Abstract
The heart is the first functioning organ to form during development. During gastrulation, the cardiac progenitors reside in the lateral plate mesoderm but maintain close contact with the underlying endoderm. In amniotes, these bilateral heart fields are initially organized as a pair of flat epithelia that move towards the embryonic midline and fuse above the anterior intestinal portal (AIP) to form the heart tube. This medial motion is typically attributed to active mesodermal migration over the underlying endoderm. In this model, the role of the endoderm is twofold: to serve as a mechanically passive substrate for the crawling mesoderm and to secrete various growth factors necessary for cardiac specification and differentiation. Here, using computational modeling and experiments on chick embryos, we present evidence supporting an active mechanical role for the endoderm during heart tube assembly. Label-tracking experiments suggest that active endodermal shortening around the AIP accounts for most of the heart field motion towards the midline. Results indicate that this shortening is driven by cytoskeletal contraction, as exposure to the myosin-II inhibitor blebbistatin arrested any shortening and also decreased both tissue stiffness (measured by microindentation) and mechanical tension (measured by cutting experiments). In addition, blebbistatin treatment often resulted in cardia bifida and abnormal foregut morphogenesis. Moreover, finite element simulations of our cutting experiments suggest that the endoderm (not the mesoderm) is the primary contractile tissue layer during this process. Taken together, these results indicate that contraction of the endoderm actively pulls the heart fields towards the embryonic midline, where they fuse to form the heart tube.
Keywords: Biomechanics, Chick embryo, Computational modeling, Endoderm, Heart development, Morphogenesis
INTRODUCTION
The heart is the first functioning organ to develop in the embryo. In avians, early cardiac progenitor cells ingress through the anterior primitive streak during gastrulation and take up residence in the lateral plate mesoderm (Rosenquist and DeHaan, 1966; Garcia-Martinez and Schoenwolf, 1993; Cui et al., 2009). They remain in close contact with the underlying endoderm (Linask and Lash, 1986; Schultheiss et al., 1995) as they form paired coherent epithelia on either side of the embryonic midline, the so-called primary heart fields (Abu-Issa and Kirby, 2007). These epithelia (i.e. the cardiogenic mesoderm) then move towards the midline, fold ‘out-of-plane’, and fuse above the anterior intestinal portal (AIP) to form the heart tube (Stalsberg and DeHaan, 1969; Linask and Lash, 1986; Kirby, 2007; Abu-Issa and Kirby, 2008; Cui et al., 2009).
Although the physical forces that drive this process remain poorly understood, it has been generally accepted that heart field motion towards the midline is primarily due to active migration (i.e. crawling) of the cardiogenic mesoderm over the underlying endoderm (DeHaan, 1963; Rosenquist and DeHaan, 1966; Linask and Lash, 1986; Trinh and Stainier, 2004). According to this model, the role of the endoderm during cardiogenesis is considered to be twofold: (1) to serve as a mechanical substrate for the crawling mesoderm and (2) to secrete a host of soluble growth factors, which induce cardiac specification and differentiation in the adjacent cardiogenic mesoderm (Schultheiss et al., 1995; Nascone and Mercola, 1995; Nascone and Mercola, 1996; Schultheiss et al., 1997; Lough and Sugi, 2000; Alsan and Schultheiss, 2002).
Here, using computational modeling and experiments with chick embryos, we show that the endoderm might also play a crucial mechanical role during cardiogenesis. Our results suggest that the endoderm actively shortens around the AIP, pulling the overlying mesoderm towards the midline. Although relative motion between the germ layers (probably associated with active migration) is evident, most of the mesodermal motion is driven by active deformations in the endoderm. Our experiments indicate that actomyosin contraction generates this endodermal shortening, as the myosin II inhibitor blebbistatin arrests any shortening and (in most cases) results in cardia bifida. Microindentation tests before and after the application of blebbistatin also show a reduction in tissue stiffness, which is associated with contraction. Finally, dissection experiments indicate a state of contraction-induced tension around the AIP, and finite element simulations of these experiments identify the endoderm as the dominant contractile tissue layer. Taken together, these results suggest that, in addition to its inductive signaling role, the endoderm also plays a crucial mechanical role during heart tube assembly.
MATERIALS AND METHODS
Embryo preparation and culture
Fertilized White Leghorn chicken eggs were incubated in a humidified, forced draft incubator at 38°C for 24-35 hours to yield embryos between Hamburger and Hamilton (HH) stages 5 and 9 (Hamburger and Hamilton, 1951). Whole embryos were harvested from the eggs using a filter paper carrier method (Voronov and Taber, 2002). The embryo and underlying vitelline membrane were kept intact, thereby preserving the stresses normally present in the tissue. Each embryo was then placed ventral side up in a 35 mm culture dish, and completely submerged under a thin layer of liquid culture media and incubated at 38°C in 95% O2 and 5% CO2. This method prevents artifacts caused by fluid surface tension, which alter the mechanical stresses in the embryo (Voronov and Taber, 2002).
In some experiments, embryos were cultured in 100 μM (–)-blebbistatin (Sigma, St Louis, MO, USA) to broadly suppress any cytoskeletal contraction dependent on myosin II. The inhibitor could be washed out by rinsing the embryo several times in PBS and then continuing the culture with new blebbistatin-free media.
Injection labeling and tracking
To measure tissue motion in both the endoderm and mesoderm around the AIP, small groups of cells (in both germ layers) were labeled at HH stage 7+/8– with the lipophilic fluorescent dye DiI (Molecular Probes, Eugene, OR, USA) mixed in a 20% sucrose solution. DiI injections were made using pulled glass micropipettes and a pneumatic pump (PicoPump PV830, World Precision Instruments). To label cardiogenic mesoderm, the tip of the injection pipette was first pierced through the superficial layer of endoderm.
Embryos were then cultured as described above. For normal embryos (n=5), bright-field and fluorescence time-lapse images were captured at ∼2-hour intervals using a Leica DMLB microscope and attached video camera (Retiga 1300). Because certain wavelengths of light disrupt the activity of blebbistatin (Kolega, 2004), images of blebbistatin-treated embryos (n=4) were captured just prior to wash-out to minimize exposure of the embryo to light. All subsequent (i.e. post-blebbistatin) images were captured at ∼2-hour intervals.
Label motion was tracked using the Manual Tracking plug-in in ImageJ. Labels were confirmed to be mesodermal if they later incorporated into the beating heart tube.
Optical coherence tomography
A Thorlabs (Newton, NJ, USA) optical coherence tomography (OCT) system with attached Nikon FN1 microscope was used to obtain cross-sectional image stacks of living embryos (n=2). Images were acquired every 5 μm across a 3 × 3 mm scanning window. Image stacks were then reconstructed into three-dimensional volumes and optically sectioned using Volocity (PerkinElmer, Waltham, MA, USA).
Microindentation and tissue stiffness
Intact embryos (n=4) were transferred to a bath of PBS at room temperature and tissue stiffness was measured using a custom-built microindentation device (Zamir et al., 2003). Briefly, the microindenter was attached to the end of a calibrated cantilever beam, the motion of which was driven by a piezoelectric motor. The measured beam deflection was then used to calculate the tissue indentation depth and applied force. As described previously (Zamir et al., 2003), the measured force-deflection data were fitted to a four-parameter exponential function, the derivative of which was used to determine the tangential tissue stiffness at 10 μm deflection. Three consecutive indentations were made at each tissue location to ensure a repeatable response. To assess the effects of contraction, each embryo was then transferred to a 35 mm culture dish and incubated in 100 μM (–)-blebbistatin in PBS for 1 to 2 hours at room temperature. Performing this incubation step at room temperature ensured that the embryo did not develop further during these experiments. Afterwards, tissue stiffness was measured again at the same locations.
Tissue microsurgery
To probe tissue stress, small linear incisions at the medial point of the AIP were made using the Gastromaster microsurgical device (Xenotek Engineering) with white tips. These experiments were performed in both a group of normal HH stage 8 embryos (n=5) and in another group of stage 8 embryos after incubation in 100 μM (–)-blebbistatin in PBS for 1 to 2 hours at room temperature (n=5). As discussed below, these linear incisions opened up to form triangularly shaped wounds (when viewed ventrally). The extent of this opening was quantified using ImageJ. A line segment was fitted to each wound edge, and the angle between the lines was measured using the Angle Tool.
Statistics
All data are reported as mean ± s.d. To compare stiffness measurements before and after treatment with blebbistatin, we used a paired t-test implemented in SigmaPlot (Systat Software, Chicago, IL, USA). Circular statistics and a two-sample Watson-Williams test were used to analyze our wound opening-angle data (Zar, 2010).
