Abstract
Dendritic cell (DC) differentiation from human CD34+ hematopoietic progenitor cells (HPCs) can be triggered in vitro by a combination of cytokines consisting of stem cell factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor α. The immune response regulatory cytokines, IL-4 and IL-13, promote DC maturation from HPCs, induce monocyte-DC transdifferentiation, and selectively up-regulate 15-lipoxygenase 1 (15-LO-1) in blood monocytes. To gain more insight into cytokine-regulated eicosanoid production in DCs we studied the effects of IL-4/IL-13 on LO expression during DC differentiation. In the absence of IL-4, DCs that had been generated from CD34+ HPCs in response to stem cell factor/granulocyte-macrophage colonystimulating factor/tumor necrosis factor α expressed high levels of 5-LO and 5-LO activating protein. However, a small subpopulation of eosinophil peroxidase+ (EOS-PX) cells significantly expressed 15-LO-1. Addition of IL-4 to differentiating DCs led to a marked and selective down-regulation of 5-LO but not of 5-LO activating protein in DCs and in EOS-PX+ cells and, when added at the onset of DC differentiation, also prevented 5-LO up-regulation. Similar effects were observed during IL-4- or IL-13-dependent monocyte-DC transdifferentiation. Down-regulation of 5-LO was accompanied by up-regulation of 15-LO-1, yielding 15-LO-1+ 5-LO-deficient DCs. However, transforming growth factor β1 counteracted the IL-4-dependent inhibition of 5-LO but only minimally affected 15-LO-1 up-regulation. Thus, transforming growth factor β1 plus IL-4 yielded large mature DCs that coexpress both LOs. Localization of 5-LO in the nucleus and of 15-LO-1 in the cytosol was maintained at all cytokine combinations in all DC phenotypes and in EOS-PX+ cells. In the absence of IL-4, major eicosanoids of CD34+-derived DCs were 5S-hydroxyeicosatetraenoic acid (5S-HETE) and leukotriene B4, whereas the major eicosanoids of IL-4-treated DCs were 15S-HETE and 5S-15S-diHETE. These actions of IL-4/IL-13 reveal a paradigm of eicosanoid formation consisting of the inhibition of one and the stimulation of another LO in a single leukocyte lineage.
Keywords: transforming growth factor β1, arachidonic acid cascade
Eicosanoids produced by the 5-lipoxygenase (5-LO) pathway are potent mediators of inflammatory and allergic tissue reactions (1). Several lines of evidence indicate that 5-LO-derived eicosanoids (2, 3) participate in the regulation of adaptive immunity (4, 5). Human epidermal Langerhans cells and dendritic cells (DCs) in the paracortex of lymphoid organs express the 5-LO pathway at marked levels in vivo (2, 3); DCs generated in vitro from CD34+ hematopoietic progenitor cells (HPCs) in response to a combination of stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor α (TNF-α) up-regulate 5-LO and 5-LO-activating protein (FLAP) genes, and the DC maturation-promoting cytokine transforming growth factor β1 (TGF-β1) enhances up-regulation of 5-LO (3); leukotriene (LT) B4 has been shown to support B and T lymphocyte activation in vitro (6); transcripts of LT receptors are expressed in lymphoid organs (unpublished data); 5-LO-deficient mice show reduced ovalbumin-dependent cellular and humoral immune responses during antigen-dependent allergenization of the lung (7); and mice deficient in the cysteinyl LT transporter multidrug resistance protein-1 have a defect in DC mobilization from skin to lymph nodes (8). Although these data associate the 5-LO pathway with the adaptive immune response, the precise molecular mechanisms of LTs action within the immune system remain elusive.
DCs are members of the family of professional antigen-presenting cells that are uniquely capable of initiating immune responses in naive lymphocytes (5). Because all human DC phenotypes examined so far constitutively express the 5-LO pathway, we suggested that DC 5-LO-derived eicosanoids may participate in the regulation of proximal steps of the immune response (3). Primary immune responses involve phenomena as diverse as antigen uptake by DCs, their subsequent migration through multiple tissue barriers, homing in lymphoid organs, and antigen presentation to T cells (reviews in refs. 2 and 3). 5-LO products could play roles in each of these events; the specific roles of the 5-LO and other LO pathways in immunity have yet to be defined. IL-4 and IL-13 (which bind to the IL-4 receptor α chain) have been shown to affect DC differentiation from both CD34+ HPCs and blood monocytes in vitro (9, 10). IL-4 is a multifunctional cytokine produced by distinct populations of T helper (TH) cells (termed TH2 cells) and other lymphocytes that have diverse biological activities in hematopoietic and nonhematopoietic tissues (9). In particular, IL-4 is required for the execution of TH2-type immune responses (i.e., B cell expansion and Ig isotype switching from IgM to IgE). Therefore, IL-4 is considered a central cytokine mediating allergic and autoimmune disorders. However, IL-4 also has been shown to exert anti-inflammatory properties in a variety of disease conditions (9). Nothing is known about the effects of IL-4 on the 5-LO pathway, but IL-4 and IL-13 up-regulate 15-LO-1 in blood monocytes and epithelial cells (11, 12). With the use of serial analyses of 17,000 genes it was found that 15-LO-1 is among the most abundantly expressed genes in DCs generated by treatment of blood monocytes with IL-4 for 6 days (13). Here we report that IL-4 inhibits 5-LO and concomitantly induces 15-LO-1 expression in differentiating DCs derived from HPCs and blood monocytes, that the combined activities of IL-4 and TGFβ1 generate 5-LO+/15-LO-1+ mature DCs, and that IL-4 also down-regulates 5-LO in 15-LO-1+ eosinophil peroxidase (EOS-PX)+ cells.
