Abstract
Over the past 20 years, high-risk human papillomavirus (HPV) infection has been established as a risk factor for developing head and neck squamous cell carcinoma, independent of tobacco and alcohol use. In particular, HPV is strongly associated with the development of oropharyngeal cancer and a small minority of oral cavity cancers. In this review, we summarize what is currently known about the biology of HPV, the mechanisms by which it effects malignant transformation, and the potential impact of HPV status on the clinical management of persons with head and neck cancer.
Keywords: HPV, oral cancer, oropharyngeal cancer
Introduction
In 2008, an estimated 47,500 people were diagnosed with head and neck cancer in the United States, representing approximately 3% of new cancer diagnoses, and an estimated 11,260 people died from this disease (Jemal et al., 2008). The vast majority of these head and neck cancers were squamous cell carcinomas. Over the past 20 years, the overall incidence of head and neck squamous cell carcinoma (HNSCC) has been declining in the United States, a decline which has been attributed to a decrease in the prevalence of smoking (Sturgis and Cinciripini, 2007). Although there has been a reduction in the overall incidence of HNSCC, an analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) data from 1975-1998 found that the incidence of tonsillar cancer increased by 2-3% annually in males under 60 yrs of age from 1975-1998 (Canto and Devesa, 2002). A more recent analysis of SEER data from 1973-2001 showed an annual increase in the incidence of oral tongue, palatine tonsil, and base-of-tongue cancers, by 2.1%, 3.9%, and 1.7%, respectively, in 20- to 44-year-old white patients, while the incidence of HNSCC at other sites declined (Shiboski et al., 2005).
Tobacco and alcohol use are the primary risk factors for HNSCC and are associated with the majority of these tumors worldwide. In addition to these traditional risk factors, high-risk human papillomaviruses (HPV), and in particular HPV-16, are recognized as independent risk factors for a subset of HNSCC and are most strongly associated with oropharyngeal squamous cell carcinomas (OPSCC) (Schwartz et al., 1998; Gillison et al., 2000; Morket al., 2001; Wiest et al., 2002; Herrero et al., 2003; Hobbs et al., 2006; Ernster et al., 2007; Andrews et al., 2008). HPV has also been associated with the pathogenesis of oral cancer; however, the association of HPV with HNSCC is strongest for oropharyngeal cancer (Gillison et al., 2000; Furniss et al., 2007; Sturgis and Cinciripini, 2007; Chaturvedi et al., 2008; Liang et al., 2008). In this review, we briefly summarize the current, generally accepted knowledge regarding the biology of HPV, and the mechanisms by which it effects malignant transformation, and subsequently focus on presenting recent research relating to the association of HPV with HNSCC, as well as on the future implications this research may have for the clinical management of persons with head and neck cancer.
Human Papillomavirus
Human papillomavirus (HPV) is a ~ 7.9-kD, circular, non-enveloped dsDNA virus that infects squamous epithelial cells. HPV infects the basal layer of epithelial cells through breaks in the epithelial surface and is maintained in the nuclei of infected basal cells (Stubenrauch and Laimins, 1999). As the basal cells divide into squamous epithelial cells, HPV-DNA replicates and attains a high copy number. The infection most often presents clinically as warts or papillomas of the skin and upper respiratory tract and as lesions of the uterine cervix (Bedell et al., 1991; Stubenrauch and Laimins, 1999). Over 120 HPV subtypes have been identified, 33% of which are known to infect the human genital tract (Longworth and Laimins, 2004). HPV is a well-documented cause of anogenital cancers and can be divided into high- and low-risk subtypes based on the propensity to cause malignancy (Walboomers et al., 1999; zur Hausen, 2000). Low-risk HPV subtypes are associated with benign warts, and high-risk subtypes are associated with dysplasia and invasive carcinomas. In cervical HPV infections, the most common low-risk HPV subtypes are HPV-6 and -11, while the most common high-risk subtypes are HPV-16, -18, -31, -33, and -45 (Munoz et al., 2003). HPV-16 and -18 are responsible for the vast majority of cervical cancers, and the mechanism of tumorigenesis has been thoroughly elucidated (Munger et al., 1989; Werness et al., 1990; Scheffner et al., 1992; McDougall, 1994; Cheng et al., 1995; Scheffner and Whitaker, 2003; McLaughlin-Drubin et al., 2005). Briefly, high-risk HPVs produce 2 oncoproteins, E6 and E7, which are necessary for viral replication. The HPV E6 protein binds and promotes the degradation of the tumor suppressor p53 by an ubiquitin-mediated pathway, diminishing the ability of the cell to undergo apoptosis. The HPV E7 protein binds and degrades the retinoblastoma protein (pRb), preventing it from inhibiting the transcription factor E2F, resulting in loss of cell cycle control. The E6 and E7 proteins also interact with a variety of other intracellular targets that have been recently reviewed (Munger et al., 2004). Cells expressing E6 have been shown to undergo structural chromosomal changes, and cells expressing E7 accumulate numerous chromosomal abnormalities and develop aneuploidy (White et al., 1994). The ultimate effect of the activity of the E6 and E7 proteins is dysregulated cell cycle progression and HPV DNA replication in infected squamous epithelial cells, and eventual oncogenesis. In the cervical cancer literature, it has been well-established that expression of viral E6 and E7 oncogenes is necessary, but not sufficient, for progression to dysplasia and, ultimately, to an HPV-associated carcinoma (Crook et al., 1989; Walboomers et al., 1999).