Computational model
Model geometry
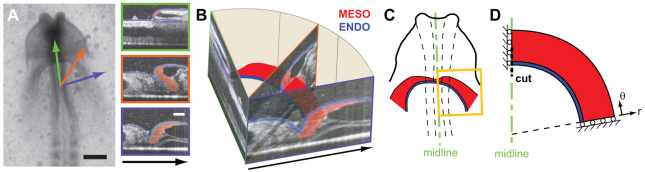
To help interpret our tissue cutting experiments, we constructed a nonlinear 2D finite element model of the endoderm and mesoderm around the AIP using COMSOL Multiphysics (Version 3.5, COMSOL AB, Providence, RI, USA). As a first approximation, we consider an idealized 2D representation of the 3D tissue geometry around the AIP (Fig. 1). OCT sections were used to visualize this geometry in living HH stage 8 embryos (Fig. 1A,B). On each section, the endoderm (blue) and cardiogenic mesoderm (red) were approximately resolved by visual inspection. The cardiogenic mesoderm was shown to be a thickened epithelium in close contact with a thin superficial layer of endoderm (Fig. 1A,B), a result consistent with previous morphological studies (Manasek, 1968; Patten, 1971; Drake et al., 1990; Kirby, 2007).
Fig. 1.
Geometry for computational model. (A) Bright-field and OCT images of HH stage 8+ embryo. OCT sections were taken through medial (green), mediolateral (orange) and lateral (purple) locations around the AIP. On each section, endoderm (blue) and cardiogenic mesoderm (red) were resolved by visual inspection. Arrows indicate orientation of each OCT section within the embryo. Scale bars: 300 μm (black); 100 μm (white). (B) OCT sections shown in A arrayed in 3D space. We consider a 2D slice through the tissue. Note that the thickness of the mesoderm (red) is greater than that of the adjacent endoderm (blue). (C) 2D projection of this slice overlaid with a schematic of HH stage 8+ embryo. (D) For our model geometry, we consider an idealized 2D representation of the tissue, and both tissue layers are modeled as concentric circular rings of pseudoelastic material. We assume bilateral symmetry relative to the embryonic midline, and the model geometry includes only the yellow boxed region in C. A polar coordinate system (r, θ) has its origin at the center of the rings. See text for further details.
For our model geometry, we consider a simplified 2D slice through the cardiogenic mesoderm and endoderm around the AIP (Fig. 1B-D). In this idealized representation, both tissue layers are modeled as concentric circular 2D rings of material in plane stress. The inner curvature of the rings represents the contour of the AIP, and we define a polar coordinate system (r, θ), the origin of which is situated at the center of the rings (Fig. 1D). As observed experimentally, the model mesoderm is thicker than the adjacent endoderm. Roller boundary conditions are specified along the medial and lateral edges of the model; the other edges are taken as traction free.
Theoretical framework
We use a continuum mechanical framework for large deformations, in combination with the Rodriguez et al. (Rodriguez et al., 1994) theory of finite volumetric growth to model the mechanics of morphogenesis. Briefly, the total deformation of a psuedoelastic body can be described by the deformation gradient tensor
 |
where I is the identity tensor, ∇ is the gradient operator in the undeformed configuration and u is the displacement vector between a material point P in the undeformed configuration and its image p in the deformed configuration. The tensor F thereby maps material points between the undeformed and deformed configurations of a body.
Contraction is simulated by negative growth, whereby F is decomposed into a contraction (or growth) tensor G and an elastic deformation gradient tensor F* by the relation F=F* · G (Rodriguez et al., 1994). The tensor G changes the zero-stress configuration of each material element (akin to thermal contraction of a passive material), and F* generates mechanical stress by both enforcing geometric compatibility between material elements and accounting for the elastic response of the material to any applied loads. This theory has been used to model several different morphogenetic processes, including head fold formation (Varner et al., 2010) and cardiac c-looping (Voronov et al., 2004; Ramasubramanian et al., 2006) in the chick embryo, cortical folding in the developing ferret brain (Xu et al., 2010), and ventral furrow formation in Drosophila (Muñoz et al., 2007; Muñoz et al., 2010).
Mechanical properties
Applied loads and mechanical deformations are coupled through the constitutive properties of the material. As a first approximation, we model both the endoderm and mesoderm as isotropic, slightly compressible, modified neo-Hookean materials characterized by the strain-energy density function
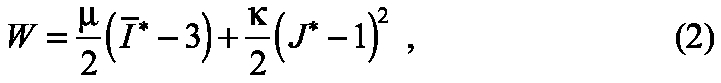 |
where μ is the small-strain shear modulus, κ is the bulk modulus, J*=det F* is the elastic volume ratio, and Ī*=J*–2/3tr (C*) is a modified first invariant of the right Cauchy-Green elastic deformation tensor C*=F*T · F*. Our assumption of slight material compressibility yields numerical solutions that converge more readily than when near incompressibility is enforced. Changing the bulk modulus (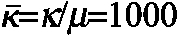 ) by an order of magnitude does not qualitatively alter our model results.
) by an order of magnitude does not qualitatively alter our model results.
The Cauchy stress tensor σ depends on F* through the relation (Taber, 2004)
 |
Stress components (σii) are normalized with respect to the passive small-strain shear modulus μp (i.e. 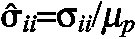 ) and reported in the convected coordinate system (r¯, θ), which is embedded in the material and deforms with it. In the undeformed configuration, (r¯, θ) is coincident with (r, θ). Details on how to implement this theoretical framework in COMSOL Multiphysics (Version 3.5) can be found in Taber (Taber, 2008).
) and reported in the convected coordinate system (r¯, θ), which is embedded in the material and deforms with it. In the undeformed configuration, (r¯, θ) is coincident with (r, θ). Details on how to implement this theoretical framework in COMSOL Multiphysics (Version 3.5) can be found in Taber (Taber, 2008).
Simulating cytoskeletal contraction
Active contraction is specified in the model by varying the components of G. We assume this contraction occurs only along the orthogonal directions er and eθ, so G=Grerer + Gθeθeθ where Gr and Gθ are contraction ratios; Gi=1 for passive material and Gi<1 specifies active contraction. As shown below, endodermal line elements shorten around the AIP (i.e. in the circumferential or θ-direction). We take Gr=1 and Gθ<1 to simulate active circumferential contraction in either the endoderm or mesoderm. Also, because contracting tissues stiffen, a concomitant material stiffening accompanies our specified contraction (i.e. μ increases as Gθ decreases). Here, we take μ=μp/Gθ, where (as a first approximation) μp is assumed equivalent in both the endoderm and mesoderm. More details for the model are provided below.
RESULTS
Approximately 24 hours into the 21-day incubation period of the chick, the head fold forms at the anterior end of the blastoderm (Varner et al., 2010) and initiates formation of the foregut and anterior intestinal portal (AIP) (Bellairs, 1953; Stalsberg and DeHaan, 1968; Varner et al., 2010). At this stage of development (i.e. HH stage 7), the cardiogenic mesoderm is organized as a pair of bilateral epithelia on either side of the embryonic midline (Stalsberg and DeHaan, 1969; Moreno-Rodriguez et al., 2006; Abu-Issa and Kirby, 2008). These heart fields then move to the midline and fuse above the AIP to form the heart tube. During this period, the mesoderm remains in close contact with the endoderm around the AIP (Fig. 1A) (Linask and Lash, 1986; Schultheiss et al., 1995).
Cardiogenic mesoderm and adjacent endoderm move together towards the midline
To measure dynamically the motion of the endoderm and mesoderm during heart tube assembly, we injected fluorescent DiI labels into both germ layers before the heart tube had formed (HH stage 7+/8–) (Fig. 2A,B). Overlapping labels were placed in the lateral region of the AIP in both the endoderm and mesoderm, and a single label was placed in the endoderm at the midline (Fig. 2A). Embryos were then cultured and labels were tracked in time as the heart tube formed (Fig. 2B-D; supplementary material Movie 1).
Fig. 2.
Tracking motion of endoderm and cardiogenic mesoderm around the AIP during heart tube assembly. (A) Schematic of representative HH stage 7+ embryo shown in B. Overlapping mesodermal (red arrowhead) and endodermal (blue arrowhead) fluorescent labels were injected in the lateral region of the AIP, and a single fluorescent label was placed in the endoderm at the medial point of the AIP (blue arrowhead). Embryos were cultured ex ovo and labels were tracked in time as the heart tube formed. The distance of both lateral labels from the midline (dM and dE for the mesoderm and endoderm, respectively) was measured at each time point. The length L between the two endodermal labels, and the separation distance dS between the (initially) adjacent labels in the endoderm and mesoderm were also measured. (B-D) Representative embryo after 0, 3 and 9 hours of incubation ex ovo. Red and blue tracks (and arrowheads) represent mesodermal and endodermal trajectories (and labels), respectively. Scale bar: 200 μm. (E) Distance of lateral labels from the midline (mesoderm, red line; endoderm, blue line), and separation distance between the labels (dashed black line) plotted as functions of time (n=5). (F) Endodermal stretch ratio around the AIP as a function of time (n=5). The distance between endodermal labels at 0 hour (L0) is used as the reference length. Error bars indicate s.d. During heart tube formation, the endoderm and cardiogenic mesoderm move together towards the midline, as the endoderm shortens around the AIP.
