Abstract
Testicular teratomas result from anomalies in germ cell development during embryogenesis. In the 129 family of inbred strains of mice, teratomas initiate around embryonic day (E) 13.5 during the same developmental period in which female germ cells initiate meiosis and male germ cells enter mitotic arrest. Here, we report that three germ cell developmental abnormalities, namely continued proliferation, retention of pluripotency, and premature induction of differentiation, associate with teratoma susceptibility. Using mouse strains with low versus high teratoma incidence (129 versus 129-Chr19MOLF/Ei), and resistant to teratoma formation (FVB), we found that germ cell proliferation and expression of the pluripotency factor Nanog at a specific time point, E15.5, were directly related with increased tumor risk. Additionally, we discovered that genes expressed in pre-meiotic embryonic female and adult male germ cells, including cyclin D1 (Ccnd1) and stimulated by retinoic acid 8 (Stra8), were prematurely expressed in teratoma-susceptible germ cells and, in rare instances, induced entry into meiosis. As with Nanog, expression of differentiation-associated factors at a specific time point, E15.5, increased with tumor risk. Furthermore, Nanog and Ccnd1, genes with known roles in testicular cancer risk and tumorigenesis, respectively, were co-expressed in teratoma-susceptible germ cells and tumor stem cells, suggesting that retention of pluripotency and premature germ cell differentiation both contribute to tumorigenesis. Importantly, Stra8-deficient mice had an 88% decrease in teratoma incidence, providing direct evidence that premature initiation of the meiotic program contributes to tumorigenesis. These results show that deregulation of the mitotic-meiotic switch in XY germ cells contributes to teratoma initiation.
Keywords: Teratoma, Pluripotency, Differentiation, Germ cell, Mouse
INTRODUCTION
Male germ cell development in the 129 family of inbred mice is an important in vivo experimental model system for studying fundamental questions about maintenance of pluripotency and induction of differentiation. Germ cells arise during embryogenesis as pluripotent primordial germ cells (PGCs) that differentiate into mature gametes and ultimately the cells and tissues of an adult organism (Aponte et al., 2005; Kunwar et al., 2006). Defects during male germ cell development can lead to the formation of testicular germ cell tumors (TGCTs), which are classified as teratomas, non-seminomas or seminomas (Almstrup et al., 2004; Looijenga et al., 2003; Oosterhuis and Looijenga, 2005; Stevens, 1966). In 129 mice, TGCTs are first evident microscopically at embryonic day (E) 15.5 as foci of pluripotent stem cells (embryonal carcinoma cells or EC cells) (Stevens, 1962). Mouse EC cells differentiate to form teratomas, disorganized cell masses consisting of embryonic and extra-embryonic tissue types at various stages of differentiation (Stevens, 1967a; Stevens, 1967b; Stevens and Hummel, 1957). The teratomas of 129 mice share many developmental characteristics with human pediatric teratomas and adult non-seminomas (Oosterhuis and Looijenga, 2005; Stevens and Hummel, 1957).
The capacity of germ cells to maintain pluripotency and to differentiate when perturbed is similar to the in vitro properties of embryonic stem (ES) cells, pluripotent stem cells derived from the inner cell mass (ICM) of the blastocyst (Smith, 2001). These developmental similarities and the shared expression of several markers have led to the hypothesis that ICM cells transition through an intermediate germ cell-like state during ES cell derivation (Chu et al., 2011; Zwaka and Thomson, 2005). Interestingly, 129, the only strain with an appreciable frequency of spontaneous TGCTs, is also the most permissive mouse strain for ES cell derivation (Smith, 2001; Stevens and Hummel, 1957; Stevens and Little, 1954). Thus, the genetic elements of strain 129 that induce the transformation of germ cells into pluripotent EC cells are likely to facilitate the efficient derivation of totipotent ES cells. In fact, we previously demonstrated that genetic factors on 129-derived chromosome 18 are essential for both increased teratoma risk and ES cell derivation efficiency (Anderson et al., 2009). Therefore, studies of testicular teratoma initiation in 129 mice not only provide insight into the etiology and pathogenesis of a common cancer in humans, but also into the genetics and cellular pathways involved in stem cell maintenance and differentiation.
It has long been hypothesized that testicular teratomas arise from germ cells that fail to become mitotically inactive in the G0 phase of the cell cycle (Matin et al., 1998; Noguchi and Stevens, 1982; Stevens, 1966; Stevens, 1967b). The decision to enter mitotic arrest versus initiate meiosis, the so-called mitotic-meiotic switch, is a crucial event in the development of the germ cell lineage (McLaren, 2000; Park and Jameson, 2005). In mice, this switch coincides with germ cell sex specification at ∼E13.5 (McLaren, 1984). In the embryonic ovary, retinoic acid induces germ cells to express Stra8, which initiates an anterior-to-posterior wave of meiotic differentiation that lasts for four days (E12.5 to E16.5) (Menke et al., 2003). During the same developmental period, Cyp26b1 inactivates retinoic acid, thereby blocking Stra8 expression in male germ cells (Bowles et al., 2006; Koubova et al., 2006; MacLean et al., 2007; Vernet et al., 2006). Nanos2 expression also inhibits Stra8 expression in male germ cells (Suzuki and Saga, 2008). Rather than initiating meiotic prophase, male germ cells remain quiescent until after birth when they re-initiate proliferation and differentiate to form the spermatogonial lineage (McLaren, 1984).
The influence of genetic modifiers on teratoma incidence in 129 mice supports the hypothesis that aberrant cell proliferation leads to tumor initiation. The Ter mutation of Dnd1 (Dnd1Ter), an engineered deletion of Dmrt1, and a Pten loss-of-function mutation increase both teratoma incidence and germ cell proliferation after E13.5 (Kimura et al., 2003; Krentz et al., 2009; Noguchi and Stevens, 1982). By contrast, deletion of Eif2s2 decreases the number of aberrantly dividing male germ cells and tumor incidence (Heaney et al., 2009; Kimura et al., 2003). Importantly, continued proliferation cannot by itself explain the cellular events that must occur to transform germ cells into EC cells and ultimately the various tissue types found in TGCTs. Retention of pluripotency is likely to play an important role. Repression of Oct4 (Pou5f1 – Mouse Genome Informatics) significantly reduces the teratoma-forming capacity of ES cells in mouse xenograph assays (Gidekel et al., 2003), and it is likely that EC cells are similarly dependent on pluripotency factors for their teratoma-forming capacity. Following initiation of G0 arrest at E13.5, male germ cells normally downregulate expression of pluripotency factors (e.g. Nanog, Sox2 and Pou5f1) (Avilion et al., 2003; Pesce et al., 1998; Yamaguchi et al., 2005). In testicular teratoma-susceptible mice, germ cells that fail to enter mitotic arrest continue to express pluripotency factors through the transition to EC cells (Cook et al., 2011; Kimura et al., 2003; Krentz et al., 2009). Importantly, signaling pathways involving POU5F1 and NANOG have been implicated in germ cell tumor initiation in humans (Clark et al., 2004; Looijenga et al., 2003; Oosterhuis and Looijenga, 2005).
Tantalizing evidence suggests that meiotic differentiation might also be involved in human and mouse testicular germ cell tumors. In humans, markers associated with both mitotic and meiotic cells, such as cyclin D2 (CCND2) and SYCP3, respectively, are expressed in EC cells and germ cell tumors (Adamah et al., 2006; Bartkova et al., 1999; Sicinski et al., 1996). In mice, Dnd1Ter mutant male germ cells express STRA8 and SYCP3, factors involved in meiotic commitment and meiotic prophase I, respectively (Cook et al., 2009). However, these factors were not expressed in EC cells. By contrast, male germ cells of Dmrt1-deficient embryos were reported to not express Stra8 (Krentz et al., 2009). Thus, whether premature expression of genes involved in meiotic differentiation contributes to tumor initiation or is an unrelated phenotype associated with certain mutations remains unresolved.
In the present study, we demonstrate that teratoma-susceptible germ cells in mice delay entry into G0 arrest, delay the repression of pluripotency, and prematurely express genes associated with pre-meiotic embryonic female and adult male germ cell differentiation. Importantly, we show that germ cell proliferation, and the expression of germ cell pluripotency and differentiation-associated factors at a specific developmental time point, E15.5, are directly correlated with increased teratoma risk. Furthermore, we demonstrate that genes involved in germ cell pluripotency (Nanog) and differentiation (Ccnd1) are co-expressed in EC cells, and that Stra8 deficiency reduces teratoma incidence. We propose that retention of pluripotency is required for the teratoma-forming capacity of EC cells and that premature expression of factors associated with germ cell differentiation contribute to the transformation of germ cells into tumorigenic EC cells. Together, our results suggest that TGCT initiation is a complex process involving several developmental abnormalities.
