Abstract
Wnt and Nodal signaling pathways are required for initial patterning of cell fates along anterior-posterior (AP) and dorsal-ventral (DV) axes, respectively, of sea urchin embryos during cleavage and early blastula stages. These mechanisms are connected because expression of nodal depends on early Wnt/β-catenin signaling. Here, we show that an important subsequent function of Wnt signaling is to control the shape of the nodal expression domain and maintain correct specification of different cell types along the axes of the embryo. In the absence of Wnt1, the posterior-ventral region of the embryo is severely altered during early gastrulation. Strikingly, at this time, nodal and its downstream target genes gsc and bra are expressed ectopically, extending posteriorly to the blastopore. They override the initial specification of posterior-ventral ectoderm and endoderm fates, eliminating the ventral contribution to the gut and displacing the ciliary band dorsally towards, and occasionally beyond, the blastopore. Consequently, in Wnt1 morphants, the blastopore is located at the border of the re-specified posterior-ventral oral ectoderm and by larval stages it is in the same plane near the stomodeum on the ventral side. In normal embryos, a Nodal-dependent process downregulates wnt1 expression in dorsal posterior cells during early gastrulation, focusing Wnt1 signaling to the posterior-ventral region where it suppresses nodal expression. These subsequent interactions between Wnt and Nodal signaling are thus mutually antagonistic, each limiting the range of the other’s activity, in order to maintain and stabilize the body plan initially established by those same signaling pathways in the early embryo.
Keywords: Strongylocentrotus purpuratus, Wnt1, Embryonic axes, Nodal, Sea urchin
INTRODUCTION
The three-dimensional coordinate system of early bilaterian embryos depends on perpendicular gradients of Wnt/β-catenin and TGFβ signaling along primary and secondary axes, respectively (Niehrs, 2010; reviewed by Angerer et al., 2011). These functions have been particularly well elucidated in the sea urchin embryo. A series of Wnt signals specifies mesoderm and endoderm at one end of the primary (animal-vegetal) axis (Logan et al., 1999; Sherwood and McClay, 1999; Wikramanayake et al., 1998; Wikramanayake et al., 2004; Croce et al., 2011) and restricts anterior neuroectoderm to the opposite end (Wei et al., 2009; Yaguchi et al., 2008; Yaguchi et al., 2006). In this way, Wnt/β-catenin-dependent patterning of the primary axis is like that along the anterior-posterior (AP) axis of most bilaterians; consequently, here we refer to the primary axis of the sea urchin embryo as the AP axis.
Wnt signaling is also required indirectly for expression of nodal, which is necessary and sufficient for production of BMP2/4 and specification of cell fates along the secondary axis (Duboc et al., 2010; Duboc et al., 2004; Flowers et al., 2004; Lapraz et al., 2009; Yaguchi et al., 2010; Saudemont et al., 2010). The use of TGFβ signals and their antagonists is conserved in other embryos to regulate cell fate specification along their dorsal-ventral (DV) axes (reviewed by De Robertis, 2008); consequently, we refer to the sea urchin secondary axis as the DV axis. An interesting double-negative regulatory device connects patterning along the AP and DV axes of sea urchin embryos. Nodal expression cannot occur until after Wnt//β-catenin activity downregulates FoxQ2, a suppressor of Nodal autoregulation (Yaguchi et al., 2008). This coordinating mechanism ensures that cell division and patterning along the AP axis have progressed sufficiently before DV polarity is imposed. Thus, the basic body plan of the embryo is established by blastula stage through the actions of Wnt/β-catenin and TGFβ, which signal along the AP and DV axes, respectively. These activities result in the specification of many different cell types along both signaling axes prior to gastrulation (reviewed by Angerer and Angerer, 2003; Davidson et al., 1998; Ettensohn and Sweet, 2000).
Nodal signaling is necessary and sufficient to impose DV polarity to cells in all three germ layers of the embryo (Duboc et al., 2010; Duboc et al., 2004) and its influence extends in a broad band imposing DV polarity on tissues that develop from anterior to posterior poles of the embryo (Duboc et al., 2010; Yaguchi et al., 2006). Where it is expressed, Nodal signaling specifies oral ectoderm on the ventral side and is required for the production of BMP2/4, which acts as a relay to specify aboral ectoderm, most of which covers the dorsal side (Duboc et al., 2004; Lapraz et al., 2009). Nodal also is required for production of Nodal and BMP antagonists that are necessary for the formation of a band of neuroectoderm tissue called the ciliary band (CB), consisting of about four rows of very tightly packed, columnar epithelial cells, which forms between the oral and aboral ectoderm territories (Cameron et al., 1993; Duboc et al., 2008; Saudemont et al., 2010; Yaguchi et al., 2010). The CB is a continuous band of cells arranged in a trapezoid that passes through the anterior neuroectoderm above the mouth, extends posteriorly down the left and right sides of the lateral ectoderm toward the blastopore and is connected on the ventral side by the posterior transverse segment.
The initial patterning of cell fates along the AP and DV axes is understood much better than how tissue specifications are subsequently maintained. Nine of the eleven Wnt genes are expressed in gastrula-stage embryos (Croce et al., 2006) and mRNAs for several, including wnt1, wnt5, wnt8 and wnt16 (Wikramanayake et al., 2004; Ferkowicz and Raff, 2001; Wei et al., 2009), have been shown to accumulate in posterior blastomeres during mesenchyme blastula stages. In addition, loss-of-function studies suggest that Wnt1, Wnt6, Wnt7, Wnt8 and Wnt16 all are required for endoderm specification and normal gastrulation (Sethi et al., 2012; Croce et al., 2011; Wikramanayake et al., 2004). During the course of a series of Wnt knockdown experiments, we observed a particularly interesting phenotype in Wnt1 morphants suggesting that Wnt1 could have functions in addition to those in endoderm development. These embryos had severe patterning defects in the posterior-ventral region of the embryo that included a striking ventral-to-dorsal shift in the position of the posterior CB. Here, we report that these defects result from an ectopic posterior shift in the DV patterning system because nodal expression extends posteriorly on the ventral side of the embryo all the way to the blastopore at the time at which gastrulation normally occurs. Thus, a second kind of crucial cross-talk between Wnt and Nodal signaling occurs during gastrulation that is essential to maintain the body plan established earlier by these same signaling systems that initiated patterning along the AP and DV axes.
MATERIALS AND METHODS
Embryo culture
Adult sea urchins (Strongylocentrotus purpuratus) were obtained from Monterey Abalone (Goleta, CA, USA) and Patrick Leahy (Pt. Loma, CA, USA) and maintained in seawater at 10°C. Embryos were cultured in artificial seawater (ASW) at 15°C.