As the AIP descended, the medial endodermal label did not move into the forming foregut pocket. Instead, it followed a posterior trajectory and maintained a similar position relative to the regressing AIP (Fig. 2B-D, medial blue lines). Both labels in the lateral region of the AIP, however, moved towards the embryonic midline (Fig. 2B-D, red and blue lateral lines) and, of these two, the label in the mesoderm (red) incorporated into the nascent heart tube. Both germ layers approached the midline at nearly identical rates (Fig. 2E), and the tracked trajectories of both labels were remarkably similar, suggesting that the motions of the cardiogenic mesoderm and adjacent endoderm are correlated (Fig. 2D). Although the initially overlapping labels moved apart as the heart tube formed (Fig. 2E), probably owing to active crawling of the mesoderm over the endoderm, the contribution of this migration to the medial motion of the mesoderm was relatively minor. The lateral mesoderm and endoderm essentially moved together towards the midline.
In addition, the endoderm shortened (i.e. narrowed) tangential to the AIP as the heart tube formed, as the distance between the medial and lateral endodermal labels decreased by >60% (Fig. 2F). At HH stage 7+ (i.e. before heart field fusion), no mesoderm is present in the medial AIP (Cui et al., 2009). As the mesoderm is not continuous across the embryonic midline, these results suggest that the medial movement of both germ layers is driven by endodermal shortening around the AIP.
Cytoskeletal contraction drives endodermal shortening around AIP
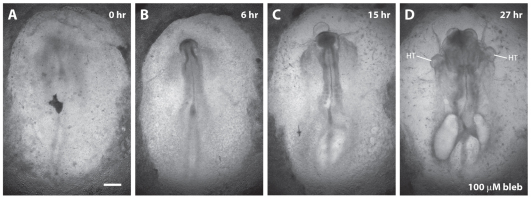
To explore the role of cytoskeletal contraction in this process, we cultured head fold stage embryos (HH stages 5-7) in 100 μM blebbistatin to suppress broadly the activity of myosin II. In these embryos, heart field fusion was impaired and complete cardia bifida often occurred (in five out of six embryos) (Fig. 3). In these cases, the foregut remained open ventrally, the AIP failed to descend and, by the end of the experiment, two asynchronously beating heart tubes were observed on either side of the embryonic midline (Fig. 3D). Proper heart field fusion thus requires actomyosin contraction and we speculated that this contraction drives the observed endodermal shortening.
Fig. 3.
Inhibiting myosin-II-dependent contraction impairs heart field fusion and causes cardia bifida. (A-D) Representative head fold stage embryo (n=6) cultured in 100 μM blebbistatin after 0 (A), 6 (B), 15 (C) and 27 (D) hours of incubation. At the end of the incubation period, two asynchronously beating heart tubes (HT) were observed on either side of the embryonic midline. Scale bar: 500 μm.
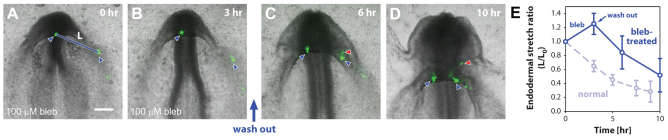
To test this idea, we repeated our injection labeling experiments in the presence of blebbistatin (Fig. 4). In these experiments, we sought to label only endodermal cells, as we were primarily concerned with deformations in the endoderm. After culture in 100 μM blebbistatin, the medial and lateral endodermal labels did not move together as before. Instead, the distance between them increased, indicating tissue relaxation (Fig. 4B,E), and the AIP did not descend as in normal embryos (compare Fig. 2C and Fig. 4B). Blebbistatin was then washed out after 3 hours, and culture was continued. After the wash-out, the labels began to approach one another (Fig. 4C,D) and the distance between them shortened at a rate comparable to that seen in normal embryos (Fig. 4E). Moreover, the AIP resumed its posterior descent.
Fig. 4.
Myosin-II-dependent contraction drives endodermal shortening around AIP. (A,B) Fluorescent labels were injected into the endoderm at medial and lateral locations around the AIP (blue arrowheads); these labels were separated by a distance L. Representative embryo cultured in 100 μM blebbistatin after 0 (A) and 3 (B) hours of incubation. After 3 hours of incubation, blebbistatin was washed out and culture was resumed. (C,D) Same embryo after 6 and 10 hours of total incubation. In this embryo, mesodermal cells adjacent to the lateral endoderm were also incidentally labeled. After wash-out, the lateral label separated into distinct mesodermal (red arrowhead) and endodermal (blue arrowhead) portions. (E) Endodermal stretch ratio around the AIP as a function of time for both blebbistatin-treated (solid line, n=4) and normal (dashed line, n=5) embryos. The distance between endodermal labels at 0 hour (L0) is used as the reference length. Error bars indicate s.d. The dashed line is identical to that shown in Fig. 2F. These results suggest that cytoskeletal contraction drives endodermal shortening around the AIP. Scale bar: 200 μm.
The resumption of normal development and contractility indicates that the foregut and heart defects observed during prolonged treatment with blebbistatin are probably not the result of irreversible toxicity or rampant cell death. These results support our hypothesis that actomyosin contraction drives endodermal shortening around the AIP.
Interestingly, during two of these experiments, mesodermal cells adjacent to the lateral endoderm were also incidentally labeled. In these two cases, when treated with blebbistatin, the lateral fluorescent label (containing both endodermal and mesodermal cells) moved away from the midline and remained a single coherent label as the tissues relaxed (Fig. 4B). After wash-out, however, this label separated into distinct mesodermal (red arrowhead) and endodermal (blue arrowhead) portions (Fig. 4C,D), as had been observed during normal development (Fig. 2). This observation suggests that when contraction is inhibited and the lateral endoderm relaxes away from the midline, the adjacent mesoderm moves with it. Moreover, this result indicates that cytoskeletal contraction is necessary for proper convergence of both the endoderm and mesoderm.
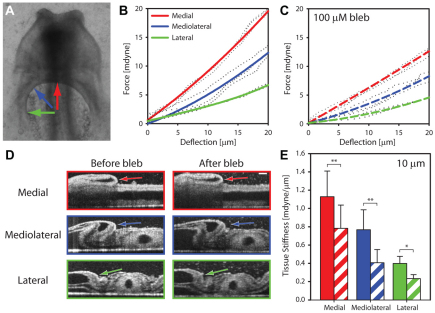
Additional physical experiments were used to confirm further the presence of contraction around the AIP. Because actively contracting tissues stiffen, we performed microindentation tests in embryos before and after blebbistatin exposure (Fig. 5). We indented medial, mediolateral and lateral locations around the AIP in normal HH stage 8 embryos and calculated force-displacement (FD) curves for each location to measure tissue stiffness locally (i.e. endoderm, mesoderm and the accompanying extracellular matrix taken together) (Fig. 5A,B). These curves were nonlinear, so the tissue stiffness (i.e. the tangential slope of each FD curve) depended on indentation depth. We therefore fitted an exponential regression curve to each set of experimental FD data, and calculated the (tangential) tissue stiffness at 10 μm to characterize the local tissue response (Fig. 5B,E). In control embryos, stiffness around the AIP decreased with distance from the midline (Fig. 5E, solid bars).
Fig. 5.
Microindentation tests indicate active cytoskeletal contraction around AIP. (A) Microindentation tests were performed at medial (red), mediolateral (blue) and lateral (green) locations around the AIP in HH stage 8+ embryos before and after incubation in 100 μM blebbistatin. Arrows indicate indentation locations. (B,C) Force-displacement (FD) curves for a representative embryo before (B) and after (C) treatment with blebbistatin. Dots represent calculated FD measurements for consecutive indentations (at each location). Solid/dashed lines are nonlinear regression curves fitted to each set of consecutive indentations. (D) Representative OCT sections of tissue geometry at each indentation location both before and after treatment with blebbistatin. Arrows indicate position of the indenter. Scale bar: 100 μm. (E) Plot of tissue stiffness at 10 μm displacement before (filled bars) and after (hatched bars) blebbistatin exposure. *P<0.05, **P<0.01 (paired t-test, n=4). Tissue stiffness decreased at each tissue location after incubation in blebbistatin. Because actively contracting tissues stiffen, these results further suggest the presence of actomyosin contraction around the AIP. Error bars represent s.d.
Contraction was then suppressed by incubating each embryo in 100 μM blebbistatin at room temperature for ∼1 to 2 hours, and the microindentation experiments were repeated. Tissue stiffness decreased at each location after incubation with blebbistatin (Fig. 5E, compare 5B and 5C). Although this result suggests that blebbistatin had suppressed active contraction in the AIP, changes in stiffness can be caused by either changes in geometry or changes in material properties. Thus, to rule out the possibility that the observed changes in stiffness were simply the result of morphological differences after contraction had been suppressed, we used OCT to image HH stage 8 embryos before and after treatment with blebbistatin (Fig. 5D). Optical sections of the tissue at each indentation location revealed only minor changes in geometry after incubation with blebbistatin, confirming that the observed decreases in stiffness were likely to be caused by changes in the local, contractile state of the tissue. Somewhat surprisingly, however, a stiffness gradient around the AIP was still present after incubation with blebbistatin (Fig. 5E).