Materials and Methods
Materials.
Avian myeloblastosis virus reverse transcriptase and Thermus aquaticus DNA polymerase were from Roche Diagnostics. Primary monoclonal antisera against CD1a and HLA-DR were from Coulter. EOS-PX was from PharMingen. Cy2-, Cy3-, phycoerythrin (PE)-, and FITC-labeled secondary antisera were from Dianova (Hamburg, Germany). Cytokines were from R & D Systems. All other reagents were from Sigma.
Cell Culture.
CD34+ HPCs were prepared to a purity of >98% from human cord blood, and 2 × 104 cells/ml were maintained in RPMI 1640 medium with supplements and cytokines as described (3). Fresh complete culture medium was added to maintain cells at a density between 3 × 105 and 5 × 105 cells/ml. Human blood monocyte purification from peripheral blood mononuclear cells involved two rounds of anti-CD14 antibody-coated microbead adsorption (Miltenyi Biotec, Bergisch Gladbach, Germany) and maintenance at 5 × 105 cells/ml in medium supplemented with GM-CSF (100 ng/ml) and IL-4 (50 ng/ml) (10).
Reverse Transcription–PCR (RT-PCR).
RT-PCR analyses were performed on RNA extracts of 1 × 105 to 2 × 105 cells as described (2). Primers for 15-LO-1 were 5′-GGG GCT GGC CGA CCT CGC TAT C-3′ (forward) and 5′-TCC TGT GCG GGG CAG CTG GAG C-3′ (reverse), giving a 432-bp product. The annealing temperature was 71°C. Results are expressed as densitometry units of 5-LO, 15-LO-1, or FLAP PCR products relative to glyceraldehyde-3-phosphate dehydrogenase PCR products. Additional parameters are available on request.
Fluorescence Immunohistochemistry.
Immunohistochemical analyses were performed as described (3) with the use of anti-5-LO antiserum, 1550. A polyclonal anti-15-LO-1 antiserum directed against rabbit 15-LO-1 antigen was generated in guinea pigs and was shown to cross-react with human 15-LO-1 at a dilution of 1:200; its specificity was determined by selective staining of EOS-PX+ cells in HPC progeny and bronchial epithelial cells of lung tissue and correlated with RT-PCR data, fluorescence-activated cell sorter (FACS), and immunoblot analyses of cultured cells. For two-color immunofluorescence slides were incubated with donkey anti-rabbit IgG F(ab′)2 conjugated with Cy3, donkey anti-guinea pig IgG F(ab′)2 conjugated with Cy3 or Cy2, and goat anti-mouse IgG F(ab′)2 conjugated with Cy2 for 1 h. Confocal laser scan microscopy was performed with a Zeiss Axiovert 100 M microscope equipped with a LSM 510 laser scanner head. Additional details are available on request.
FACS Analyses.
FACS analyses of LOs were performed as described for 5-LO (3). For cell lineage identification of both LOs, we combined a rabbit anti-5-LO antiserum with a guinea pig anti-15-LO-1 antiserum, with the use of a FACSCalibur flow cytometer (Becton Dickinson). Data were recorded with the use of the cellquest program (Becton Dickinson).
Assays.
Immunoblots were performed on 10 μg total cell protein as described (3). Eicosanoid formation in intact cells was determined in response to 10 μM Ca2+ ionophore A23187 and 40 μM arachidonic acid or in cell-free systems in the presence of 40 μM arachidonic acid as described (12).
Results
IL-4 Down-Regulates 5-LO During Leukopoiesis of SCF/GM-CSF/TNF-α-Treated CD34+ HPCs.