HPV-Positive HNSCC Tumor Biology
A model for the genetic and transcriptional development of head and neck cancers related to tobacco and alcohol use has been previously established (Califano et al., 1996; Ha et al., 2003). In HNSCC caused by these traditional risk factors, p53 is commonly mutated (Ahomadegbe et al., 1995; Carlos de Vicente et al., 2004), and 9p21-22 is lost early in carcinogenesis, resulting in loss of the tumor suppressor p16 (Herman et al., 1995; Reed et al., 1996; Worsham et al., 2006; Kim et al., 2007). p16 functions by associating with the cyclin 1-cyclin-dependent kinase 4/cyclin-dependent kinase 6 (CDK4/CDK6) complex to block phosphorylation of pRb, allowing pRb to control cell-cycle regulation by associating with the transcription factor E2F (Serrano et al., 1993). In contrast, HPV-positive HNSCC has decreased expression of wild-type p53, due to inactivation and degradation by E6. Additionally, these tumors do not show p16 depletion, since loss of 9p21-22 does not typically occur in these tumors. Instead, loss of cell-cycle regulation occurs when HPV E7 inactivates host cell pRb, preventing regulation of E2F (Li et al., 1994; Hara et al., 1996; Begum et al., 2005; Licitra et al., 2006; Perrone et al., 2006; Ragin et al., 2006). This represents a distinct molecular phenotype and a unique mechanism of tumorigenesis independent of the mutagenic effects of tobacco and alcohol.
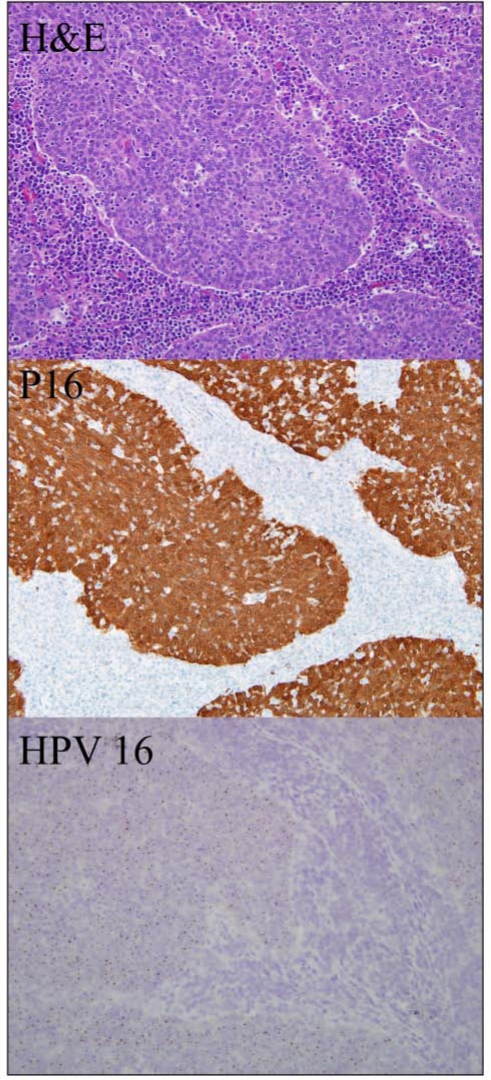
In addition to having a distinct etiology, HPV-positive HNSCCs are histologically distinct from HPV-negative tumors. In contrast to the non-HPV-related HNSCC, which are usually moderately differentiated and keratinizing, HPV-associated HNSCC are consistently poorly differentiated and non-keratinizing, and have a distinct ‘basaloid’ appearance (Wilczynski et al., 1998; Gillison et al., 2000; El-Mofty and Lu, 2003) (Fig. 1). These tumors also differ at the genetic level. A large proportion of HPV-positive HNSCCs have HPV DNA integrated into the host cell genome (Gillison et al., 2000; Wiest et al., 2002; Hafkamp et al., 2003; Kim et al., 2007). HPV-positive HNSCCs demonstrate significantly lower levels of chromosomal mutations and loss than do HPV-negative tumors (Braakhuis et al., 2004; Smeets et al., 2006).
Figure 1.
Oropharyngeal squamous cell carcinoma evaluated by routine hematoxylin and eosin staining (box 1), p16 immunostaining (box 2), and HPV-16 in situ hybridization (box 3). Tumor islands demonstrate characteristic basaloid appearance and lymphocytic infiltration.