Endoderm (not mesoderm) is the primary contractile tissue layer
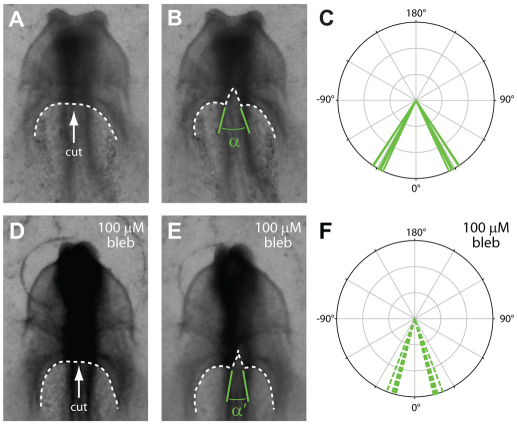
Active contraction tangential to the AIP would tend to generate tension in that direction. To estimate the mechanical stress in the tissue, we made a small linear incision at the medial point of the AIP. In normal embryos, the resulting wounds immediately opened (with a mean opening angle of 59±3°), indicating a state of tension (Fig. 6A-C). Measuring the immediate tissue behavior precluded the possibility that an active healing response had affected our results. When similar cuts were made in embryos after treatment in blebbistatin for ∼1 hour, the wounds still opened but to a significantly lesser extent (with a mean opening angle of 33±3°) (Fig. 6D-F). These results indicate that the observed tension tangential to the AIP is at least partly due to actomyosin contraction.
Fig. 6.
Tension around the AIP decreases when contraction is suppressed. (A-C) Small linear incisions were made at the medial point of the AIP in normal HH stage 8 embryos (n=5). Dashed white lines indicate the contour of the AIP before (A) and after (B) cutting. Opening of wound indicates tension along the AIP. (C) Resulting wound geometry was characterized by the opening angle α59±3°. (D-F) Similar incisions were made in HH stage 8 embryos after incubation with 100 μM blebbistatin (n=4). Dashed white lines indicate the contour of the AIP before (D) and after (E) cutting. (F) Wound geometry was characterized by the opening angle α′=33±3°. Wounds opened to a significantly lesser extent than in control embryos (P<0.001, two-sample Watson-Williams test), indicating a reduction in tissue tension after blebbistatin exposure.
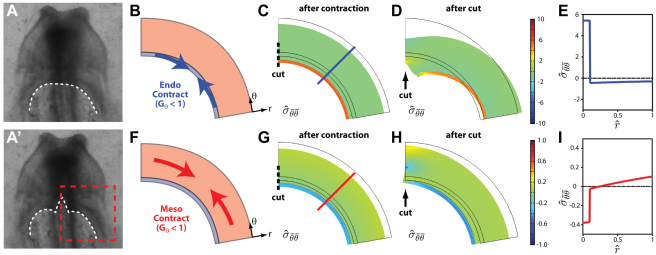
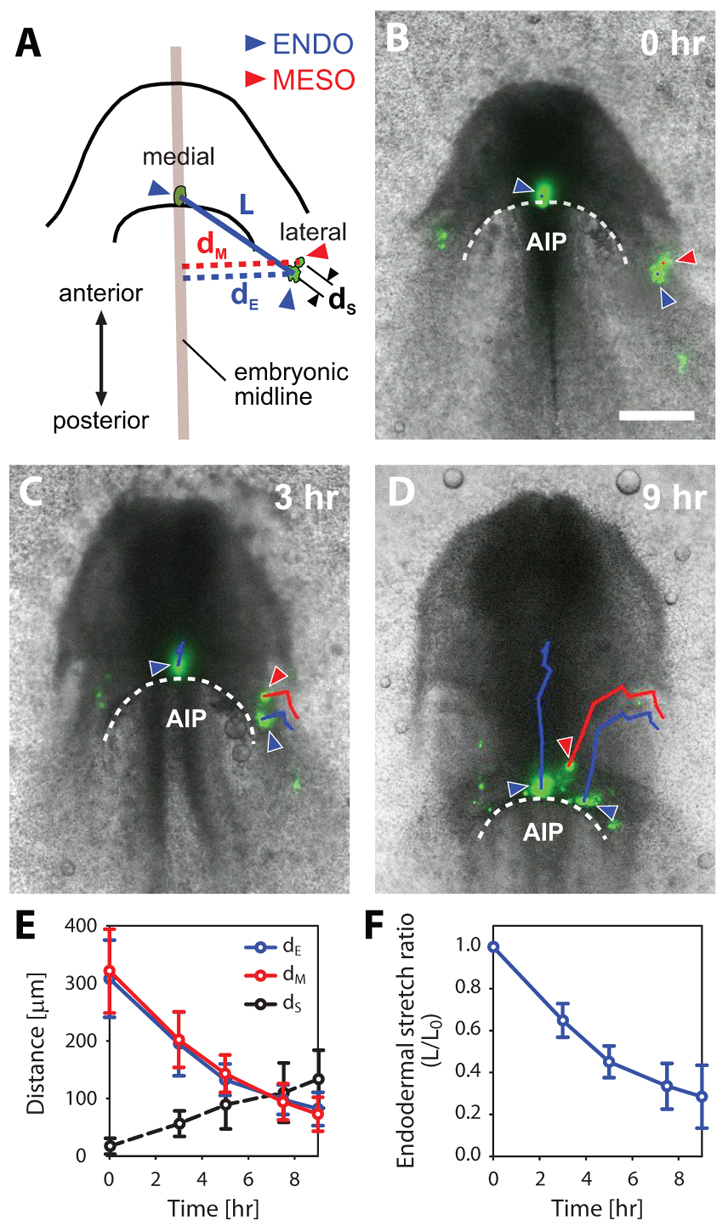
To determine whether the mesoderm or endoderm is the dominant contractile tissue layer during this process, we constructed a 2D nonlinear finite element model of both germ layers around the AIP (see Materials and methods for details). Briefly, we consider an idealized 2D representation of the tissue (Fig. 1). In this 2D slice, the endoderm and mesoderm are modeled as concentric circular rings of pseudoelastic material (Fig. 1D); the AIP corresponds to the inner curvature of the rings. We also specify a polar coordinate system (r, θ) such that the r- and θ-directions run parallel and perpendicular, respectively, to the radii of the rings.
Active contraction is specified in the model only along the θ-direction (i.e. by assigning Gθ<1 with Gr=1), because we observed endodermal shortening tangential to the AIP (Fig. 2). Our cutting experiments are then simulated by modifying the boundary conditions along the midline (i.e. switching a portion from roller to free) to create a linear incision at the medial point of the AIP (Fig. 7C,G, dotted lines). Contraction was specified in either the endoderm, the mesoderm or both layers, and the simulated wound geometry was compared with that observed experimentally.
Fig. 7.
Computational model indicates endoderm as primary contractile tissue layer. (A,A′) Deformed shape of the AIP before (A) and after (A′) cutting (same images as in Fig. 6A,B). (B-D) When contraction is specified in the endoderm only (B) and an incision is simulated at the midline (C), the cut opens as observed experimentally (D). The model AIP curls posteriorly and qualitatively matches the deformed contour of the AIP in our cutting experiments (compare white contour inside dashed red box in A with geometry in D. (E) When the endoderm contracts, the (convected) circumferential Cauchy stresses are compressive in the mesoderm and tensile in the endoderm (computed along blue line in C. (F-H) When contraction is simulated in the mesoderm only (F), the model cut (G) fails to open up (H), and the shape of the AIP is not curled posteriorly as it is in experiments. (I) In this case, the endoderm is in compression and the mesoderm is in tension (computed along red line in G). Agreement between the experiments and model in D, but not in H, indicates that the endoderm (not the mesoderm) is the primary contractile tissue layer. r is the normalized radial distance across the rings, where 0 represents the inner curvature.
During our cutting experiments, the contour of the AIP curled posteriorly and increased in curvature in the neighborhood of the cut (compare Fig. 7A,A′). In our model, when we specify contraction in the endoderm only and simulate an incision at the midline (Fig. 7B-D), the cut opens, as observed experimentally. In addition, the model AIP curls posteriorly and qualitatively matches the deformed contour of the AIP in our cutting experiments (compare white contour inside dashed red box in Fig. 7A′ with 7D). The model, however, does not capture the nearly straight wound edges observed experimentally. When contraction is simulated in the mesoderm only, the model wound fails to open, and the shape of the AIP does not match that seen in experiments (Fig. 7F-H).
Both of these models have the same overall shape before cutting is simulated. These contrasting results are therefore due to differences in stress across the tissue. When the endoderm contracts, the circumferential stresses (in the θ-direction) are compressive in the mesoderm and tensile in the endoderm (Fig. 7E). If the mesoderm contracts, however, the trend is reversed, i.e. the endoderm is in compression and the mesoderm is in tension (Fig. 7I). In this second case, compressive stresses in the endoderm drive wound closure.
