Abstract
While effective therapies are available for some types of craniofacial pain, treatments for deep-tissue craniofacial pain such as temporomandibular disorders are less efficacious. Several ion channels and receptors which are prominent in craniofacial nociceptive mechanisms have been identified on trigeminal primary afferent neurons. Many of these receptors and channels exhibit unusual distributions compared with extracranial regions. For example, expression of the ATP receptor P2X3 is strongly implicated in nociception and is more abundant on trigeminal primary afferent neurons than analogous extracranial neurons, making them potentially productive targets specifically for craniofacial pain therapies. The initial part of this review therefore focuses on P2X3 as a potential therapeutic target to treat deep-tissue craniofacial pain. In the trigeminal ganglion, P2X3 receptors are often co-expressed with the nociceptive neuropeptides CGRP and SP. Therefore, we discuss the role of CGRP and SP in deep-tissue craniofacial pain and suggest that neuropeptide antagonists, which have shown promise for the treatment of migraine, may have wider therapeutic potential, including the treatment of deep-tissue craniofacial pain. P2X3, TRPV1, and ASIC3 are often co-expressed in trigeminal neurons, implying the formation of functional complexes that allow craniofacial nociceptive neurons to respond synergistically to altered ATP and pH in pain. Future therapeutics for craniofacial pain thus might be more efficacious if targeted at combinations of P2X3, CGRP, TRPV1, and ASIC3.
Keywords: trigeminal, nociception, muscle, TMD, neuropeptides
Introduction
Chronic deep-tissue pain in the craniofacial region remains a prevalent clinical problem that often responds poorly to currently available analgesics. Evidence continues to grow indicating that craniofacial pain mechanisms differ from pain mechanisms in other body regions, and that specialized craniofacial tissue types—such as tooth, periodontal ligament, jaw joint, and dura—exhibit unusual nociceptor phenotypes or distinctive nociceptive receptor distributions. For example, the ATP receptor P2X3 is restricted to primary afferent neurons and is much more abundant on neurons relaying nociceptive feedback from deep craniofacial tissues than on analogous extracranial neurons. We propose that differences such as this may provide fruitful targets that could be exploited to direct therapies specifically at craniofacial pain. The initial part of this review therefore focuses on P2X3 as a potential therapeutic target to treat deep-tissue craniofacial pain.
The neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP) play a pivotal role in the processing of nociceptive information arising from various peripheral tissues and are co-expressed with P2X3 receptors. Thus, recent neuropeptide antagonists which show promise in treating migraine headache may have a much wider therapeutic potential, including the treatment of craniofacial pain. Transient receptor potential channels (TRPV) and acid-sensing ion channels (ASICs) can also be concurrently activated with the P2X3 receptor. Therefore, we review the most prominent potential peripheral therapeutic targets for deep-tissue craniofacial pain: the ATP receptor P2X3, neuropeptides, TRPV, and ASICs.
ATP, P2X3 Receptors, and Pain
When ATP has been applied to the skin in human studies, painful burning sensations have been reported (Hamilton et al., 2000). The injection of ATP analogues into the hindpaw of rats has also been reported to elicit nociceptive paw-lifting behavior (Hamilton et al., 1999), while intrathecal administration of ATP analogues produces thermal hyperalgesia (Tsuda et al., 1999). These responses indicate that ATP can evoke pain which is now known to be mediated via ATP purinoceptors (for review, see Hwang and Oh, 2007; Wirkner et al., 2007). Receptors activated by ATP can be divided into P2X and P2Y families, based upon differences in receptor structure and their signal transduction mechanisms (Gever et al., 2006). P2X receptors are a family of ionotropic receptors activated by ATP (for review, see Khakh and North, 2006). One member of this group, the P2X3 receptor, has been implicated specifically in nociception (reviewed by Hwang and Oh, 2007; Wirkner et al., 2007). Since these P2X3 receptors are restricted to peripheral nociceptive sensory neurons (Vulchanova et al., 1997; Bradbury et al., 1998), they are promising targets for pain therapies. P2Y receptors are G-protein-coupled metabotropic receptors (Fischer and Krugel, 2007). Compared with the P2X receptors, the tissue distribution of P2Y receptors is quite diverse, and the role of these P2Y receptors in pain has been less-well-studied. Of the 8 cloned P2Y receptors, P2Y1 and P2Y2 receptors have been implicated in nociception (Okada et al., 2002). Particularly, in the trigeminal ganglion and dorsal root ganglion (DRG), P2Y1 is present in small-diameter neurons (Ruan and Burnstock, 2003) and is co-expressed with TRPV channel TRPV1 and P2X3 receptors (Gerevich and Illes, 2004; Burnstock, 2007). P2Y2 receptor is also expressed in small-diameter DRG neurons (Moriyama et al., 2003; Stucky et al., 2004) and may play a role in nociceptive transmission through TRPV1 receptors (Moriyama et al., 2003; Malin et al., 2008). Further studies are necessary to elucidate the contributions of P2Y receptors to craniofacial pain transmission.
Recent studies have indicated that P2X3, P2X2/3 receptors are involved in a variety of pain states, including neuropathic pain (Sharp et al., 2006; Shinoda et al., 2007). Neuropathic pain is a complex, chronic pain state that is usually accompanied by tissue injury involving lesion(s) of the peripheral or central nervous system. P2X3 receptor activation has also been implicated in inflammatory pain (Seino et al., 2006). Inflammatory pain results from an insult to the integrity of tissues at a cellular level, which involves multiple chemical mediators. These mediators are from tissue cells and blood vessels as well as fibroblasts and mast cells. The ‘chemical soup’ of inflammatory mediators can directly affect peripheral nociceptive nerve terminals and cause long-term changes that sensitize them. Overt tissue damage releases a variety of substances from the cytosol, including ATP. Since ATP directly activates P2X3 receptors, it has been proposed that ATP released from tissue damage activates P2X3 receptors in vivo. Measurements of intracellular ATP and in vitro simulations show that sufficient ATP is released to activate neuronal P2X3 receptors (Cook and McCleskey, 2002). The release of ATP from damaged tissue may be particularly relevant for deep craniofacial tissues, since ATP could be released during tissue damage caused by condylar displacement, masticatory muscle myofiber damage, or dental restoration.
Neurons expressing P2X3 receptors also interact with glial cells. For example, nerve stimulation evokes ATP release from the somata of DRG neurons, which leads to the release of TNF-α from satellite cells and an increased excitability of P2X3 neurons (Zhang et al., 2007). Processes such as these suggest that sensitization may occur not only in the CNS, but also within the ganglion (Thalakoti et al., 2007).
In the trigeminal ganglion, nociceptors possess two types of desensitizing currents in response to ATP (Cook and McCleskey, 1997), suggesting that these neurons express different types of the P2X subunit. One type of ATP current rapidly desensitizes and is attributable to P2X3, while another current is largely sustained and likely represents a P2X3 heteromultimer (Connor et al., 2005). In large neurons, P2X3 receptors appear to be restricted to the cell body (Vulchanova et al., 1997; Ramer et al., 2001), implying that P2X3 is involved in intraganglionic signaling and cross-excitation among sensory neurons (Matsuka et al., 2001). Current amplitude through P2X3 channels is altered by inflammatory acidification (Gerevich et al., 2007), and inflammatory mediators such as substance P and bradykinin sensitize nociceptors through P2X3 ion channels (Paukert et al., 2001). ATP also is released concurrently with substance P from neuronal somata in the trigeminal ganglia (Matsuka et al., 2001), suggesting that ATP responses could be modulated within the ganglion. Prostaglandin E2 (PGE2), another inflammatory mediator, has no effect on P2X2/3 responses but potentiates ATP currents mediated by homomeric P2X3 receptors (Wang et al., 2007).