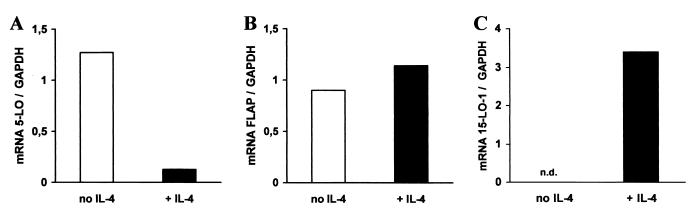
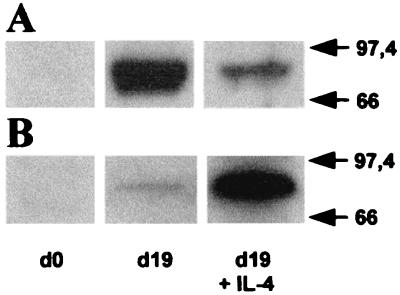
Exposure of HPCs to SCF/GM-CSF/TNF-α yields a major population of 5-LO+ DCs consisting of three main populations, monocytic DCs, Langerhans-type DCs, and a mixed DC phenotype, and two smaller 5-LO+ cell populations, myeloid/neutrophil precursors and EOS-PX+ cells (3). In the first set of experiments, we added IL-4 to 5-LO+ progeny that had been induced to differentiate into DCs in the presence of SCF/GM-CSF/TNF-α for 8 days. By this time leukopoiesis had been initiated and significant up-regulation of 5-LO and FLAP expression had occurred (3, 10). 5-LO transcript levels were determined 7 days thereafter. IL-4 caused a major decrease in 5-LO transcript levels (Fig. 1A). In contrast, FLAP transcripts that also had been up-regulated during the first 8 days of the DC differentiation pathway (3) remained largely unaffected by IL-4 (Fig. 1B). IL-4 caused a decrease in 5-LO transcripts by >90% of the control level (Fig. 1A). In a second experiment, IL-4 was added together with SCF/GM-CSF/TNF-α to freshly isolated HPCs, and transcript levels were determined 15 days later. IL-4 prevented up-regulation of 5-LO but not of FLAP transcripts (not shown). However, kinetic studies showed that 5-LO transcript inhibition was absent or, in other experiments, less pronounced within the initial 5–7 days of DC differentiation, i.e., when generation of DCs from HPCs in response to cytokines is known to be initiated (10). This observation indicated that the action of IL-4 on 5-LO requires ongoing leukopoiesis. 5-LO transcript inhibition was accompanied by the inhibition of 5-LO protein as determined by immunoblots (Fig. 2A). As IL-4 promotes DC differentiation from CD34+ HPCs, these data raised the possibility that DCs are among the target cells of IL-4.
Figure 1.
IL-4 down-regulates 5-LO transcripts in CD34+ HPCs. HPCs were maintained in SCF/GM-CSF/TNF-α for 15 days (empty columns) or in SCF/GM-CSF/TNF-α for 8 days and additional IL-4 for 7 days (filled columns). Transcripts for 5-LO (A), FLAP (B), and 15-LO-1 (C) were determined by RT-PCR. Data are expressed as ratios of densitometry units of 5-LO (27 cycles), FLAP (25 cycles), or 15-LO-1 (30 cycles) transcripts relative to glyceraldehyde-3-phosphate dehydrogenase transcripts (23 cycles). n.d., not detectable.
Figure 2.
IL-4 down-regulates 5-LO protein and up-regulates 15-LO-1 protein during DC differentiation. HPCs were cultured as described in Fig. 1, except that IL-4 was added to freshly prepared HPCs. After 19 days, 5-LO (A) and 15-LO-1 (B) immunoblots were prepared from 10 μg total cell protein. Molecular weight standards are indicated at the right.
IL-4 Up-Regulates 15-LO-1 in HPC-Derived Leukocytes.
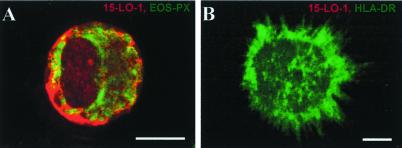
Transcript levels of 15-LO-1 were determined in freshly isolated HPCs and its SCF/GM-CSF/TNF-α-derived progeny in the absence of added IL-4. We observed significant up-regulation of 15-LO-1 transcripts (data not shown). When eicosanoid production in response to Ca2+ ionophore A23187 and arachidonic acid was determined, (15S,5Z,8Z,11Z,13E)-15-hydroxy-5,8,11,13-eicosatetraenoic acid (15S-HETE) and lower amounts of 12S-HETE were formed in addition to 5S-HETE and LTB4 (see below). These results showed that 15-LO-1 is up-regulated during DC differentiation by SCF/GM-CSF/TNF-α. To identify the 15-LO-1+ leukocyte lineage, cell type markers and anti-15-LO-1 antisera were used. 15-LO-1 protein expression was found to be restricted to EOS-PX+ cells (Fig. 3A), whereas none of several DC phenotypes expressed 15-LO-1 (Fig. 3B; additional data not shown). The expression levels of 15-LO-1 protein in EOS-PX+ cells were significant, as it was detectable in immunoblots of 10 μg of protein extracted from the total progeny (Fig. 2B). These data showed that the majority of HPC progeny were 5-LO+/15-LO-1− and that a small EOS-PX+ population expressed 15-LO-1 at significant levels. It is noteworthy that 15-LO-2 (14, 15) transcripts were also detectable in the HPC progeny but not in freshly isolated CD34+ HPCs. No attempts were made to identify the cells that express 15-LO-2.
Figure 3.
15-LO-1 is expressed exclusively in EOS-PX+ cells but not in DCs of HPC progeny. HPCs were cultured in SCF/GM-CSF/TNF-α for 15 days. Fluorescence immunohistochemistry was performed as described in Materials and Methods, with the use of anti-15-LO-1 antiserum (red, Cy3) (A and B), EOS-PX (Cy2, green, A), and HLA-DR (Cy2, green, B). (Bars = 10 μm.)