These two models can be considered to be paradigmatic cases as all of the contraction is specified in either one germ layer or the other. Intermediate models, which include different ratios of contractility between the two layers, yield intermediate wound geometries between those shown in Fig. 7D and 7H (supplementary material Fig. S1).
Taken together, these results suggest that, during heart tube formation, the endoderm is the dominant contractile tissue layer. This contraction generates tension in the endoderm, which pulls the cardiogenic mesoderm towards the midline.
DISCUSSION
Morphogenesis is fundamentally a physical process, as mechanical forces deform developing tissues in a coordinated way to create biological form (Taber, 1995; Lecuit and Lenne, 2007; Gjorevski and Nelson, 2010; Davidson, 2011). In recent years, groups of developmental biologists, physicists and engineers have been paying renewed attention to the mechanics of morphogenesis: how forces are generated in the embryo (Hutson et al., 2003; Rauzi et al., 2008; Martin et al., 2009; Martin, 2010; Wozniak and Chen, 2009), how those forces are integrated into tissue-level deformations (Ramasubramanian et al., 2006; Chen and Brodland, 2008; Martin et al., 2010; Varner et al., 2010; Brodland et al., 2010) and how they might regulate both cytoskeletal dynamics (Bertet et al., 2004; Fernandez-Gonzalez et al., 2009; Pouille et al., 2009; Zhou et al., 2010; Filas et al., 2011) and regional gene expression (Farge, 2003; Desprat et al., 2008). Here, we have characterized some of the mechanical forces that drive heart tube assembly in the avian embryo.
Physical forces that shape the heart tube are poorly understood
Much work has been done to map the regions of the embryo that are destined to form the heart (Rawles, 1943; Stalsberg and DeHaan, 1969; Redkar et al., 2001; Cui et al., 2009), and many of the important genetic and molecular factors that drive cardiac specification and differentiation have been identified (Olson and Srivastava, 1996; Yutzey and Kirby, 2002; Buckingham et al., 2005; Abu-Issa and Kirby, 2007). The mechanical forces that physically drive formation of the primitive heart tube, however, remain relatively uncharacterized (Taber, 2006).
Several investigators have speculated that a combination of forces probably drives heart tube assembly (Stalsberg and DeHaan, 1969; Linask and Lash, 1986; Drake and Jacobson, 1988; Meilhac and Buckingham, 2010). In particular, the mechanism by which the bilateral fields of cardiogenic mesoderm move towards the midline and fuse to form the heart tube has garnered the most experimental attention. Early evidence in avian embryos suggested that this convergence is driven largely by active crawling of the mesoderm over the underlying endoderm (DeHaan, 1963; Linask and Lash, 1986; Linask and Lash, 1988a; Linask and Lash, 1988b), and the possibility that other physical mechanisms also might contribute to this process, for the most part, faded from view. A notable exception, however, is the study by Wiens (Wiens, 1996), who speculated that cytoskeletal contraction within the mesoderm itself might drive its convergence towards the midline. Our results support this contraction idea, but suggest that the source of contraction is the endoderm rather than the mesoderm.
Endoderm has generally been considered to be an inductive substrate
Mounting evidence has established a clear signaling role for the endoderm during early cardiogenesis (Nascone and Mercola, 1996; Lough and Sugi, 2000; Brand, 2003). Removal of (or defects in) the endoderm can produce abnormal heart development (Orts-Llorca, 1963; Rosenquist, 1970), and the endoderm is necessary for the initiation and maintenance of several cardiac transcription factors (Alsan and Schultheiss, 2002). Others have shown, however, that explants of cardiogenic mesoderm removed post-gastrulation are still capable of expressing a host of cardiac-specific genes in the absence of endoderm (Gannon and Bader, 1995; Du et al., 2003). Even so, the endoderm is at least transiently required to generate beating heart tissue (Gannon and Bader, 1995). Furthermore, mesoderm not fated to contribute to the heart can be induced to express cardiac marker genes by co-culture with endoderm normally adjacent to the cardiogenic mesoderm (Schultheiss et al., 1995). This endoderm expresses the cardiac inducing factors BMP-2 (Schultheiss et al., 1997; Andrée et al., 1998) and FGF-8 (Alsan and Schultheiss, 2002), as well as the Frizzled-related protein Crescent (Marvin et al., 2001), which inhibits Wnt signaling, an antagonist of cardiogenesis.
Such evidence has contributed to the generally accepted view that the role of the endoderm during heart tube formation is to serve as an inductive substrate for the actively crawling mesoderm, secreting various growth factors that induce cardiac specification and differentiation in the mesoderm as it migrates past.
Endoderm actively contracts to pull cardiogenic mesoderm towards the midline
In the present study, we have used a combination of computational modeling and ex ovo experiments with chick embryos to show that the endoderm also plays a crucial mechanical role during cardiogenesis. Our results suggest that the endoderm around the AIP actively contracts and pulls the bilateral fields of cardiogenic mesoderm towards the midline, enabling them to fuse and form the heart tube properly.
Dynamically tracking the motions of the mesoderm and endoderm during heart tube assembly revealed that both layers move towards the midline together (Fig. 2). Although relative movement between the two germ layers was observed (Fig. 2E), possibly reflecting active migration, its contribution to the overall convergence of the cardiogenic mesoderm was relatively minor. The motions of the endodermal labels in these experiments are consistent with the pioneering work of Rosenquist (Rosenquist, 1966) and Stalsberg and DeHaan (Stalsberg and DeHaan, 1968), who used tritiated thymidine labeling and iron oxide particles, respectively, to track endodermal displacements. Although both of these studies reported endodermal shortening around the AIP, neither dynamically measured the concomitant motion of the cardiogenic mesoderm or investigated the mechanical stresses present in the tissue. Even so, Stalsberg and DeHaan (Stalsberg and DeHaan, 1968) proposed a simple mechanical model for foregut morphogenesis, contending that the posterior descent of the AIP is driven by tension around the AIP, a tension that is generated by elongation of the notochord and regression of Henson’s node.
Here, however, we have shown that myosin-II-based cytoskeletal contraction drives both endodermal shortening and AIP descent, and that inhibiting this contraction with blebbistatin can lead to cardia bifida and abnormal foregut morphogenesis (Fig. 3). Notably, both elongation of the notochord and regression of Henson’s node occur relatively normally in these embryos even with the observed heart and foregut defects. We therefore suggest an alternative mechanism to that of Stalsberg and DeHaan (Stalsberg and DeHaan, 1968). Rather than deformations caused by forces at a distance (i.e. notochordal elongation, etc.), local contraction around the AIP itself drives endodermal shortening and thereby both AIP descent and heart field convergence.
Wei et al. (Wei et al., 2001) have reported cardia bifida in chick embryos treated with the Rho kinase inhibitor Y27632, which also suppresses cytoskeletal contraction. They concluded, however, that the observed heart and foregut defects were not due to a lack of contractility, as treatment with the myosin light chain kinase (MLCK) inhibitor ML-9 did not reproduce the observed abnormalities. Previous work in our laboratory, however, has shown that the similar but more specific MLCK inhibitor ML-7 (Bain et al., 2003) did not significantly reduce either myosin regulatory light chain phosphorylation or tissue stiffness in looping chick hearts (Rémond et al., 2006). It is thus possible that ML-9 did not completely suppress cytoskeletal contraction, and that cardia bifida produced by treatment with Y27632 was (at least in part) due to attenuated contraction.
To determine whether the cardiogenic mesoderm or the adjacent endoderm is the dominant contractile layer during heart tube assembly, we constructed a 2D computational model of both germ layers around the AIP (Fig. 1). When a linear incision is simulated at the midline, the deformed shape of the AIP matches our cutting experiments only if contraction is included (predominantly) in the endoderm (Fig. 7). The model predicts endodermal tension and mesodermal compression, a result consistent with previous work in our laboratory that reported mesodermal compression in the fusing omphalomesenteric veins of HH stage 10 chick hearts (Voronov et al., 2004). Furthermore, preliminary data from our laboratory has estimated a small-strain shear modulus of 50–100 Pa for the tissue around the AIP (V.D.V., unpublished). Our model thus predicts peak stresses in the contracting endoderm that are between 300 and 600 Pa, which are in the order of the contractile stresses generated within a coherent and collectively migrating cell sheet (Trepat et al., 2009). We suggest, therefore, that active contraction around the AIP is primarily endodermal in origin, not mesodermal as postulated by Wiens (Wiens, 1996).
Taken together, these results suggest an essential mechanical role for the endoderm during heart tube assembly (Fig. 8). Instead of just serving as a passive, secretory substrate for the crawling cardiogenic mesoderm (Fig. 8C), the endoderm around the AIP actively contracts and pulls (i.e. convects) the heart fields towards the midline (Fig. 8D), enabling them to fuse and form the heart tube. How this contraction is spatially distributed and how this distribution might contribute to the observed morphogenetic movements remain open questions. In particular, it would be interesting to determine whether the observed stiffness gradient around the AIP (Fig. 5E) is due to spatial variations in tissue geometry, mechanical properties, actomyosin contractility, or the amount of cross-linking within the extracellular matrix (ECM).