Risk Factors for HPV-Positive HNSCC
As well as having distinct molecular characteristics, HPV-positive HNSCCs are associated with a risk factor profile that differs from the profile associated with non-HPV-associated HNSCC. Unlike HPV-negative HNSCC, the major risk factors for HPV-positive HNSCC are not tobacco or alcohol, but are instead related to sexual history (Kreimer et al., 2004; Gillison et al., 2008). The risk for HPV-positive HNSCC increases with increasing numbers of both oral and vaginal sexual partners, a history of genital warts, and a younger age at first intercourse (Schwartz et al., 1998; Kreimer et al., 2004). In a recent analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) data from 1973-2002, a diagnosis of cervical cancer was found to confer an increased risk for development of a second primary oropharyngeal cancer (oropharynx SIR = 2.7; tonsil SIR = 3.1), without increasing the risk of developing oral cavity or other cancers (Rose Ragin and Taioli, 2008). Additionally, an increased risk of tonsillar cancer has been observed in the husbands of women with documented history of cervical dysplasia or cancer (Hemminki et al., 2000). Further evidence of the association of HPV-positive cancer with sexual behavior comes from a recent case-control study of 100 persons with oropharyngeal cancer and 200 control individuals, which found that development of oropharyngeal cancer is associated with a high lifetime number of vaginal sex partners (≥ 26) and with a high lifetime number of oral sex partners (≥ 6) (odds ratios 3.1 and 3.4, respectively) (D’Souzaet al., 2007). Most recently, a case-control study of 240 individuals with oropharyngeal cancer, 92 of whom had HPV-16-positive HNSCC, found that the risk of developing an HPV-16-positive HNSCC increased with increasing numbers of oral sex partners, as well as with increased marijuana use (Gillison et al., 2008). Neither of these studies found an association between tobacco or alcohol use and the development of HPV-positive HNSCC (D’Souza et al., 2007; Gillison et al., 2008).
Association of HPV with HNSCC
As previously mentioned, the association between HNSCC and HPV is strongest for OPSCC, specifically for cancers of the palatine and lingual tonsils (Schwartz et al., 1998; Gillison et al., 2000; Mork et al., 2001; Wiest et al., 2002; Herrero et al., 2003; Ernster et al., 2007). A recent meta-analysis of 17 studies found that HPV is most strongly associated with tonsillar cancer (OR 15.1, 95% CI 6.8-33.7), is intermediate for oropharyngeal cancer in general (OR 4.3, 95% CI 2.1-8.9), and is weakest for oral cancer (OR 2.0, 95% CI 1.0-4.2) (Hobbs et al., 2006). Additionally, oral infection with high-risk HPV has been associated with a dramatically increased risk of developing oropharyngeal cancer when adjusted for alcohol and tobacco use (OR 230; 95% CI 44-1200) (Hansson et al., 2005). A recent study by the International Agency for Research on Cancer (IARC) found HPV in 3.9% (95% CI, 2.5-5.3) of oral cavity tumors and 18.3% of OPSCC (Herrero et al., 2003).
HPV-16 seropositivity, as an indicator of HPV exposure, is associated with an increased risk of developing HPV-positive oropharyngeal cancers, as well as a weaker association with oral cancer (Herrero et al., 2003; Furniss et al., 2007). In a landmark paper, researchers performed a nested case-control study using serum samples collected from 900,000 people in Norway, Finland, and Sweden (Mork et al., 2001). From this registry, the study identified 292 individuals diagnosed with HNSCC and compared them with 1568 matched control individuals. After adjustment for smoking, the overall adjusted odds ratio for development of a HNSCC at any site in the setting of HPV-16 seropositivity was modest (OR = 2.1, 95% CI =1.4-3.2). When analyzed for specific subsites in the head and neck, however, HPV-16 seropositivity was most strongly associated with increased risk of oropharyngeal cancer (OR = 14.4; 95% CI = 3.6-58.1) and was more weakly associated with risk of developing a cancer of the oral cavity (OR 3.6, 95% CI = 0.5-26.3) (Mork et al., 2001).
A recent case-control study of persons diagnosed with OPSCC found that HPV-16 seropositivity was strongly associated with OPSCC in people without a history of smoking or drinking (OR = 33.6; 95% CI = 3.3-84.8), as well as in individuals with a history of drinking and smoking (OR = 19.4; 95% CI = 13.3-113.9) (D’Souza et al., 2007).