MATERIALS AND METHODS
Mice
129S1/SvImJ (JR#002448) and FVB/NJ (JR#001800) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). 129/SvImJ mice homosomic for the Chr19MOLF/Ei chromosome substitution (M19) were obtained from our research colony (Matin et al., 1999). The germ cell-specific Oct4ΔPE:GFP (Oct4::GFP) transgene (Yoshimizu et al., 1999; Youngren et al., 2005) was backcrossed onto the 129/SvImJ, FVB/NJ and M19 backgrounds to establish congenic lines (Heaney et al., 2009). An engineered deletion of Stra8 (Stra8KO) was backcrossed onto a 129/Sv inbred background for at least ten generations to establish a congenic strain. Stra8KO mice were PCR genotyped as previously described (Baltus et al., 2006). All protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Timed matings and embryonic gonad dissections
For immunohistochemistry and fluorescence-assisted cell sorting (FACS), wild-type females were bred to males homozygous for the Oct4::GFP transgene to produce FVB, 129 or M19 transgenic embryos. For meiotic chromosome spreads, wild-type mice were bred to produce FVB, 129 or M19 embryos. E0.5 was assumed to be noon of the day the vaginal plug was observed. Pregnant females were euthanized by cervical dislocation and gonads were removed from embryos in ice-cold PBS. Embryos older than E14.5 were decapitated prior to dissection. PCR genotyping for Sry identified the sex of E12.5 embryos (Heaney et al., 2009). Gonad morphology identified the sex of E13.5 to E16.5 embryos.
Fluorescence-assisted cell sorting
FACS with the Oct4::GFP transgene has been previously described (Heaney et al., 2009; Molyneaux et al., 2003). Briefly, gonads were digested in 0.25% trypsin (Invitrogen, Carlsbad, CA, USA) for 15 minutes at 37°C. Tissues were triturated into single-cell suspensions and filtered through a 40 μm nylon mesh cell strainer (BD Biosciences, Franklin Lakes, NJ, USA). The mesh was washed with 2% bovine serum albumen (BSA) in PBS and the cells were kept on ice until FACS with the BD Biosciences FACSAria system. The Oct4::GFP transgene was used to sort GFP-positive PGCs from GFP-negative somatic cells, which typically yielded 8000 GFP-positive germ cells (98% purity) from both gonads of a single embryo (Heaney et al., 2009; Molyneaux et al., 2003).
Quantitative real-time PCR expression analysis
Germ cell RNA was prepared using the RNeasy Micro Kit (Qiagen, Valencia, CA, USA). RNA was reverse transcribed with the SuperScript First-Strand Synthesis System (Invitrogen). Quantitative real-time PCR (qPCR) was performed with the Chromo4 real-time PCR system (MJ Research/BioRad, Hercules, CA, USA) and the DyNAmo HS Sybr Green qPCR kit (Fisher Scientific) using manufacturer’s suggested protocols. Serial dilutions of wild-type adult testis cDNA were used to generate standard curves for each primer set. Expression was normalized to the ubiquitously expressed housekeeping gene Rpl7 as previously described (Heaney et al., 2009; Jeong et al., 2005). Female germ cell expression data from all strains was pooled. Significant differences in expression between male FVB germ cells (control) and male M19, male 129 and female germ cells were tested with unpaired t-tests with P values corrected for multiple testing. See supplementary material Table S1 for qPCR primer sequences.
Immunohistochemistry
Gonads were removed from E13.5 to E16.5 embryos and processed for sectioning and immunohistochemistry as previously described (Heaney et al., 2009). Sections were incubated with a 1:500 dilution of rabbit polyclonal anti-KI67 (ab15580, Abcam, Cambridge, MA, USA), a 1:100 dilution of rabbit polyclonal anti-NANOG (IHC-00205, Bethyl Laboratories, Montgomery, TX, USA) or a 1:100 dilution of rabbit monoclonal anti-CCND1 (ab16663, Abcam) antibody overnight at 4°C. For some experiments, sections were co-incubated with a 1:400 dilution of rat monoclonal anti-E-cadherin (13-900, Invitrogen) or a 1:500 dilution of mouse monoclonal anti-phosphoSer139-Histone H2A.X (γH2A.X) (05-636, Fisher Scientific) antibody. For secondary detection, sections were incubated with a 1:400 dilution of goat anti-rabbit Alexa Fluor 555 (A-21429), a 1:400 dilution of goat anti-rat Alexa Fluor 633 (A-21094, Invitrogen) or a 1:400 dilution of goat anti-mouse Alexa Fluor 633 (A-21052, Invitrogen) antibody for 2 hours at room temperature. Nuclei were counterstained with DAPI. Images were taken with a Leica SP2 Confocal Microscope.
Cell counts
Oct4::GFP-positive germ cells positive or negative for KI67, NANOG or CCND1 immunostaining were counted as previously described (Heaney et al., 2009). At E15.5 and E16.5, co-labeling for E-cadherin was used to exclude Oct4::GFP-positive EC cells from germ cell assays. Significant differences in the percentage of KI67, CCND1 or NANOG-positive germ cells were tested with unpaired t-tests with P values corrected for multiple testing.
Cell spreads for meiotic chromosome analysis
Gonads were removed at E14.5 and E16.5 and chromosome spreads were processed for immunohistochemistry as previously described (Anderson et al., 2008). Spreads were incubated with a 1:100 dilution of mouse monoclonal anti-SYCP3 (ab97672, Abcam) or a 1:100 dilution of rabbit polyclonal anti-SYCP1 (ab15090, Abcam) antibody overnight at 4°C. For secondary detection, spreads were incubated in blocking solution containing a 1:400 dilution of goat anti-mouse Alexa Fluor 555 (A-21424, Invitrogen) or goat anti-rabbit Alexa Fluor 555 (A-21429, Invitrogen) antibody for 2 hours at room temperature. Nuclei were counterstained with DAPI.
Tumor surveys
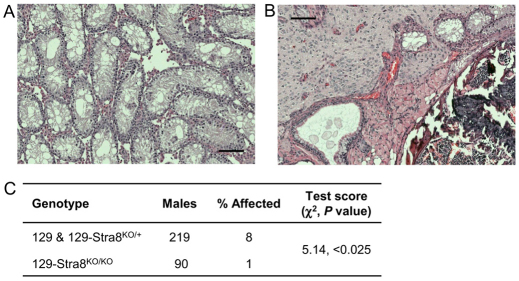
Crosses between 129 mice heterozygous for Stra8KO were used to produce wild-type (129), heterozygous knockout (129-Stra8KO/+) and homozygous knockout (129-Stra8KO/KO) male offspring to survey for teratomas. Males at least 1 month of age were necropsied prior to genotyping and testes were visually and histologically examined for tumors, which are readily detected at this age (Lam et al., 2004; Matin et al., 1999). A χ2 contingency table was used to assess statistical differences between the number of teratoma-affected control (129 wild type and 129-Stra8KO/+) and experimental (129-Stra8KO/KO) progeny.
Histology
Tissues were fixed in 10% buffered formalin, embedded in paraffin, sectioned (5 μm), and stained with Hematoxylin and Eosin. Images were taken with the Leica SCN400 Slide Scanner and processed with SlidePath Digital Image Hub and Adobe Photoshop CS5.
RESULTS
Delayed entry into G0 arrest associates with increased testicular teratoma risk
To test whether germ cell proliferation at a specific developmental time point associates with increased teratoma risk, we examined germ cell proliferation in two teratoma susceptible strains, the 129-Chr19MOLF/Ei chromosome substitution strain (M19) and the 129/SvImJ (129) inbred strain, and a teratoma-resistant strain, FVB/NJ (FVB). M19 mice, in which both copies of chromosome 19 are derived from the MOLF/Ei inbred strain, have a high risk of developing teratomas (80% of males affected) (Matin et al., 1999). By contrast, 129 inbred mice have a low risk of developing teratomas (8% of males affected) (Stevens, 1967b). Importantly, most M19 and 129 germ cells develop normally (Matin et al., 1999; Stevens, 1967b). Thus, the developmental characteristics of teratoma-susceptible germ cells that do not transform to EC cells can also be studied in M19 and 129 mice.
We first assayed germ cell proliferation at E13.5 and E14.5 by immunostaining Oct4::GFP transgenic FVB, 129 and M19 embryonic testes with the proliferation marker KI67 (Fig. 1; supplementary material Fig. S1). At E13.5, the percentage of proliferating Oct4::GFP-positive germ cells was similar in the embryonic testes of all strains. At E14.5, the percentage of proliferating germ cells decreased in all strains, but the incidence of germ cell proliferation was statistically higher in teratoma-susceptible gonads (13% proliferating in FVB and 40-46% proliferating in 129 and M19). Importantly, the percentage of proliferating germ cells in 129 and M19 testes at E14.5 was statistically similar, suggesting that the occurrence of proliferation at this time point does not influence teratoma risk.
