO (control) or fisetin (87.5 μM) for 2 h and total protein extracts were immunoblotted as detailed in Materials and Methods for α-tubulin and acetylated α-tubulin. A representative immunoblot is shown from 3 independent experiments in panel A. Panel B shows the histogram of the mean ratio of acetylated to total α-tubulin (mean ± SEM) in fisetin-treated cells compared to control cells. The asterisks indicate a statistical significance (P<0.0005) using the Student t test with the Welch correction.
Discussion
Although several flavonoids present in the human diet have been shown to be of interest in cancer prevention or therapy, their structure-activity relationships (SAR) for cytotoxicity, antiangiogenic and antivascular activity are still poorly understood (12,13). The purpose of this work was therefore to study the SAR of a series of 24 minimally substituted flavonoids with regard to their cytotoxicity against cancer cell lines (B16 melanoma and Lewis lung carcinoma), and their effect on the morphology of endothelial cells, as an indicator of potential antiangiogenic or antivascular action.
Concerning the cytotoxic activity of flavonoids on cancer cells, it was of interest to note that the B16 melanoma cells and the Lewis lung carcinoma cells yielded similar IC50 values indicating that, at least for these two cell lines, no preferential activity toward a particular cancer cell type was noted. With regard to the SAR needed for cytotoxicity, the 6-OH and the 4′-OH positions were shown to be involved in the increase in cytotoxicity. Similarly the methoxylated group at the 7 position and the 3′,4′-dihydroxylation also led to an increase in cytotoxicity. The presence of the C2-C3 double bond was shown to be essential for activity because its saturation led to a marked decrease in activity. It was also found that a hydroxyl group at the 3 position could significantly increase cytotoxic activity when no other group was present on the flavonoid molecule. Our results concerning the increase in cytotoxicity with 3′,4′ dihydroxylation are in agreement with previous studies (16,25) who have also identified the importance of these positions for cytotoxicity. However, in our series, we have not seen a decrease in cytotoxic activity with the 5-OH, nor an increase in cytotoxicity with the 5′-hydroxylation as reported previously (25), probably due to the different cancer cell lines used.
It is also of interest that the SAR noted for cytotoxicity in this work are similar to the SAR concerning the antioxidant potential and the inhibition of topoisomerases 1 and 2 (12). For example, the 3′,4′-dihydroxylation, the C2-C3 double bond, and the hydroxylation in 3, were all found important for antioxidant and anti-topoisomerase activity, and were also found important for cytotoxicity in the present work. Similarly, glycosylation was also found to decrease the flavonoid effects on antioxidant and topoisomerases activities, and it was also found to markedly decrease the cytotoxicity in this study, probably by decreasing the lipid solubility needed to cross cell membranes.
As mentioned above we were also interested to study the effects of flavonoids on endothelial cells because they are the target of antiangiogenic and antivascular agents and that certain flavonoids have been shown to selectively disrupt the tumor vasculature (15). In addition, endothelial cells grown in vitro are considered as good predictors of in vivo antivascular effects (26). Our results have shown that several flavonoids were indeed morphologically active on endothelial cells, although the effect was not the expected rounding up, as observed for example with combretastatin and its analogues (27), but instead presented the rapid formation of a vast network of cell extensions and filopodias, at non cytotoxic concentrations. The morphologically active flavonoids identified in this work (i.e., fisetin, apigenin, kaempferol, quercetin and morin) presented some structural similarities, i.e., hydroxylation on ring A at carbon 7, and hydroxylation on ring B at the 4′ position.
Comparing the SAR needed for morphological activity with the ones previously mentioned for cytotoxic activity, it was apparent that the 2 effects did not require the same pattern of substituents, as the most morphologically active flavonoids were not the most cytotoxic ones. For example, the 3′,4′-dihydroxyflavone was one of the most cytotoxic compound but was without morphologic effects on endothelial cells, and conversely, the most morphologically active flavonoids were not the most cytotoxic. These observations indicate that cytotoxicity and morphologic activities are not sharing the same signaling networks.