Unlike DCs, EOS-PX+ cells, which constitute a minor cell population under DC differentiation conditions (Fig. 3 and see Fig. 5), express 15-LO-1 constitutively, i.e., in the absence of added IL-4/IL-13 (Fig. 3A). In addition, with the use of RT-PCR, we failed to detect endogenous IL-4/IL-13 transcripts during the DC differentiation pathway. These data support the hypothesis that unknown factors control the expression of 15-LO-1 in EOS-PX+ cells. We next determined the effects of IL-4 on 15-LO-1 transcripts during SCF/GM-CSF/TNF-α-dependent DC differentiation. IL-4 caused a marked increase in 15-LO-1 transcripts in IL-4 treated cells compared with control cells (Fig. 1C). IL-4 also induced 15-LO-1 protein (Fig. 2B). These data showed that IL-4 up-regulates 15-LO-1 during a time window that roughly correlates with its inhibitory action on 5-LO. As for the inhibitory action on 5-LO, these data raised the possibility that 15-LO-1 up-regulation occurred in DCs.
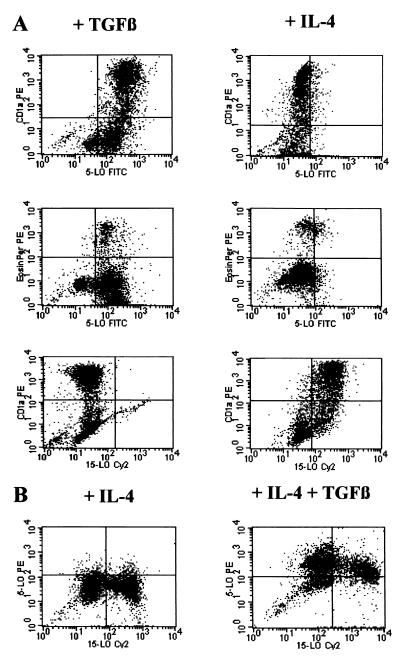
Figure 5.
5-LO and 15-LO-1 FACS analyses identify IL-4 targets as DCs. 5-LO and 15-LO-1 FACS analyses were performed as described (3). HPCs were cultured in SCF/GM-CSF/TNF-α/IL-4 (Right, A; Left, B), SCF/GM-CSF/TNF-α/TGF-β1 (Left, A), or SCF/GM-CSF/TNF-α/IL-4/TGF-β1 (Right, B) for 14 days.
Distinct Activities of IL-4 and TGF-β1 on 5-LO and 15-LO-1.
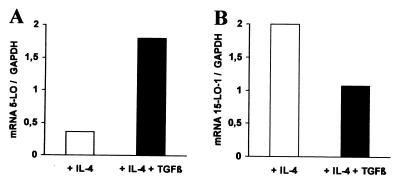
The IL-4 effect on 5-LO prevailed over the up-regulating effects of SCF/GM-CSF/TNF-α, resulting in reduction of 5-LO transcript and protein levels by ≈90% (Figs. 1 and 2). This inhibitory activity of IL-4 was of interest in relation to the previously reported up-regulation of 5-LO by TGF-β1 (3). As both IL-4 and TGF-β1 are potent in vitro DC differentiation-promoting cytokines (reviewed in refs. 2 and 3), we quantified 5-LO transcript expression during DC differentiation in response to both IL-4 and TGF-β1. TGF-β1 caused a state of resistance to the down-regulation of 5-LO by IL-4. Thus, when IL-4 was added together with TGF-β1, high levels of 5-LO transcripts and protein were maintained (Fig. 4A and data not shown). In addition, TGFβ1 mitigated somewhat 15-LO-1 expression caused by IL-4, albeit to a much lesser extent (Fig. 4B). These results show separable activities of IL-4 and TGF-β1 on 5- and 15-LO-1 during in vitro leukopoiesis from HPCs.
Figure 4.
IL-4 and TGF-β1 differentially affect 5- and 15-LO-1 expression. HPCs were cultured in SCF/GM-CSF/TNF-α/IL-4 (empty columns) or SCF/GM-CSF/TNF-α/IL-4/TGFβ1 (filled columns) for 14 days. Transcripts for 5-LO (A) and 15-LO-1 (B) were determined by RT-PCR as described in Materials and Methods.
5- and 15-LO-1 FACS Analyses Identify IL-4 and TGF-β1 Targets as 5-LO+/15-LO-1+ DCs.