Fig. 1.
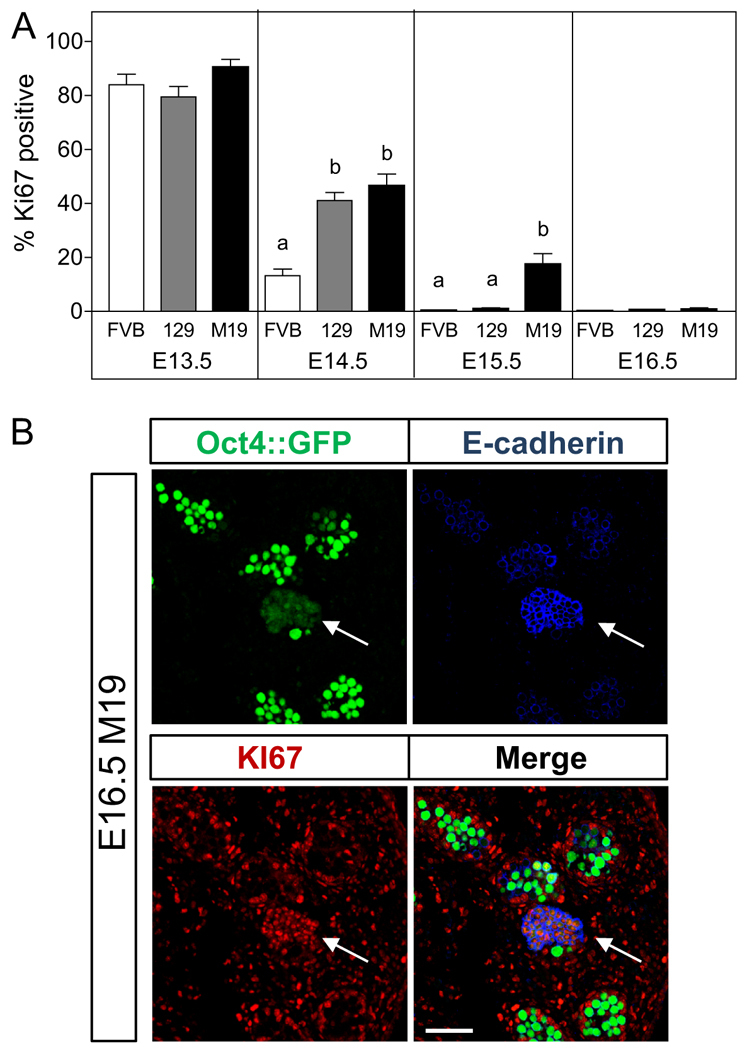
Teratoma-susceptibility germ cells delayed entry into G0 arrest. Male germ cell proliferation at E15.5 associates with increased teratoma risk. (A) Oct4::GFP transgenic FVB (teratoma-resistant), 129 (low teratoma risk) and M19 (high teratoma risk) embryonic testes were sectioned and immunostained for the nuclear mitotic marker KI67 (E13.5 and E14.5) or KI67 and the EC cell marker E-cadherin to exclude EC cells from the germ cell counts (E15.5 and E16.5). Data are plotted as the percentage of KI67-positive germ cells ± s.e.m. (n=6-8). Means without a common letter differ, P<0.05. (B) Confocal microscopy images of an E16.5 M19 testis immunostained for KI67 and E-cadherin. A proliferating cluster of cells with an EC cell-like morphology, positive for E-cadherin and expressing low levels of the Oct4::GFP transgene is noted (arrows). E-cadherin-negative, Oct4::GFP-positive germ cells are not expressing KI67. Scale bar: 75 μm.
Having found no differences in germ cell proliferation in 129 and M19 testes through E14.5, we assayed germ cell proliferation at E15.5 and E16.5. For these assays, Oct4::GFP transgenic embryonic testes were co-labeled with KI67 and the EC cell marker E-cadherin. EC cells, which are transformed and no longer represent the developmental characteristics of germ cells, first appear at E15.5 and are often mistakenly included in germ cell assays because they express markers common to embryonic germ cells (e.g. endogenous OCT4 and TRA98) (Kimura et al., 2003; Krentz et al., 2009). E-cadherin is expressed early in germ cell development, but is downregulated in male germ cells at E15.5, whereas EC cells continue to express E-cadherin (Cook et al., 2009; Di Carlo and De Felici, 2000). In addition, the Oct4::GFP transgene is expressed at low levels in EC cells (Yeom et al., 1996). Thus, E-cadherin and Oct4::GFP expression can be used to identify and exclude EC cell foci (Fig. 1B; supplementary material Fig. S1C,D). At E15.5, <1% of 129 and FVB germ cells were KI67-positive (Fig. 1A). By contrast, 25% of M19 germ cells were proliferative. Therefore, teratoma risk increases with the incidence of germ cell proliferation at E15.5, suggesting that the occurrence of aberrant germ cell proliferation at this specific time point increases the risk of germ cell transformation into EC cells. Interestingly, only 2% of M19 germ cells were KI67-positive at E16.5 (Fig. 1A), which was not significantly different from the percentage of germ cells proliferating in E16.5, 129 or FVB testes. Thus, at E16.5, most germ cells in teratoma-susceptible strains become quiescent regardless of tumor risk, suggesting that germ cells that have not transformed by this time point develop normally.
Delayed repression of pluripotency associates with increased teratoma risk
Downregulation of pluripotency in male germ cells coincides with the induction of G0 arrest (Avilion et al., 2003; Yamaguchi et al., 2005). Because differences in germ cell proliferation in 129 (low teratoma risk) and M19 (high teratoma risk) embryonic testes were only observed at E15.5, we investigated whether pluripotency gene expression increased with teratoma risk only at E15.5. Oct4 and Nanog expression was examined in teratoma-resistant and susceptible germ cells isolated by FACS with the Oct4::GFP transgene. Endogenous Oct4 expression was similar in teratoma-resistant and teratoma-susceptible germ cells at all embryonic time points tested, with no observable decrease in expression from E13.5 to E15.5 (Fig. 2A). By contrast, Nanog expression increased with teratoma risk (Fig. 2B). At E13.5, Nanog was similarly expressed in teratoma-resistant and -susceptible germ cells. By E14.5, Nanog expression decreased in all male germ cells but was significantly higher (and similar) in teratoma-susceptible 129 and M19 germ cells. Importantly, at E15.5, Nanog expression decreased in 129 male germ cells to levels similar to those in FVB male germ cells, but remained significantly increased in M19 male germ cells. As expected, decreased Oct4 and Nanog expression was observed in E13.5 to E15.5 female germ cells, coinciding with initiation of the meiotic program (Fig. 2A,B) (Massari and Murre, 2000; Pesce et al., 1998).
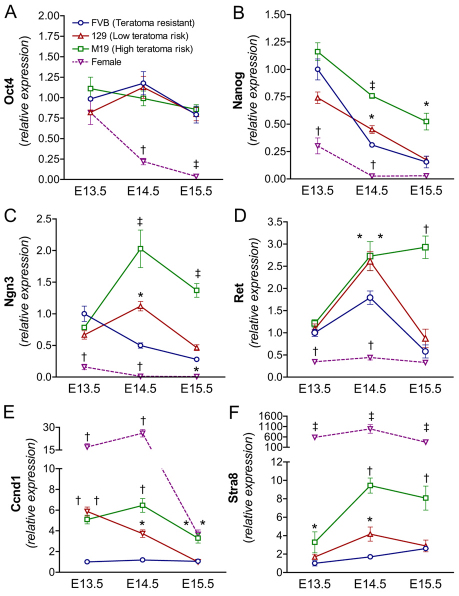
Fig. 2.
Teratoma-susceptible germ cells express markers of germ cell pluripotency and male germ cell differentiation. (A-F) Teratoma-susceptible germ cells express pluripotency (A,B) and differentiation (C-F) markers. Oct4::GFP transgenic FVB, 129 and M19 germ cells from E13.5-E15.5 gonads were FACS-isolated and gene expression was analyzed by qPCR (n=4-15). Female germ cell expression data from all strains was statistically similar and pooled. Gene expression at all embryonic time points is plotted relative to expression in E13.5 FVB male germ cells. *, P<0.05; †, P<0.005; ‡, P<0.0005.
