Abstract
Candida-associated denture stomatitis (CADS) is a significant clinical concern. We developed rechargeable infection-responsive antifungal denture materials for potentially managing the disease. Polymethacrylic acid (PMAA) was covalently bound onto diurethane dimethacrylate denture resins in the curing step. The PMAA resins bound cationic antifungal drugs such as miconazole and chlorhexidine digluconate (CG) through ionic interactions. The anticandidal activities of the drug-containing PMAA-resin discs were sustained for a prolonged period of time (weeks and months). Drug release was much faster at acidic conditions (pH 5) than at pH 7. Drugs bound to the denture materials could be “washed out” by treatment with EDTA, and the drug-depleted resins could be recharged with the same or a different class of anticandidal drugs. These results suggest clinical potential of the newly developed antifungal denture materials in the management of CADS and other infectious conditions.
Keywords: antifungal denture, infection-responsive, rechargeable, Candida-associated denture stomatitis
Introduction
Candida colonization and biofilm formation often lead to Candida-associated denture stomatitis (CADS), a common recurring disease that affects approximately 11% to 67% of denture wearers (Arendorf and Walker, 1987; Radford et al., 1999; Douglass et al., 2002; Ramage et al., 2004; Perezous et al., 2005). Candida infection, especially in immunocompromised/medically compromised patients, has been linked to dental caries, periodontal diseases, gastrointestinal/pleuropulmonary infections, and even death (Webb et al., 1998; Golecka et al., 2006). The use of antifungal denture materials has been proposed as one strategy to control CADS. The general principle is to impregnate dentures with drugs that release from the device and inhibit microbial growth. A high antifungal concentration can be achieved under the denture and its nearby mucosa, generally exceeding the minimum inhibitory concentration (MIC) of the susceptible species. The effectiveness of these antifungal dentures for short-term use (e.g., days to weeks) has been examined in several studies (Schneid, 1992; Matsuura et al., 1997; Chow et al., 1999; Lefebvre et al., 2001; Lin et al., 2003; Abe et al., 2004; Yoshinari et al., 2006; Redding et al., 2009).
However, because the current impregnating approaches cannot incorporate enough drugs into dentures to maintain the MIC for extended periods, there are still no antifungal dentures that can offer long-term protection (e.g., months to years). Further, the antifungal-releasing patterns are not controlled by whether active infection is present. These denture materials have an initially high antifungal release with a subsequent exponential decrease in drug release. After a short period of time, the drugs in the dentures are depleted, and the inhibitory effects are lost.
Because a denture will be worn for years and CADS is a recurring disease, long-term antifungal dentures that can initiate and/or cease drug release based on clinical infection would have significant health benefits. Thus, we developed infection-responsive antifungal denture materials in which covalently bound poly(methacrylic acid) (PMAA) serves as a rechargeable drug carrier. Herein we first report the formulation and fabrication of these innovative denture materials, and their anticandidal activity in vitro.
Materials & Methods
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Candida albicans (C. albicans; ATCC 10231) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA).
Fabrication of Denture Materials
Denture discs (diameter, 13 mm) were prepared by co-polymerization of methacrylic acid (MAA) with diurethane dimethacrylate (DUDMA) in aluminum molds. MAA weight percentage to DUDMA varied at 0% (control), 5%, 10%, and 20%, and the weight percentage of the initiator (azobisisobutyronitrile) to the monomers (DUDMA plus MAA) was 1%. Polymerization was carried out at 70°C for 3 hrs. We examined the specimens visually to ensure that they were free of voids.
Drug Loading
A series of PMAA-based discs was immersed in either 5% miconazole ethanol solution or 10% chlorhexidine digluconate (CG) aqueous solution for 24 hrs at room temperature under constant shaking. The discs were rinsed individually with water (3x, 10 mL, and 1 min for each rinse), air-dried, and stored in a desiccator. The drugs bound to the discs were extracted with de-ionized water in a Soxhlet extraction apparatus for 24 hrs. Drug content in the extraction solution was measured at λ = 220 nm for miconazole or λ = 253 nm for CG (Senel et al., 2000; Bhalekar et al., 2009). Each test was repeated 5 times.