Simply detecting HPV in tumor cells or detecting serum markers for HPV infection, however, does not demonstrate that HPV is involved in the pathogenesis of a given tumor (Snijders et al., 2003). As we described earlier in this review, the cervical cancer literature has established that transcriptionally active HPV is necessary for HPV-associated oncogenesis. This same principle holds true for HPV-associated HNSCC. Markers for transcriptionally active HPV include p16 over-expression, and expression of E6 and E7 proteins (Wiest et al., 2002; Weinberger et al., 2006). HPV has been identified in 0-100% of oral cavity pre-malignant lesions (Chang et al., 1991; Holladay and Gerald, 1993; Fouret et al., 1995; Franceschi et al., 1996; Bouda et al., 2000; Sand et al., 2000; Mork et al., 2001; Ha et al., 2002), as well as in 0-100% of oral cancers (Greer et al., 1990; Mao et al., 1996; Matzow et al., 1998; Miguel et al., 1998; Bouda et al., 2000); however, the majority of studies relied on PCR for detection and did not quantitate HPV viral load or identify markers of HPV transcriptional activity. PCR is a highly sensitive technique that can amplify exceedingly small fragments of HPV DNA, resulting in detection of non-pathologic HPV infections or sample contamination. A variety of other techniques has been used to detect HPV, including Southern blot, quantitative PCR, and in situ hybridization. A meta-analysis of studies using different techniques of HPV detection found higher rates of tumor HPV positivity in studies that relied on non-quantitative PCR for HPV detection, compared with other methods, implying a higher rate of false-positive results (Miller and White, 1996). Recent studies utilizing quantitative PCR and/or in situ hybridization have found HPV in only a small proportion of oral cavity squamous cell cancers, while consistently detecting HPV in 40-60% of oropharyngeal tumors (Schwartz et al., 1998; Gillison et al., 2000; Klussmann et al., 2003; Ritchie et al., 2003; Syrjänen, 2005; Kim et al., 2007; Fakhry et al., 2008).
Prognosis of HPV-Positive HNSCC
In addition to having distinct histopathologic and molecular characteristics, HPV-positive HNSCC carries a better prognosis than HPV-negative HNSCC. Multiple studies have shown that persons with HPV-positive oropharyngeal cancer are more responsive to treatment and have better rates of disease-specific survival than those with HPV-negative oropharyngeal cancer (Gillison et al., 2000; Friesland et al., 2001; Sisk et al., 2002; Li et al., 2003; Ritchie et al., 2003; Licitra et al., 2006; Weinberger et al., 2006; Kumar et al., 2007, 2008).
The molecular markers that differentiate HPV-positive tumors from HPV-negative tumors have been shown to correlate with tumor prognosis. An association between improved survival and transcriptionally active HPV infection exists and has been demonstrated by elevated p16 levels in HPV-infected tumor cells (Klussmann et al., 2003; Licitra et al., 2006; Reimers et al., 2007). Recently, p16 over-expression in OPSCC, as a marker for transcriptionally active HPV, has been associated with 79% 5-year survival, compared with 20% 5-year survival in individuals with HPV-negative tumors, and 18% 5-year survival in persons with HPV-16-positive tumors with normal levels of p16 expression (P = 0.0095) (Weinberger et al., 2006). Additionally, a prospective study of 66 individuals with stage III and IV OPSCC study found that tumor p16 over-expression was significantly associated with overall survival (p = 0.001) and disease-specific survival (P = 0.003) (Kumar et al., 2008). Another recent study demonstrated similarly improved 5-year survival rates in individuals with HPV-positive tonsillar cancers compared with those with HPV-negative tumors (71% vs. 36%; p = 0.023) (Charfi et al., 2008).
A recent multi-center prospective trial evaluating treatment response and survival in 96 persons with oropharyngeal or laryngeal carcinoma demonstrated that those with HPV-positive oropharyngeal tumors had higher response rates to induction chemotherapy and to chemoradiation therapy than those with HPV-negative tumors (Fakhry et al., 2008). In this study, the overall two-year survival rate for persons with HPV-positive tumors was 95% (95% CI = 87%-100%), compared with a two-year survival rate of 62% (95% CI = 49%-74%) for persons with HPV-negative tumors (Fakhry et al., 2008). Additionally, the study found that individuals with HPV-positive tumors had a better response to induction chemotherapy than those with HPV-negative tumors.
The mechanism of improved survival in HPV-associated HNSCC is still unclear. This survival benefit has been attributed to an enhanced radiosensitivity of HPV-positive tumors (Lindel et al., 2001), an improved apoptotic response secondary to the presence of unmutated p53 in HPV-associated tumors (Butzet al., 1996), and a reduced risk of developing a second primary tumor, since these individuals tend not to have a history of smoking and drinking and therefore lack the field cancerization characteristic of persons with tobacco- and alcohol-related HNSCC (Gillison et al., 2000).
Clinical Implications
Although HPV-associated HNSCC has a relatively good disease-free survival rate, a subset of persons develops recurrence of their cancer after treatment and dies from their disease. For these individuals, a screening test that takes advantage of the unique markers associated with HPV infection could be beneficial for the detection of disease persistence or of early disease recurrence. A recent study nested within a longitudinal cohort study of 59 persons with histologically confirmed HNSCC evaluated salivary rinsing as a possible screening test for recurrence of HPV-positive tumors after treatment. In this cohort, detection of HPV E6 and E7 copy number by RT-PCR from salivary rinses had a specificity of 50% and sensitivity of 100% for detection of oropharyngeal cancer recurrence (Chuang et al., 2008). This study, however, encompassed a small number of persons, and a larger study is warranted to determine the value of salivary rinsing as a screening test for tumor recurrence.