Expression of P2X3 in the DRG is largely restricted to non-peptidergic, unmyelinated primary afferent nociceptors (Novakovic et al., 1999), while in the trigeminal ganglion about one-third of P2X3 neurons contain a marker for myelinated axons (Staikopoulos et al., 2007), indicating that many trigeminal P2X3 neurons posses thinly myelinated axons with conduction velocities faster than those of P2X3 DRG neurons.
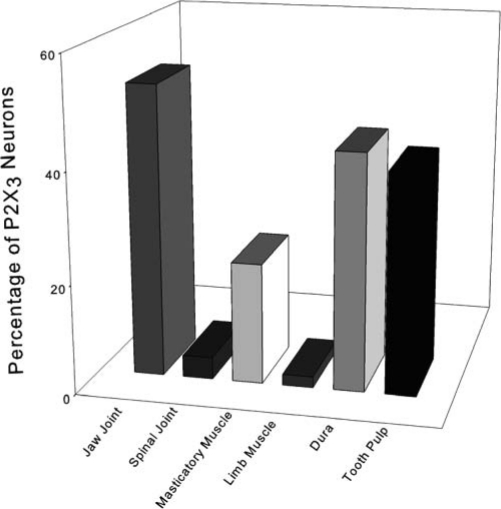
The proportion of neurons expressing the P2X3 receptor varies substantially, depending upon the type of peripheral target tissue in which the peripheral P2X3 axon resides. For instance, while almost half of cutaneous DRG neurons express P2X3, very few DRG joint afferent neurons express P2X3 (Bradbury et al., 1998) (Fig. 1). This differential expression implies that limb joint inflammation is not prominently mediated via ATP.
Figure 1.
Comparison of the proportion of primary afferent neurons that express the P2X3 receptor. Note that a high percentage of neurons projecting to deep craniofacial tissues expresses P2X3 receptors, while very few analogous extracranial neurons express P2X3.
There are even more dramatic differences between cranial and spinal cord neurons projecting to the same type of peripheral target tissue. For example, less than 5% of DRG neurons projecting to joint tissues express P2X3, while more than 50% of jaw joint neurons express P2X3 (Ichikawa et al., 2004). Large differences are also present for P2X3 muscle afferent neurons. In limb muscles, only 2% of muscle afferent neurons express P2X3 (Bradbury et al., 1998), while more than 20% of craniofacial muscle afferent neurons are immunopositive for P2X3 (Ambalavanar et al., 2005). The percentage of P2X3 craniofacial muscle afferent neurons is reported to be even higher (55%) when electrophysiological techniques are used (Connor et al., 2005). Thus, ATP is strongly implicated as a major component in craniofacial muscle and joint nociception, but not in limb muscle and joint nociception. The dramatic disparity exhibited for joint and muscle does not appear to exist for superficial tissues. Approximately 22-37% of cutaneous DRG afferent neurons express P2X3 (Bradbury et al., 1998), while a similar percentage (16-30%) of trigeminal cutaneous neurons express P2X3 (Ichikawa and Sugimoto, 2004; Ambalavanar et al., 2005). Thus, the abundance of P2X3 expression is similar on neurons projecting from superficial tissues, while neurons expressing P2X3 receptors are much more abundant in deep tissues of the craniofacial region than in deep tissues of the extracranial region (Fig. 2). This distribution suggests that activation of P2X3 receptors contributes modestly to cutaneous pain throughout the body, while activation of P2X3 receptors by ATP is a major player, specifically in deep-tissue craniofacial pain.
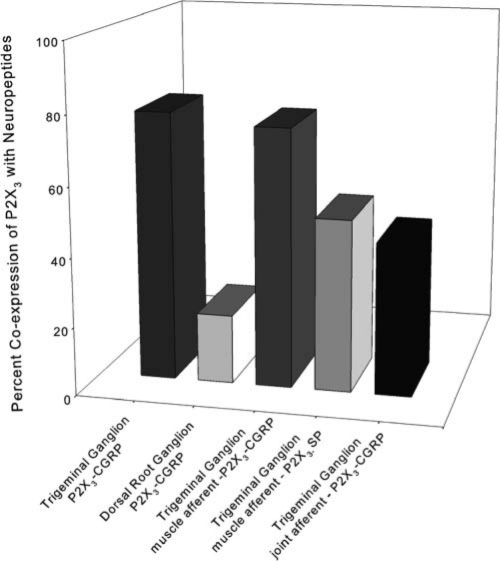
Figure 2.
Co-expression of P2X3 receptor and neuropeptides in primary afferent neurons. Note the frequent co-expression of neuropeptides with P2X3 in craniofacial primary afferent neurons supplying deep tissues. In contrast to this, very few dorsal root ganglion neurons co-express peptides with P2X3.
P2X3 Receptors are Abundant In Deep Craniofacial Tissue and May Provide Selective Targets for Deep-Tissue Craniofacial Pain
Teeth
P2X3 receptors are present on tooth afferent neurons (Ichikawa and Sugimoto, 2004). Within the tooth, these fibers are located in the sub-odontoblastic plexus of Raschkow, the odontoblastic layer, and advance into the predentin and dentin (Alavi et al., 2001). Since about 30-40% of tooth neuronal afferents express P2X3, and large inward currents can be evoked in tooth pulp afferent neurons by ATP (Cook and McCleskey, 1997), P2X3 receptors are likely to be substantial contributors to nociceptive responses from the teeth. Few tooth pulp afferents co-express TRPV1 (formerly vanilloid receptor subtype I), but most co-express TRPV2 (formerly vanilloid receptor 1-like receptor, VRL-1) (Ichikawa and Sugimoto, 2004). Thus, future interventional therapies might attenuate pain by blocking neuronal responses evoked by ATP released from cell damage and inflammation within the teeth. Antagonists for P2X3/P2X2/3 could even have therapeutic potential as a prophylactic intervention for dental procedures.
P2X Receptors on Jaw Joint Afferent Neurons
A large proportion of neurons (about 50%) that supply the jaw joint express P2X receptors (Ichikawa et al., 2004). Injection of α,βmeATP, a P2X1,3,2/3 receptor agonist, also produced nociceptive responses in animals with inflamed and non-inflamed jaw joints (Shinoda et al., 2005). This response was likely mediated by P2X receptors, since the reduced pain pressure threshold was blocked by a P2X1,3,2/3,1/5 receptor antagonist (Shinoda et al., 2005). Since inflammation is frequently present in some phases of TMD involving the temporomandibular joint (Mejersjo and Wenneberg, 2008; Tanaka et al., 2008), it is noteworthy that P2X3 expression increases in jaw joint afferents following joint inflammation (Shinoda et al., 2005). Within the population of P2X3 expressing joint afferent neurons, more than 40% co-express CGRP (Ichikawa et al., 2004). Since exposure of cultured trigeminal neurons to CGRP enhances ATP currents and reduces P2X3 desensitization (Fabbretti et al., 2006), this co-expression suggests analogous interactions between P2X3 and CGRP in jaw joint afferent neurons. Thus, both CGRP and P2X3 antagonists may prove efficacious in treating joint-based TMD.
Masticatory Muscle Pain, mTMD, and P2X3 Receptors
Persons with TMD exhibit altered central nociceptive processing (Maixner et al., 1998; Sarlani et al., 2004), which is thought to be initially triggered from a peripheral source. One prime candidate for this insult is nociceptive input from muscle afferents, since it is particularly potent at generating CNS wind-up (Wall and Woolf, 1984). More specifically, oral parafunctional behavior that increases muscle tension is a good predictor of the level of jaw-muscle pain in persons with TMD (Glaros et al., 2005). Since parafunctional behavior can occur for prolonged periods, and masticatory muscles have an impaired ability to repair (Pavlath et al., 1998), myofiber damage, subsequent inflammation, and increased primary afferent drive constitute a potential source to initiate or exacerbate mTMD.