Drug Releasing
Disc specimens loaded with miconazole or CG were immersed individually in 10 mL buffer containing physiological concentrations of electrolytes in saliva with slight viscosity (0.375 g/L CaCl2•2H2O, 0.125 g/L MgCl2•6H2O, 1.2 g/L KCl, 0.85 g/L NaCl, 2.5 g/L NaHPO4•12H2O, 1 g/L sorbine acid, 5 g/L carboxylmethylcellulose sodium, and 43 g/L sorbitol solution) at neutral pH (Raum et al., 2007) or pH = 5 (adjusted with citric acid; Yap et al., 2004). The specimens were shaken at 37°C and 40 RPM. The buffer was changed daily. Periodically, the quantity of drugs released from each specimen was determined by UV measurements. Each test was repeated 5 times.
Antifungal/Anti-biofilm Efficacy
The antifungal efficacy of the discs was assessed by the Kirby-Bauer (KB) technique. The drug-containing discs for each drug, at various drug release times as described above, were rinsed with 10 mL phosphate-buffered saline (PBS) and placed onto the surface of a yeast and mold (YM) agar plate (Fisher Scientific, Pittsburgh, PA, USA) containing overnight culture of 1 mL C. albicans (108-109 CFU). After incubation at 37°C for 24 hrs, the inhibition zone was measured with a ruler.
To test anti-biofilm activity, we immersed PMAA-based discs loaded with miconazole or CG in 10 mL buffer containing 108-109 CFU/mL of C. albicans. The mixture was gently shaken at 37°C for 1 hr. The discs were gently washed with PBS (3x, 10 mL), immersed individually in 10 mL YM broth, and incubated at 37°C for 3 days with constant low-speed shaking. Afterward, half of the discs were rinsed with 0.1 M sodium cacodylate buffer (SCB), fixed with 3% glutaraldehyde, and prepared for scanning electron microscopy (SEM; Hitachi S-3200N) study with dehydration/critical-point-drying and sputter-coating with gold (Cao et al., 2009). The other half were washed with PBS (3x, 10 mL), sonicated individually for 5 min, and vortexed for 1 min in 10 mL PBS. The recoverable Candida were determined by CFU after incubation of the serially diluted Candida PBS solution on YM agar at 37°C for 24 hrs.
Drug Quenching, Drug Recharging, and Drug Switching
To test drug “quenching” (wash-out), we immersed drug- containing discs individually in 5% EDTA aqueous solution for 8 hrs at room temperature. The resin-to-quenching-solution weight ratio was 1:50. The discs were washed with distilled water and air-dried. The quantity of remaining drugs in the disc was determined by Soxhlet extraction and UV measurement.
In testing rechargeability, either the quenched resin discs were recharged with drugs again, e.g., discs previously loaded with miconazole were either recharged with miconazole or switched to CG, or discs originally charged with CG were recharged with either CG or miconazole. The amount of recharged drugs on the discs was determined with Soxhlet extraction and UV measurement.
The data were expressed as means ± standard deviations, and p values for comparison of drug-loading capacity were calculated by one-way analysis of variance (ANOVA) followed by a post hoc Bonferroni test.
Results
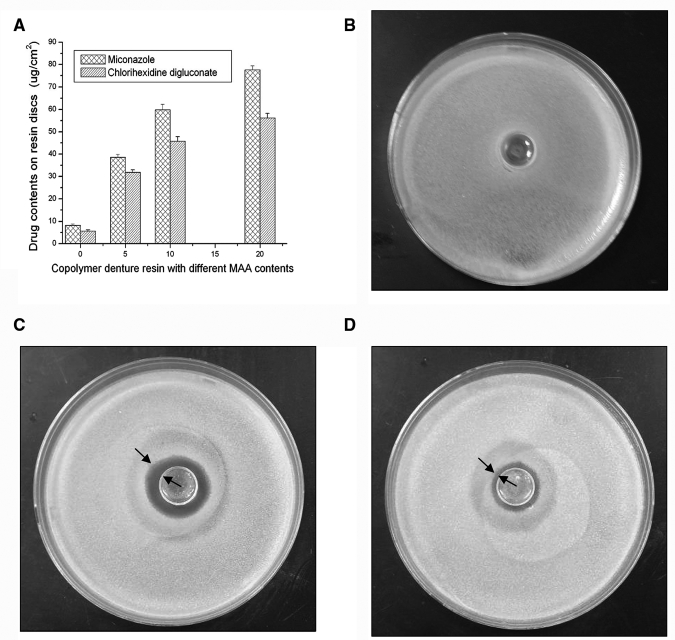
The DUDMA discs without MAA absorbed 8.2 ± 0.7 µg/cm2 of miconazole or 5.6 ± 0.6 µg/cm2 of CG (n = 5), respectively. In the DUDMA-MAA copolymers, drug-binding capacity increased with the increase of MAA content (Fig. 1A). With 10% of PMAA, the discs absorbed 59.8 ± 2.5 µg/cm2 of miconazole or 45.7 ± 2.1 µg/cm2 of CG, respectively (n = 5; p < 0.001 vs. the DUDMA control). The 10% PMAA resin discs were used for the subsequent studies.