Because fisetin was the most morphologically active compound, we were interested to study further its mechanism of action. The confocal analysis of fisetin-treated cells showed the loss of the hair-like aspect of the microtubule network and dense peripheral actin filaments distribution observed in control cells. This fisetin-induced alteration of the distribution of microtubules and actin filaments was indicative of a different mechanism of action compared to other standard agents. Indeed, the comparison between fisetin, the most morphologically active flavonoid in our series, and standard agents found to induce endothelial cell morphology changes, did show significant differences. While colchicine, combretastatin and docetaxel induced a rapid cellular rounding up, as expected with these tubulin acting agents, fisetin, on the contrary induced an increase in cellular asymmetry with numerous large extensions and filopodias. In addition, the morphological effects of the anti-actin cytochalasin D were very different from those induced by fisetin. Indeed, cytochalasin D did not present the large extensions induced by fisetin, but instead a partial cellular retraction which revealed several thin filopodias. Collectively, these data indicate that fisetin has an effect on both microtubule and actin filament organization, although it is difficult to determine which effect is preceding the other (28). Because it has been reported that microtubules may organize independently of actin filaments, and that actin filaments need microtubules to maintain a normal organization (29), fisetin could therefore act first on the organization of microtubules through stabilization, and secondly on the organization of actin filaments. The fisetin-induced dramatic increase in cell asymmetry thus appears to be the result of an alteration of the whole cytoskeleton organization. Our data indicate an original mechanism of action of fisetin which markedly differ from standard anti-tubulin or anti-actin compounds.
Because the microscopic observation of the cytoskeleton of the fisetin-treated cells was hinting to a microtubule stabilization, these cells were then submitted to a cold-induced depolymerization test, and it was found that microtubules from the fisetin-treated cells were more resistant than control cells. We next investigated a possible post-translational modification of α-tubulin involved in microtubule stabilization and we found that the degree of α-tubulin acetylation following fisetin treatment was rapidly and significantly increased. It is of interest that similar morphological changes have been observed with neuronal cells during the process of differentiation (30), but to our knowledge, no such changes have been reported for endothelial cells. Falconer et al. (30) have demonstrated that retinoic acid can induce a new array of acetylated microtubules arranged in a bundle of parallel microtubules which were resistant to depolymerization. Similarly, we found that fisetin-treated cell extensions also presented parallel microtubules arranged in bundles and were more stable because of their resistance to depolymerization. The increased microtubule stability assessed by the cold test has already been shown to be correlated with acetylated microtubules (31). It can also be noted that fisetin not only could delay microtubules depolymerization, but could also induce the formation of small aggregates indicating an incomplete depolymerization process. However, it should be mentioned that in addition to α-tubulin acetylation, the fisetin-induced increase in microtubule stability could also be due to other factors involved in microtubule stabilization, such as microtubule associated proteins (MAP) like tau, MAP1B, and MAP2c (32–34).
The cellular studies reported herein have to be put in perspective, because flavonoid bioavailability is in general highly variable and relatively low after oral administration of the aglycone, in part due to extensive hepatic and intestinal metabolism (reviewed in (35)). However, it can be stressed that improving metabolic stability of these compounds is feasible (through methylation for example), and could be a useful approach for improving oral bioavailability and therefore the in vivo effects of these molecules (36). Concerning fisetin, concentration of 10 μM could be reached in vivo in mice after intraperitoneal injection of a non toxic dose, thus demonstrating that potentially efficacious concentrations, close to the ones reported here in cellular experiments, can indeed be achieved in vivo (Touil et al., unpublished data).
The flavonoid induced morphological effects on endothelial cells at non cytotoxic concentrations reported in this study are probably involved in the therapeutic mechanism of action of some flavonoids. Although the increase in asymmetry of endothelial cells induced by fisetin is not the usual rounding up observed with antivascular agents of the combretastatin class of agents, it could nonetheless act on the vasculature. For example, certain flavonoids, including fisetin, have been reported to possess antiangiogenic activity in vitro (37) and in vivo (38). In addition, the morphologically active flavonoids identified in the present work could be useful agents in the study of microtubule dynamics and post-translational modifications of α-tubulin. In conclusion, this study disclosed important SAR that could guide the choice of nutritional flavonoids or the rational synthesis of novel flavonoids with improved cytotoxic, antiangiogenic and/or antivascular properties useful in cancer therapy and other diseases where pathologic angiogenesis is involved.