Microinjection of morpholino antisense oligonucleotides (MOs)
Eggs were prepared as described previously (Wei et al., 2009). Approximately 2 pl of solution containing 22.5% glycerol and morpholinos (Gene-Tools, Eugene, OR, USA) were injected. For Wnt1MO1 and Wnt1MO2, the concentrations were 1.0 mM and 0.4 mM, respectively. For NodalMO, the concentration was 0.6 mM (Wei et al., 2009). The morpholino sequences are as follows: Wnt1MO1, ACGCTACAAACCACTCAAGTTTCAT; Wnt1MO2, ATCCTCATCAAAACTAACTCCAAGA; and NodalMO, GATGTCTCAGCTCTCTGAAATGTAG.
Whole-mount in situ hybridization
Embryos were fixed, hybridized and stained as described previously (Minokawa et al., 2004) except that each RNA in situ hybridization (ISH) probe was purified with a Qiagen QiaQuik PCR column after adding EDTA to a concentration of 10 mM. wnt1, endo16, nodal and nk1 probes were labeled with digoxygenin and detected with alkaline phosphatase (AP). The gsc probe was labeled with FITC and detected with Cy3-TSA. Two-color fluorescent in situ hybridization (FISH) was carried out as described previously (Yaguchi et al., 2008). The bra probe was labeled with FITC and detected with Cy3-TSA and the endo16 probe was labeled with digoxygenin and detected with FITC-TSA. The concentration of probes was 0.1 ng/μl.
KikGR fluorescence protein labeling
The labeling of the PVC and posterior half of embryos by photoactivating KikGR (green to red) was carried out as described (Wei et al., 2011). Briefly, KikGR RNA (0.5 μg/μl), synthesized in vitro with the mMessageMachine kit (Ambion), was injected into fertilized eggs. At 24 hours post-fertilization, the red KikGR fluorescence in the posterior half of the embryo was obtained via photoactivation by a 3-second UV light exposure with a Zeiss microscope (Axiovert 200M). After 2 days of further development, embryos were photographed with an ApoTome unit (Zeiss).
Detection of apoptotic cells by caspase 3 immunostaining
Apoptosis was induced in embryos with 1 mM cadmium chloride (Sigma-Aldrich) in sea water to provide a positive control for caspase 3 detection. Wnt1MO-injected and uninjected embryos were stained for caspase 3 at 34, 44 and 72 hours after fertilization using caspase 3 antibody (Invitrogen, 1:200 dilution).
Immunohistochemistry
Embryos were fixed in 2% formaldehyde. Primary antibodies were incubated overnight at 4°C using the following dilutions: Serotonin, 1:1000 (Sigma, St Louis, MO, USA); Endo1, 1:500 (Wessel et al., 1989); Vasa, 1:2000 (Voronina et al., 2008); Gsc, 1:500 (Angerer et al., 2001); 6a9, 1:25 (Ettensohn and McClay, 1988); Spec1, 1:1000 (Wikramanayake et al., 1995); Hnf6, 1:500 (Nakajima et al., 2004); and SoxB1, 1:1000 (Kenny et al., 1999). Bound primary antibodies were detected by incubation with Alexa-coupled secondary antibodies for 1 hour and nuclei were stained with DAPI.
RESULTS
Wnt1 morphants have dramatic posterior-ventral defects
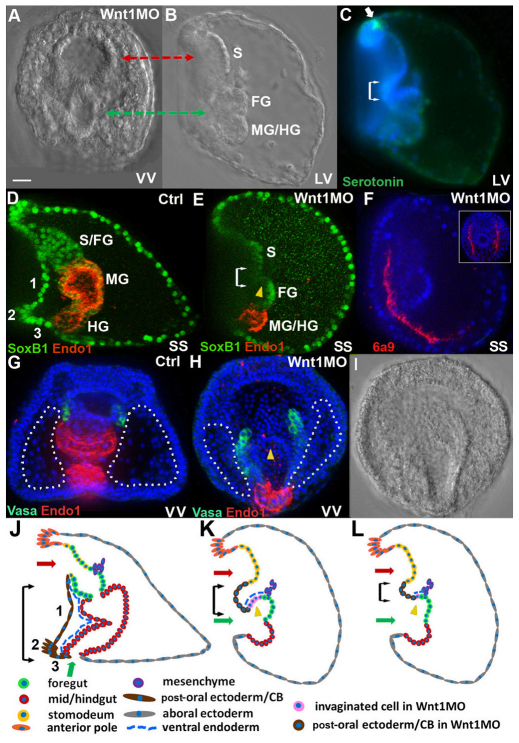
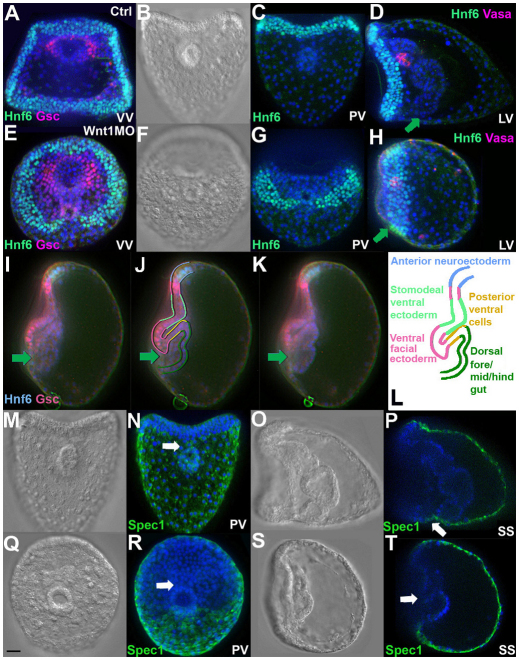
Loss of Wnt1 produces a striking distortion of ventral structures in the pluteus larva. In Wnt1 morphants there are two apparent openings or ‘in-pocketings’ in the same ventral plane (Fig. 1A,B, red and green arrows), separated from each other by only a small number of cells (see also Fig. 1K,L). The anterior opening is actually a large indentation in the stomodeal region located just posterior to the anterior neuroectoderm, which is identified by serotonergic neurons (Fig. 1C, green) (Yaguchi et al., 2000). The posterior opening corresponds to the blastopore, as it is adjacent to cells labeled by the posterior gut marker Endo1 (Fig. 1D,G control and 1E,H morphant, red) (Wessel et al., 1989). However, unlike as in normal embryos, this posterior opening connects to a small pocket of tissue. The gut marker Endo1 was expressed only in cells on the dorsal side of this pocket (the ventral region is indicated by yellow arrowheads in Fig. 1E,H,K,L and by the pink cells in 1K,L). Instead of forming a tube, the endodermal tissue consists of Endo1-positive cells that form a groove. The anterior cells in this groove correspond to foregut (FG) because they express SoxB1 (Fig. 1D,E, green) (Wei et al., 2011). In addition to the reduction of ventral endoderm, the gut tissue is reduced in size because of defects in endoderm development that occur several days earlier during the early mesenchyme blastula stage (Sethi et al., 2012). This partially formed gut does not reach the stomodeum and no mouth forms. By comparison, in normal embryos, the mouth and blastopore (Fig. 1J, red and green arrows, respectively) are separated by post-oral ectoderm (Fig. 1D,J, region 1), the posterior or post-oral transverse CB (Fig. 1D,J, region 2) and the supra-anal ectoderm (Fig. 1D,J, region 3) so that the two openings are oriented at 90 degrees with respect to each other rather than in the same plane as in Wnt1 morphants.