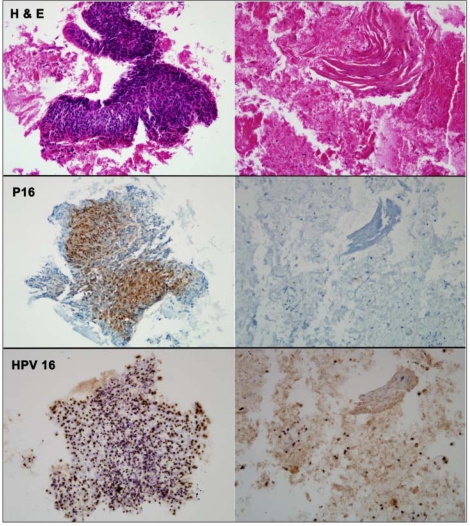
Another potentially useful clinical application is in the identification of the site of primary tumor in HNSCC presenting as a neck mass without a known primary site of origin. Neck mass of unknown primary origin constitutes 1-5% of HNSCC diagnoses, and even after complete clinical, endoscopic, and radiographic evaluation, the primary site of origin may not be identified (Schmalbach and Miller, 2007; Wong, 2008). Markers of HPV-associated tumors have been utilized in recent studies addressing this problem. Evaluation of fine-needle aspiration biopsies of neck metastases by in situ hybridization for HPV (Begum et al., 2007; Zhang et al., 2008) and by immunohistochemistry for p16 overexpression (Begum et al., 2007) have been shown to be predictive of an oropharyngeal primary tumor (Fig. 2). Additionally, a recent study demonstrated that the finding of cystic lymph nodes correlates strongly with HPV positivity by in situ hybridization (Goldenberg et al., 2008). These studies demonstrate that detection of HPV in tumor tissue can help localize the primary site of tumor origin for individuals who present with regional metastasis.
Figure 2.
Fine-needle aspirate of a metastatic squamous cell carcinoma evaluated by routine hematoxylin and eosin staining (row 1), p16 immunostaining (row 2), and HPV in situ hybridization (row 3). A fragment of viable tumor (left column) is strongly p16-positive and HPV16-positive. In areas of cellular degeneration (right column), the tumor cells lose their p16 immunoreactivity, but retain their HPV16 hybridization signals. (Previously published in Begum et al., 2007. Used with permission.)
Finally, the introduction of the HPV vaccine Gardasil® (targeted against HPV-16, -18, -6, and -11) in June of 2006, and the impending FDA approval of Cervarix® (targeted against HPV-16 and -18), if widely used, could slow or even reverse the trend of increasing prevalence of OPSCC seen in the United States over the past 30 years. These vaccines are highly effective at preventing cervical intra-epithelial neoplasia and cervical cancer (Harper et al., 2004; Villa, 2006; Paavonen et al., 2007), but it remains to be seen how widely they will be used and if their use has an effect on the incidence of HNSCC.
Conclusion
Our understanding of the association between HPV infection and HNSCC has been evolving for the past 20 years. Although initial data supported a strong association between HPV infection and both oral cavity and oropharyngeal cancer, it has become clear that, in the head and neck, HPV infection is predominantly associated with carcinogenesis in the oropharynx.
The knowledge gained about the biology of HPV-positive HNSCC is now beginning to be translated clinically and is being used to give patients prognoses, to direct treatment, and to guide follow-up. Further research has the potential to provide reliable screening tests for detection of tumor recurrence in persons treated for HPV-positive HNSCC, and the potential exists for the development of a screening test similar to the Papanicolaou test, which could be used to screen individuals at high risk for developing HPV-associated HNSCC.
Footnotes
Dr. Califano is supported by a Clinical Innovator Award from the Flight Attendant Medical Research Institute and by a NIDCR and NCI SPORE grant, P50 CA96784, as well as by NCI-supported R21CA1 35635-01, R21CA126055, and P20 CA118782.