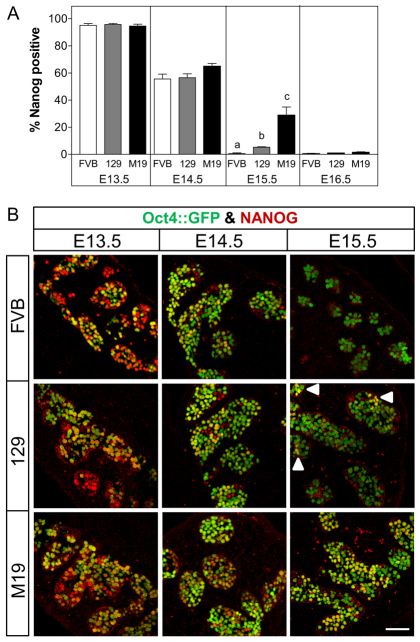
Next, we tested whether the number of NANOG-expressing germ cells increases with teratoma risk (Fig. 3). At E13.5 and E14.5, the percentage of NANOG-positive germ cells was similar in teratoma-resistant and -susceptible testes. Interestingly, at E15.5, the percentage of NANOG-positive germ cells decreased substantially in all testes; however, 30% of M19 germ cells remained NANOG-positive whereas only 1-5% of 129 and FVB germ cells were NANOG-positive. Curiously, at E16.5, only 1% of M19 germ cells were NANOG-positive, which was statistically similar to the percentage of NANOG-positive germ cells in 129 and FVB testes. Therefore, teratoma risk increases with the occurrence of NANOG-positive germ cells at E15.5 and germ cells that have not transformed by E16.5 repress Nanog expression.
Fig. 3.
Teratoma-susceptible germ cells retain pluripotency. NANOG expression at E15.5 associates with increased teratoma risk. (A) Oct4::GFP transgenic FVB, 129 and M19 embryonic testes were sectioned and immunostained for NANOG (E13.5 and E14.5) or NANOG and E-cadherin (E15.5 and E16.5). Data are plotted as the percentage of NANOG-positive germ cells ± s.e.m. (n=6-8). Means without a common letter differ, P<0.05. (B) Confocal microscopy images of E13.5-E15.5 testis immunostained for NANOG. At E15.5 FVB germ cells are NANOG-negative, most 129 germ cells are NANOG-negative, and many M19 germ cells are NANOG-positive. Small groups of NANOG-positive germ cells are noted (arrowheads). Scale bar: 75 μm.
Premature expression of male germ cell differentiation factors associates with increased teratoma risk
In addition to regulating pluripotency gene expression, entry into G0 arrest might also serve to prevent inappropriate germ cell responses to signals produced by somatic cells within the developing testis. Because teratoma-susceptible germ cells continue to actively divide until E15.5, we tested whether a failure to enter mitotic arrest is followed by premature expression of genes associated with spermatogonial differentiation. E13.5 to E15.5 germ cells were examined for the expression of neurogenin 3 (Ngn3; Neurog3 – Mouse Genome Informatics) and the ret receptor (Ret), which are expressed in undifferentiated adult spermatogonia (As, Ap and Aal) (Peters, 1970; Yoshida et al., 2004; Yoshida et al., 2006), and cyclin D1 (Ccnd1), which is expressed in neonatal and adult spermatogonia but not embryonic male germ cells (Beumer et al., 2000). Our expression analysis revealed that all three spermatogonial markers are induced in teratoma-susceptible germ cells (Fig. 2C-E). At E13.5, the expression of Ngn3 and Ret was similar in teratoma-resistant and -susceptible male germ cells. However, the expression of Ccnd1 in 129 and M19 male germ cells was significantly higher than in FVB male germ cells at E13.5. By E14.5, all three markers were significantly increased in 129 and M19 germ cells relative to FVB germ cells. By contrast, at E15.5 expression of all three differentiation-associated factors in 129 male germ cells had decreased to levels similar to those observed in FVB male germ cells. However, expression of all three genes remained significantly increased in M19 male germ cells (Fig. 2C-E). Therefore, as observed with germ cell proliferation and Nanog expression, teratoma risk increases with the expression of male germ cell differentiation-associated genes at E15.5.
Ccnd1 was also strongly expressed at E13.5 and E14.5 in female germ cells and decreased substantially at E15.5 when most female germ cells entered meiosis (Fig. 2E). Similar to its association with pre-meiotic spermatogonia in the adult testis, cyclin D1 expression might be associated with pre-meiotic embryonic female germ cells. Furthermore, expression of Ccnd1 in teratoma-susceptible germ cells and female germ cells at the same developmental time points implies that a shared signal induces Ccnd1 expression and possibly pre-meiotic germ cell differentiation. However, Ret and Ngn3 were not induced in female germ cells (Fig. 2C,D), suggesting that if a common signal induces germ cell differentiation, male germ cell identity is retained in teratoma-susceptible embryos.
Pre-meiotic female germ cells and teratoma-susceptible male germ cells express CCND1
Aberrant expression of cyclin D1 in teratoma-susceptible male germ cells has both developmental and tumorigenic implications (Deshpande et al., 2005). Therefore, we characterized the kinetics of CCND1 expression during the developmental stages associated with the mitotic-meiotic switch and teratoma initiation. We first examined the expression of CCND1 in female germ cells. At E12.5, germ cells of most female gonads did not express CCND1. However, some female gonads contained small groups of CCND1-positive germ cells at the anterior or posterior end (Fig. 4). By E13.5, we observed a strong induction of CCND1 expression in germ cells throughout the developing ovary (Fig. 4). Curiously, from E14.5 to E15.5 expression of CCND1 in female germ cells was lost in an anterior-to-posterior wave, so that by E15.5 only a few germ cells at the posterior end of the developing ovary expressed CCND1 (Fig. 4). Similar patterns of CCND1 expression were found in FVB, 129 and M19 female germ cells. Co-labeling of E14.5 and E15.5 female gonads for CCND1 and γH2A.X, a marker of DNA double strand breaks produced during meiotic prophase (Paull et al., 2000), revealed that meiotic female germ cells did not express CCND1 (supplementary material Fig. S2). Therefore, CCND1 expression is induced at ∼E13.5 in pre-meiotic female germ cells and is downregulated during meiotic initiation.
Fig. 4.
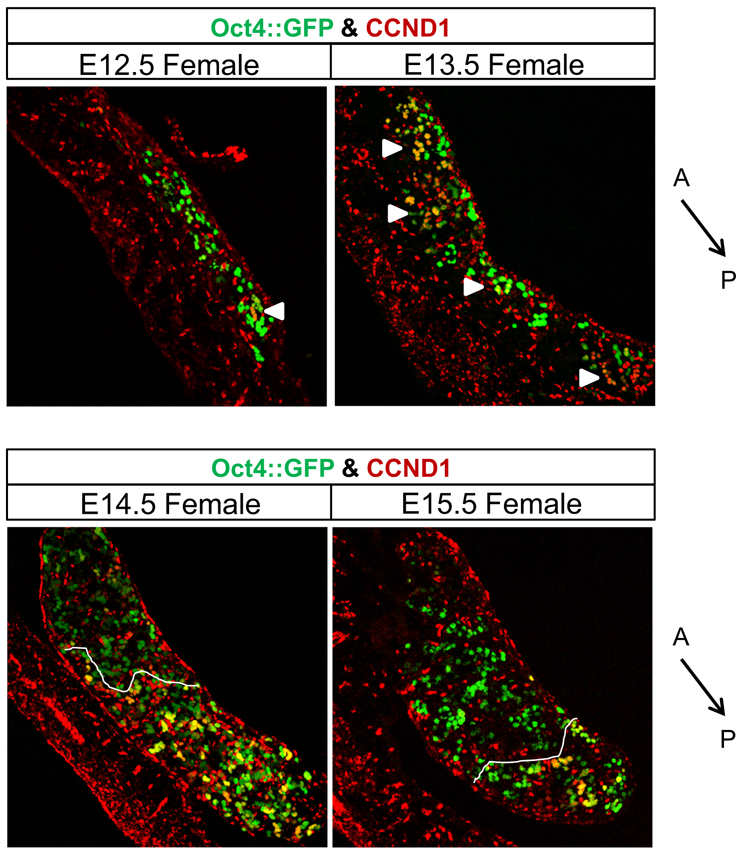
Pre-meiotic female germ cells express CCND1. CCND1 is expressed prior to meiosis and is repressed in an anterior-to-posterior wave. Confocal microscopy images of E12.5 to E15.5, Oct4::GFP transgenic ovaries immunostained for CCND1. Dotted lines indicate the boundary at which germ cells transition from CCND1-negative to CCND1-positive. Small groups of CCND1-positive germ cells are noted (arrowheads). The anterior (A) to posterior (P) axis is indicated. Scale bars: 150 μm.