Evidence of inflammation in TMDs involving myalgia (mTMD) is limited, perhaps because affected individuals are typically examined long after the initiation of the disorder, when a precipitating event involving inflammation may have subsided. However, levels of SP in the masseter muscle are reportedly reduced in persons with mTMD (Kehl et al., 2006), which is consistent with decreased intramuscular levels of SP in animal studies following inflammation (Galeazza et al., 1995). Some have argued that muscle inflammation is not involved in orofacial muscle pain, because it is not relieved by ibuprofen (Svensson et al., 1997). In muscle pain, however, PGE2 levels do not temporally correlate with pain intensity, indicating that PGE2 is not the major algesic substance involved in muscle pain (Tegeder et al., 2002). Further, cyclo-oxygenase inhibitors such as ibuprofen are ineffective in relieving muscle pain (Donnelly et al., 1990). Thus, the failure of ibuprofen to alleviate orofacial muscle pain should not be interpreted as evidence for a lack of inflammation in masticatory muscle pain.
Purinergic receptors in primary afferent neurons may be of particular importance for muscle pain. Intramuscular infusion of ATP, for instance, evokes pain (Mork et al., 2003), and muscle nociceptors can be activated by ATP (Reinohl et al., 2003). Further evidence that purinergic receptors are involved in muscle pain comes from the observation that non-specific P2X antagonists reduce nocifensive behavior in animals experiencing muscle pain (Shinoda et al., 2008).
Previous studies on tissue inflammation have specifically associated P2X receptors with craniofacial nociceptive behavior (Oliveira et al., 2005). It is also known that P2X3 receptors are present on masseter muscle afferent neurons (Ambalavanar et al., 2005), and that currents characteristic of P2X3 receptors can be activated in some masseter afferents (Connor et al., 2005). Following CFA-induced masseter inflammation, the number of P2X3 muscle afferent neurons increases (Ambalavanar et al., 2005), while P2X3 muscle afferents increase 15 days following repetitive muscle contraction (Shinoda et al., 2008), indicating a role for P2X3 in muscle inflammation. One potential source of ATP to activate P2X3 receptors is ATP released from the cytosol of damaged cells. In co-culture systems, action potentials and inward currents are evoked in nociceptors by ATP when nearby cells are mechanically damaged (Cook and McCleskey, 2002). In muscle, the concentration of ATP within myofibers is approximately 10 mM (Stewart et al., 1994), a concentration which readily activates muscle primary afferent neurons in vivo (Reinohl et al., 2003), thus demonstrating that sufficient ATP is present within myofibers to activate muscle afferent neurons. Since parafunctional muscle contraction mechanically damages myofibers (Lovering and De Deyne, 2004), it is possible that ATP released from damaged myofibers can activate P2X3 receptors on muscle nociceptors.
Temporomandibular disorders are about 1.5-2 times more prevalent in females than in males, and 80% of persons seeking treatment for TMD are females (Dworkin et al., 1990). While the origin of these gender differences is not known, TMD pain in women is highest at times of low estrogen (LeResche et al., 2003). Several studies have indicated that the central processing of sensory feedback is altered once TMD has become well-established (Sarlani et al., 2004). It is not known, however, whether the different effects of sex hormones on TMD and pain emerge only after these central transformations have occurred, or whether they are present during the initial development of this disorder. Here we suggest several peripheral mechanisms that may contribute to the sex differences in TMD. [For a more encompassing review. see Warren and Fried (2001), Sarlani and Greenspan (2005), and Craft (2007).] One possibility is that primary afferent neurons are modulated by sex hormones, and, in fact, very brief exposure of DRG neurons to estradiol decreases ATP-mediated currents (Chaban et al., 2003; Ma et al., 2005). While this finding is consistent with the observation that greater pain is present when estrogen is low, more prolonged exposure of DRG neurons to estradiol apparently does not affect P2X currents (Xu et al., 2008). Trigeminal ganglion neurons, however, do possess estrogen receptors (Bereiter et al., 2005), and estradiol modulates the responses of brainstem neurons evoked by injection of ATP into the jaw joint (Tashiro et al., 2007).
Levels of nerve growth factor (NGF) rise in inflamed tissue (Woolf et al., 1994; Spears et al., 2005). The presence of NGF also leads to enhanced up-regulation of P2X3 receptors (Simonetti et al., 2006). While NGF interacts with estrogen in a tissue-dependent manner, in some tissues estrogen increases NGF (Bjorling et al., 2002). Thus, gender effects involving craniofacial pain and P2X3 receptors could be mediated directly on P2X3 or indirectly via NGF. Nerve growth factor can also stimulate CGRP expression (Lindsay and Harmar, 1989), and CGRP expression up-regulates P2X3 receptors (Fabbretti et al., 2006). Thus, sex, craniofacial pain, and P2X3 may interact through sex hormones acting on NGF, leading to increased CGRP and subsequent P2X3 expression (Fig. 3).
Figure 3.
Schematic representation of the inter-relationships between the P2X3 receptor and tissue injury, nerve growth factor, and CGRP. Note that sex hormones may directly or indirectly modulate the expression of the P2X3 receptor in primary afferent neurons.
Persons with muscular TMDs report pain associated with mastication (Winocur et al., 2001; Gavish et al., 2002), and females with muscular TMD report more pain following chewing than do males (Karibe et al., 2003). Analysis of existing data also suggests that sex hormones make muscles of females less susceptible to mechanical damage (Tiidus, 2003). Thus, estrogen may alter muscle damage and subsequent extracellular ATP concentration (for review, see Kendall and Eston, 2002).
P2X Receptors and Headache
A large percentage of trigeminal afferent neurons that innervate the dura express P2X receptors. About half of dura afferents express P2X3, P2X2, or both (Staikopoulos et al., 2007). It has recently been shown that exposure of trigeminal ganglion neurons to CGRP up-regulates the expression of P2X3 receptors (Fabbretti et al., 2006). The level of nerve growth factor (NGF) is also increased in the cerebrospinal fluid of persons with some types of headache (Sarchielli et al., 2001), and neutralization of NGF decreases P2X3 receptor activity on trigeminal ganglion neurons (D’Arco et al., 2007). Thus, CGRP antagonists and anti-NGF agents may prove to be useful targets to modulate P2X3 receptor function.
Peripheral Trigeminal Nerve Damage
The trigeminal nerve is routinely encountered during dental procedures, during which it can be damaged and produce nerve dysfunction (Sandstedt and Sorensen, 1995; Robert et al., 2005). Injury to the inferior alveolar nerve, for instance, produces enhanced nocifensive behavior and cutaneous mechanical allodynia (Saito et al., 2008). Following inferior alveolar nerve injury, P2X3 receptors are also up-regulated in the trigeminal ganglion (Eriksson et al., 1998). Some of the responses exhibited following damage to branches of the trigeminal nerve appear to differ from those of other nerves, leading to the suggestion that new therapeutic approaches need to be developed to treat injury-induced trigeminal pain (Robinson et al., 2004). These differences include differences in the time-course of ectopic discharge after injury, involvement of sympathetic fibers, and changes in the expression of some neuropeptides, including galanin and vasoactive intestinal peptide. Whether P2X receptor antagonists or agonists have therapeutic potential for pain and nerve recovery remains to be determined.