References
- Ahomadegbe JC, Barrois M, Fogel S, Le Bihan ML, Douc-Rasy S, Duvillard P, et al. (1995). High incidence of p53 alterations (mutation, deletion, overexpression) in head and neck primary tumors and metastases; absence of correlation with clinical outcome. Frequent protein overexpression in normal epithelium and in early non-invasive lesions. Oncogene 10:1217-1227 [PubMed] [Google Scholar]
- Andrews E, Seaman WT, Webster-Cyriaque J. (2008). Oropharyngeal carcinoma in non-smokers and non-drinkers: a role for HPV. Oral Oncol (in press). [DOI] [PubMed] [Google Scholar]
- Bedell MA, Hudson JB, Golub TR, Turyk ME, Hosken M, Wilbanks GD, et al. (1991). Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J Virol 65:2254-2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum S, Cao D, Gillison M, Zahurak M, Westra WH. (2005). Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res 11:5694-5699 [DOI] [PubMed] [Google Scholar]
- Begum S, Gillison ML, Nicol TL, Westra WH. (2007). Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 13:1186-1191 [DOI] [PubMed] [Google Scholar]
- Bouda M, Gorgoulis VG, Kastrinakis NG, Giannoudis A, Tsoli E, Danassi-Afentaki D, et al. (2000). “High risk” HPV types are frequently detected in potentially malignant and malignant oral lesions, but not in normal oral mucosa. Mod Pathol 13:644-653 [DOI] [PubMed] [Google Scholar]
- Braakhuis BJ, Snijders PJ, Keune WJ, Meijer CJ, Ruijter-Schippers HJ, Leemans CR, et al. (2004). Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst 96:998-1006 [DOI] [PubMed] [Google Scholar]
- Butz K, Geisen C, Ullmann A, Spitkovsky D, Hoppe-Seyler F. (1996). Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. Int J Cancer 68:506-513 [DOI] [PubMed] [Google Scholar]
- Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. (1996). Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res 56:2488-2492 [PubMed] [Google Scholar]
- Canto MT, Devesa SS. (2002). Oral cavity and pharynx cancer incidence rates in the United States, 1975-1998. Oral Oncol 38:610-617 [DOI] [PubMed] [Google Scholar]
- Carlos de Vicente J, Junquera Gutierrez LM, Zapatero AH, Fresno Forcelledo MF, Hernandez-Vallejo G, Lopez Arranz JS. (2004). Prognosticsignificance of p53 expression in oral squamous cell carcinoma without neck node metastases. Head Neck 26:22-30 [DOI] [PubMed] [Google Scholar]
- Chang F, Syrjänen S, Kellokoski J, Syrjänen K. (1991). Human papillomavirus (HPV) infections and their associations with oral disease. J Oral Pathol Med 20:305-317 [DOI] [PubMed] [Google Scholar]
- Charfi L, Jouffroy T, de Cremoux P, Le Peltier N, Thioux M, Freneaux P, et al. (2008). Two types of squamous cell carcinoma of the palatine tonsil characterized by distinct etiology, molecular features and outcome. Cancer Lett 260:72-78 [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. (2008). Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 26:612-619 [DOI] [PubMed] [Google Scholar]
- Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. (1995). Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev 9:2335-2349 [DOI] [PubMed] [Google Scholar]
- Chuang AY, Chuang TC, Chang S, Zhou S, Begum S, Westra WH, et al. (2008). Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol 44:915-919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T, Morgenstern JP, Crawford L, Banks L. (1989). Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. EMBO J 8:513-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. (2007). Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356:1944-1956 [DOI] [PubMed] [Google Scholar]
- El-Mofty SK, Lu DW. (2003). Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol 27:1463-1470 [DOI] [PubMed] [Google Scholar]
- Ernster JA, Sciotto CG, O‘Brien MM, Finch JL, Robinson LJ, Willson T, et al. (2007). Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope 117:2115-2128 [DOI] [PubMed] [Google Scholar]
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. (2008). Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261-269 [DOI] [PubMed] [Google Scholar]
- Fouret P, Martin F, Flahault A, Saint-Guily JL. (1995). Human papillomavirus infection in the malignant and premalignant head and neck epithelium. Diagn Mol Pathol 4:122-127 [DOI] [PubMed] [Google Scholar]
- Franceschi S, Munoz N, Bosch XF, Snijders PJ, Walboomers JM. (1996). Human papillomavirus and cancers of the upper aerodigestive tract: a review of epidemiological and experimental evidence. Cancer Epidemiol Biomarkers Prev 5:567-575 [PubMed] [Google Scholar]
- Friesland S, Mellin H, Munck-Wikland E, Nilsson A, Lindholm J, Dalianis T, et al. (2001). Human papilloma virus (HPV) and p53 immunostaining in advanced tonsillar carcinoma—relation to radiotherapy response and survival. Anticancer Res 21(1B):529-534 [PubMed] [Google Scholar]
- Furniss CS, McClean MD, Smith JF, Bryan J, Nelson HH, Peters ES, et al. (2007). Human papillomavirus 16 and head and neck squamous cell carcinoma. Int J Cancer 120:2386-2392 [DOI] [PubMed] [Google Scholar]
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. (2000). Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92:709-720 [DOI] [PubMed] [Google Scholar]
- Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. (2008). Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 100:407-420 [DOI] [PubMed] [Google Scholar]
- Goldenberg D, Begum S, Westra WH, Khan Z, Sciubba J, Pai SI, et al. (2008). Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck 30:898-903 [DOI] [PubMed] [Google Scholar]
- Greer RO, Jr, Eversole LR, Crosby LK. (1990). Detection of human papillomavirus-genomic DNA in oral epithelial dysplasias, oral smokeless tobacco-associated leukoplakias, and epithelial malignancies. J Oral Maxillofac Surg 48:1201-1205 [DOI] [PubMed] [Google Scholar]