We reported (3) that >50% of the progeny were 5-LO+ DCs after prolonged exposure to SCF/GM-CSF/TNF-α and that TGF-β1 enhanced this percentage by up to >80% (HLA-DR+ cells), 64% of which were CD1a+ (Fig. 5A; additional data not shown). In the absence of TGF-β1 IL-4 suppressed 5-LO positivity to <5% of all cells (Fig. 5). Other FACS and immunohistochemical analyses showed that IL-4 suppressed 5-LO in CD1a+, HLA-DR+, CD80+, and CD86+ DCs (Fig. 5A; additional data not shown). Moreover, IL-4 suppressed 5-LO in EOS-PX+ cells (Fig. 5A). Thus, major target cells of IL-4 with respect to 5-LO suppression are DCs. However, the IL-4 effect extends to EOS-PX+ cells. More work is required to demonstrate that mature tissue eosinophils and other mature leukocytes down-regulate 5-LO expression in response to IL-4 (see below). To identify the target lineages of IL-4 with respect to 15-LO-1 expression, a 15-LO-1 FACS assay was established. As determined by immunohistochemistry, 15-LO-1 protein was exclusively found in EOS-PX+ cells but not in DCs (compare Fig. 3 A and B), and TGF-β1 did not alter the lineage distribution of 15-LO-1 protein, as no CD1a+ cells expressed 15-LO-1 in TGF-β1-treated DCs (Fig. 5A). However, IL-4 up-regulated 15-LO-1 in CD1a+ DCs and additional DC phenotypes (Fig. 5A). These data indicated that IL-4 caused down-regulation of 5-LO and up-regulation of 15-LO-1 in the same DCs. Indeed, combined two-color FACS analyses showed that a large proportion of CD1a+ DCs generated in the presence of IL-4 without added TGF-β1 express 15-LO-1 but no 5-LO (Fig. 5B) and that both cytokines generated double 5-LO+/15-LO-1+ DCs (Fig. 5B).
Confocal Laser Scan Microscopy Reveals Separate 5-LO and 15-LO-1 Compartments in DCs Generated in the Presence of IL-4 and TGF-β1.
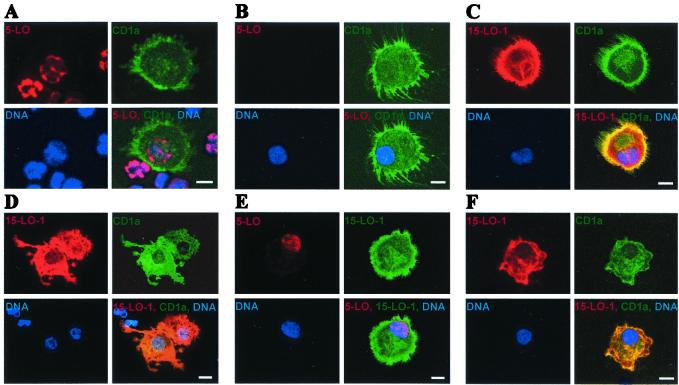
To study the effects of IL-4 on cellular compartments of each LO, we applied confocal laser scan microscopy. IL-4 eliminated 5-LO protein from the nuclei of DCs that had been generated by SCF/GM-CSF/TNF-α (Fig. 6 A and B). Concomitantly, IL-4 caused cytoplasmic expression of 15-LO-1 in several morphological DC phenotypes: in DCs with extensive networks of fine dendrites, in DCs with large cytoplasmic protrusions (Fig. 6 C and D), and in CD1a+ DCs without networks of long dendrites (not shown). 15-LO-1 appeared to be homogeneously distributed throughout the cytoplasm and was clearly seen in the dendrites (Fig. 6 C and D). When TGF-β1 was present, 5-LO+/15-LO-1+ DCs were generated that expressed 5-LO in the nucleus and 15-LO-1 in the cytosol (Fig. 6E). These data showed that 5-LO has a strict nuclear localization in all DC phenotypes, that IL-4 eliminates 5-LO protein from its nuclear compartment when TGF-β1 is absent, that 15-LO-1 is constitutively present in the cytosol of EOS-PX+ cells and induced in the cytosol of IL-4-derived DCs, and that the nuclear and cytosolic compartments of both LOs are maintained in IL-4 plus TGF-β1-treated DCs.
Figure 6.
Localization of 5-LO and 15-LO-1 in HPC-derived DCs and of 15-LO-1 in monocyte-derived DCs. HPCs were cultured in SCF/GM-CSF/TNF-α (A), SCF/GM-CSF/TNF-α/IL-4 (B–D), and SCF/GM-CSF/TNF-α/TGF-β1/IL-4 (E) for 14 days. Monocyte-derived DCs (F) were generated as described in Materials and Methods by treatment with GM-CSF + IL-4 for 7 days. Secondary antisera or DNA (Hoechst 33258) stains were performed as indicated in individual frames. The lower right frame of each set represents a merge of secondary antisera and DNA stain. (Bars = 10 μm.)
IL-4 Triggers a Switch from 5S-HETE Production to 15S-HETE and 5S-15S-diHETE in DCs.