Deep-tissue Craniofacial Peptidergic Afferent Neurons Co-express P2X3
While P2X3 neurons in the DRG rarely express neuropeptides, there is extensive co-expression of P2X3 and neuropeptides in trigeminal neurons, suggesting a far greater interaction between neuropeptides and P2X3 in deep craniofacial neurons. Out of the population of DRG neurons that express CGRP, only 14-26% express P2X3 (Bradbury et al., 1998; Ruan et al., 2005). In trigeminal neurons expressing CGRP, about 80% co-express P2X3 (Fabbretti et al., 2006). We found that 75% of the CGRP and about 50% of the SP muscle afferent population in the trigeminal ganglion express P2X3 (Ambalavanar et al., 2005). About half of jaw joint afferents express both P2X3 and CGRP (Ichikawa et al., 2004). Thus, a striking difference exists between the percentage of peptidergic neurons that co-express P2X3 in the trigeminal ganglion and DRG (Fig. 2). This extensive co-localization of P2X3 with neuropeptides likely reflects interactions between neuropeptides and P2X3 in trigeminal neurons. For instance, exposure of trigeminal neurons to CGRP increases trafficking of P2X3 receptors to the cell membrane (Fabbretti et al., 2006).
Encouraging Progress Treating Deep-Tissue Pain with Neuropeptide Antagonists
Peripheral receptor activation through neuropeptide release is an important effector mechanism coupled with nociceptor stimulation. In particular, calcitonin gene-related peptide (CGRP) and substance P (SP) are specifically implicated in nociceptive mechanisms involving deep tissues (Schaible, 2004), including muscle (Reinert et al., 1998). The widespread involvement of these neuropeptides in deep-tissue pain is exciting, because the recent development of neuropeptide antagonists to treat migraine headache may have broader therapeutic applications, including deep-tissue craniofacial pain.
Calcitonin Gene-related Peptide
Calcitonin gene-related peptide is associated with orofacial inflammation (Hutchins et al., 2000; Carleson et al., 2004; Ambalavanar et al., 2006). For example, levels of CGRP are elevated in the trigeminal ganglia following craniofacial inflammation, including the jaw joint (Devor, 1991) and masseter muscle (Carleson et al., 2004; Ambalavanar et al., 2006). In persons with temporomandibular disorders, a positive correlation has also been reported between CGRP levels in the temporomandibular joint and the severity of joint pain (Amano et al., 1986). The up-regulation of CGRP production, therefore, is a characteristic feature of craniofacial deep-tissue pain.
The significance of CGRP in craniofacial pain has been further substantiated by studies where blockade of CGRP transmission resulted in anti-nociceptive effects (Olesen et al., 2004; Ambalavanar et al., 2006; Ho et al., 2008). In particular, the clinical effectiveness of a potent CGRP antagonist for acute treatment of migraine headache (Olesen et al., 2004; Ho et al., 2007) represents a potential therapeutic venue for other chronic pain disorders, such as TMD. Intravenous injection of the CGRP antagonist BIBN4096 and an orally active non-peptide antagonist, MK-0974 (Ho et al., 2008), has shown tremendous promise in the treatment of migraine headache. These studies indicate that neuropeptide antagonists can be safe for human use and may prove useful in treating TMD in which levels of CGRP are elevated.
Substance P
Substance P is present in about 20% of trigeminal ganglion neurons (Hokfelt et al., 1975) and is associated with orofacial inflammation and pain. For instance, tooth pulp is supplied by SP-containing neurons, and SP mediates inflammation and pain arising from the tooth pulp (Sabino et al., 2002). Levels of SP are also increased in arthritic temporomandibular joints (Carleson et al., 1997) and trigeminal ganglia following jaw joint inflammation (Hutchins et al., 2000). Masseter muscle afferent neurons also express SP, and this SP is up-regulated following muscle inflammation (Ambalavanar et al., 2006). Additional evidence supporting a role for NK1 in craniofacial vasoregulation and nociception is the fact that activation of NK1 increases blood flow in the human temporalis muscle (Tuxen et al., 1989). Although many studies show the anti-nociceptive effects of NK1 receptor antagonists, several clinical trials have failed to demonstrate the analgesic efficacy of these compounds in humans (reviewed by Hill and Oliver, 2007), though they have been successful in the treatment of other diverse conditions, including depression, chemotherapy-induced emesis, and inflammatory bowel disease (Duffy, 2004). Future studies are necessary to evaluate the usefulness of NK1 receptor antagonists to treat craniofacial deep-tissue pain.
Neuropeptides Interact with Growth Factors and P2X3
Nociception involves multiple interactions between and among growth factors, neuropeptides, and nociceptive receptors. For instance, NGF not only induces increased levels of CGRP and SP (Skoff and Adler, 2006), but also increases P2X3 expression (Simonetti et al., 2006; D’Arco et al., 2007). Because inflammation and partial nerve-injury-induced hyperalgesia are partly dependent upon NGF production (Boucher et al., 2000), increased NGF levels following injury or inflammation in craniofacial tissue may increase neuropeptide synthesis, subsequent release, and P2X3-mediated ATP currents (Fig. 3). P2X3 receptor synthesis and trafficking can also be up-regulated by CGRP independent of NGF (Fabbretti et al., 2006).
Transient Receptor Potential Channels: TRPV1
The mammalian transient receptor potential channels represent a family of ion channels subdivided into 6 subfamilies (for review, see Vennekens et al., 2008). Of these receptors, the vanilloid receptors TRPV1, TRPV2, TRPV3, and TRPV4 are involved in nociception and thermo-sensation. TRPV2 (formerly vanilloid receptor-like receptor VRL1) is a non-selective ligand-gated cation channel that responds to noxious heat (> 52°C) (Caterina et al., 1999) and membrane stretch, as well as osmolarity changes (Muraki et al., 2003). Compared with TRPV1, however, TRPV2 may serve diverse physiological functions, and its therapeutic potential in hyperalgesia remains unclear (Szallasi et al., 2007). Similarly, studies on the role of TRPV3 and TRPV4 in pain are inconsistent (for review, see Szallasi et al., 2007; Vennekens et al., 2008).
TRPV1 (formerly vanilloid receptor VR1) (reviewed by Szallasi et al., 2007), a voltage-dependent cation channel, is highly expressed in nociceptive primary afferent neurons and is gated by capsaicin, noxious heat (> 45°C), acidic pH (< 5.3), components of endogenous inflammatory mediators (bradykinin, PGE2), and anandamide (Vellani et al., 2001). Recently, several TRPV1 antagonists have been reported to alleviate or reverse mechanical and thermal hyperalgesia associated with inflammatory pain (Gavva et al., 2005; Jia et al., 2005; Szallasi et al., 2007). In some persons, the oral TRPV1 antagoninst SB705498 (Chizh et al., 2007) is effective in alleviating capsaicin- and UV-induced hyperalgesia. These encouraging pre-clinical and clinical findings indicate that TRPV1 can be a potential target for the treatment of human pain conditions.
Evidence for the involvement of TRPV1 receptors in craniofacial pain comes from studies on tooth pulp (Rodd et al., 2007; Kim et al., 2008) as well as meningeal afferents (Dux et al., 2003; Geppetti et al., 2008). Tooth pulp and jaw joint afferents express TRPV1 (see Table). In addition, TRPV1 plays a role in thermal hyperalgesia and cold pain following inflammation of the face (Pei et al., 2007). Persons with burning mouth syndrome also show a positive correlation between TRPV1 expression and pain score (Yilmaz et al., 2007).
Table.