Fig. 1.
The structure of sea urchin larvae lacking Wnt1. The Wnt1 morphant phenotype was produced consistently with either of two morpholinos (see Materials and methods). The dose used for each morpholino was chosen to yield a high rate of embryo survival and a consistent phenotype that was observed in >95% of embryos in at least 20 batches of embryos. (A,B) A Wnt1 morphant at the 4-day larval stage, ventral (A) and lateral (B) views, showing a large indentation in the stomodeal region (red arrow) and the blastoporal opening (green arrow) in the same ventral plane. (C) Lateral view of the embryo in B, stained for serotonin (green, white arrow) and nuclei (DAPI, blue). (D,E) Control and Wnt1 morphant 4-day larvae stained for SoxB1, which is present in nuclei of ectoderm and foregut (green), and Endo1 (red), which marks mid- and hindgut. (F) A 3-day Wnt1 morphant in which the 6a9 monoclonal antibody (red) labels primary mesenchyme cells that secrete the skeletal spicules; DAPI (blue), lateral view. Inset: oral view showing both spicules. (G,H) Control larva (G) and Wnt1 morphant (H), ventral views, stained for Vasa (green), which labels the small micromere descendants that populate the coelomic pouches of larvae, Endo1 (red) and DAPI (blue). Dotted line indicates posterior-ventral ectoderm. (I) DIC image of the larva in H, showing that the blastoporal opening is cup-shaped and joins a trough of endodermal tissue that is continuous with the oral ectoderm and is open to the medium. (J-L) Schematic of tissues in control embryos (J) and Wnt1 morphants (K,L). Double arrows in C,E,J-L indicate the posterior ventral corner (PVC) region. Yellow arrowheads in E,H,K,L mark invaginated tissue from the ventral side of the embryo. Green arrows indicate the blastopore. Red arrows indicate the stomodeal region. CB, ciliary band; FG, foregut; HG, hindgut; LV, lateral view; MG, midgut; S, stomodeum; SS, sagittal section; VV, ventral view; Wnt1MO, Wnt1 morpholino; 1, post-oral ectoderm; 2, post-oral transverse CB; 3, supra-anal ectoderm. Scale bar: 20 μm.
Because the regions altered in Wnt1 morphants include posterior-ventral ectoderm (Fig. 1D,J, regions 1-3) and ventral endoderm (Fig. 1J,K, blue dashed line), which are located in the posterior-ventral corner of the embryo, we will refer to the region collectively as the PVC. In sagittal optical sections of the Wnt1 morphants shown in Fig. 1C,E, the PVC (double arrows) appears to be reduced to varying degrees compared with normal embryos (Fig. 1K,L). However, in ventral views of Wnt1 morphants, the density of cells in the posterior half of the oral ectoderm is greater (Fig. 1G,H, compare the density of DAPI-stained nuclei within the regions outlined by dotted lines) and extends more posteriorly on left and right sides of the blastopore. These observations raise the possibility that cells normally contributing to the PVC region are instead part of the oral epithelium.
Despite these striking ventral abnormalities, the positions of different cell types along the AP axis appear to be relatively normal. There are correctly positioned serotonergic neurons at the anterior end (Fig. 1C, green) and the posterior-most small micromeres, marked by Vasa staining, develop normally and, in three-day larvae, are located appropriately next to the anterior endoderm (Voronina et al., 2008) (Fig. 1G versus 1H, green). In addition, differentiating skeletogenic mesenchyme cells, stained with the monoclonal antibody 6a9 (Ettensohn and McClay, 1988), are able to assemble within the blastocoel as in normal embryos (Fig. 1F, lateral view, inset shows ventral view; red).
In summary, there are drastic alterations in the development of many cell types in the PVC of sea urchin larvae lacking Wnt1, as shown in Fig. 1K,L. These changes raise the possibility that a defect in a major patterning system occurs in embryos lacking Wnt1.
Early axial polarities are normal in Wnt1 morphants
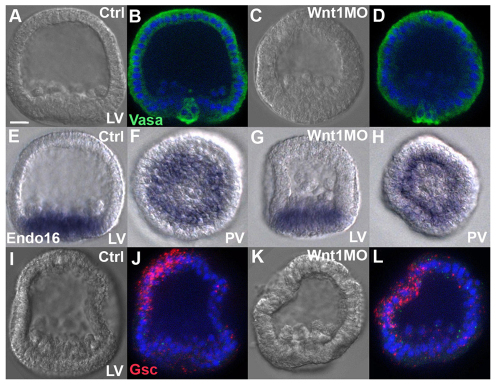
To examine early patterning along the AP and DV axes in Wnt1 morphants, we compared the expression of three markers in normal embryos and Wnt1 morphants at mesenchyme blastula stages: (1) Vasa protein (Fig. 2A-D), which marks the posterior-most cells; (2) endo16 mRNA (Fig. 2E-H), which is present throughout posterior endomesodermal cells (Nocente-McGrath et al., 1989; Ransick et al., 1993); and (3) gsc mRNA (Fig. 2I-L), which accumulates early in the oral ectoderm in response to Nodal signaling (Duboc et al., 2004), and is necessary and sufficient to activate expression of oral ectoderm-specific genes (Angerer et al., 2001; Saudemont et al., 2010). At the mesenchyme blastula stage, all three markers are expressed normally in Wnt1 morphants. Vasa expression is restricted to cells at the posterior end of the primary axis (Fig. 2B,D), and endo16 expression is posterior and radially symmetric around the AP axis as in the normal embryo (Fig. 2F,H), although the number of endo16-positive cells is reduced, as expected (Sethi et al., 2012). Furthermore, although the defects seen in Wnt1 morphants appear to be primarily on the ventral side of larvae, gsc expression is correctly localized in this region at blastula stages (Fig. 2J,L). As shown above (Fig. 1F), skeletogenic mesenchyme cells are correctly distributed with respect to the AP and DV axes in Wnt1 morphants, in response to ectoderm cues established before gastrulation that direct them to the posterior-ventral region where skeletogenesis is initiated (Ettensohn, 1990). Thus, these assays suggest that, although endoderm specification during blastula stages is altered in Wnt1 morphants, most of the initial patterning events along the AP and DV axes are normal at this stage in embryos lacking Wnt1.