Acknowledgments
Supported by the Institut National de la Recherche et de la Santé Médicale (Inserm), by the Centre National de la Recherche Scientifique (CNRS), and by the French Institut National du Cancer (INCa, 92513 Boulogne-Billancourt, France). We thank Johanne Seguin, Sébastien David, Else Hotin, and Aurélie Chiche for their expert technical assistance in cellular experiments. We also wish to thank Dr. Virginie Escriou for her assistance with confocal microscopy.
Abbreviations used
- SAR
structure-activity relationships
- HUVEC
human umbilical vein endothelial cells
- EA·hy 926
immortalized HUVEC line
- VEGF
vascular endothelial growth factor
- PDGF
platelet-derived growth factor
- DAPI
4′,6-diamidino-2-phenyindole
- DMEM
Dulbecco’s modified medium
- DMSO
dimethylsulfoxide
- MTT
1-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium
- FITC
fluorescein isothiocyanate
- EC50
effective concentration for cell shape change relative to controls
- IC50
inhibitory concentration for 50% of cells
- PBS
phosphate buffered saline
- BSA
bovine serum albumin
- TRITC
tetramethylrhodamine isothiocyanate
Footnotes
This study was presented in part at the XXVIIth Forum de cancérologie, Paris, June 26-27, 2007: Touil YS, Fellous A, Scherman D, Chabot GG. Effets des flavonoïdes sur la morphologie des cellules endothélials par stabilisation des microtubules. Bull. Cancer 94, 551, 2007 (abstr No. 84).
References
- 1.Kerbel RS. Molecular origins of cancer: Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Taraboletti G, Margosio B. Antiangiogenic and antivascular therapy for cancer. Curr Opin Pharmacol. 2001;1:378–384. doi: 10.1016/s1471-4892(01)00065-0. [DOI] [PubMed] [Google Scholar]
- 5.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 8.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 9.Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer. 2004;100:2491–2499. doi: 10.1002/cncr.20299. [DOI] [PubMed] [Google Scholar]
- 10.Siemann DW. Vascular targeting agents. Horizons in cancer therapeutics: From bench to bedside. 2002;3:4–15. [Google Scholar]
- 11.Aherne SA, O’Brien NM. Dietary flavonols. chemistry, food content, and metabolism. Nutrition. 2002;18:75–81. doi: 10.1016/s0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Lazaro M. Flavonoids as anticancer agents: structure-activity relationship study. Curr Med Chem Anticancer Agents. 2002;2:691–714. doi: 10.2174/1568011023353714. [DOI] [PubMed] [Google Scholar]
- 13.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 14.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 15.Hill S, Williams KB, Denekamp J. Vascular collapse after flavone acetic acid: a possible mechanism of its anti-tumour action. Eur J Cancer Clin Oncol. 1989;25:1419–1424. doi: 10.1016/0277-5379(89)90099-0. [DOI] [PubMed] [Google Scholar]
- 16.Monasterio A, Urdaci MC, Pinchuk IV, Lopez-Moratalla N, Martinez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50:90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 17.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouis D, Hospers GA, Meijer C, Molema G, Mulder NH. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis. 2001;4:91–102. doi: 10.1023/a:1012259529167. [DOI] [PubMed] [Google Scholar]
- 19.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 20.Verschueren H, Houben B, De Braekeleer J, De Wit J, Roggen D, et al. Methods for computer assisted analysis of lymphoid cell shape and motility, including Fourier analysis of cell outlines. J Immunol Methods. 1993;163:99–113. doi: 10.1016/0022-1759(93)90244-2. [DOI] [PubMed] [Google Scholar]