Expression of TRPV and ASICs in Trigeminal Ganglion Neurons Innervating Different Craniofacial Tissues and Their Co-expression with CGRP and P2X3
| Receptor/Channel | TG Neurons (%) | Cutaneous (%) | Tooth Pulp (%) | Jaw Joint (%) | Muscle* (%) |
|---|---|---|---|---|---|
| TRPV1 (VR-1) | 201 | 261 | 81 | 254 | 666 |
| TRPV2 (VRL-1) | 142 | 92 | 362 | 414 | — |
| ASIC3 | 23 | 13 | 33 | — | 646 |
| TRPV1 & P2X3 | 35% of P2X33 | 163 | 63 | — | 506 |
| TRPV1 & CGRP | 571 | 181 | 1001 | 38% of CGRP4 | — |
| TRPV2 & P2X3 | 9% of P2X33 | 83 | 233 | - | — |
| TRPV2 & CGRP | — | 252 | 452 | 30% of CGRP4 | — |
| ASIC3 & CGRP | 265 | 37% of CGRP5 | 36% of CGRP5 | — | — |
| TRPV1, ASIC3 & P2X3 | — | - | - | — | 56 |
Electrophysiological study.
Recent studies demonstrated that TRPV1 may play a role specifically in muscle pain (Hoheisel et al., 2004; Rau et al., 2007). Injection of capsaicin into the masseter muscle causes pain in humans (Sohn et al., 2000), and masseter muscle afferent neurons in the trigeminal ganglion express TRPV1 receptors (Connor et al., 2005), suggesting that TRPV1 may be involved in craniofacial muscle pain.
The P2X3 receptor is sometimes co-localized with TRPV1 (Table). In the trigeminal ganglion, about 35% of P2X3 neurons express VR1 (Ichikawa et al., 2004; Ichikawa and Sugimoto, 2004). In contrast, approximately 75% of P2X3 DRG neurons express TRPV1 (Guo et al., 1999). The differences in overlap of these receptors may indicate a differential interaction or co-activation of different receptors.
Acid-Sensing Ion Channels (ASICs)
Activation of acid-sensing ion channels (ASICs) on primary afferent neuron terminals may be at least partly responsible for the sensitization of neurons during hyperalgesia, since low tissue pH occurs in muscle during exhaustive exercise, ischemic muscle contraction, and inflammation (Issberner et al., 1996; Reeh and Steen, 1996). Acid-sensing ion channel 3 (ASIC3) receptors are present in trigeminal ganglion neurons that innervate the masseter muscle, tooth pulp, and facial skin (Ichikawa and Sugimoto, 2002) (see Table). Nearly all trigeminal ganglion P2X3 muscle afferent neurons also express ASIC3 (Connor et al., 2005), implying that tissue pH is a relevant stimulus for craniofacial neurons.
Acidic saline injected into limb muscles activates a proportion of muscle afferents (Hoheisel et al., 2004) and produces hyperalgesia (Sluka et al., 2001). Even though ASIC3 channels are present on craniofacial muscle afferents (Connor et al., 2005; Ambalavanar et al., 2007), injection of acidic saline into the masseter muscle does not produce hyperalgesia or alter CGRP or SP expression (Ambalavanar et al., 2007). While in vitro studies indicate that trigeminal ganglion (Connor et al., 2005) and DRG neurons (Hoheisel et al., 2004; Molliver et al., 2005) respond to very small changes in pH, injection of acidic saline into the masseter muscle in vivo failed to evoke nociceptive responses (Ambalavanar et al., 2007).
The co-expression of P2X3 and ASIC3 also appears to vary considerably between the cranial and extracranial regions. In the trigeminal ganglion, nearly every P2X3 primary muscle afferent neuron also expresses ASIC3 (Connor et al., 2005). In contrast, less than 5% of DRG neurons express both P2X3 and ASIC3 (Molliver et al., 2005).
Interactions Between Craniofacial Nociceptive Receptors and Channels
Many primary afferent neurons co-express more than 2 nociceptive receptors or channels. For instance, more than 50% of masseter muscle afferent neurons in the trigeminal ganglion express not only TRPV1, but also P2X3 as well as ASIC3 receptors in the same neuron (Connor et al., 2005). CGRP is also highly co-expressed with TRPV1 in the trigeminal ganglion (Dux et al., 2003). Previous studies demonstrated that the release of CGRP is induced by low pH in trigeminal ganglion neurons (Goodis et al., 2006), and that ASIC3 receptors are co-localized with CGRP (Ichikawa and Sugimoto, 2002). Thus, the role of ASIC receptors in pain processing may be influenced by their subunit composition and the contributions of TRPV1 receptors in proton sensitivity (Ugawa et al., 2002; Poirot et al., 2006). Thus, it seems likely that concurrent activation of different molecular receptors in trigeminal ganglion neurons occurs during normal and pathological states. This type of interaction has recently been shown in DRG neurons, which respond primarily to a combination of muscle metabolites (Light et al., 2008). Further studies with physiologically relevant stimuli are necessary to determine whether the co-expression of nociceptive receptors and channels in trigeminal neurons conveys a synergistic action of 2 or more receptors and represents functional complexes.
Therapeutic Possibilities
The therapeutic potential of providing pain therapies by blocking P2X3 receptors may initially appear limited, since P2X3 receptors exhibit rather long-lasting desensitization (Cook et al., 1998; Cook and McCleskey, 2002). However, heteromultimers containing P2X3 exhibit sustained ATP currents (Connor et al., 2005), and thus may be productive therapeutic targets. The development of the selective P2X3 receptor antagonist, 5-[[[(3-Phenoxyphenyl)methyl][(1S)-1,2,3,4-tetrahydro-1-1naphthalenyl]amino]carbonyl]-1,2,4-benzenetricarboxylic acid sodium salt hydrate, A-317491, represents a milestone in the development of purinergic-based pain therapies (Jarvis et al., 2002). This antagonist reduces thermal and mechanical hyperalgesia in CFA pain models (Jarvis et al., 2002; Wu et al., 2004). An important characteristic of A-317491 is that it exhibits limited penetration into the CNS (Wu et al., 2004), and thus may be suited for the selective targeting of peripheral pain. Development of additional P2X3 receptor antagonists—such as the endogenous peptide spinorphin, which shows potent antagonism at the human P2X3 receptor (Jung et al., 2007)—is clearly warranted. Slow ATP responses in P2X2/3 DRG and nodose ganglion neurons can also be inhibited by cannabinoids (Krishtal et al., 2006), which may also have potential for modulating deep-tissue craniofacial pain. Since P2X3 receptors are much more abundant on neurons supplying deep craniofacial tissue, and P2X3 receptors have prominent interactions with CGRP, TRPV1, and ASIC, they currently appear to be very promising therapeutic targets for deep craniofacial pain.
Sustained exposure of trigeminal neurons to CGRP not only enhances ATP currents through P2X3 receptors, but also accelerates the recovery of P2X3 receptors from desensitization (Fabbretti et al., 2006). Thus, CGRP antagonists have therapeutic potential, not only because of their ability to reduce plasma extravasation significantly, but also because they can block the facilitatory effects of CGRP on P2X3 receptors. CGRP and P2X3 antagonists, perhaps in combination, are therefore strong candidate targets for craniofacial pain, while antagonists for SP currently appear less promising.
Unfortunately, little information is available on the use of TRPV1 antagonists to alleviate deep-tissue craniofacial pain. With the recent advances in the understanding of TRPV1 physiology and pharmacology, together with the availability of potent, selective TRPV1 antagonists, future studies should lead to further understanding of the role of TRPV1 receptors and of the TRPV1 receptor as a potential therapeutic target for the treatment of craniofacial pain, including TMD. Complete, selective antagonists for ASIC3 are not currently available (Hayes et al., 2007). Studies are needed to develop selective ASIC3 antagonists and to determine whether ASIC3 currents can be modulated indirectly via other receptors.