Fig. 2.
AP and DV axial patterning are normal at mesenchyme blastula stage in Wnt1 morphants. (A-D) Control (A,B) and Wnt1 morphant (C,D) 24-hour sea urchin embryos stained for Vasa (green) and DNA (DAPI, blue). (E-H) Chromogenic ISH detecting endo16 mRNA in control embryos (E,F) and Wnt1 morphants (G,H). (I-L) FISH detecting gsc mRNA (red) in control embryos (I,J) and Wnt1 morphants (K,L). Blue, DAPI. LV, lateral view; PV, posterior view. Scale bar: 20 μm.
Wnt1 is required for correct DV patterning at the gastrula stage
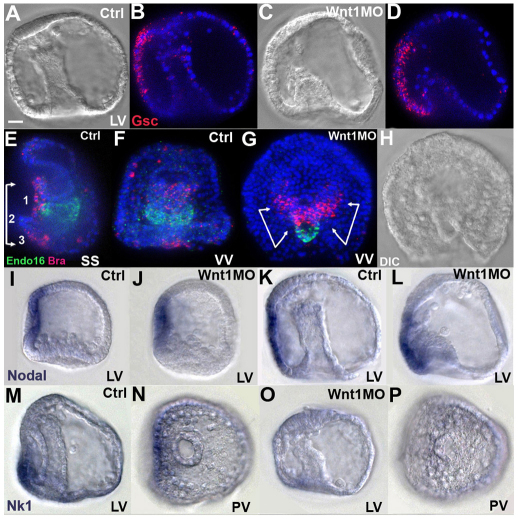
Although gsc expression is normal in mesenchyme blastulae of Wnt1 morphants (Fig. 2), a striking change occurs during gastrulation, when it extends posteriorly through the PVC region all the way to the blastopore (Fig. 3A,B control versus 3C,D morphant). In these gastrulating embryos, the ventral side of the gut already is detectably reduced relative to the dorsal side (Fig. 3C,D). In addition, in Wnt1 morphant three-day larvae, bra is expressed ectopically in a single, uninterrupted patch that extends all the way to the blastopore (Fig. 3G, magenta) through the region that normally gives rise to the posterior transverse CB and supra-anal ectoderm (Fig. 1, PVC regions 2 and 3). By contrast, in normal embryos, the patches of bra-expressing cells in the oral ectoderm and blastoporal regions are separated by this CB segment, as previously described (Gross and McClay, 2001) (Fig. 3E sagittal view, 3F oral view). As illustrated in Fig. 1L and indicated by the double-arrow brackets in Fig. 3G, in morphants, the group of cells expressing bra protrudes outwards from the oral epithelium. Because gsc and bra function early in the Nodal-dependent oral ectoderm gene regulatory network, these observations suggest that nodal itself might also be expressed ectopically in the PVC region of Wnt1 morphants, but only after blastula stages. This is exactly the case: at the mesenchyme blastula stage, nodal is expressed normally in Wnt1 morphants (Fig. 3I control versus 3J morphant), but at the gastrula stage, it extends posteriorly to the blastopore (Fig. 3K control versus 3L morphant). Thus, Wnt1 is required to prevent a significant posterior shift in the domain of Nodal expression and, consequently, in the position of the DV axis patterning system. As a result, the specification of cells exposed to ectopic Nodal signaling changes. In addition to the gain of gsc and bra expression, expression of the PVC marker nk1 (Minokawa et al., 2004) is lost [Fig. 3, control (Fig. 3M lateral, 3N posterior) versus Wnt1 morphants (Fig. 3O lateral, 3P posterior)].
Fig. 3.
Posterior-ventral cell fate changes in Wnt1 morphants during gastrulation. (A-D) FISH detecting gsc mRNA (B,D; red) in a 36-hour control sea urchin embryo (A,B) and a Wnt1 morphant (C,D). Blue, DAPI. (E-H) Two-color FISH detecting bra (red) and endo16 (green) expression in a control 3-day larva (E,F) and Wnt1 morphant (G,H). Blue, DAPI. (I-L) Chromogenic ISH detecting nodal mRNA in controls (I,K) and Wnt1 morphants (J,L) at mesenchyme blastula (I,J, 28-hour) and gastrula (K,L, 36-hour) stages. (M-P) Chromogenic ISH detecting nk1 mRNA in controls (M,N) and Wnt1 morphants (O,P). LV, lateral view; PV, posterior view; SS, sagittal section; VV, ventral view. Scale bar: 20 μm.
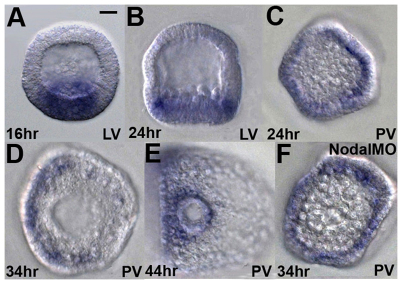
These observations indicate that Wnt1 activity is required to prevent ectopic operation of the Nodal-dependent gene regulatory network in the PVC during gastrulation, raising the question of where Wnt1 is expressed at that time. wnt1 transcripts accumulate in posterior cells at blastula stages (Fig. 4A,B, lateral views) in a radially symmetric torus (Fig. 4C, posterior view). However, between late mesenchyme blastula and early gastrula stages, they fade from the dorsal side (Fig. 4D, 34 hour; 4E, 44 hour; posterior views) in a process that is dependent on Nodal function, because dorsal clearance fails to occur in Nodal morphants (Fig. 4F, posterior view). The lack of wnt1 expression polarity when Nodal is knocked down is expected, as no DV patterning occurs in the absence of this signal (Duboc et al., 2004; Flowers et al., 2004). The Nodal-dependent downregulation of wnt1 expression in the dorsal ectoderm is likely to involve activity of the Nodal-dependent relay signal, BMP2/4, which is active in this region of the embryo (Lapraz et al., 2009; Duboc et al., 2010). Furthermore, Nodal is also unlikely to suppress directly wnt1 expression on the ventral side, because there is no ectopic wnt1 expression there in Nodal morphants (data not shown). The fact that Wnt1 is concentrated on the ventral side (Fig. 4D,E) is consistent with its role in suppressing nodal expression in the PVC.
Fig. 4.
Restriction of Wnt1 expression to the ventral side during gastrulation requires Nodal. (A-C) At blastula stages (16 hour and 24 hour), wnt1 mRNA is detected in a ring near the posterior end of the embryo. (D,E) At gastrula stages (34 hour and 44 hour), wnt1 mRNA expression is strongly enriched in ventral cells near the posterior end. (F) In a Nodal morphant, wnt1 mRNA accumulation is radialized at the posterior end at gastrula stage (compare D and F). LV, lateral view; PV, posterior view. Scale bar: 20 μm.