- Ha PK, Pai SI, Westra WH, Gillison ML, Tong BC, Sidransky D, et al. (2002). Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res 8:1203-1209 [PubMed] [Google Scholar]
- Ha PK, Benoit NE, Yochem R, Sciubba J, Zahurak M, Sidransky D, et al. (2003). A transcriptional progression model for head and neck cancer. Clin Cancer Res 9:3058-3064 [PubMed] [Google Scholar]
- Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, Ramaekers FC, et al. (2003). A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer 107:394-400 [DOI] [PubMed] [Google Scholar]
- Hansson BG, Rosenquist K, Antonsson A, Wennerberg J, Schildt EB, Bladstrom A, et al. (2005). Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta Otolaryngol 125:1337-1344 [DOI] [PubMed] [Google Scholar]
- Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. (1996). Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 16:859-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. (2004). Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757-1765 [DOI] [PubMed] [Google Scholar]
- Hemminki K, Dong C, Frisch M. (2000). Tonsillar and other upper aerodigestive tract cancers among cervical cancer patients and their husbands. Eur J Cancer Prev 9:433-437 [DOI] [PubMed] [Google Scholar]
- Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, et al. (1995). Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res 55:4525-4530 [PubMed] [Google Scholar]
- Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. (2003). Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 95:1772-1783 [DOI] [PubMed] [Google Scholar]
- Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, Thomas SJ. (2006). Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol 31:259-266 [DOI] [PubMed] [Google Scholar]
- Holladay EB, Gerald WL. (1993). Viral gene detection in oral neoplasms using the polymerase chain reaction. Am J Clin Pathol 100:36-40 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. (2008). Cancer statistics, 2008. CA Cancer J Clin 58:71-96 [DOI] [PubMed] [Google Scholar]
- Kim SH, Koo BS, Kang S, Park K, Kim H, Lee KR, et al. (2007). HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer 120:1418-1425 [DOI] [PubMed] [Google Scholar]
- Klussmann JP, Gultekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, et al. (2003). Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 162:747-753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, et al. (2004). Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis 189:686-698 [DOI] [PubMed] [Google Scholar]
- Kumar B, Cordell KG, Lee JS, Prince ME, Tran HH, Wolf GT, et al. (2007). Response to therapy and outcomes in oropharyngeal cancer are associated with biomarkers including human papillomavirus, epidermal growth factor receptor, gender, and smoking. Int J Radiat Oncol Biol Phys 69(2 Suppl):109S-111S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. (2008). EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol 26:3128-3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Thompson CH, O’Brien CJ, McNeil EB, Scolyer RA, Cossart YE, et al. (2003). Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer 106:553-558 [DOI] [PubMed] [Google Scholar]
- Li Y, Nichols MA, Shay JW, Xiong Y. (1994). Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res 54:6078-6082 [PubMed] [Google Scholar]
- Liang XH, Lewis J, Foote R, Smith D, Kademani D. (2008). Prevalence and significance of human papillomavirus in oral tongue cancer: the Mayo Clinic experience. J Oral Maxillofac Surg 66:1875-1880 [DOI] [PubMed] [Google Scholar]
- Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, et al. (2006). High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24:5630-5636 [DOI] [PubMed] [Google Scholar]
- Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. (2001). Human papillomavirus positive squamous cell carcinoma of the oropharynx:a radiosensitive subgroup of head and neck carcinoma. Cancer 92:805-813 [DOI] [PubMed] [Google Scholar]
- Longworth MS, Laimins LA. (2004). Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev 68:362-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao EJ, Schwartz SM, Daling JR, Oda D, Tickman L, Beckmann AM. (1996). Human papilloma viruses and p53 mutations in normal pre-malignant and malignant oral epithelia. Int J Cancer 69:152-158 [DOI] [PubMed] [Google Scholar]
- Matzow T, Boysen M, Kalantari M, Johansson B, Hagmar B. (1998). Low detection rate of HPV in oral and laryngeal carcinomas. Acta Oncol 37:73-76 [DOI] [PubMed] [Google Scholar]
- McDougall JK. (1994). Immortalization and transformation of human cells by human papillomavirus. Curr Top Microbiol Immunol 186:101-119 [DOI] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Bromberg-White JL, Meyers C. (2005). The role of the human papillomavirus type 18 E7 oncoprotein during the complete viral life cycle. Virology 338:61-68 [DOI] [PubMed] [Google Scholar]
- Miguel RE, Villa LL, Cordeiro AC, Prado JC, Sobrinho JS, Kowalski LP. (1998). Low prevalence of human papillomavirus in a geographic region with a high incidence of head and neck cancer. Am J Surg 176:428-429 [DOI] [PubMed] [Google Scholar]
- Miller CS, White DK. (1996). Human papillomavirus expression in oral mucosa, premalignant conditions, and squamous cell carcinoma: a retrospective review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 82:57-68 [DOI] [PubMed] [Google Scholar]
- Mork J, Lie AK, Glattre E, Clark S, Hallmans G, Jellum E, et al. (2001). Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 344:1125-1131 [DOI] [PubMed] [Google Scholar]