Fig. 8.
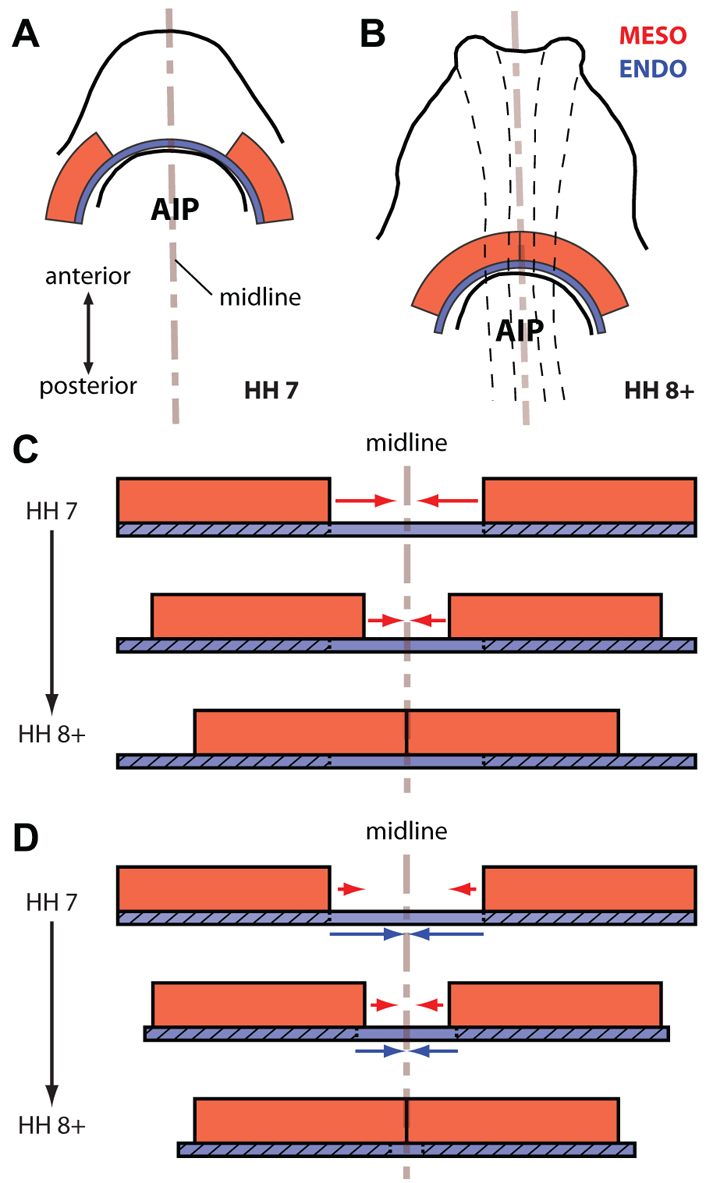
Endoderm actively contracts to pull the cardiogenic mesoderm towards the midline. (A) Schematic of HH stage 7 embryo. The cardiogenic mesoderm (red) is organized as a pair of bilateral epithelia that are separated on either side of the embryonic midline and remain in close contact with the underlying endoderm (blue). (B) Schematic of HH stage 8+ embryo. As the AIP descends, the cardiogenic mesoderm moves towards the midline and fuses to begin forming the early heart tube. Dashed black lines represent the neural tube. (C) Investigators have suggested that the endoderm serves primarily as an inductive substrate for the actively crawling mesoderm (red arrows). (D) Our results suggest that the endoderm also has a distinct mechanical role in early cardiogenesis; it actively contracts (blue arrows) to pull the cardiogenic mesoderm towards the midline. Although relative motion (red arrows) occurs between the endoderm and mesoderm during this process (probably owing to collective migration), this motion is much less than the convection caused by contraction.
Other recent studies of avian embryogenesis have shown that cell displacements are similarly convected by ECM (i.e. substrate) motion during the processes of primitive streak formation (Zamir et al., 2008), gastrulation (Zamir et al., 2006) and axial elongation (Bénazéraf et al., 2010). The interface between the cardiogenic mesoderm and adjacent endoderm is textured with an abundant ECM containing fibronectin, laminin and collagen types I and IV (Linask and Lash, 1986; Drake et al., 1990; Wiens, 1996). The two tissue layers are thus intimately coupled, with numerous interdigitating cell processes extending through the layer of ECM (Linask and Lash, 1986). Contractile forces generated in the endoderm could thus be plausibly transmitted to the overlying mesoderm, and we speculate that ECM deforms with the contracting endoderm.
Both our label-tracking experiments and model, however, are limited to two dimensions and fail to accurately capture the out-of-plane folding that both tissue layers undergo during this process. Although our results offer qualitative insight into the mechanics of heart tube formation, further experimental and computational work is needed to characterize more fully the 3D nature of this problem. Imaging technologies such as OCT (Filas et al., 2007) or dynamic wide-field fluorescence imaging (Zamir et al., 2006; Zamir et al., 2008; Sato et al., 2010) are sure to prove useful tools in this regard.
In addition, it would be interesting to investigate whether mesodermal migration might provide a redundant mechanism for heart field convergence. Although the contracting endoderm pulls the mesoderm towards the midline during normal development, if myosin-II activity is inhibited specifically in the endoderm, might elevated mesodermal migration compensate for a lack of endodermal contractility?
It also remains to be seen whether endodermal contraction proceeds in the pulsatile, ratchet-like manner described during both Drosophila ventral furrow formation (Martin et al., 2009) and dorsal closure (Solon et al., 2009). The endoderm shortens so dramatically (>60%) as the AIP descends (Fig. 2F) that it seems reasonable that the contracting cytoskeleton might need to stabilize or remodel at intermediate configurations (akin to airway smooth muscle cells) to accomplish an active shortening of this magnitude (Fredberg et al., 1997). These stabilized configurations could explain the observed residual tension in the AIP after treatment with blebbistatin (Fig. 6D-F). Remodeling of the ECM between the endoderm and mesoderm might also contribute to such stabilizations.
In addition, it remains a possibility that, once they have moved to the midline, the fusing cardiogenic mesoderm might (much like a zipper) provide a driving force behind AIP descent (Moreno-Rodriguez et al., 2006). Recent work, however, has shown that ectopic expression of Wnt3a can generate a cardia bifida phenotype without any apparent defects in AIP descent or foregut morphogenesis (Yue et al., 2008). Moreover, because the AIP begins its descent well before there is any mesoderm at the midline, we speculate that even after heart field fusion, endodermal contraction continues to drive the posterior descent of the AIP.
Role for endodermal contractility in cardia bifida mutants?
Several gene mutants produce a cardia bifida phenotype (Meilhac and Buckingham, 2010) and it is unclear whether any are due to suppressed levels of endodermal contractility. An intriguing possibility is the Gata4–/– mouse mutant, which has both cardia bifida and abnormal foregut morphogenesis (Kuo et al., 1997; Molkentin et al., 1997). GATA4 is normally expressed in both the cardiogenic mesoderm and adjacent endoderm. In the intriguing mosaic experiments of Narita et al. (Narita et al., 1997), however, wild-type (Gata4+/+) endoderm alone was sufficient to rescue both the cardia bifida and abnormal foregut phenotype in embryos that otherwise consisted only of Gata4–/– cells. GATA4 thus seems to be required in the endoderm, not the mesoderm, for proper heart tube assembly (Narita et al., 1997; Watt et al., 2004). As GATA4 has also been shown to regulate the expression of cytoskeletal proteins (Molkentin et al., 1994), it might be involved in contractility and, consequently, endodermal shortening around the AIP. It would be interesting to investigate the role of actomyosin contractility in the these mutants.
In zebrafish, other types of genetic perturbations produce both endodermal defects and cardia bifida, such as one-eyed pinhead (Schier et al., 1997) and casanova (sox32 – Zebrafish Information Network) (Alexander et al., 1999). Intuiting a possible physical role for the endoderm here, however, is decidedly more problematic, as it seems to play a somewhat different role in cardiogenesis. Several recent studies have suggested that heart field convergence in zebrafish is in fact largely driven by active crawling of cardiomyocytes towards the midline (Holtzman et al., 2007), a process probably regulated by fibronectin (Trinh and Stainier, 2004; Garavito-Aguilar et al., 2010). How deformations in the endoderm might contribute to this process remains unclear. Other recent work in ascidian embryos, however, has suggested a possible morphogenetic role for the endoderm during heart progenitor convergence (Ragkousi et al., 2011).
In conclusion, we propose an active mechanical role for the endoderm during heart tube formation. This work constitutes a new step towards characterizing some of the mechanical forces that shape the vertebrate heart. How these forces are regulated by (or in turn regulate) the various molecular and genetic factors involved in cardiogenesis remains an exciting avenue of research to explore, as we seek to connect genetic and molecular mechanisms of development with the mechanics of morphogenesis.