Wnt1 signaling maintains the position of the CB along the DV axis
In Wnt1 morphants, nodal expression expands into the PVC where the posterior transverse CB normally differentiates after being specified during the mesenchyme blastula stage (Poustka et al., 2007; Saudemont et al., 2010). To determine whether ectopic Nodal signaling overrides this specification, we monitored the expression of Hnf6, a transcription factor expressed specifically in the CB (Otim et al., 2004; Poustka et al., 2007; Yaguchi et al., 2010). In control three-day larvae (Fig. 5A-D), the posterior transverse CB is located on the ventral side of the blastopore. By contrast, in Wnt1 morphants (Fig. 5E-H), there is a striking dorsal shift in its position. In addition, there is often a discontinuity where the blastopore forms (Fig. 5E,G). In lateral views (Fig. 5D,H), the shift of the CB relative to the AP axis is easily visualized by comparing it with the positions of anterior cells expressing Vasa and the blastopore at the posterior pole (green arrows). As a consequence of this shift, the blastopore is next to the oral facial epithelium and much closer to the stomodeal ectoderm, as shown in Fig. 5E.
Fig. 5.
Ectoderm marker expression reveals shifts in the positions of oral, aboral and ciliary band territories. (A-K) Nuclei in CB cells are labeled with anti-Hnf6 (green) and in A,E,I-K those in the oral ectoderm are immunostained for Gsc (magenta); all nuclei are labeled with DAPI (blue); D,H are labeled with Vasa antibody (magenta). (A-D) Control embryos; (E-K) Wnt1 morphants. DIC (B,F) and immunostained (C,G) images show that the posterior transverse CB segment is ventral to the blastopore in control embryos and dorsal in morphants. In B,C,F,G, ventral is at the top. In D,H-K, ventral is to the left. Green arrows in D,H-K mark the position of the blastopore. (I-K) Sagittal focal planes reveal that the folded oral epithelium is connected to invaginated tissue in a continuous sheet, as illustrated in L. (M-T) Control embryos (M-P) and Wnt1 morphants (Q-T) immunostained for Spec1 (green). White arrows in N and P point to Spec1 staining in the supra-anal ectoderm on the ventral side of the blastopore in controls; white arrows in R and T mark the ventral side of the blastopore in morphants, showing that the boundary of Spec1 expression shifts to the dorsal side of the blastopore. VV, ventral view; PV, posterior view; LV, lateral view; SS, sagittal section. Scale bar: 20 μm.
The position of the CB in the normal embryo is completely dependent on Nodal signaling (Bradham et al., 2009; Yaguchi et al., 2010; Saudemont et al., 2010), which, along with BMP2/4, specifies the fates of oral and aboral ectoderm, respectively, and, consequently, the position of the CB at the border between these territories. The change in the CB position in Wnt1 morphants could result from changes in PVC cell fates to oral ectoderm through ectopic Nodal signaling. This appears to be the case, because expression of the Nodal-dependent transcription factor Gsc extends throughout the PVC region, all the way to the rim of the blastopore (Fig. 5E), and even occurs in several invaginated cells on the ventral side of the gut (Fig. 5I-L, different focal planes). This ectopic Gsc expression includes cells in the supra-anal ectoderm, a small patch of aboral ectoderm on the ventral side of the gut in normal embryos, i.e. between the posterior transverse CB and the blastopore (PVC region 3, Fig. 1). These cells normally express Spec1 (Lynn et al., 1983; Wikramanayake et al., 1995) (Fig. 5N,P, white arrows). However, in Wnt1 morphants, there are no Spec1-expressing cells on the ventral side of the blastopore (Fig. 5R,T, white arrows). This, along with the loss of nk1 expression from the PVC, indicates that the fates of cells of the prospective supra-anal ectoderm have been altered.
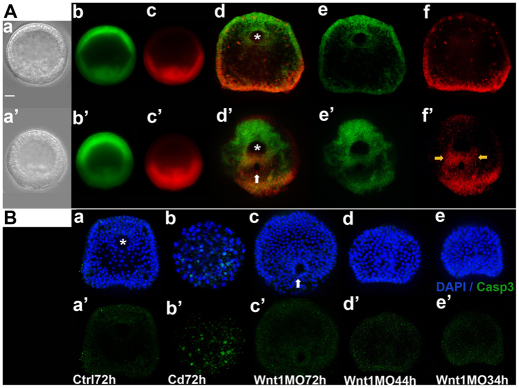
Although all of these observations strongly suggest that loss of Wnt1 causes cells in the PVC to change fates, an alternative interpretation for the loss of PVC markers is that cells in this region are lost in Wnt1 morphants. To examine this possibility, we introduced KikGR, the photoactivatable dye we used previously (Wei et al., 2011; Tsutsui et al., 2005), to label the cells in the posterior half of early mesenchyme blastulae red (Fig. 6Aa-c control and 6Aa′-c′ Wnt1 morphant). Ventral views of larvae show that the stomodeal ectoderm and anterior ectoderm are green (Fig. 6Ad,e) whereas the posterior transverse CB and other posterior-ventral tissues, including the blastoporal endoderm, are red (Fig. 6Ad,f). In Wnt1 morphants (Fig. 6Ad′-f′), ectoderm is also green and red cells are detected adjacent to the blastopore (Fig. 6Ad′, white arrow), in posterior dorsal cells (Fig. 6Ad′,f′, bottom) and in a ventral patch between the stomodeum (green) and the blastopore (red) (Fig. 6Af′, yellow arrows). These are the cells in which Gsc and bra are ectopically expressed and where the CB would form in normal embryos. These observations indicate that many progeny of red PVC cells in Wnt1 morphants were included in the oral ectoderm. We checked further for loss of PVC cells by apoptosis in Wnt1 morphants, by looking for caspase 3 expression at blastula, gastrula and larval stages (Fig. 6B). Because apoptosis is very rare in normal sea urchin embryos (Vega Thurber and Epel, 2007) (Fig. 6Ba,a′), we first verified that the caspase 3 antibody recognizes sea urchin caspase 3 by inducing apoptosis in 3-day embryos with cadmium treatment (Agnello et al., 2007) (Fig. 6Bb,b′). We detected no apoptotic cells in Wnt1 morphants either at early (34 hour; Fig. 6Be,e′) or late (44 hour; Fig. 6Bd,d′) gastrula stages or in 3-day-old larvae (Fig. 6Bc,c′). Together, these observations strongly support the interpretation that PVC cells are not lost in Wnt1 morphants and that the phenotype results from changes in fate specification of most, if not all, of the cells in the PVC by ectopic Nodal signaling.
Fig. 6.