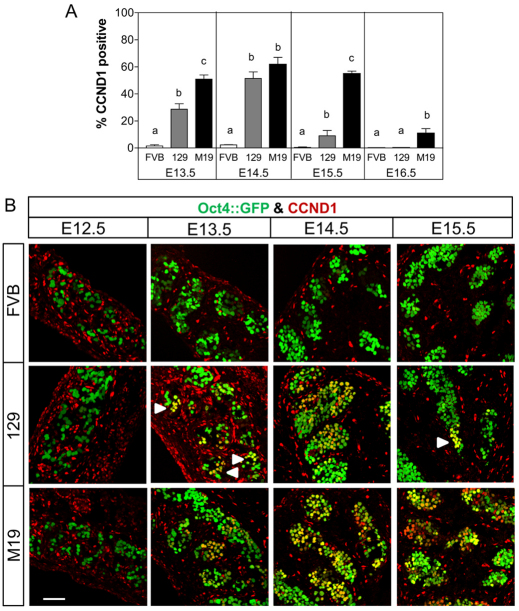
We next examined the expression of CCND1 in teratoma-resistant and -susceptible male germ cells. CCND1 expression was not observed in male germ cells at E12.5 (Fig. 5B). However, as observed in the female gonad, CCND1 expression was induced at E13.5 in 28% of 129 and 51% of M19 male germ cells (Fig. 5). By E14.5, the majority (50-60%) of 129 and M19 male germ cells expressed CCND1 (Fig. 5). Interestingly, CCND1 expression was dramatically downregulated in 129 male germ cells at E15.5 and E16.5 (10% and 1% positive, respectively) (Fig. 5). By contrast, 50% of E15.5 and 10% of E16.5 M19 male germ cells expressed CCND1 (Fig. 5). In both 129 and M19 testes, there was no evidence of an anterior-to-posterior wave of CCND1 repression. In agreement with our RNA expression data, CCND1 protein expression was rarely observed in FVB male germ cells (0-3%) at any embryonic time points tested (Fig. 5). Therefore, as observed with the proliferation and pluripotency markers, the percentage of germ cells expressing CCND1 at E15.5 increases with teratoma risk and germ cells that have not transformed into EC cells by E16.5 repress Ccnd1 expression and probably develop normally.
Fig. 5.
Teratoma-susceptible germ cells ectopically express CCND1. CCND1 expression at E15.5 associates with increased teratoma risk. (A) Oct4::GFP transgenic FVB, 129 and M19 embryonic testes were sectioned and immunostained for CCND1 (E13.5 and E14.5) or CCND1 and E-cadherin (E15.5 and E16.5). Data are plotted as the percentage of CCND1-positive germ cells ± s.e.m. (n=6-8). Means without a common letter differ, P<0.05. (B) Confocal microscopy images of E12.5 to E15.5 testis immunostained for CCND1. At E14.5, teratoma-resistant, FVB germ cells are CCND1-negative and teratoma-susceptible germ cells are mostly cyclin D1-positive. At E15.5, FVB are CCND1-negative, most 129 germ cells are CCND1-negative, and several M19 germ cells are CCND1-positive. Small groups of CCND1-positive germ cells are noted (arrowheads). Scale bar: 75 μm.
EC cells co-express NANOG and CCND1
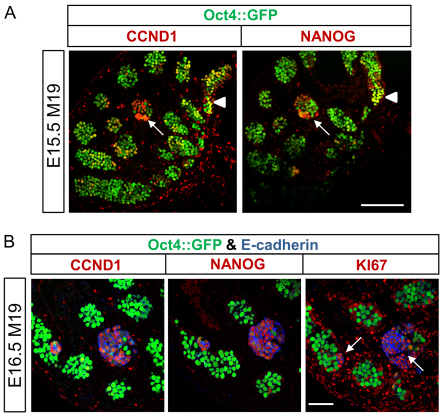
Retention of pluripotency and induction of germ cell differentiation demonstrate that the mitotic-meiotic switch and normal male germ cell development are disrupted in teratoma-susceptible gonads. However, whether two independent populations of abnormal germ cells, one pluripotent and one prematurely differentiating, or a single population of abnormal germ cells that expresses both pluripotency and differentiation factors exists within teratoma-susceptible gonads was unclear. Thus, to test whether these disparate factors are co-expressed in germ cells, we examined NANOG and CCND1 expression in serial sections of Oct4::GFP transgenic, E15.5 M19 testes. Importantly, NANOG and CCND1 were expressed in germ cells within similar regions of the developing tubules and in small clusters of cells with weak Oct4::GFP expression and morphology consistent with nascent EC cells (Fig. 6A).
Fig. 6.
CCND1 and NANOG are co-expressed in proliferating teratoma-susceptible germ cells and EC cells. (A) Confocal microscopy images of serial sections of an Oct4::GFP transgenic, E15.5 M19 testis immunostained for CCND1 or NANOG. Tubules with germ cells expressing both CCND1 and NANOG are noted (arrowhead). A cluster of cells with EC cell-like morphology and co-expressing CCND1 and NANOG is indicated (arrow). Scale bar: 150 μm. (B) Confocal microscopy images of serial sections of an Oct4::GFP transgenic, E16.5 M19 testis immunostained for CCND1, NANOG, or KI67 and E-cadherin. E-cadherin-positive EC cell foci with low levels of Oct4::GFP and expressing CCND1, NANOG and KI67 are noted (arrows). Oct4::GFP-positive, E-cadherin-negative germ cells are not expressing CCND1, NANOG or KI67. Scale bar: 75 μm.
To corroborate that pluripotency and germ cell differentiation-associated factors are co-expressed in EC cells, serial sections of E16.5 M19 gonads were immunostained for E-cadherin and either CCND1 or NANOG (Fig. 6B). In agreement with our previous assays, few Oct4::GFP-positive, E-cadherin-negative germ cells expressed CCND1 or NANOG at E16.5 (Fig. 6B). However, CCND1 and NANOG colocalized to E-cadherin-positive EC cell foci with weak Oct4::GFP expression (Fig. 6B; supplementary material Fig. S3A). In addition, expression of the KI67 proliferation marker localized to the same EC cell foci as CCND1 and NANOG (Fig. 6B; supplementary material Fig. S3A). As with all other time points assayed, few teratoma-resistant FVB germ cells expressed CCND1 or NANOG at E16.5 (Fig. 3A, Fig. 5A; supplementary material Fig. S3B). Therefore, markers of germ cell differentiation and pluripotency colocalize to proliferative EC cells, suggesting that induction of genes associated with both premature differentiation and pluripotency contribute to tumor stem cell formation. Furthermore, restriction of CCND1 and NANOG to proliferating EC cell foci at E16.5 demonstrates that as most teratoma-susceptible germ cells enter G0 arrest (Fig. 1A), expression of pluripotency and differentiation factors is repressed (Fig. 3A, Fig. 5A).
Stra8 expression in teratoma-susceptible male germ cells influences teratoma susceptibility
We tested whether teratoma-susceptible male germ cells are induced to initiate the meiotic program. In the adult testis, Stra8 is required for the initiation of meiosis (Anderson et al., 2008; van Pelt et al., 1995; Wang and Kim, 1993). Thus, we assayed the expression of Stra8 in E13.5 to E15.5 germ cells (Fig. 2E). At all embryonic time points, Stra8 expression was significantly increased in M19 (high teratoma risk) germ cells compared with FVB (teratoma-resistant) germ cells. By contrast, Stra8 expression was significantly increased in 129 (low teratoma risk) germ cells only at E14.5. As with the other markers of germ cell differentiation, Stra8 expression in 129 male germ cells reduced to levels similar to those in FVB male germ cells at E15.5. Therefore, Stra8 expression at E15.5 increases with teratoma risk.
To test whether premature Stra8 expression during male germ cell development contributes to tumor initiation, we surveyed 129 male mice harboring an engineered deletion of Stra8 (Stra8KO) for teratomas. The Stra8KO allele has been shown previously to be a null mutation that blocks the meiotic commitment of embryonic female and adult male germ cells in homozygotes (Stra8KO/KO) (Fig. 7A) (Anderson et al., 2008; Baltus et al., 2006; Koubova et al., 2006). Heterozygotes (Stra8KO/+) are phenotypically normal. 129-Stra8KO/+ mice were intercrossed to produce wild-type (129), 129-Stra8KO/+ and homozygous (129-Stra8KO/KO) offspring. As expected, surveys of 129 and 129-Stra8KO/+ males revealed a teratoma incidence similar to the expected 129 incidence (not shown) and the two groups were pooled as controls. Importantly, the teratoma incidence of 129-Stra8KO/KO males was decreased by 88% compared with 129 and 129-Stra8KO/+ siblings (Fig. 7C), with one teratoma observed in 90 males examined (Fig. 7B). Therefore, Stra8 expression and possibly the initiation of meiosis influence teratoma susceptibility.
Fig. 7.