Supplementary Material
Acknowledgments
We thank the members of the Taber lab, especially Judy A. Fee, for their constructive questions and comments. We also thank David Wilson for his insight regarding the GATA-4 null mouse mutants.
Footnotes
Funding
This work was supported by grants from the National Institutes of Health [R01 GM075200 and R01 HL083393 to L.A.T.]; and a grant from the American Heart Association [09PRE2060795 to V.D.V.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.073486/-/DC1
References
- Abu-Issa R., Kirby M. L. (2007). Heart field: from mesoderm to heart tube. Annu. Rev. Cell Dev. Biol. 23, 45–68 [DOI] [PubMed] [Google Scholar]
- Abu-Issa R., Kirby M. L. (2008). Patterning of the heart field in the chick. Dev. Biol. 319, 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J., Rothenberg M., Henry G. L., Stainier D. Y. (1999). Casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 215, 343–357 [DOI] [PubMed] [Google Scholar]
- Alsan B. H., Schultheiss T. M. (2002). Regulation of avian cardiogenesis by Fgf8 signaling. Development 129, 1935–1943 [DOI] [PubMed] [Google Scholar]
- Andrée B., Duprez D., Vorbusch B., Arnold H. H., Brand T. (1998). BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech. Dev. 70, 119–131 [DOI] [PubMed] [Google Scholar]
- Bain J., McLauchlan H., Elliott M., Cohen P. (2003). The specificities of protein kinase inhibitors: an update. Biochem. J. 371, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellairs R. (1953). Studies on the development of the foregut in the chick. II. The morphogenetic movements. J. Embryol. Exp. Morphol. 1, 369–385 [Google Scholar]
- Bénazéraf B., Francois P., Baker R. E., Denans N., Little C. D., Pourquié O. (2010). A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466, 248–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C., Sulak L., Lecuit T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671 [DOI] [PubMed] [Google Scholar]
- Brand T. (2003). Heart development: molecular insights into cardiac specification and early morphogenesis. Dev. Biol. 258, 1–19 [DOI] [PubMed] [Google Scholar]
- Brodland G. W., Conte V., Cranston P. G., Veldhuis J., Narasimhan S., Hutson M. S., Jacinto A., Ulrich F., Baum B., Miodownik M. (2010). Video force microscopy reveals the mechanics of ventral furrow invagination in Drosophila. Proc. Natl. Acad. Sci. USA 107, 22111–22116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M., Meilhac S., Zaffran S. (2005). Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 6, 826–835 [DOI] [PubMed] [Google Scholar]
- Chen X., Brodland G. W. (2008). Multi-scale finite element modeling allows the mechanics of amphibian neurulation to be elucidated. Phys. Biol. 5, 015003 [DOI] [PubMed] [Google Scholar]
- Cui C., Cheuvront T. J., Lansford R. D., Moreno-Rodriguez R. A., Schultheiss T. M., Rongish B. J. (2009). Dynamic positional fate map of the primary heart-forming region. Dev. Biol. 332, 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L. A. (2011). Embryo mechanics: balancing force production with elastic resistance during morphogenesis. Curr. Top. Dev. Biol. 95, 215–241 [DOI] [PubMed] [Google Scholar]
- DeHaan R. L. (1963). Migration patterns of the precardiac mesoderm in the early chick embryo. Exp. Cell Res. 29, 544–560 [DOI] [PubMed] [Google Scholar]
- Desprat N., Supatto W., Pouille P.-A., Beaurepaire E., Farge E. (2008). Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 15, 470–477 [DOI] [PubMed] [Google Scholar]
- Drake C. J., Jacobson A. G. (1988). A survey by scanning electron microscopy of the extracellular matrix and endothelial components of the primordial chick heart. Anat. Rec. 222, 391–400 [DOI] [PubMed] [Google Scholar]
- Drake C. J., Davis L. A., Walters L., Little C. D. (1990). Avian vasculogenesis and the distribution of collagens I, IV, laminin, and fibronectin in the heart primordia. J. Exp. Zool. 255, 309–322 [DOI] [PubMed] [Google Scholar]
- Du A., Sanger J. M., Linask K. K., Sanger J. W. (2003). Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev. Biol. 257, 382–394 [DOI] [PubMed] [Google Scholar]
- Farge E. (2003). Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 13, 1365–1377 [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R., de Matos Simoes S., Röper J.-C., Eaton S., Zallen J. A. (2009). Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filas B. A., Efimov I. R., Taber L. A. (2007). Optical coherence tomography as a tool for measuring morphogenetic deformation of the looping heart. Anat. Rec. 290, 1057–1068 [DOI] [PubMed] [Google Scholar]
- Filas B. A., Bayly P. V., Taber L. A. (2011). Mechanical stress as a regulator of cytoskeletal contractility and nuclear shape in embryonic epithelia. Ann. Biomed. Eng. 39, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredberg J. J., Inouye D., Miller B., Nathan M., Jafari S., Raboudi S. H., Butler J. P., Shore S. A. (1997). Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am. J. Respir. Crit. Care Med. 156, 1752–1759 [DOI] [PubMed] [Google Scholar]
- Gannon M., Bader D. (1995). Initiation of cardiac differentiation occurs in the absence of anterior endoderm. Development 121, 2439–2450 [DOI] [PubMed] [Google Scholar]
- Garavito-Aguilar Z. V., Riley H. E., Yelon D. (2010). Hand2 ensures an appropriate environment for cardiac fusion by limiting Fibronectin function. Development 137, 3215–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez V., Schoenwolf G. C. (1993). Primitive-streak origin of the cardiovascular system in avian embryos. Dev. Biol. 159, 706–719 [DOI] [PubMed] [Google Scholar]
- Gjorevski N., Nelson C. M. (2010). The mechanics of development: Models and methods for tissue morphogenesis. Birth Defects Res. C Embryo Today 90, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H. L. (1951). A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 [PubMed] [Google Scholar]
- Holtzman N. G., Schoenebeck J. J., Tsai H.-J., Yelon D. (2007). Endocardium is necessary for cardiomyocyte movement during heart tube assembly. Development 134, 2379–2386 [DOI] [PubMed] [Google Scholar]
- Hutson M. S., Tokutake Y., Chang M.-S., Bloor J. W., Venakides S., Kiehart D. P., Edwards G. S. (2003). Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 300, 145–149 [DOI] [PubMed] [Google Scholar]
- Kirby M. L. (2007). Cardiac Development. Oxford: Oxford University Press; [Google Scholar]
- Kolega J. (2004). Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem. Biophys. Res. Commun. 320, 1020–1025 [DOI] [PubMed] [Google Scholar]
- Kuo C. T., Morrisey E. E., Anandappa R., Sigrist K., Lu M. M., Parmacek M. S., Soudais C., Leiden J. M. (1997). GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 11, 1048–1060 [DOI] [PubMed] [Google Scholar]
- Lecuit T., Lenne P.-F. (2007). Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 8, 633–644 [DOI] [PubMed] [Google Scholar]
- Linask K. K., Lash J. W. (1986). Precardiac cell migration: fibronectin localization at mesodermendoderm interface during directional movement. Dev. Biol. 114, 87–101 [DOI] [PubMed] [Google Scholar]
- Linask K. K., Lash J. W. (1988a). A role for fibronectin in the migration of avian precardiac cells. I. Dose-dependent effects of fibronectin antibody. Dev. Biol. 129, 315–323 [DOI] [PubMed] [Google Scholar]
- Linask K. K., Lash J. W. (1988b). A role for fibronectin in the migration of avian precardiac cells. II. Rotation of the heart-forming region during different stages and its effects. Dev. Biol. 129, 324–329 [DOI] [PubMed] [Google Scholar]
- Lough J., Sugi Y. (2000). Endoderm and heart development. Dev. Dyn. 217, 327–342 [DOI] [PubMed] [Google Scholar]
- Manasek F. J. (1968). Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J. Morphol. 125, 329–365 [DOI] [PubMed] [Google Scholar]
- Martin A. C. (2010). Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev. Biol. 341, 114–125 [DOI] [PubMed] [Google Scholar]
- Martin A. C., Kaschube M., Wieschaus E. F. (2009). Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C., Gelbart M., Fernandez-Gonzalez R., Kaschube M., Wieschaus E. F. (2010). Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin M. J., Di Rocco G., Gardiner A., Bush S. M., Lassar A. B. (2001). Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 15, 316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac S. M., Buckingham M.E. (2010). The behavior of cells that form the myocardial compartments of the vertebrate heart. In Heart Development and Regeneration, Vol. 1 (ed. Rosenthal N., Harvery R. P.), pp. 195–217 Burlington, MA: Elsevier/Academic Press; [Google Scholar]