The altered PVC does not result from extensive cell loss in Wnt1 morphants. (A) PVC progeny are present in the ectoderm between the blastoporal and stomodeal regions in 3-day old Wnt1 morphants. The posterior half of the embryo was labeled red by photoactivating KikGR at early mesenchyme blastula stage (control embryo, Aa-c; Wnt1 morphant, Aa′-c′) and photographed at 3 days post-fertilization to monitor the positions of red cells in the PVC (same control embryo, Ad-f; same Wnt1 morphant, Ad′-f′). The yellow arrows in Af′ indicate the PVC progeny between the blastopore and stomodeal regions. (B) No caspase 3 (green) is detected in Wnt1 morphants. (Ba,a′) Control 3-day embryo; (Bb,b′) cadmium chloride-treated 3-day embryo; (Bc-e,c′-e′) Wnt1 morphants at the indicated times. Asterisks mark mouth in (Ad,Ba) and stomodeal ectoderm in (Ad′). White arrows indicate blastopore in (Ad′,Bc). Scale bar: 20 μm.
DISCUSSION
Here, we report an unexpected, but essential interplay between the two major signaling pathways that pattern sea urchin embryos along their primary (AP) and secondary (DV) axes. Many hours after the perpendicular signaling systems, Wnt/β-catenin and Nodal/BMP, have initially specified the major tissue territories along these axes, signaling via Wnt1 continues to play a crucial role in maintaining these axial specifications. This is because it is necessary to prevent nodal expression in posterior ventral cells of the embryo (Fig. 7A,B). This is crucial because Nodal is a powerful signal that not only drives the specification of the oral facial epithelium where it is expressed by activating expression of genes in the oral ectoderm gene regulatory network, such as gsc and bra, but also is required non-cell-autonomously, to impose DV polarity on all three germ layers (Duboc et al., 2010). Its expansion posteriorly in Wnt1 morphants leads to a significant re-alignment of oral and aboral ectoderm tissues that differentiate along the DV axis (Fig. 7B). Thus, in order to maintain the initial axial patterning of the embryo, this study shows that there is an important continuing requirement to restrict Nodal’s remarkable power to change cell fates many hours after their specification.
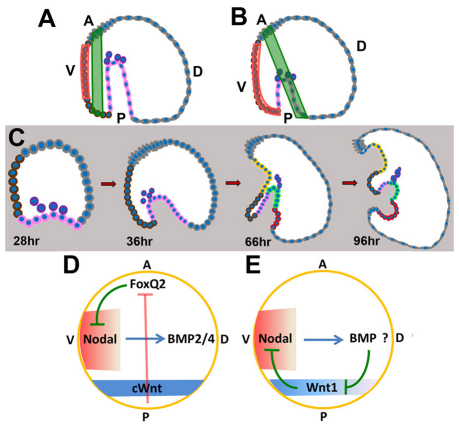
Fig. 7.
Model: Wnt1-mediated suppression of nodal expression maintains patterning orientation along the DV axis. (A,B) Diagrams of the expansion of oral ectoderm (orange) and the shift of the CB (green) with respect to the AP axis in control (A) and Wnt1 morphant (B) embryos. In B, Nodal expression (orange) extends ectopically toward the blastopore. (C) Time course of development from mesenchyme blastula (28-hour), gastrula (36-hour), early pluteus larva (66-hour) and late pluteus larva (96-hour) stages in Wnt1 morphants, illustrating positions of different cell types in a mid-sagittal section. Color codes are given in Fig. 1. (D) Initiation of AP and DV axial patterning by Wnt/β-catenin and Nodal signals and interactions between these signals at early stages. Wnt/β-catenin eliminates FoxQ2 from all but the anterior-most ectoderm. FoxQ2 suppresses expression of nodal, which, along with its target gene, bmp2/4, is necessary and sufficient for DV axial patterning. This double-negative regulatory device coordinates early AP and DV axis patterning. (E) Interactions between Wnt1 and Nodal signaling during gastrulation. Nodal-dependent processes, possibly working through BMP2/4, eliminate Wnt1 expression from dorsal posterior cells but not from those on the ventral side. Conversely, ventral Wnt1-dependent processes suppress nodal expression in posterior ventral cells, which is required to maintain the correct orientation of patterning along the DV axis. A, anterior; D, dorsal; P, posterior; V, ventral.
There are multiple cell fate changes in embryos lacking Wnt1. Expression of nk1 in the PVC and spec1 in supra-anal ectoderm is lost, as is presumptive ventral endoderm, as no cells on the ventral side of the small, partial gut express endoderm markers. Some of the few ventral cells that do invaginate express Gsc and might have oral ectoderm characteristics, whereas the fate of others is unknown (Fig. 7B,C, 66-hour and 96-hour, pink cells). The effect of ectopic nodal expression is revealed most strikingly by the significant shift of the CB, which always forms adjacent to the nodal expression domain (Fig. 7B). During this re-positioning of the CB, in most of the embryos, the CB is interrupted where the blastopore forms. Because the Nodal-dependent oral ectoderm gene regulatory network is activated throughout the region that normally would give rise to posterior CB, supra-anal ectoderm and some ventral-side gut, the site of invagination is now next to the posterior edge of the re-specified posterior-ventral oral epithelium (Fig. 7C, 96-hour; see also Fig. 5I-L). This leads to a pronounced curvature of the AP morphological axis, which extends from the anterior neuroectoderm to the blastopore. This bending might be facilitated by the lack of a continuous band of rigid, columnar ectoderm between the blastopore and the post-oral ectoderm as well as the replacement of the PVC by a flexible epithelial sheet. In any case, the failure to confine nodal expression to oral ectoderm in Wnt1 morphants clearly has catastrophic effects on patterning along the axes of the embryo.
The Wnt1-dependent mechanism that suppresses nodal expression is not yet identified at the molecular level. Although it is possible that Wnt/β-catenin signaling directly affects regulators of nodal transcription, it is also possible that it could work indirectly through other factors known to restrict Nodal expression to the oral ectoderm. These currently include Lefty, which is a direct antagonist of Nodal signaling (Duboc et al., 2008), BMP2/4 (Saudemont et al., 2010; Yaguchi et al., 2010), endocytic activity (Ertl et al., 2011), and the presence of sulfated proteoglygans in the extracellular environment that are thought to control Nodal diffusion (Bergeron et al., 2010). There also could be several intermediate steps, because there is an interval of ∼5 hours between mesenchyme blastula and gastrula stages when the Wnt1 effect on nodal expression occurs.