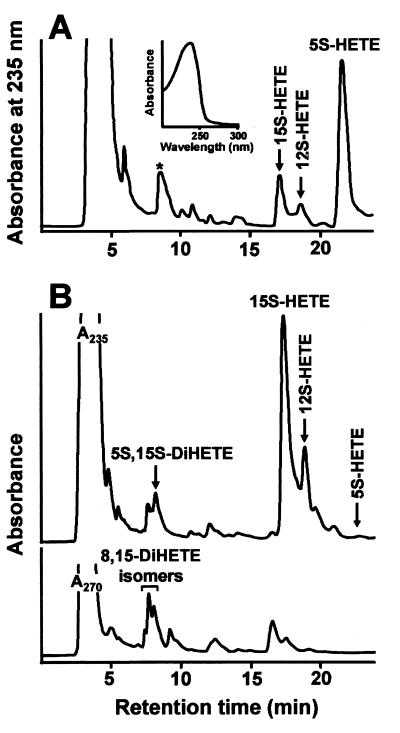
Intact progeny of HPCs treated with SCF/GM-CSF/TNF-α produce significant amounts of 5-HETE and LTB4, and TGF-β1 enhances the amounts of 5-LO products by a factor of 3–4 (3). To study HETE formation in intact cells we added arachidonic acid and Ca2+ ionophore A23187 to DCs that had been differentiating in the presence of TGF-β1 but in the absence of IL-4. The major eicosanoid in these cells was 5S-HETE (Fig. 7). The UV spectrum of the major oxygenation product indicated a typical conjugated diene chromophore with an absorbance maximum at 235 nm (Fig. 7A, Inset). It is noteworthy that we did not observe formation of significant amounts of 5,15-diHETE when IL-4 was absent. The peak labeled with an asterisk in the chromatogram (Fig. 7A), which migrated in the di-HETE region, may be due to products of nonenzymatic LTA4 hydrolysis (conjugated triene chromophore in UV spectroscopy) 15S-HETE was also formed, at amounts that ranged around 15% of the amount of 5-LO products (Fig. 7A). In contrast, when IL-4 was present during DC differentiation, 5S-HETE production was greatly reduced and the major eicosanoid was 15(S)-HETE (Fig. 7B). This represented a 27-fold stimulation of 15S-HETE formation. In addition to 15S-HETE, IL-4-treated DCs formed 12-HETE (5Z,8Z,10E,14Z)-12-hydroxy-5,8,1,14-eicosatetraenoic acid) and 5S,15S-diHETE (5S,15S,6E,8Z,11Z,13E)-5,15-hydroxy-6,8,11,13-eicosatetraenoic acid). This product pattern is compatible with the sole action of 15-LO-1 (16). Interestingly, however, we also found transcripts of 15-LO-2 (14) in all CD34+-derived progeny but not in freshly isolated HPCs, indicating up-regulation of 15-LO-2 in this culture system (not shown). Recording the chromatogram at 270 nm, we found small amounts of LTB4. In addition, various 8,15-diHETE isomers were observed that may originate from secondary oxygenation of 15-HETE by 15-LO-1. This observation is consistent with earlier reports that pure rabbit 15-LO-1 can oxygenate 15-HETE to 5,15-diH(P)ETE and 8,15-diH(P)ETE (16). The formation of 5S-15S-HETE also may originate from the concerted action of residual 5-LO and 15-LO-1. As the IL-4 plus TGF-β1-treated DCs coexpressed both LOs and because it has been proposed that lipoxins can be produced by the subsequent action of 15-LO-1 and 5-LO (1), we studied the possibility that lipoxins were formed. The amounts of conjugated tetraenes, determined at 300 nm, including lipoxins, however, did not exceed 1% of the recorded eicosanoids (not shown). Thus, DC coexpression of 15-LO-1 and 5-LO did not generate significant amounts of lipoxins in response to Ca2+ ionophore and arachidonic acid despite significant coexpression of both LOs in single DCs (Figs. 5–7).
Figure 7.
IL-4 triggers a switch in eicosanoid production from 5S-HETE to 15S-HETE and 5S-15S-diHETE. HPCs were cultured in the presence of SCF/GM-CSF/TNF-α/TGF-β1 (A) or SCF/GM-CSF/TNF-α/IL-4 (B) for 14 days. Eicosanoid formation in intact cells was performed and analyzed as described in Materials and Methods.
IL-4 Down-Regulates 5-LO and Up-Regulates 15-LO-1 Expression in Human Blood Monocytes During Monocyte-DC Transdifferentiation.
IL-4/IL-13 are the only known cytokines that can trigger transdifferentiation of blood monocytes into DCs and induce 15-LO-1 in blood monocytes (11, 12, 17). Recently, it has been shown that a fraction of phagocytic mouse blood monocytes, when injected s.c., can home in on T lymphocyte-rich areas of draining lymph nodes and concomitantly undergo a DC differentiation pathway, supporting the hypothesis that monocyte-DC transdifferentiation can occur in vivo (18). We therefore studied the possibility that IL-4 and IL-13 suppress 5-LO transcripts and protein during the monocyte-DC transdifferentiation pathway. For this purpose, we purified human blood monocytes and maintained them in the presence of GM-CSF in the absence and presence of IL-4 or IL-13. Whereas GM-CSF-treated monocytes maintained a constant or somewhat elevated level of 5-LO expression for 9 days, IL-4 down-regulated 5-LO transcript levels within 3 days, which amounted to a 92% inhibition by day 9 when compared with the control cells. Similar effects were obtained with IL-13. Although 5-LO protein was detected for 5–7 days after the addition of IL-4 (indicating a considerably long half-life of 5-LO protein), it also became undetectable between days 7 and 9, as determined by immunohistochemistry (not shown). Concomitantly, IL-4-treated monocytes acquired a DC phenotype, as evidenced by the expression of several DC markers, including CD1a, as reported by others (10, 17). Concomitant with suppression of 5-LO by IL-4 or IL-13, the cytokines induced a major rise in 15-LO-1 expression, causing a LO switch similar to that in differentiating DCs (Fig. 6F).