- Molkentin J. D., Kalvakolanu D. V., Markham B. E. (1994). Transcription factor GATA-4 regulates cardiac muscle-specific expression of the alpha-myosin heavy-chain gene. Mol. Cell. Biol. 14, 4947–4957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin J. D., Lin Q., Duncan S. a., Olson E. N. (1997). Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 11, 1061–1072 [DOI] [PubMed] [Google Scholar]
- Moreno-Rodriguez R. A., Krug E. L., Reyes L., Villavicencio L., Mjaatvedt C. H., Markwald R. R. (2006). Bidirectional fusion of the heart-forming fields in the developing chick embryo. Dev. Dyn. 235, 191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz J. J., Barrett K., Miodownik M. (2007). A deformation gradient decomposition method for the analysis of the mechanics of morphogenesis. J. Biomech. 40, 1372–1380 [DOI] [PubMed] [Google Scholar]
- Muñoz J. J., Conte V., Miodownik M. (2010). Stress-dependent morphogenesis: continuum mechanics and truss systems. Biomech. Model. Mechanobiol. 9, 451–467 [DOI] [PubMed] [Google Scholar]
- Narita N., Bielinska M., Wilson D. B. (1997). Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev. Biol. 189, 270–274 [DOI] [PubMed] [Google Scholar]
- Nascone N., Mercola M. (1995). An inductive role for the endoderm in Xenopus cardiogenesis. Development 121, 515–523 [DOI] [PubMed] [Google Scholar]
- Nascone N., Mercola M. (1996). Endoderm and cardiogenesis new insights. Trends Cardiovasc. Med. 6, 211–216 [DOI] [PubMed] [Google Scholar]
- Olson E. N., Srivastava D. (1996). Molecular pathways controlling heart development. Science 272, 671–676 [DOI] [PubMed] [Google Scholar]
- Orts-Llorca F. (1963). Influence of the endoderm on heart differentiation during the early stages of development of the chicken embryo. Wilhelm Roux’s Arch. Entwicklungsmech. Organ. 154, 533–551 [DOI] [PubMed] [Google Scholar]
- Patten B. M. (1971). Early embryology of the chick. New York: McGraw-Hill; [Google Scholar]
- Pouille P.-A., Ahmadi P., Brunet A.-C., Farge E. (2009). Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal. 2, ra16 [DOI] [PubMed] [Google Scholar]
- Ragkousi K., Beh J., Sweeney S., Starobinska E., Davidson B. (2011). A single GATA factor plays discrete, lineage specific roles in ascidian heart development. Dev. Biol. 352, 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubramanian A., Latacha K. S., Benjamin J. M., Voronov D. A., Ravi A., Taber L. A. (2006). Computational model for early cardiac looping. Ann. Biomed. Eng. 34, 1355–1369 [DOI] [PubMed] [Google Scholar]
- Rauzi M., Verant P., Lecuit T., Lenne P.-F. (2008). Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401–1410 [DOI] [PubMed] [Google Scholar]
- Rawles M. E. (1943). The heart-forming areas of the early chick blastoderm. Physiol. Zool. 16, 22–43 [Google Scholar]
- Redkar A., Montgomery M., Litvin J. (2001). Fate map of early avian cardiac progenitor cells. Development 128, 2269–2279 [DOI] [PubMed] [Google Scholar]
- Rémond M. C., Fee J. A., Elson E. L., Taber L. A. (2006). Myosin-based contraction is not necessary for cardiac c-looping in the chick embryo. Anat. Embryol. (Berl.) 211, 443–454 [DOI] [PubMed] [Google Scholar]
- Rodriguez E. K., Hoger A., McCulloch A. D. (1994). Stress-dependent finite growth in soft elastic tissues. J. Biomech. 27, 455–467 [DOI] [PubMed] [Google Scholar]
- Rosenquist G. C. (1966). A radioautographic study of labeled grafts in the chick blastoderm. Development from primitive streak stages to stage 12. Carnegie Inst. Wash. Contrib. Embryol. 38, 71–110 [Google Scholar]
- Rosenquist G. C. (1970). Cardia bifida in chick embryos: anterior and posterior defects produced by transplanting tritiated thymidine-labeled grafts medial to the heart-forming regions. Teratology 3, 135–142 [DOI] [PubMed] [Google Scholar]
- Rosenquist G. C., DeHaan R. L. (1966). Migration of precardiac cells in the chick embryo: a radioautographic study. Carnegie Inst. Wash. Contrib. Embryol. 38, 111–121 [Google Scholar]
- Sato Y., Poynter G., Huss D., Filla M. B., Czirok A., Rongish B. J., Little C. D., Fraser S. E., Lansford R. (2010). Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PLoS ONE 5, e12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier A. F., Neuhauss S. C., Helde K. A., Talbot W. S., Driever W. (1997). The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development 124, 327–342 [DOI] [PubMed] [Google Scholar]
- Schultheiss T. M., Xydas S., Lassar A. B. (1995). Induction of avian cardiac myogenesis by anterior endoderm. Development 121, 4203–4214 [DOI] [PubMed] [Google Scholar]
- Schultheiss T. M., Burch J. B., Lassar A. B. (1997). A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 11, 451–462 [DOI] [PubMed] [Google Scholar]
- Solon J., Kaya-Copur A., Colombelli J., Brunner D. (2009). Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137, 1331–1342 [DOI] [PubMed] [Google Scholar]
- Stalsberg H., DeHaan R. L. (1968). Endodermal movements during foregut formation in the chick embryo. Dev. Biol. 18, 198–215 [DOI] [PubMed] [Google Scholar]
- Stalsberg H., DeHaan R. L. (1969). The precardiac areas and formation of the tubular heart in the chick embryo. Dev. Biol. 19, 128–159 [DOI] [PubMed] [Google Scholar]
- Taber L. A. (1995). Biomechanics of growth, remodeling, and morphogenesis. Appl. Mech. Rev. 48, 487 [Google Scholar]
- Taber L. A. (2004). Nonlinear Theory Of Elasticity: Applications In Biomechanics. River Edge, NJ: World Scientific; [Google Scholar]
- Taber L. A. (2006). Biophysical mechanisms of cardiac looping. Int. J. Dev. Biol. 50, 323–332 [DOI] [PubMed] [Google Scholar]
- Taber L. A. (2008). Theoretical study of Beloussov’s hyper-restoration hypothesis for mechanical regulation of morphogenesis. Biomech. Model. Mechanobiol. 7, 427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepat X., Wasserman M. R., Angelini T. E., Millet E., Weitz D. A., Butler J. P., Fredberg J. J. (2009). Physical forces during collective cell migration. Nature Physics 5, 426–430 [Google Scholar]
- Trinh L. A., Stainier D. Y. R. (2004). Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell 6, 371–382 [DOI] [PubMed] [Google Scholar]
- Varner V. D., Voronov D. A., Taber L. A. (2010). Mechanics of head fold formation: investigating tissue-level forces during early development. Development 137, 3801–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov D. A., Taber L. A. (2002). Cardiac looping in experimental conditions: effects of extraembryonic forces. Dev. Dyn. 224, 413–421 [DOI] [PubMed] [Google Scholar]
- Voronov D. A., Alford P. W., Xu G., Taber L. A. (2004). The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev. Biol. 272, 339–350 [DOI] [PubMed] [Google Scholar]
- Watt A. J., Battle M. A., Li J., Duncan S. A. (2004). GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc. Natl. Acad. Sci. USA 101, 12573–12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Roberts W., Wang L., Yamada M., Zhang S., Zhao Z., Rivkees S. A., Schwartz R. J., Imanaka-Yoshida K. (2001). Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development 128, 2953–2962 [DOI] [PubMed] [Google Scholar]
- Wiens D. J. (1996). An alternative model for cell sheet migration on fibronectin during heart formation. J. Theor. Biol. 179, 33–39 [DOI] [PubMed] [Google Scholar]
- Wozniak M. A., Chen C. S. (2009). Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Knutsen A. K., Dikranian K., Kroenke C. D., Bayly P. V., Taber L. A. (2010). Axons pull on the brain, but tension does not drive cortical folding. J. Biomech. Eng. 132, 071013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q., Wagstaff L., Yang X., Weijer C., Münsterberg A. (2008). Wnt3a-mediated chemorepulsion controls movement patterns of cardiac progenitors and requires RhoA function. Development 135, 1029–1037 [DOI] [PubMed] [Google Scholar]
- Yutzey K. E., Kirby M. L. (2002). Wherefore heart thou? Embryonic origins of cardiogenic mesoderm. Dev. Dyn. 223, 307–320 [DOI] [PubMed] [Google Scholar]
- Zamir E. A., Srinivasan V., Perucchio R., Taber L. A. (2003). Mechanical asymmetry in the embryonic chick heart during looping. Ann. Biomed. Eng. 31, 1327–1336 [DOI] [PubMed] [Google Scholar]
- Zamir E. A., Czirók A., Cui C., Little C. D., Rongish B. J. (2006). Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proc. Natl. Acad. Sci. USA 103, 19806–19811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E. A., Rongish B. J., Little C. D. (2008). The ECM moves during primitive streak formation – computation of ECM versus cellular motion. PLoS Biol. 6, e247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. H. (2010). Biostatistical Analysis, 5th edn. Upper Saddle River, NJ: Prentice-Hall/Pearson; [Google Scholar]
- Zhou J., Kim H. Y., Wang J. H.-C., Davidson L. A. (2010). Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development 137, 2785–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.