The regions in which wnt1 and nodal are expressed are not immediately adjacent but instead are separated from each other by a few rows of cells at mesenchyme blastula and gastrula stages (supplementary material Fig. S1, double arrow). If these cells correspond to the future CB, which is likely, then the edges of the nodal and wnt1 expression domains would border the anterior and posterior sides of the posterior transverse CB, respectively. Wnt1-dependent signaling emanating from this point might prevent the spread of nodal expression by interfering in some way with its autoregulatory machinery (Bolouri and Davidson, 2009; Duboc et al., 2004). This could explain, at least on the ventral side of the embryo, why the posterior ectoderm is not converted to oral ectoderm when Nodal is misexpressed (Duboc et al., 2004; Lapraz et al., 2009; Saudemont et al., 2010; Yaguchi et al., 2010). However, under these conditions, Nodal can affect the identity of posterior ectoderm because the PVC marker nk1 is also expressed ectopically in the dorsal posterior region of the embryo (Saudemont et al., 2010; Yaguchi et al., 2010). The work presented here shows that in the normal embryo Wnt1 signaling is an important component of the mechanisms that protect PVC fates.
This is the first demonstration that Wnt signaling affects the borders of the nodal expression domain. It is possible that Wnt signaling through ligands other than Wnt1 might also suppress nodal expression in posterior cells at earlier stages because, in blastulae, nodal expression does not expand in Wnt1 morphants. However, during gastrulation, it is clear that Wnt1 is the only Wnt required to prevent the later posterior ventral expression of nodal.
At early stages, Wnt/β-catenin signaling is required not only to set up the ectoderm, endoderm and mesoderm germ layers along the AP axis (Logan et al., 1999; Sherwood and McClay, 1999; Peter and Davidson, 2010; Peter and Davidson, 2011; and references cited therein), but also to allow operation of the Nodal signaling pathway (Yaguchi et al., 2008) to pattern these territories along the DV axis (reviewed by Angerer et al., 2011). Wnt/β-catenin signaling eliminates expression of genes required for anterior neuroectoderm development including one, foxQ2, that suppresses Nodal autoregulation (Yaguchi et al., 2008) (Fig. 7D). Thus, initiating patterning along both AP and DV axes depends on outputs from both of these pathways and it is coordinated by a net positive interaction between them. By contrast, at later stages, the interactions between these axial patterning systems are mutually antagonistic (Fig. 7E). When gastrulation begins, wnt1 is downregulated on the dorsal side in a process that indirectly depends on Nodal signaling as it does not occur in Nodal morphants. Conversely, nodal expression is suppressed on the ventral side by a Wnt1-dependent mechanism (Fig. 7E). These continued interactions between Wnt and Nodal signaling thereby maintain the initial axial patterning that established the body plan of the embryo.
Supplementary Material
Acknowledgments
We thank Drs Robert Burke, Garry Wessel and David McClay for antibodies; Dr Sarah Knox for anti-caspase 3; and other members of our lab group, Adi Sethi and Diane Adams, for helpful discussions.
Footnotes
Funding
This work was supported by the Division of Intramural Research in the National Institute for Dental and Craniofacial Research of the National Institutes of Health [ZO1 DE000712]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.075051/-/DC1
References
- Agnello M., Filosto S., Scudiero R., Rinaldi A. M., Roccheri M. C. (2007). Cadmium induces an apoptotic response in sea urchin embryos. Cell Stress Chaperones 12, 44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer L., Yaguchi S., Angerer R., Burke R. (2011). The evolution of nervous system patterning: insights from sea urchin development. Development 138, 3613–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer L. M., Angerer R. C. (2003). Patterning the sea urchin embryo: gene regulatory networks, signaling pathways, and cellular interactions. Curr. Top. Dev. Biol. 53, 159–198 [DOI] [PubMed] [Google Scholar]
- Angerer L. M., Oleksyn D. W., Levine A. M., Li X., Klein W. H., Angerer R. C. (2001). Sea urchin goosecoid function links fate specification along the animal-vegetal and oral-aboral embryonic axes. Development 128, 4393–4404 [DOI] [PubMed] [Google Scholar]
- Bergeron K. F., Xu X., Brandhorst B. P. (2010). Oral-aboral patterning and gastrulation of sea urchin embryos depend on sulfated glycosaminoglycans. Mech. Dev. 128, 71–89 [DOI] [PubMed] [Google Scholar]
- Bolouri H., Davidson E. H. (2009). The gene regulatory network basis of the “community effect”, and analysis of a sea urchin embryo example. Dev. Biol. 340, 170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradham C. A., Oikonomou C., Kuhn A., Core A. B., Modell J. W., McClay D. R., Poustka A. J. (2009). Chordin is required for neural but not axial development in sea urchin embryos. Dev. Biol. 328, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. A., Britten R. J., Davidson E. H. (1993). The embryonic ciliated band of the sea urchin, Strongylocentrotus purpuratus derives from both oral and aboral ectoderm. Dev. Biol. 160, 369–376 [DOI] [PubMed] [Google Scholar]
- Croce J., Duloquin L., Lhomond G., McClay D. R., Gache C. (2006). Frizzled5/8 is required in secondary mesenchyme cells to initiate archenteron invagination during sea urchin development. Development 133, 547–557 [DOI] [PubMed] [Google Scholar]
- Croce J., Range R., Wu S.-Y., Miranda E., Lhomond G., Peng J. C., Lepage T., McClay D. R. (2011). Wnt6 activates endoderm in the sea urchin gene regulatory network. Development 138, 3297–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Cameron R. A., Ransick A. (1998). Specification of cell fate in the sea urchin embryo: summary and some proposed mechanisms. Development 125, 3269–3290 [DOI] [PubMed] [Google Scholar]
- De Robertis E. M. (2008). Evo-devo: variations on ancestral themes. Cell 132, 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboc V., Rottinger E., Besnardeau L., Lepage T. (2004). Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev. Cell 6, 397–410 [DOI] [PubMed] [Google Scholar]
- Duboc V., Lapraz F., Besnardeau L., Lepage T. (2008). Lefty acts as an essential modulator of Nodal activity during sea urchin oral-aboral axis formation. Dev. Biol. 320, 49–59 [DOI] [PubMed] [Google Scholar]
- Duboc V., Lapraz F., Saudemont A., Bessodes N., Mekpoh F., Haillot E., Quirin M., Lepage T. (2010). Nodal and BMP2/4 pattern the mesoderm and endoderm during development of the sea urchin embryo. Development 137, 223–235 [DOI] [PubMed] [Google Scholar]
- Ertl R. P., Robertson A. J., Saunders D., Coffman J. A. (2011). Nodal-mediated epigenesis requires dynamin-mediated endocytosis. Dev. Dyn. 240, 704–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettensohn C. A. (1990). The regulation of primary mesenchyme cell patterning. Dev. Biol. 140, 261–271 [DOI] [PubMed] [Google Scholar]
- Ettensohn C. A., McClay D. R. (1988). Cell lineage conversion in the sea urchin embryo. Dev. Biol. 125, 396–409 [DOI] [PubMed] [Google Scholar]