- Munger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. (1989). Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 8:4099-4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. (2004). Mechanisms of human papillomavirus-induced oncogenesis. J Virol 78:11451-11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. (2003). Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348:518-527 [DOI] [PubMed] [Google Scholar]
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. (2007). Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 369:2161-2170; erratum in Lancet 370:1414, 2007 [DOI] [PubMed] [Google Scholar]
- Perrone F, Suardi S, Pastore E, Casieri P, Orsenigo M, Caramuta S, et al. (2006). Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin Cancer Res 12:6643-6651; erratum in Clin Cancer Res 13:4313, 2007 [DOI] [PubMed] [Google Scholar]
- Ragin CC, Taioli E, Weissfeld JL, White JS, Rossie KM, Modugno F, et al. (2006). 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer 95:1432-1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, et al. (1996). High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res 56:3630-3633 [PubMed] [Google Scholar]
- Reimers N, Kasper HU, Weissenborn SJ, Stutzer H, Preuss SF, Hoffmann TK, et al. (2007). Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer 120:1731-1738 [DOI] [PubMed] [Google Scholar]
- Ritchie JM, Smith EM, Summersgill KF, Hoffman HT, Wang D, Klussmann JP, et al. (2003). Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer 104:336-344 [DOI] [PubMed] [Google Scholar]
- Rose Ragin CC, Taioli E. (2008). Second primary head and neck tumor risk in patients with cervical cancer—SEER data analysis. Head Neck 30:58-66 [DOI] [PubMed] [Google Scholar]
- Sand L, Jalouli J, Larsson PA, Hirsch JM. (2000). Human papilloma viruses in oral lesions. Anticancer Res 20(2B):1183-1188 [PubMed] [Google Scholar]
- Scheffner M, Whitaker NJ. (2003). Human papillomavirus-induced carcinogenesis and the ubiquitin-proteasome system. Semin Cancer Biol 13:59-67 [DOI] [PubMed] [Google Scholar]
- Scheffner M, Munger K, Huibregtse JM, Howley PM. (1992). Targeted degradation of the retinoblastoma protein by human papillomavirus E7-E6 fusion proteins. EMBO J 11:2425-2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbach CE, Miller FR. (2007). Occult primary head and neck carcinoma. Curr Oncol Rep 9:139-146 [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Daling JR, Doody DR, Wipf GC, Carter JJ, Madeleine MM, et al. (1998). Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst 90:1626-1636 [DOI] [PubMed] [Google Scholar]
- Serrano M, Hannon GJ, Beach D. (1993). A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366:704-707 [DOI] [PubMed] [Google Scholar]
- Shiboski CH, Schmidt BL, Jordan RC. (2005). Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer 103:1843-1849 [DOI] [PubMed] [Google Scholar]
- Sisk EA, Soltys SG, Zhu S, Fisher SG, Carey TE, Bradford CR. (2002). Human papillomavirus and p53 mutational status as prognostic factors in head and neck carcinoma. Head Neck 24:841-849 [DOI] [PubMed] [Google Scholar]
- Smeets SJ, Braakhuis BJ, Abbas S, Snijders PJ, Ylstra B, van de Wiel MA, et al. (2006). Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene 25:2558-2564 [DOI] [PubMed] [Google Scholar]
- Snijders PJ, van den Brule AJ, Meijer CJ. (2003). The clinical relevance of human papillomavirus testing: relationship between analytical and clinical sensitivity. J Pathol 201:1-6 [DOI] [PubMed] [Google Scholar]
- Stubenrauch F, Laimins LA. (1999). Human papillomavirus life cycle: active and latent phases. Semin Cancer Biol 9:379-386 [DOI] [PubMed] [Google Scholar]
- Sturgis EM, Cinciripini PM. (2007). Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer 110:1429-1435 [DOI] [PubMed] [Google Scholar]
- Syrjänen S. (2005). Human papillomavirus (HPV) in head and neck cancer. J Clin Virol 32(Suppl 1):59-66 [DOI] [PubMed] [Google Scholar]
- Villa LL. (2006). Prophylactic HPV vaccines: reducing the burden of HPV-related diseases. Vaccine 24(Suppl 1):23-28 [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12-19 [DOI] [PubMed] [Google Scholar]
- Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. (2006). Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 24:736-747 [DOI] [PubMed] [Google Scholar]
- Werness BA, Levine AJ, Howley PM. (1990). Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248(4951): 76-79 [DOI] [PubMed] [Google Scholar]
- White AE, Livanos EM, Tlsty TD. (1994). Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev 8:666-677 [DOI] [PubMed] [Google Scholar]
- Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. (2002). Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 21:1510-1517 [DOI] [PubMed] [Google Scholar]
- Wilczynski SP, Lin BT, Xie Y, Paz IB. (1998). Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol 152:145-156 [PMC free article] [PubMed] [Google Scholar]
- Wong RJ. (2008). Current status of FDG-PET for head and neck cancer. J Surg Oncol 97:649-652 [DOI] [PubMed] [Google Scholar]
- Worsham MJ, Chen KM, Tiwari N, Pals G, Schouten JP, Sethi S, et al. (2006). Fine-mapping loss of gene architecture at the CDKN2B (p15INK4b), CDKN2A (p14ARF, p16INK4a), and MTAP genes in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 132:409-415 [DOI] [PubMed] [Google Scholar]
- Zhang MQ, El-Mofty SK, Davila RM. (2008). Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: a tool for identifying the site of an occult head and neck primary. Cancer 114:118-123 [DOI] [PubMed] [Google Scholar]
- zur Hausen H. (2000). Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst 92:690-698 [DOI] [PubMed] [Google Scholar]