Figure 1.
Drug-binding capability and anticandidal activity of the new PMAA-based resins. (A) Drug contents on resin discs with different weight percentages of PMAA (%), showing significant drug-binding capacity increases with the increase of PMAA contents (p < 0.001), and zone-of-inhibition results of (B) the original DUDMA disc, (C) PMAA-based DUDMA disc containing 10% of PMAA with 59.8 ± 2.5 µg/cm2 of absorbed miconazole, and (D) PMAA-based disc containing 10% of PMAA with 45.73 ± 2.1 µg/cm2 of absorbed CG.
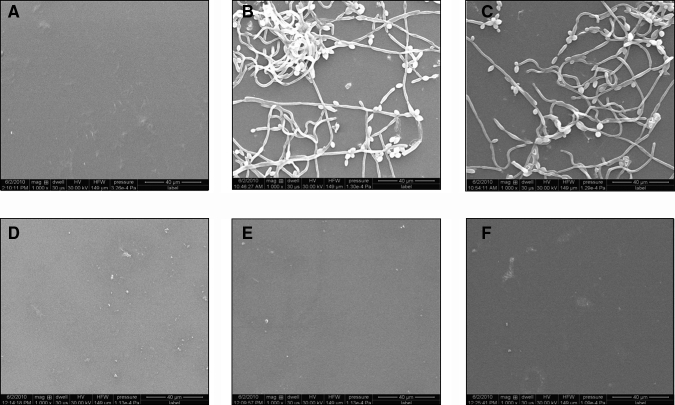
In zone-of-inhibition tests, the DUDMA discs did not provide any measurable inhibitory zones (Fig. 1B). In contrast, the PMAA-based disc containing miconazole produced a zone of 5.0 ± 0.4 mm in diameter, and the disc containing CG generated a zone of 2.0 ± 0.2 mm (Figs. 1C, 1D). In anti-biofilm tests, the control discs were covered with Candida biofilm after 3 days of incubation. However, the PMAA-based drug-containing discs showed no adherent cells/biofilm (Figs. 2B, 2C vs. 2E, 2F). These observations have been further confirmed with recoverable Candida colonies on the discs. While (26.0 ± 4.7) x 104 CFU/cm2 of C. albicans could be recovered from the DUDMA control discs, no C. albicans could be recovered from the drug-containing discs.
Figure 2.
Representative SEM images of 5 independent experiments (magnification: 1000x) showing that although biofilm formed on DUDMA discs, PMAA-based drug-containing discs inhibited Candida biofilm: (A) DUDMA disc before immersion in C. albicans, (B) representative image 1 of DUDMA disc after immersion in C. albicans for 3 days, (C) representative image 2 of DUDMA disc after immersion in C. albicans for 3 days, (D) PMAA-based disc containing 59.8 ± 2.5 µg/cm2 of miconazole before immersion in C. albicans, (E) representative image 1 of PMAA-based disc containing 59.8 ± 2.5 µg/cm2 of miconazole after immersion in C. albicans for 3 days, and (F) representative image 2 of PMAA-based disc containing 59.8 ± 2.5 µg/cm2 of miconazole after immersion in C. albicans for 3 days. Recoverable microbial level from the DUDMA discs was (26.0 ± 4.7) x 104 CFU/cm2 (n = 5). From the PMAA-based disc containing 59.8 ± 2.5 µg/cm2 of miconazole; however, no recoverable adherent C. albicans could be detected (n = 5).