- Ettensohn C. A., Sweet H. C. (2000). Patterning the early sea urchin embryo. Curr. Top. Dev. Biol. 50, 1–44 [DOI] [PubMed] [Google Scholar]
- Ferkowicz M. J., Raff R. A. (2001). Wnt gene expression in sea urchin development: heterochronies associated with the evolution of developmental mode. Evol. Dev. 3, 24–33 [DOI] [PubMed] [Google Scholar]
- Flowers V. L., Courteau G. R., Poustka A. J., Weng W., Venuti J. M. (2004). Nodal/activin signaling establishes oral-aboral polarity in the early sea urchin embryo. Dev. Dyn. 231, 727–740 [DOI] [PubMed] [Google Scholar]
- Gross J. M., McClay D. R. (2001). The role of Brachyury (T) during gastrulation movements in the sea urchin Lytechinus variegatus. Dev. Biol. 239, 132–147 [DOI] [PubMed] [Google Scholar]
- Kenny A. P., Kozlowski D., Oleksyn D. W., Angerer L. M., Angerer R. C. (1999). SpSoxB1, a maternally encoded transcription factor asymmetrically distributed among early sea urchin blastomeres. Development 126, 5473–5483 [DOI] [PubMed] [Google Scholar]
- Lapraz F., Besnardeau L., Lepage T. (2009). Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-chordin signaling network. PLoS Biol. 7, e1000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. Y., Miller J. R., Ferkowicz M. J., McClay D. R. (1999). Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 126, 345–357 [DOI] [PubMed] [Google Scholar]
- Lynn D. A., Angerer L. M., Bruskin A. M., Klein W. H., Angerer R. C. (1983). Localization of a family of mRNAS in a single cell type and its precursors in sea urchin embryos. Proc. Natl. Acad. Sci. USA 80, 2656–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokawa T., Rast J. P., Arenas-Mena C., Franco C. B., Davidson E. H. (2004). Expression patterns of four different regulatory genes that function during sea urchin development. Gene Expr. Patterns 4, 449–456 [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Kaneko H., Murray G., Burke R. D. (2004). Divergent patterns of neural development in larval echinoids and asteroids. Evol. Dev. 6, 95–104 [DOI] [PubMed] [Google Scholar]
- Niehrs C. (2010). On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development 137, 845–857 [DOI] [PubMed] [Google Scholar]
- Nocente-McGrath C., Brenner C. A., Ernst S. G. (1989). Endo16, a lineage-specific protein of the sea urchin embryo, is first expressed just prior to gastrulation. Dev. Biol. 136, 264–272 [DOI] [PubMed] [Google Scholar]
- Otim O., Amore G., Minokawa T., McClay D. R., Davidson E. H. (2004). SpHnf6, a transcription factor that executes multiple functions in sea urchin embryogenesis. Dev. Biol. 273, 226–243 [DOI] [PubMed] [Google Scholar]
- Peter I. S., Davidson E. H. (2010). The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev. Biol. 340, 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter I. S., Davidson E. H. (2011). A gene regulatory network controlling the embryonic specification of endoderm. Nature 474, 635–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustka A. J., Kuhn A., Groth D., Weise V., Yaguchi S., Burke R. D., Herwig R., Lehrach H., Panopoulou G. (2007). A global view of gene expression in lithium and zinc treated sea urchin embryos: new components of gene regulatory networks. Genome Biol. 8, R85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransick A., Ernst S., Britten R. J., Davidson E. H. (1993). Whole mount in situ hybridization shows Endo 16 to be a marker for the vegetal plate territory in sea urchin embryos. Mech. Dev. 42, 117–124 [DOI] [PubMed] [Google Scholar]
- Saudemont A., Haillot E., Mekpoh F., Bessodes N., Quirin M., Lapraz F., Duboc V., Rottinger E., Range R., Oisel A., et al. (2010). Ancestral regulatory circuits governing ectoderm patterning downstream of Nodal and BMP2/4 revealed by gene regulatory network analysis in an echinoderm. PLoS Genet. 6, e1001259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A. J., Angerer R. C., Angerer L. M. (2012). Sequential signaling crosstalk regulates endomesoderm segregation in sea urchin embryos. Science 335, 590–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood D. R., McClay D. R. (1999). LvNotch signaling mediates secondary mesenchyme specification in the sea urchin embryo. Development 126, 1703–1713 [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Karasawa S., Shimizu H., Miyawaki A. (2005). Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 6, 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega Thurber R., Epel D. (2007). Apoptosis in early development of the sea urchin, Strongylocentrotus purpuratus. Dev. Biol. 303, 336–346 [DOI] [PubMed] [Google Scholar]
- Voronina E., Lopez M., Juliano C. E., Gustafson E., Song J. L., Extavour C., George S., Oliveri P., McClay D., Wessel G. (2008). Vasa protein expression is restricted to the small micromeres of the sea urchin, but is inducible in other lineages early in development. Dev. Biol. 314, 276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Yaguchi J., Yaguchi S., Angerer R. C., Angerer L. M. (2009). The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development 136, 1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Angerer R. C., Angerer L. M. (2011). Direct development of neurons within foregut endoderm of sea urchin embryos. Proc. Natl. Acad. Sci. USA 108, 9143–9147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel G. M., Goldberg L., Lennarz W. J., Klein W. H. (1989). Gastrulation in the sea urchin is accompanied by the accumulation of an endoderm-specific mRNA. Dev. Biol. 136, 526–536 [DOI] [PubMed] [Google Scholar]
- Wikramanayake A. H., Brandhorst B. P., Klein W. H. (1995). Autonomous and non-autonomous differentiation of ectoderm in different sea urchin species. Development 121, 1497–1505 [DOI] [PubMed] [Google Scholar]
- Wikramanayake A. H., Huang L., Klein W. H. (1998). beta-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc. Natl. Acad. Sci. USA 95, 9343–9348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikramanayake A. H., Peterson R., Chen J., Huang L., Bince J. M., McClay D. R., Klein W. H. (2004). Nuclear beta-catenin-dependent Wnt8 signaling in vegetal cells of the early sea urchin embryo regulates gastrulation and differentiation of endoderm and mesodermal cell lineages. Genesis 39, 194–205 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Kanoh K., Amemiya S., Katow H. (2000). Initial analysis of immunochemical cell surface properties, location and formation of the serotonergic apical ganglion in sea urchin embryos. Dev. Growth Differ. 42, 479–488 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Burke R. D. (2006). Specification of ectoderm restricts the size of the animal plate and patterns neurogenesis in sea urchin embryos. Development 133, 2337–2346 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Angerer R. C., Angerer L. M. (2008). A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev. Cell 14, 97–107 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Angerer R. C., Angerer L. M., Burke R. D. (2010). TGFbeta signaling positions the ciliary band and patterns neurons in the sea urchin embryo. Dev. Biol. 347, 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.