Stra8 is a modifier of teratoma susceptibility. (A,B) Photomicrographs of a Hematoxylin and Eosin stained 6-week-old 129-Stra8KO/KO testis lacking meiotic germ cells (A), and 6-week-old 129-Stra8KO/KO testis with a teratoma consisting of neuronal, muscle, secretory epithelial, bone marrow, bone, and cartilage (B). (C) χ2 analysis of the teratoma survey of 129 wild-type and 129-Stra8KO/+ (control) and 129-Stra8KO/KO (experimental) siblings reveals that Stra8 deficiency reduces teratoma incidence by 88%. Scale bars: 100 μm.
Teratoma-susceptible male germ cells initiate meiotic prophase
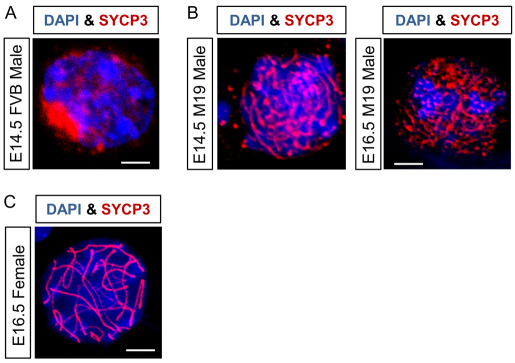
Next, we asked whether the aberrant expression of Stra8 is sufficient to induce male germ entry into meiosis. If so, then the chromosomes of teratoma-susceptible male germ cells should be decorated with the synaptonemal complexes associated with meiotic prophase. At E14.5, synaptonemal complex protein 3 (SYPC3) localized to the nucleoli of teratoma-resistant, FVB and most teratoma-susceptible M19 germ cells (Fig. 8A; supplementary material Fig. S4A). However, in <1% of E14.5 and E16.5 teratoma-susceptible M19 germ cells, SYCP3 associated with chromosomes in a pattern consistent with leptotene to early zygotene stages of meiotic prophase (Fig. 8A,B).
Fig. 8.
Teratoma-susceptible germ cells initiate but do not complete prophase I of meiosis. Chromosome spreads from E14.5 and E16.5 gonads were immunostained for DAPI and SYCP3 and imaged by confocal microscopy. (A) An E14.5 male, FVB germ cell nucleus with pre-lepotene, nucleolar localization of Sycp3. (B) At E14.5 and E16.5, some male M19 germ cell nuclei have a leptotene to early zygotene SYCP3 distribution with no evidence of chromosome pairing or synapsis. (C) An SYCP3-immunostained, E16.5 M19 female germ cell in the pachytene stage of meiotic prophase. Scale bars: 5 μm.
Interestingly, unlike female germ cells, a SYCP3 distribution consistent with chromosome pairing and synapsis during later stages of meiotic prophase (late zygotene and pachytene) was not observed in teratoma-susceptible male germ cells (Fig. 8C). Thus, E16.5 chromosomes were immunostained for synaptonemal complex protein 1 (SYCP1) to test whether teratoma-susceptible male germ cells progress through the late stages of meiotic prophase. Importantly, SYCP1 associated with chromosomes of female germ cells but not teratoma-susceptible M19 male germ cells (supplementary material Fig. S4B). Therefore, teratoma-susceptible male germ cells do not complete meiotic prophase. Whether meiotic male germ cells become apoptotic or revert to mitotic cells and contribute to germ cell tumor initiation remains to be determined.
DISCUSSION
Male germ cell proliferation and retention of pluripotency after the mitotic-meiotic switch at E13.5 has been shown to be associated with increased teratoma susceptibility (Matin et al., 1998; Noguchi and Stevens, 1982; Stevens, 1966; Stevens, 1967b). Our analyses of germ cell development in low and high teratoma risk strains of mice not only agree with these observations, but also identify E15.5 as the specific time point at which germ cell proliferation and pluripotency increase with teratoma risk. As with ES cells, the teratoma forming-capacity of EC cells is dependent upon their initial pluripotency (Gidekel et al., 2003). Because EC cells first appear at E15.5 (Stevens, 1962; Stevens, 1967b), retention of proliferation and pluripotency through this time point is probably necessary to establish a tumor stem cell population.
Our results also demonstrate that teratoma-susceptible germ cells are induced to express genes associated with pre-meiotic embryonic female and adult male germ cells (e.g. Ccnd1, Stra8, Ngn3 and Ret) following the failure to enter mitotic arrest. Importantly, despite the expression of some markers of adult male germ cells, it is unlikely that teratoma-susceptible embryonic male germ cells differentiate into bona fide spermatogonia. Ccnd1 expression initiated at the same developmental time point in pre-meiotic female and teratoma-susceptible germ cells, implying that a signal shared by the female and teratoma-susceptible male gonad induces differentiation. Thus, teratoma-susceptible germ cells appear to adopt some characteristics of pre-meiotic female germ cells. In fact, the rapid commitment of some teratoma-susceptible male germ cells to a meiotic fate is more reminiscent of female germ cell differentiation than the prolonged proliferation/differentiation steps of spermatogenesis. Furthermore, we demonstrated that NANOG and CCND1 are co-expressed in teratoma-susceptible germ cells. Mouse spermatogonia and meiotically differentiating female germ cells do not express detectable levels of NANOG (Yamaguchi et al., 2005). Thus, teratoma-susceptible male germ cells acquire some but not all characteristics of germ cells differentiating towards a meiotic fate.
As observed with proliferation and pluripotency, the expression of germ cell differentiation markers decreases substantially in 129 male germ cells at E15.5 but remains elevated in M19 male germ cells. Given the tenfold increased risk of teratoma formation in M19 mice, expression of germ cell differentiation markers beyond E15.5 might be required for the transformation of germ cells into EC cells. Whether all or a subset of these genes influence teratoma risk remains to be determined. However, our expression analyses and teratoma surveys suggest that at least two differentiation-associated factors, Ccnd1 and Stra8, contribute to tumor initiation.
Ectopic expression of Ccnd1 in teratoma-susceptible male germ cells and EC cells might play a central role in inducing tumor initiation and maintaining pluripotency. D-type cyclins are normally expressed in response to mitogenic signals and activate cyclin-dependent kinases, which phosphorylate and inactivate retinoblastoma (pRB) to induce expression of genes required for G1 to S phase cell cycle transition (Deshpande et al., 2005). As with inactivating mutations of pRB, ectopic expression of cyclin D1 is sufficient to induce tumorigenesis by promoting growth factor independence, quiescent cells to re-enter the cell cycle, and rapid progression through the G1-S cell cycle transition (Deshpande et al., 2005; Musgrove et al., 1994). Importantly, rapid transition from the G1 to S phase might also facilitate the maintenance of pluripotency (Filipczyk et al., 2007; Singh and Dalton, 2009). A short G1 and long S phase promotes the euchromatic state of chromatin in pluripotent cells and inhibits differentiation, which preferentially occurs during G1 in EC cells (Herrera et al., 1996; Jonk et al., 1992; Mummery et al., 1987). Therefore, ectopic Ccnd1 expression might be required for, not only the neoplastic transformation of germ cells, but also the retention of pluripotency by germ cells and EC cells. Previous studies of human EC cells and TGCTs support a role for Ccnd1 in stem cell maintenance and teratoma development. Inhibition of cyclin D1 reduces the proliferative capacity of EC cells, CCND1 expression decreases with germ cell tumor differentiation, and CCND1 expression increases with chemoresistance to cisplatin (Freemantle et al., 2007; Noel et al., 2010). Thus, understanding the contributions of CCND1 to testicular teratoma pathogenesis has both developmental and clinical relevance.
Stra8 is required for the initiation of the meiotic program and its expression is tightly regulated to ensure that entry into meiosis is induced at the appropriate developmental time points (Bowles et al., 2006; Koubova et al., 2006). Thus, the premature induction of Stra8 expression in teratoma-susceptible embryonic male germ cells might be indicative of a breakdown in the regulation of germ cell fate. Furthermore, the reduced teratoma incidence of 129-Stra8KO/KO mice implies that premature initiation of the meiotic program is directly involved in tumor susceptibility. Whether entry into meiosis and reversion to mitosis contributes to teratoma susceptibility, as observed in Drosophila and Caenorhabditis elegans (Biedermann et al., 2009; Parisi et al., 2001; Sugimura and Lilly, 2006), remains to be determined in the mouse model. The molecular function of Stra8 also remains to be resolved, although it appears that it functions as a transcription factor (Tedesco et al., 2009). Thus, it is possible that if Stra8 is expressed at an inappropriate developmental time point and in a cell type that has retained pluripotency, gene expression changes might be induced that do not result in meiotic initiation but instead lead to tumorigenesis.