Discussion
Our data demonstrate that IL-4/IL-13 inhibit 5-LO and concomitantly up-regulate 15-LO-1 gene expression in DCs. This effect is evidence of a novel paradigm in eicosanoid formation consisting of cytokine-specific down-regulation of one and up-regulation of another major LO in a single leukocyte lineage. The LO switch applies to differentiating DCs derived from proliferating CD34+ HPCs and to nondividing peripheral blood monocytes during DC transdifferentiation. Moreover, IL-4 inhibits 5-LO in EOS-PX+ cells, raising the possibility that additional IL-4-responsive leukocyte lineages may also be involved. Whereas several cytokines and cytokine combinations have been shown to enhance 5-LO expression (3), IL-4/IL-13 demonstrate inhibitory activity toward this LO. However, IL-4 acts distinctively on each LO, and both actions depend on the cytokine environment. Regarding 5-LO inhibition, IL-4 dominates over the 5-LO up-regulating activities brought about by SCF/GM-CSF/TNF-α, yielding 5-LO-deficient DCs (Fig. 6B) or 5-LO-deficient EOS-PX+ cells (Fig. 5A), yet TGF-β1 counteracts IL-4, yielding double 5-LO+/15-LO-1+ DCs (Figs. 5B and 6E). In contrast, 15-LO-1 up-regulation in response to GM-CSF/TNF-α is restricted to EOS-PX+ cells, and the IL-4-selective 15-LO-1 up-regulation in DCs (Figs. 3, 5, and 6) is not greatly affected by TGF-β1 (Fig. 4B).
We failed to detect significant amounts of transcripts of IL-4, IL-13, 15-LO-1, or 15-LO-1 protein-expressing cells in normal human lymph nodes. Therefore, the IL-4/IL-13-dependent LO switch in DCs may not occur in normal lymphoid organs but may be induced during lymphocyte activity in connection with an ongoing immune reaction mediated by TH2 lymphocytes (19, 20) or in allergic/autoimmune disorders at sites of tissue destruction, all of which are known to be associated with strong up-regulation of IL-4/IL-13 gene expression (20).
IL-4 is a pleiotropic cytokine that is not constitutively expressed, but its production requires receptor-mediated activation events. IL-4 has multiple effects on hematopoietic and extrahematopoietic tissues (9) and is induced in selected leukocytes such as CD4+ TH2 and TH3 lymphocytes, γ/δ T lymphocytes, basophils, mast cells, eosinophils, and mouse NK1.1 T lymphocytes in an antigen/microbe-specific fashion (21). Interestingly, some TH3-type lymphocytes, also termed regulatory T cells, are producers of IL-4 and TGF-β1 and, by that token, are capable of down-regulating an ongoing autoimmune response such as in experimental autoimmune encephalomyelitis, a model of multiple sclerosis in humans (21). Several physiological functions of IL-4 within the immune response have been defined: IL-4 is essential in the activation of naive T cells to TH2 cells, thereby powerfully suppressing the appearance of INF-γ-producing TH1 cells (9); and IL-4 controls Ig class switching from IgM to IgE in B cells (9). Production of IgE and the balance between TH1 and TH2 cells play critical roles in the development of protective immunity against parasitic infections and in the pathogenesis of autoimmune disorders in several animal models (20). This dual property of IL-4 is supported by studies of double IL-4/IL-13-deficient mice, IL-4 α-chain receptor-deficient mice, and other cytokine-deficient mouse models and by clinical observations of the effect of IL-4 in a treatment regimen (9).
What may be the function of the IL-4-dependent LO switch observed in DCs and other leukocytes? At least three possibilities merit consideration: (i) The fact that IL-4/IL-13 are not expressed constitutively but function as central TH2-type immune response regulatory cytokines indicates a role of 15-LO-1 in the execution of this response; we are currently studying the possibility that 15-LO-1 is induced in DCs or other immune cells during a TH2 immune response in lymph nodes in vivo and are attempting to determine whether this response depends on the action of IL-4/IL-13. Additional immune responses, i.e., TH3-type responses mediated by regulatory T cells, are also associated with production of IL-4 and, interestingly, TGFβ1, thereby mediating tolerance to unself antigens (21). For both of these events down-regulation of 5-LO and up-regulation of 15-LO-1 should be studied at the site of lymphocyte activation (lymph node) and at sites of lymphocyte activity (peripheral tissue). (ii) A possible direct connection between IL-4-induced 15-LO-1 (or its presumed mouse homologue, 12/15-LO) and the ligand-activated nuclear receptor peroxisome proliferator-activated receptor γ1 has recently been identified in IL-4-treated human blood monocytes in vitro and mouse peritoneal macrophages of normal and 12/15-LO-deficient mice (22). The IL-4-induced 12/15-LO in these cells was found to be required for the expression of a macrophage scavenger receptor, CD36, and the products of 15-LO, 13-hydroxyoctadecanoic acid, and 15-HETE stimulated expression of CD36 when added at micromolar concentrations; this finding was preliminary evidence that products of the IL-4-induced 15-LO-1 pathway may function as endogenous ligands of peroxisome proliferator-activated receptor γ1 (22); as for different types of lymphocyte activities (above), however, it will be important to demonstrate that a 15-LO-1 requirement for the biological activity of this receptor exists in vivo; (iii) finally, 5-LO suppression in leukocytes of the innate and adaptive immune systems may represent a hitherto unrecognized component of the antiinflammatory activities of IL-4 or constitute a component of an IL-4-dependent antiinflammatory feedback mechanism in vivo, as LTs are potent mediators of inflammatory and allergic reactions in peripheral tissues.