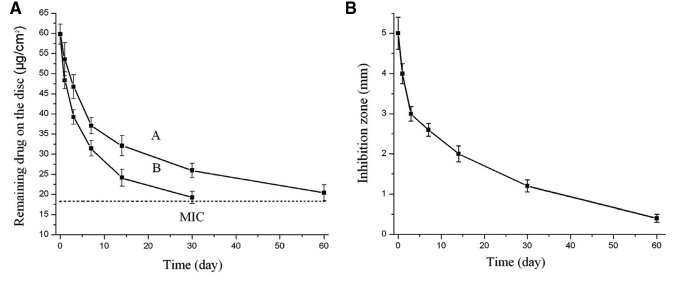
The drug release kinetic assay demonstrated a sustained release of miconazole from the PMAA-based resins. At neutral pH, the disc still contained 18.22 ± 1.2 µg/cm2 of miconazole after 60 days of immersion in the buffer (Fig. 3A, curve A). Miconazole release was about two times faster in an acidic environment (pH = 5). In parallel, the discs were removed from the buffer at indicated times for Candida inhibition tests. The inhibition zone decreased as the releasing period was extended (Fig. 3A, curve B), and reduced to 0.4 ± 0.1 mm after 60 days (pH = 7). Based on drug release kinetics and the Candida inhibition curve, 18.22 ± 1.2 µg/cm2 was defined as the minimum inhibitory content (MIC) of absorbed miconazole on the discs to provide a clear Candida inhibition zone.
Figure 3.
Sustained drug release and anticandidal effects of the new PMAA-based resins charged with miconazole. (A) Release curves of miconazole from the PMAA-based discs containing 10% of PMAA showing pH-sensitive drug release: curve A, neutral pH, and curve B, pH = 5.0 (original miconazole content, 59.8 ± 2.5 µg/cm2); and (B) effects of drug-releasing periods from the PMAA-based denture materials on inhibition zone size against the test Candida cells. The resin contained 10% of PMAA and had 59.8 ± 2.5 µg/cm2 of absorbed miconazole at the beginning of the test. The pH of the immersing solution was neutral.
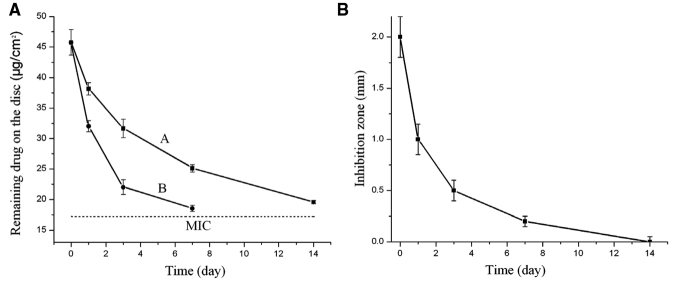
Similar pH-sensitive drug release kinetics was observed in the testing of PMAA-based resins with CG (Fig. 4). The MIC for the CG-containing PMAA disc was estimated at 17.24 ± 0.2 µg/cm2. CG content in the disc was still above MIC after 14 days of release at neutral pH. However, at pH 5, CG on the discs reached MIC after 7 days of release.
Figure 4.
Sustained drug release and anticandidal effects of the new PMAA-based resins charged with chlorhexidine digluconate (CG). (A) Release curves of CG from the PMAA-based discs containing 10% of PMAA, showing pH-sensitive drug release: curve A, neutral pH, and curve B, pH = 5.0 (original CG content, 45.73 ± 2.1 µg/cm2); and (B) effects of drug-releasing periods from the PMAA-based denture materials on inhibition zone size against the test Candida cells. The resin contained 10% of PMMA and had 45.73 ± 2.1 µg/cm2 of CG at the beginning of the test. The pH of the immersion solution was neutral.
To test whether drug-loaded PMAA-based resins can be quenched, we treated miconazole- or CG-containing discs with EDTA for 8 hrs. The remaining concentrations of miconazole on the PMAA-based disc decreased to 4.1 ± 2.9 µg/cm2 (i.e., 93.1% of the original miconazole was “washed out”). Similarly, after the EDTA treatment, the remaining CG on the disc was 2.1 ± 1.2 µg/cm2 (95.4 % of the original CG was “washed out”).
After the quenching treatment, the miconazole-depleted discs were re-immersed in miconazole solution for 24 hrs and achieved 61.8 ± 2.1 µg/cm2 of recharged miconazole (n = 5), which generated a Candida inhibition zone of 5.1 ± 0.3 mm (image not shown). Similarly, the quenched CG discs were again recharged with CG, and achieved 47.5 ± 2.1 µg/cm2 of CG (n = 5), which generated an inhibition zone of 2.2 ± 0.3 mm.