- 21.Shelanski ML, Gaskin F, Cantor CR. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fellous A, Francon J, Lennon AM, Nunez J. Microtubule assembly in vitro. Purification of assembly-promoting factors. Eur J Biochem. 1977;78:167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- 23.Zavala F, Guenard D, Robin JP, Brown E. Structure-antitubulin activity relationship in steganacin congeners and analogues. Inhibition of tubulin polymerization in vitro by (+/−)-isodeoxypodophyllotoxin. J Med Chem. 1980;23:546–549. doi: 10.1021/jm00179a014. [DOI] [PubMed] [Google Scholar]
- 24.Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- 25.Beutler JA, Hamel E, Vlietinck AJ, Haemers A, Rajan P, et al. Structure-activity requirements for flavone cytotoxicity and binding to tubulin. J Med Chem. 1998;41:2333–2338. doi: 10.1021/jm970842h. [DOI] [PubMed] [Google Scholar]
- 26.Galbraith SM, Chaplin DJ, Lee F, Stratford MR, Locke RJ, et al. Effects of combretastatin A4 phosphate on endothelial cell morphology in vitro and relationship to tumour vascular targeting activity in vivo. Anticancer Res. 2001;21:93–102. [PubMed] [Google Scholar]
- 27.Dupeyre G, Chabot GG, Thoret S, Cachet X, Seguin J, et al. Synthesis and biological evaluation of (3,4,5-trimethoxyphenyl)indol-3-ylmethane derivatives as potential antivascular agents. Bioorg Med Chem. 2006;14:4410–4426. doi: 10.1016/j.bmc.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 28.Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–477. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Gotlieb AI. Microtubules regulate aortic endothelial cell actin microfilament reorganization in intact and repairing monolayers. Histol Histopathol. 2005;20:455–465. doi: 10.14670/HH-20.455. [DOI] [PubMed] [Google Scholar]
- 30.Falconer MM, Vielkind U, Brown DL. Establishment of a stable, acetylated microtubule bundle during neuronal commitment. Cell Motil Cytoskeleton. 1989;12:169–180. doi: 10.1002/cm.970120306. [DOI] [PubMed] [Google Scholar]
- 31.Schatten G, Simerly C, Asai DJ, Szoke E, Cooke P, et al. Acetylated alpha-tubulin in microtubules during mouse fertilization and early development. Dev Biol. 1988;130:74–86. doi: 10.1016/0012-1606(88)90415-0. [DOI] [PubMed] [Google Scholar]
- 32.Hanemaaijer R, Ginzburg I. Involvement of mature tau isoforms in the stabilization of neurites in PC12 cells. J Neurosci Res. 1991;30:163–171. doi: 10.1002/jnr.490300117. [DOI] [PubMed] [Google Scholar]
- 33.Mack TG, Koester MP, Pollerberg GE. The microtubule-associated protein MAP1B is involved in local stabilization of turning growth cones. Mol Cell Neurosci. 2000;15:51–65. doi: 10.1006/mcne.1999.0802. [DOI] [PubMed] [Google Scholar]
- 34.Gamblin TC, Nachmanoff K, Halpain S, Williams RC., Jr Recombinant microtubule-associated protein 2c reduces the dynamic instability of individual microtubules. Biochemistry. 1996;35:12576–12586. doi: 10.1021/bi961135d. [DOI] [PubMed] [Google Scholar]
- 35.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 36.Walle T, Wen X, Walle UK. Improving metabolic stability of cancer chemoprotective polyphenols. Expert Opin Drug Metab Toxicol. 2007;3:379–388. doi: 10.1517/17425255.3.3.379. [DOI] [PubMed] [Google Scholar]
- 37.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, et al. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 38.Joussen AM, Rohrschneider K, Reichling J, Kirchhof B, Kruse FE. Treatment of corneal neovascularization with dietary isoflavonoids and flavonoids. Exp Eye Res. 2000;71:483–487. doi: 10.1006/exer.2000.0900. [DOI] [PubMed] [Google Scholar]