Concluding Remarks
Chronic craniofacial pain often leads to long-term alterations in central nociceptive processing (i.e., central sensitization). While these transformations can result in a state in which pain becomes independent of peripheral input, the initiation of these central transformations is likely to involve a peripheral stimulating event or trigger. In the craniofacial region, P2X3 receptors are restricted to primary afferent neurons and are particularly abundant on neurons relaying nociceptive feedback from deep craniofacial tissues. Thus, transmission through P2X3 neurons represents one prominent pathway by which nociceptive signaling from deep craniofacial tissues could be conveyed to the central nervous system. The recent development of specific P2X3 antagonists which do not readily cross the blood-brain barrier thus might be particularly effective in reducing nociceptive feedback from deep craniofacial tissues and attenuating peripheral triggers that might evoke central sensitization. Therapeutics directed at NGF and CGRP may also be promising therapeutic targets for deep-tissue craniofacial pain, since not only are they involved in peripheral nociceptive mechanisms, but they also up-regulate P2X3 receptors. The potential for TRPV1 antagonists to treat craniofacial pain needs further study, while the role of ASIC3 in craniofacial pain remains problematic. Thus, P2X3 and CGRP antagonists currently appear to be the most promising potential targets to treat deep-tissue craniofacial pain.
Footnotes
This work is supported by NIH RO1DE15386, NIH RO1DE10132 (to DD), and NIH RO3DE016795 (to RA).
References
- Alavi AM, Dubyak GR, Burnstock G. (2001). Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res 80:476-483 [DOI] [PubMed] [Google Scholar]
- Amano N, Hu JW, Sessle BJ. (1986). Responses of neurons in feline trigeminal subnucleus caudalis (medullary dorsal horn) to cutaneous, intraoral, and muscle afferent stimuli. J Neurophysiol 55:227-243 [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Moritani M, Dessem D. (2005). Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain 117:280-291 [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Moritani M, Moutanni A, Gangula P, Yallampalli C, Dessem D. (2006). Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain 120:53-68 [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Yallampalli C, Yallampalli U, Dessem D. (2007). Injection of adjuvant but not acidic saline into craniofacial muscle evokes nociceptive behaviors and neuropeptide expression. Neuroscience 149:650-659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter DA, Cioffi JL, Bereiter DF. (2005). Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol 50:971-979 [DOI] [PubMed] [Google Scholar]
- Bjorling DE, Beckman M, Clayton MK, Wang ZY. (2002). Modulation of nerve growth factor in peripheral organs by estrogen and progesterone. Neuroscience 110:155-167 [DOI] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. (2000). Potent analgesic effects of GDNF in neuropathic pain states. Science 290:124-127 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. (1998). The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12:256-268 [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2007). Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471-1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleson J, Bileviciute I, Theodorsson E, Appelgren B, Appelgren A, Yousef N, et al. (1997). Effects of adjuvant on neuropeptide-like immunoreactivity in the temporomandibular joint and trigeminal ganglia. J Orofac Pain 11:195-199 [PubMed] [Google Scholar]
- Carleson J, Lundeberg T, Appelgren B. (2004). Muscle and brain changes of calcitonin gene-related peptide in experimentally induced unilateral rat masseter myositis. J Orofac Pain 18:246-252 [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. (1999). A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436-441 [DOI] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. (2003). Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience 118:941-948 [DOI] [PubMed] [Google Scholar]
- Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, et al. (2007). The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 132:132-141 [DOI] [PubMed] [Google Scholar]
- Connor M, Naves LA, McCleskey EW. (2005). Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Mol Pain 1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. (1997). Desensitization, recovery and Ca(2+)-dependent modulation of ATP-gated P2X receptors in nociceptors. Neuropharmacology 36:1303-1308 [DOI] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. (2002). Cell damage excites nociceptors through release of cytosolic ATP. Pain 95:41-47 [DOI] [PubMed] [Google Scholar]
- Cook SP, Rodland KD, McCleskey EW. (1998). A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J Neurosci 18:9238-9244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM. (2007). Modulation of pain by estrogens. Pain 132(Suppl 1):S3-S12 [DOI] [PubMed] [Google Scholar]
- D’Arco M, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, et al. (2007). Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci 27:8190-8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. (1991). Neuropathic pain and injured nerve: peripheral mechanisms. Br Med Bull 47:619-630 [DOI] [PubMed] [Google Scholar]
- Donnelly AE, Maughan RJ, Whiting PH. (1990). Effects of ibuprofen on exercise-induced muscle soreness and indices of muscle damage. Br J Sports Med 24:191-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy RA. (2004). Potential therapeutic targets for neurokinin-1 receptor antagonists. Expert Opin Emerg Drugs 9(1):9-21 [DOI] [PubMed] [Google Scholar]
- Dux M, Santha P, Jancso G. (2003). Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J Physiol 552(Pt 3):859-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SF, Huggins KH, LeResche L, Von Korf M, Howard J, Truelove E, et al. (1990). Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc 120:273-281 [DOI] [PubMed] [Google Scholar]
- Eriksson J, Bongenhielm U, Kidd E, Matthews B, Fried K. (1998). Distribution of P2X3 receptors in the rat trigeminal ganglion after inferior alveolar nerve injury. Neurosci Lett 254:37-40 [DOI] [PubMed] [Google Scholar]
- Fabbretti E, D’Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. (2006). Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci 26:6163-6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Krugel U. (2007). P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem 14:2429-2455 [DOI] [PubMed] [Google Scholar]
- Galeazza MT, Garry MG, Yost HJ, Strait KA, Hargreaves KM, Seybold VS. (1995). Plasticity in the synthesis and storage of substance P and calcitonin gene-related peptide in primary afferent neurons during peripheral inflammation. Neuroscience 66:443-458 [DOI] [PubMed] [Google Scholar]
- Gavish A, Winocur E, Menashe S, Halachmi M, Eli I, Gazit E. (2002). Experimental chewing in myofascial pain patients. J Orofac Pain 16:22-28 [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, et al. (2005). AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313:474-484 [DOI] [PubMed] [Google Scholar]
- Geppetti P, Nassini R, Materazzi S, Benemei S. (2008). The concept of neurogenic inflammation. BJU Int 101(Suppl 3):2-6 [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Illes P. (2004). P2Y receptors and pain transmission. Purinergic Signal 1:3-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerevich Z, Zadori ZS, Koles L, Kopp L, Milius D, Wirkner K, et al. (2007). Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. J Biol Chem 282:33949-33957 [DOI] [PubMed] [Google Scholar]
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. (2006). Pharmacology of P2X channels. Pflügers Arch 452:513-537 [DOI] [PubMed] [Google Scholar]
- Glaros AG, Williams K, Lausten L. (2005). The role of parafunctions, emotions and stress in predicting facial pain. J Am Dent Assoc 136:451-458 [DOI] [PubMed] [Google Scholar]
- Goodis HE, Poon A, Hargreaves KM. (2006). Tissue pH and temperature regulate pulpal nociceptors. J Dent Res 85:1046-1049 [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. (1999). Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11:946-958 [DOI] [PubMed] [Google Scholar]
- Hamilton SG, Wade A, McMahon SB. (1999). The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol 126:326-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. (2000). ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgesia. Brain 123 (Pt 6):1238-1246 [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. (2007). Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581(Pt 3):1271-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RG, Oliver KR. (2007). Neuropeptide and kinin antagonists. Handbk Exp Pharmacol 177:181-216 [DOI] [PubMed] [Google Scholar]
- Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. (2008). Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 70:1304-1312 [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Reinohl J, Unger T, Mense S. (2004). Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain 110:149-157 [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Kellerth JO, Nilsson G, Pernow B. (1975). Experimental immunohistochemical studies on the localization and distribution of substance P in cat primary sensory neurons. Brain Res 100:235-252 [DOI] [PubMed] [Google Scholar]
- Hutchins B, Spears R, Hinton RJ, Harper RP. (2000). Calcitonin gene-related peptide and substance P immunoreactivity in rat trigeminal ganglia and brainstem following adjuvant-induced inflammation of the temporomandibular joint. Arch Oral Biol 45:335-345 [DOI] [PubMed] [Google Scholar]
- Hwang SW, Oh U. (2007). Current concepts of nociception: nociceptive molecular sensors in sensory neurons. Curr Opin Anaesthesiol 20:427-434 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. (2000). Vanilloid receptor 1-like receptor-immunoreactive primary sensory neurons in the rat trigeminal nervous system. Neuroscience 101:719-725 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. (2001). VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res 890:184-188 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. (2002). The co-expression of ASIC3 with calcitonin gene-related peptide and parvalbumin in the rat trigeminal ganglion. Brain Res 943:287-291 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. (2004). The co-expression of P2X3 receptor with VR1 and VRL-1 in the rat trigeminal ganglion. Brain Res 998:130-135 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Fukunaga T, Jin HW, Fujita M, Takano-Yamamoto T, Sugimoto T. (2004). VR1-, VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain Res 1008:131-136 [DOI] [PubMed] [Google Scholar]
- Issberner U, Reeh PW, Steen KH. (1996). Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett 208:191-194 [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, et al. (2002). A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA 99:17179-17184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, McLeod RL, Hey JA. (2005). TRPV1 receptor: a target for the treatment of pain, cough, airway disease and urinary incontinence. Drug News Perspect 18:165-171 [DOI] [PubMed] [Google Scholar]
- Jung KY, Moon HD, Lee GE, Lim HH, Park CS, Kim YC. (2007). Structure-activity relationship studies of spinorphin as a potent and selective human P2X(3) receptor antagonist. J Med Chem 50:4543-4547 [DOI] [PubMed] [Google Scholar]
- Karibe H, Goddard G, Gear RW. (2003). Sex differences in masticatory muscle pain after chewing. J Dent Res 82:112-116 [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Besspiata DA, Rankin AM, Lenton PA, Schiffman EL. (2006). Muscle and synovial fluid substance P levels are positively correlated in human musculoskeletal pain (abstract). ASA Abstr #A505 [Google Scholar]
- Kendall B, Eston R. (2002). Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med 32:103-123 [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. (2006). P2X receptors as cell-surface ATP sensors in health and disease. Nature 442:527-532 [DOI] [PubMed] [Google Scholar]
- Kim SM, Kim J, Kim E, Hwang SJ, Shin HK, Lee SE. (2008). Local application of capsaicin alleviates mechanical hyperalgesia after spinal nerve transection. Neurosci Lett 433:199-204 [DOI] [PubMed] [Google Scholar]
- Krishtal O, Lozovaya N, Fedorenko A, Savelyev I, Chizhmakov I. (2006). The agonists for nociceptors are ubiquitous, but the modulators are specific: P2X receptors in the sensory neurons are modulated by cannabinoids. Pflügers Arch 453:353-360 [DOI] [PubMed] [Google Scholar]
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. (2003). Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain 106:253-261 [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. (2008). Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X and TRPV1. J Neurophysiol 100:1184-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM, Harmar AJ. (1989). Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 337:362-364 [DOI] [PubMed] [Google Scholar]
- Lovering RM, De Deyne PG. (2004). Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol 286:C230-C238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Rong W, Dunn PM, Burnstock G. (2005). 17beta-estradiol attenuates alpha, beta-meATP-induced currents in rat dorsal root ganglion neurons. Life Sci 76:2547-2558 [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. (1998). Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain 76:71-81 [DOI] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Richard KH, Reynolds IJ, Albers KM, Molliver DC. (2008). Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y(2). Pain 138:484-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuka Y, Neubert JK, Maidment NT, Spigelman I. (2001). Concurrent release of ATP and substance P within the guinea pig trigeminal ganglia in vivo. Brain Res 915:248-255 [DOI] [PubMed] [Google Scholar]
- Mejersjo C, Wenneberg B. (2008). Diclofenac sodium and occlusal splint therapy in TMJ osteoarthritis: a randomized controlled trial. J Oral Rehabil 35:729-738 [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. (2005). ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, et al. (2003). Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci 23:6058-6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mork H, Ashina M, Bendtsen L, Olesen J, Jensen R. (2003). Experimental muscle pain and tenderness following infusion of endogenous substances in humans. Eur J Pain 7:145-153 [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, et al. (2003). TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93:829-838 [DOI] [PubMed] [Google Scholar]
- Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, et al. (1999). Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain 80:273-282 [DOI] [PubMed] [Google Scholar]
- Okada M, Nakagawa T, Minami M, Satoh M. (2002). Analgesic effects of intrathecal administration of P2Y nucleotide receptor agonists UTP and UDP in normal and neuropathic pain model rats. J Pharmacol Exp Ther 303:66-73 [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. (2004). Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350:1104-1110 [DOI] [PubMed] [Google Scholar]
- Oliveira MC, Parada CA, Veiga MC, Rodrigues LR, Barros SP, Tambeli CH. (2005). Evidence for the involvement of endogenous ATP and P2X receptors in TMJ pain. Eur J Pain 9:87-93 [DOI] [PubMed] [Google Scholar]
- Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, et al. (2001). Inflammatory mediators potentiate ATP-gated channels through the P2X(3) subunit. J Biol Chem 276:21077-21082 [DOI] [PubMed] [Google Scholar]
- Pavlath GK, Thaloor D, Rando TA, Cheong M, English AW, Zheng B. (1998). Heterogeneity among muscle precursor cells in adult skeletal muscles with differing regenerative capacities. Dev Dyn 212:495-508 [DOI] [PubMed] [Google Scholar]
- Pei L, Lin CY, Dai JP, Yin GF. (2007). Facial pain induces the alteration of transient receptor potential vanilloid receptor 1 expression in rat trigeminal ganglion. Neurosci Bull 23:92-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot O, Berta T, Decosterd I, Kellenberger S. (2006). Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol 576(Pt 1):215-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer MS, Bradbury EJ, McMahon SB. (2001). Nerve growth factor induces P2X(3) expression in sensory neurons. J Neurochem 77:864-875 [DOI] [PubMed] [Google Scholar]
- Rau KK, Jiang N, Johnson RD, Cooper BY. (2007). Heat sensitization in skin and muscle nociceptors expressing distinct combinations of TRPV1 and TRPV2 protein. J Neurophysiol 97:2651-2662 [DOI] [PubMed] [Google Scholar]
- Reeh PW, Steen KH. (1996). Tissue acidosis in nociception and pain. Prog Brain Res 113:143-151 [DOI] [PubMed] [Google Scholar]
- Reinert A, Kaske A, Mense S. (1998). Inflammation-induced increase in the density of neuropeptide-immunoreactive nerve endings in rat skeletal muscle. Exp Brain Res 121:174-180 [DOI] [PubMed] [Google Scholar]
- Reinohl J, Hoheisel U, Unger T, Mense S. (2003). Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett 338:25-28 [DOI] [PubMed] [Google Scholar]
- Robert RC, Bacchetti P, Pogrel MA. (2005). Frequency of trigeminal nerve injuries following third molar removal. J Oral Maxillofac Surg 63:732-735 [DOI] [PubMed] [Google Scholar]