Our results demonstrate that when the decision to enter mitotic arrest is not executed properly, pluripotency is retained and differentiation-associated genes are induced. These observations suggest that male germ cell commitment to mitotic arrest has two important developmental roles, namely to facilitate the transition of pluripotent PGCs into unipotent germ-line stem cells, and to prevent lineage-restricted germ-line stem cells from receiving signals that induce premature differentiation. Previous studies in ES and germ cells support our conclusion that mitotic arrest has a dual role in regulating male germ cell development. In both germ and ES cells, cell cycle arrest is accompanied by the reprogramming of pluripotency genes (Avilion et al., 2003; Pesce et al., 1998; Singh and Dalton, 2009; Wang and Blelloch, 2009; Yamaguchi et al., 2005). In addition, G0 arrest at E13.5 prevents the induction of the meiotic differentiation program in male germ cells (McLaren and Southee, 1997; Trautmann et al., 2008).
Importantly, our results also suggest that co-expression of pluripotency and differentiation-associated factors through E15.5 can alter germ cell fate from spermatogenic to tumorigenic. However, not all germ cells that express pluripotency and differentiation-associated factors lose germ cell identity and transform into EC cells. Germ cells that have not transformed by E16.5 become quiescent and downregulate pluripotency- and differentiation-associated genes. We propose that these germ cells retain or regain germ-line stem cell identity and develop normally to establish spermatogenesis within teratoma-susceptible testes. Understanding the developmental processes that control these germ cell fate decisions in teratoma-susceptible mice will help guide future studies in humans, and may provide new targets for the diagnosis and treatment of human TGCTs.
Supplementary Material
Acknowledgments
We thank the Case Comprehensive Cancer Center Flow Cytometry Core for assistance with cell sorting. We would also like to acknowledge the use of the Leica SCN400 Slide Scanner and the Leica AOBS confocal in the Genetics Department Imaging Facility at Case Western Reserve University made available through a National Center for Research Resources (NIH-NCRR) Shared Instrument Grants (RR031842 and RR017980).
Footnotes
Funding
This work was supported by National Institutes of Health grants [HD059945 to J.D.H. and CA75056 to J.H.N.]; the Howard Hughes Medical Institute [D.C.P.]; and the Jerome and Florence Brill Fellowship [E.L.A.]. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.076851/-/DC1
References
- Adamah D. J., Gokhale P. J., Eastwood D. J., Rajpert, De-Meyts E., Goepel J., Walsh J. R., Moore H. D., Andrews P. W. (2006). Dysfunction of the mitotic:meiotic switch as a potential cause of neoplastic conversion of primordial germ cells. Int. J. Androl. 29, 219–227 [DOI] [PubMed] [Google Scholar]
- Almstrup K., Hoei-Hansen C. E., Wirkner U., Blake J., Schwager C., Ansorge W., Nielsen J. E., Skakkebaek N. E., Rajpert-de M. E., Leffers H. (2004). Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 64, 4736–4743 [DOI] [PubMed] [Google Scholar]
- Anderson E. L., Baltus A. E., Roepers-Gajadien H. L., Hassold T. J., de Rooij D. G., van Pelt A. M., Page D. C. (2008). Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 105, 14976–14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. D., Nelson V. R., Tesar P. J., Nadeau J. H. (2009). Genetic factors on mouse chromosome 18 affecting susceptibility to testicular germ cell tumors and permissiveness to embryonic stem cell derivation. Cancer Res. 69, 9112–9117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte P. M., van Bragt M. P., de Rooij D. G., van Pelt A. M. (2005). Spermatogonial stem cells: characteristics and experimental possibilities. APMIS 113, 727–742 [DOI] [PubMed] [Google Scholar]
- Avilion A. A., Nicolis S. K., Pevny L. H., Perez L., Vivian N., Lovell-Badge R. (2003). Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltus A. E., Menke D. B., Hu Y. C., Goodheart M. L., Carpenter A. E., de Rooij D. G., Page D. C. (2006). In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat. Genet. 38, 1430–1434 [DOI] [PubMed] [Google Scholar]
- Bartkova J., Rajpert-de M. E., Skakkebaek N. E., Bartek J. (1999). D-type cyclins in adult human testis and testicular cancer: relation to cell type, proliferation, differentiation, and malignancy. J. Pathol. 187, 573–581 [DOI] [PubMed] [Google Scholar]
- Beumer T. L., Roepers-Gajadien H. L., Gademan I. S., Kal H. B., de Rooij D. G. (2000). Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol. Reprod. 63, 1893–1898 [DOI] [PubMed] [Google Scholar]
- Biedermann B., Wright J., Senften M., Kalchhauser I., Sarathy G., Lee M. H., Ciosk R. (2009). Translational repression of cyclin E prevents precocious mitosis and embryonic gene activation during C. elegans meiosis. Dev. Cell 17, 355–364 [DOI] [PubMed] [Google Scholar]
- Bowles J., Knight D., Smith C., Wilhelm D., Richman J., Mamiya S., Yashiro K., Chawengsaksophak K., Wilson M. J., Rossant J., et al. (2006). Retinoid signaling determines germ cell fate in mice. Science 312, 596–600 [DOI] [PubMed] [Google Scholar]
- Chu L. F., Surani M. A., Jaenisch R., Zwaka T. P. (2011). Blimp1 expression predicts embryonic stem cell development in vitro. Curr. Biol. 21, 1759–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. T., Rodriguez R. T., Bodnar M. S., Abeyta M. J., Cedars M. I., Turek P. J., Firpo M. T., Reijo Pera R. A. (2004). Human STELLAR, NANOG, and GDF3 genes are expressed in pluripotent cells and map to chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells 22, 169–179 [DOI] [PubMed] [Google Scholar]
- Cook M. S., Coveney D., Batchvarov I., Nadeau J. H., Capel B. (2009). BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev. Biol. 328, 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. S., Munger S. C., Nadeau J. H., Capel B. (2011). Regulation of male germ cell cycle arrest and differentiation by DND1 is modulated by genetic background. Development 138, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A., Sicinski P., Hinds P. W. (2005). Cyclins and cdks in development and cancer: a perspective. Oncogene 24, 2909–2915 [DOI] [PubMed] [Google Scholar]
- Di Carlo A., De Felici M. (2000). A role for E-cadherin in mouse primordial germ cell development. Dev. Biol. 226, 209–219 [DOI] [PubMed] [Google Scholar]
- Filipczyk A. A., Laslett A. L., Mummery C., Pera M. F. (2007). Differentiation is coupled to changes in the cell cycle regulatory apparatus of human embryonic stem cells. Stem Cell Res. 1, 45–60 [DOI] [PubMed] [Google Scholar]
- Freemantle S. J., Vaseva A. V., Ewings K. E., Bee T., Krizan K. A., Kelley M. R., Hattab E. M., Memoli V. A., Black C. C., Spinella M. J., et al. (2007). Repression of cyclin D1 as a target for germ cell tumors. Int. J. Oncol. 30, 333–340 [PubMed] [Google Scholar]
- Gidekel S., Pizov G., Bergman Y., Pikarsky E. (2003). Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell 4, 361–370 [DOI] [PubMed] [Google Scholar]
- Heaney J. D., Michelson M. V., Youngren K. K., Lam M. Y., Nadeau J. H. (2009). Deletion of eIF2beta suppresses testicular cancer incidence and causes recessive lethality in agouti-yellow mice. Hum. Mol. Genet. 18, 1395–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R. E., Chen F., Weinberg R. A. (1996). Increased histone H1 phosphorylation and relaxed chromatin structure in Rb-deficient fibroblasts. Proc. Natl. Acad. Sci. USA 93, 11510–11515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y. J., Choi H. W., Shin H. S., Cui X. S., Kim N. H., Gerton G. L., Jun J. H. (2005). Optimization of real time RT-PCR methods for the analysis of gene expression in mouse eggs and preimplantation embryos. Mol. Reprod. Dev. 71, 284–289 [DOI] [PubMed] [Google Scholar]
- Jonk L. J., de Jonge M. E., Kruyt F. A., Mummery C. L., van der Saag P. T., Kruijer W. (1992). Aggregation and cell cycle dependent retinoic acid receptor mRNA expression in P19 embryonal carcinoma cells. Mech. Dev. 36, 165–172 [DOI] [PubMed] [Google Scholar]
- Kimura T., Suzuki A., Fujita Y., Yomogida K., Lomeli H., Asada N., Ikeuchi M., Nagy A., Mak T. W., Nakano T. (2003). Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 130, 1691–1700 [DOI] [PubMed] [Google Scholar]
- Koubova J., Menke D. B., Zhou Q., Capel B., Griswold M. D., Page D. C. (2006). Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 103, 2474–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz A. D., Murphy M. W., Kim S., Cook M. S., Capel B., Zhu R., Matin A., Sarver A. L., Parker K. L., Griswold M. D., et al. (2009). The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc. Natl. Acad. Sci. USA 106, 22323–22328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar P. S., Siekhaus D. E., Lehmann R. (2006). In vivo migration: a germ cell perspective. Annu. Rev. Cell Dev. Biol. 22, 237–265 [DOI] [PubMed] [Google Scholar]