Acknowledgments
We are grateful to the nurses of the Departments of Gynecology of the Universities of Heidelberg and Jena for providing cord blood. Antiserum 1550 was prepared by Drs. Y.-Y. Zhang, T. Hammarberg, and H. Okamoto. This study was supported by the German Research Council (Ha 1083/12-1), the German Bundesministerium für Forschung und Technologie (01GB 9401/6), grants from the European Union concerted actions (BMH4-CT96-0229 and BMH4-CT98-3191), the Verband für Klinische Forschung Jena, the Swedish Medical Research Council (03X-217), and the Stiftung für Verhalten und Umwelt (VERUM).
Abbreviations
- DC
dendritic cell
- EOS-PX
eosinophil peroxidase
- FACS
fluorescence activated cell sorter
- FLAP
5-LO-activating protein
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HETE
hydroxyeicosatetraenoic acid
- HPC
hematopoietic progenitor cells
- LO
lipoxygenase
- LT
leukotriene
- RT-PCR
reverse transcription–PCR
- TH lymphocyte
T helper lymphocyte
- TNF
tumor necrosis factor
- TGF
transforming growth factor
- SCF
stem cell factor
References
- 1.Samuelsson B, Dahlén S-E, Lindgren J A, Rouzer C A, Serhan C N. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 2.Spanbroek R, Stark H-J, Janssen-Timmen U, Kraft S, Hildner M, Andl T, Bosch F-X, Fusenig N E, Bieber T, Rådmark O, et al. Proc Natl Acad Sci USA. 1998;95:663–668. doi: 10.1073/pnas.95.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spanbroek R, Hildner M, Steinhilber D, Fusenig N, Yoneda K, Rådmark O, Samuelsson B, Habenicht A J R. Blood. 2000;96:3857–3865. [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman R M. Nature (London) 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Brière F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Annu Rev Immunol. 2000;18:767–813. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 6.Yamaoka K A, Dugas B, Paul-Eugene N, Mencia-Huerta J M, Braquet P, Kolb J P. Cell Immunol. 1994;156:124–1134. doi: 10.1006/cimm.1994.1158. [DOI] [PubMed] [Google Scholar]
- 7.Funk C D. In: Molecular and Cellular Basis of Inflammation. Serhan C N, Ward P A, editors. Totowa, NJ: Humana; 1999. pp. 109–125. [Google Scholar]
- 8.Robbiani D F, Finch R A, Jäger D, Muller W A, Sartorelli A C, Randolph G J. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 9.Nelms K, Keegan A D, Zamorano J, Ryan J J, Paul W E. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 10.Caux C, Massacrier C, Dubois B, Valladeau J, Dezutter-Dambuyant C, Durand I, Schmitt D, Saeland S. J Leukocyte Biol. 1999;66:781–791. doi: 10.1002/jlb.66.5.781. [DOI] [PubMed] [Google Scholar]
- 11.Conrad D J, Kuhn H, Mulkins M, Highland E, Sigal E. Proc Natl Acad Sci USA. 1992;89:217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinckmann R, Topp M, Zalán I, Heydeck D, Ludwig P, Kühn H, Berdel K, Habenicht A J R. Biochem J. 1996;318:305–312. doi: 10.1042/bj3180305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto S-I, Suzuki T, Dong H-Y, Nagai S, Yamazaki N, Matsushima K. Blood. 1999;94:845–852. [PubMed] [Google Scholar]
- 14.Brash A R, Boeglin W E, Chang M S. Proc Natl Acad Sci USA. 1997;94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kühn H, Thiele B J. FEBS Lett. 1999;449:7–11. doi: 10.1016/s0014-5793(99)00396-8. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz K, Borngräber S, Anton M, Kühn H. Biochemistry. 1998;37:15327–15335. doi: 10.1021/bi9816204. [DOI] [PubMed] [Google Scholar]
- 17.Geissmann F, Prost C, Monnet J-P, Dy M, Brousse N, Hermine O. J Exp Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randolph G J, Inaba K, Robbiani D F, Steinman R M, Muller W A. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 19.Wills-Karp M. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 20.Mosman T R, Sad S. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 21.Weiner H. Annu Rev Med. 1997;48:341–351. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 22.Huang J T, Welch J S, Ricote M, Binder C J, Willson T M, Kelly C, Witztum J L, Funk C D, Conrad D, Glass C K. Nature (London) 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]