- Robinson PP, Boissonade FM, Loescher AR, Smith KG, Yates JM, Elcock C, et al. (2004). Peripheral mechanisms for the initiation of pain following trigeminal nerve injury. J Orofac Pain 18:287-292 [PubMed] [Google Scholar]
- Rodd HD, Morgan CR, Day PF, Boissanade FM. (2007). Pulpal expression of TRPV1 in molar incisor hypomineralisation. Eur Arch Paediatr Dent 8:184-188 [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Burnstock G. (2003). Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol 120:415-426 [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Birder LA, de Groat WC, Tai C, Roppolo J, Buffington CA, et al. (2005). Localization of P2X and P2Y receptors in dorsal root ganglia of the cat. J Histochem Cytochem 53:1273-1282 [DOI] [PubMed] [Google Scholar]
- Sabino MA, Honore P, Rogers SD, Mach DB, Luger NM, Mantyh PW. (2002). Tooth extraction-induced internalization of the substance P receptor in trigeminal nucleus and spinal cord neurons: imaging the neurochemistry of dental pain. Pain 95:175-186 [DOI] [PubMed] [Google Scholar]
- Saito K, Hitomi S, Suzuki I, Masuda Y, Kitagawa J, Tsuboi Y, et al. (2008). Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J Neurophysiol 99:2251-2263 [DOI] [PubMed] [Google Scholar]
- Sandstedt P, Sorensen S. (1995). Neurosensory disturbances of the trigeminal nerve: a long-term follow-up of traumatic injuries. J Oral Maxillofac Surg 53:498-505 [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Floridi A, Gallai V. (2001). Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology 57:132-134 [DOI] [PubMed] [Google Scholar]
- Sarlani E, Greenspan JD. (2005). Why look in the brain for answers to temporomandibular disorder pain? Cells Tissues Organs 180:69-75 [DOI] [PubMed] [Google Scholar]
- Sarlani E, Grace EG, Reynolds MA, Greenspan JD. (2004). Evidence for up-regulated central nociceptive processing in patients with masticatory myofascial pain. J Orofac Pain 18:41-55 [PubMed] [Google Scholar]
- Schaible HG. (2004). Spinal mechanisms contributing to joint pain. Novartis Found Symp 260:4-22 [PubMed] [Google Scholar]
- Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, et al. (2006). The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain 123:193-203 [DOI] [PubMed] [Google Scholar]
- Sharp CJ, Reeve AJ, Collins SD, Martindale JC, Summerfield SG, Sargent BS, et al. (2006). Investigation into the role of P2X(3)/P2X(2/3) receptors in neuropathic pain following chronic constriction injury in the rat: an electrophysiological study. Br J Pharmacol 148:845-852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y. (2005). Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain 116:42-51 [DOI] [PubMed] [Google Scholar]
- Shinoda M, Kawashima K, Ozaki N, Asai H, Nagamine K, Sugiura Y. (2007). P2X3 receptor mediates heat hyperalgesia in a rat model of trigeminal neuropathic pain. J Pain 8:588-597 [DOI] [PubMed] [Google Scholar]
- Shinoda M, Ozaki N, Sugiura Y. (2008). Involvement of ATP and its receptors on nociception in rat model of masseter muscle pain. Pain 134:148-157 [DOI] [PubMed] [Google Scholar]
- Simonetti M, Fabbro A, D’Arco M, Zweyer M, Nistri A, Giniatullin R, et al. (2006). Comparison of P2X and TRPV1 receptors in ganglia or primary culture of trigeminal neurons and their modulation by NGF or serotonin. Mol Pain 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoff AM, Adler JE. (2006). Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol 197:430-436 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. (2001). Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 24:37-46 [DOI] [PubMed] [Google Scholar]
- Sohn MK, Graven-Nielsen T, Arendt-Nielsen L, Svensson P. (2000). Inhibition of motor unit firing during experimental muscle pain in humans. Muscle Nerve 23:1219-1226 [DOI] [PubMed] [Google Scholar]
- Spears R, Dees LA, Sapozhnikov M, Bellinger LL, Hutchins B. (2005). Temporal changes in inflammatory mediator concentrations in an adjuvant model of temporomandibular joint inflammation. J Orofac Pain 19:34-40 [PubMed] [Google Scholar]
- Staikopoulos V, Sessle BJ, Furness JB, Jennings EA. (2007). Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience 144:208-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LC, Deslauriers R, Kupriyanov VV. (1994). Relationships between cytosolic [ATP], [ATP]/[ADP]and ionic fluxes in the perfused rat heart: A 31P, 23Na and 87Rb NMR study. J Mol Cell Cardiol 26:1377-1392 [DOI] [PubMed] [Google Scholar]
- Stucky CL, Medler KA, Molliver DC. (2004). The P2Y agonist UTP activates cutaneous afferent fibers. Pain 109:36-44 [DOI] [PubMed] [Google Scholar]
- Svensson P, Houe L, Arendt-Nielsen L. (1997). Effect of systemic versus topical nonsteroidal anti-inflammatory drugs on postexercise jaw-muscle soreness: a placebo-controlled study. J Orofac Pain 11:353-362 [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. (2007). The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 6:357-372 [DOI] [PubMed] [Google Scholar]
- Tanaka E, Detamore MS, Mercuri LG. (2008). Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res 87:296-307 [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Milam SB, Bereiter DA. (2007). Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in female rats. J Neurophysiol 98:3242-3253 [DOI] [PubMed] [Google Scholar]
- Tegeder L, Zimmermann J, Meller ST, Geisslinger G. (2002). Release of algesic substances in human experimental muscle pain. Inflamm Res 51:393-402 [DOI] [PubMed] [Google Scholar]
- Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, et al. (2007). Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache 47:1008-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiidus PM. (2003). Influence of estrogen on skeletal muscle damage, inflammation, and repair. Exerc Sport Sci Rev 31:40-44 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Ueno S, Inoue K. (1999). In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha,beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system. Br J Pharmacol 127:449-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxen C, Jensen K, Kjeldsen M, Edvinsson L, Jansen I, Olesen J. (1989). Effect of neurokinin A on human temporal muscle blood flow. Peptides 10:921-924 [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. (2002). Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest 110:1185-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. (2001). Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 534(Pt 3):813-825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R, Owsianik G, Nilius B. (2008). Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des 14:18-31 [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, et al. (1997). Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology 36:1229-1242 [DOI] [PubMed] [Google Scholar]
- Wall PD, Woolf CJ. (1984). Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol 356:443-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li GW, Huang LY. (2007). Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Mol Pain 3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MP, Fried JL. (2001). Temporomandibular disorders and hormones in women. Cells Tissues Organs 169:187-192 [DOI] [PubMed] [Google Scholar]
- Winocur E, Gavish A, Finkelshtein T, Halachmi M, Gazit E. (2001). Oral habits among adolescent girls and their association with symptoms of temporomandibular disorders. J Oral Rehabil 28:624-629 [DOI] [PubMed] [Google Scholar]
- Wirkner K, Sperlagh B, Illes P. (2007). P2X3 receptor involvement in pain states. Mol Neurobiol 36:165-183 [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. (1994). Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 62:327-331 [DOI] [PubMed] [Google Scholar]
- Wu G, Whiteside GT, Lee G, Nolan S, Niosi M, Pearson MS, et al. (2004). A-317491, a selective P2X3/P2X2/3 receptor antagonist, reverses inflammatory mechanical hyperalgesia through action at peripheral receptors in rats. Eur J Pharmacol 504:45-53 [DOI] [PubMed] [Google Scholar]
- Xu S, Cheng Y, Keast JR, Osborne PB. (2008). 17{beta}-Estradiol activates ER{beta} signalling and inhibits TRPV1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology 149:5540-5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Z, Renton T, Yiangou Y, Zakrzewska J, Chessell IP, Bountra C, et al. (2007). Burning mouth syndrome as a trigeminal small fibre neuropathy: increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci 14:864-871 [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Wang C, Huang LY. (2007). Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA 104:9864-9869 [DOI] [PMC free article] [PubMed] [Google Scholar]