- Lam M. Y., Youngren K. K., Nadeau J. H. (2004). Enhancers and suppressors of testicular cancer susceptibility in single- and double-mutant mice. Genetics 166, 925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looijenga L. H., Stoop H., de Leeuw H. P., de Gouveia Brazao C. A., Gillis A. J., van Roozendaal K. E., van Zoelen E. J., Weber R. F., Wolffenbuttel K. P., van Dekken H., et al. (2003). POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 63, 2244–2250 [PubMed] [Google Scholar]
- MacLean G., Li H., Metzger D., Chambon P., Petkovich M. (2007). Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 148, 4560–4567 [DOI] [PubMed] [Google Scholar]
- Massari M. E., Murre C. (2000). Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20, 429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Collin G. B., Varnum D. S., Nadeau J. H. (1998). Testicular teratocarcinogenesis in mice-a review. APMIS 106, 174–182 [DOI] [PubMed] [Google Scholar]
- Matin A., Collin G. B., Asada Y., Varnum D., Nadeau J. H. (1999). Susceptibility to testicular germ-cell tumours in a 129.MOLF-Chr 19 chromosome substitution strain. Nat. Genet. 23, 237–240 [DOI] [PubMed] [Google Scholar]
- McLaren A. (1984). Meiosis and differentiation of mouse germ cells. Symp. Soc. Exp. Biol. 38, 7–23 [PubMed] [Google Scholar]
- McLaren A. (2000). Germ and somatic cell lineages in the developing gonad. Mol. Cell Endocrinol. 163, 3–9 [DOI] [PubMed] [Google Scholar]
- McLaren A., Southee D. (1997). Entry of mouse embryonic germ cells into meiosis. Dev. Biol. 187, 107–113 [DOI] [PubMed] [Google Scholar]
- Menke D. B., Koubova J., Page D. C. (2003). Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev. Biol. 262, 303–312 [DOI] [PubMed] [Google Scholar]
- Molyneaux K. A., Schaible K., Wylie C. (2003). GP130, the shared receptor for the LIF/IL6 cytokine family in the mouse, is not required for early germ cell differentiation, but is required cell-autonomously in oocytes for ovulation. Development 130, 4287–4294 [DOI] [PubMed] [Google Scholar]
- Mummery C. L., van Rooijen M. A., van den Brink S. E., de Laat S. W. (1987). Cell cycle analysis during retinoic acid induced differentiation of a human embryonal carcinoma-derived cell line. Cell Differ. 20, 153–160 [DOI] [PubMed] [Google Scholar]
- Musgrove E. A., Lee C. S., Buckley M. F., Sutherland R. L. (1994). Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc. Natl. Acad. Sci. USA 91, 8022–8026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel E. E., Yeste-Velasco M., Mao X., Perry J., Kudahetti S. C., Li N. F., Sharp S., Chaplin T., Xue L., McIntyre A., et al. (2010). The association of CCND1 overexpression and cisplatin resistance in testicular germ cell tumors and other cancers. Am. J. Pathol. 176, 2607–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Stevens L. C. (1982). Primordial germ cell proliferation in fetal testes in mouse strains with high and low incidences of congenital testicular teratomas. J. Natl. Cancer Inst. 69, 907–913 [PubMed] [Google Scholar]
- Oosterhuis J. W., Looijenga L. H. (2005). Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 5, 210–222 [DOI] [PubMed] [Google Scholar]
- Parisi M. J., Deng W., Wang Z., Lin H. (2001). The arrest gene is required for germline cyst formation during Drosophila oogenesis. Genesis. 29, 196–209 [DOI] [PubMed] [Google Scholar]
- Park S. Y., Jameson J. L. (2005). Minireview: transcriptional regulation of gonadal development and differentiation. Endocrinology 146, 1035–1042 [DOI] [PubMed] [Google Scholar]
- Paull T. T., Rogakou E. P., Yamazaki V., Kirchgessner C. U., Gellert M., Bonner W. M. (2000). A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10, 886–895 [DOI] [PubMed] [Google Scholar]
- Pesce M., Wang X., Wolgemuth D. J., Scholer H. (1998). Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech. Dev. 71, 89–98 [DOI] [PubMed] [Google Scholar]
- Peters H. (1970). Migration of gonocytes into the mammalian gonad and their differentiation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 259, 91–101 [DOI] [PubMed] [Google Scholar]
- Sicinski P., Donaher J. L., Geng Y., Parker S. B., Gardner H., Park M. Y., Robker R. L., Richards J. S., McGinnis L. K., Biggers J. D., et al. (1996). Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384, 470–474 [DOI] [PubMed] [Google Scholar]
- Singh A. M., Dalton S. (2009). The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell 5, 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G. (2001). Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 17, 435–462 [DOI] [PubMed] [Google Scholar]
- Stevens L. (1962). Testicular teratomas in fetal mice. J. Natl. Cancer Inst. 28, 247–267 [PubMed] [Google Scholar]
- Stevens L. (1966). Development of resistance to teratocarcinogenesis by primordial germ cells in mice. J. Natl. Cancer Inst. 37, 859–867 [PubMed] [Google Scholar]
- Stevens L. (1967a). Origin of testicular teratomas from primordial germ cells in mice. J. Natl. Cancer Inst. 38, 549–552 [PubMed] [Google Scholar]
- Stevens L. (1967b). The biology of teratomas. Adv. Morphog. 6, 1–31 [DOI] [PubMed] [Google Scholar]
- Stevens L., Little C. C. (1954). Spontaneous testicular teratomas in an inbred strain of mice. Proc. Natl. Acad. Sci. USA 40, 1080–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L., Hummel K. (1957). A description of spontaneous congenital testicular teratomas in strain 129 mice. J. Natl. Cancer Inst. 18, 719–747 [PubMed] [Google Scholar]
- Sugimura I., Lilly M. A. (2006). Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev. Cell 10, 127–135 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Saga Y. (2008). Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 22, 430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco M., La Sala G., Barbagallo F., De Felici M., Farini D. (2009). STRA8 shuttles between nucleus and cytoplasm and displays transcriptional activity. J. Biol. Chem. 284, 35781–35793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann E., Guerquin M. J., Duquenne C., Lahaye J. B., Habert R., Livera G. (2008). Retinoic acid prevents germ cell mitotic arrest in mouse fetal testes. Cell Cycle 7, 656–664 [DOI] [PubMed] [Google Scholar]
- van Pelt A. M., van Dissel-Emiliani F. M., Gaemers I. C., van der Burg M. J., Tanke H. J., de Rooij D. G. (1995). Characteristics of A spermatogonia and preleptotene spermatocytes in the vitamin A-deficient rat testis. Biol. Reprod. 53, 570–578 [DOI] [PubMed] [Google Scholar]
- Vernet N., Dennefeld C., Rochette-Egly C., Oulad-Abdelghani M., Chambon P., Ghyselinck N. B., Mark M. (2006). Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 147, 96–110 [DOI] [PubMed] [Google Scholar]
- Wang Y., Blelloch R. (2009). Cell cycle regulation by MicroRNAs in embryonic stem cells. Cancer Res. 69, 4093–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Kim K. H. (1993). Vitamin A-deficient testis germ cells are arrested at the end of S phase of the cell cycle: a molecular study of the origin of synchronous spermatogenesis in regenerated seminiferous tubules. Biol. Reprod. 48, 1157–1165 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Kimura H., Tada M., Nakatsuji N., Tada T. (2005). Nanog expression in mouse germ cell development. Gene Expr. Patterns 5, 639–646 [DOI] [PubMed] [Google Scholar]
- Yeom Y. I., Fuhrmann G., Ovitt C. E., Brehm A., Ohbo K., Gross M., Hubner K., Scholer H. R. (1996). Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122, 881–894 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Takakura A., Ohbo K., Abe K., Wakabayashi J., Yamamoto M., Suda T., Nabeshima Y. (2004). Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev. Biol. 269, 447–458 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Sukeno M., Nakagawa T., Ohbo K., Nagamatsu G., Suda T., Nabeshima Y. (2006). The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133, 1495–1505 [DOI] [PubMed] [Google Scholar]
- Yoshimizu T., Sugiyama N., De Felice M., Yeom Y. I., Ohbo K., Masuko K., Obinata M., Abe K., Scholer H. R., Matsui Y. (1999). Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev. Growth Differ. 41, 675–684 [DOI] [PubMed] [Google Scholar]
- Youngren K. K., Coveney D., Peng X., Bhattacharya C., Schmidt L. S., Nickerson M. L., Lamb B. T., Deng J. M., Behringer R. R., Capel B., et al. (2005). The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 435, 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaka T. P., Thomson J. A. (2005). A germ cell origin of embryonic stem cells? Development 132, 227–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.