For drug-switching tests, the CG-quenched discs were recharged with miconazole, resulting in 58.9 ± 3.1 µg/cm2 of bound miconazole. Alternatively, the miconazole-quenched discs were recharged with CG, and achieved 45.2 ± 3.0 µg/cm2 of bound CG (n = 5).
Discussion
Antifungal denture materials have been described in several studies (Schneid, 1992; Matsuura et al., 1997; Chow et al., 1999; Lefebvre et al., 2001; Lin et al., 2003; Abe et al., 2004; Yoshinari et al., 2006; Redding et al., 2009). However, most antifungal dentures have short antifungal action that cannot be controlled based on clinical needs. We developed rechargeable infection-responsive antifungal denture materials by copolymerizing DUDMA with MAA (Sun, 2010). DUDMA resins are rapidly gaining popularity as denture materials which contain 2 vinyl double bonds, allowing for cross-linking of MAA into the denture resins. We used miconazole and CG as the model drugs, since miconazole gel has already been used to treat oral Candida infection, and CG mouthwash is known to have anticandidal activity.
The presence of up to 10% PMAA did not negatively affect the physical properties and biocompatibility of the original DUDMA resins (data not shown). The DUDMA resin itself has very low drug-binding capacity (Fig. 1A). After copolymerization with MAA, the drugs could be bound onto the denture materials through ionic interactions with the carboxylate groups (-COO-) of PMAA. Thus, drug-binding capacity increased significantly with the increase of PMAA content, resulting in potent antifungal effects (Figs. 1, 2).
The ionic interactions between the denture materials and the drugs led to a sustained drug release for weeks (CG) to months (miconazole). Since drug release was controlled by ionic interactions between the -COO- groups and the drugs, and -COO- content was affected by pH (-COOH ↔ -COO-), the drug release rate from the PMAA-based denture materials was pH-sensitive (Figs. 3A, 4A). This was explored as a biological “switch” in the infection-responsive drug delivery system: that is, while the normal oral environment has neutral pH, oral cavities infected with Candia have acidic conditions (Samaranayake et al., 1983; Nikawa et al., 1994). Therefore, the new anticandidal dentures are clinically expected to adjust drug release rates based on whether Candida is present and on the severity of the infections. With the same PMAA content, discs containing miconazole had much longer antifungal duration than discs containing CG (Fig. 3 vs. Fig. 4), which could be attributed to the higher water solubility of CG than miconazole.
Drugs on the new denture materials could be “washed out” by EDTA, a widely used chelating agent with 4 carboxylate groups on each molecule, which would compete with PMAA on the denture for the drugs. Under our quenching conditions, there were 30 times more carboxylate groups on EDTA than the carboxylate groups on the PMAA. Therefore, most of the drugs on the discs were “washed out” or “quenched” upon treatment with EDTA.
With these new denture materials, the released drugs could be repeatedly recharged to resume their antifungal activities. In subsequent recharging, drugs could be changed/switched, without affecting the drug-binding capacity. This unique “click-on/click-off” drug delivery feature is expected to revolutionize the management of CADS. For high-risk patients, the PMAA-containing denture can be worn as a conventional denture if CADS is not present. If CADS occurs, the dentures can be charged with drugs to initiate the antifungal therapy. When the infection is cleared, the dentures can be washed with EDTA to quench the therapeutic effects when no further drug release is needed. If CADS recurs, drugs can be recharged to re- initiate the treatment. In subsequent recharging, drugs can be changed/switched.
In conclusion, rechargeable infection-responsive antifungal denture materials were developed by the covalent binding of MAA onto DUDMA denture resins. The new denture materials bind and then slowly release antifungal drugs, leading to sustained, pH-sensitive drug release and prolonged antifungal effects. Drug release can be quenched by EDTA, and re-initiated by drug recharging based on disease conditions and/or the individual patient’s needs. In subsequent recharging, drugs can be changed/switched to potentially enhance inhibitory potency and/or reduce the risk of microbial resistance.
Acknowledgments
This study was sponsored by NIH, NIDCR (R03DE018735).
References
- Abe Y, Ishii M, Takeuchi M, Ueshige M, Tanaka S, Akagawa Y. (2004). Effect of saliva on an antimicrobial tissue conditioner containing silver-zeolite. J Oral Rehabil 31:568-573 [DOI] [PubMed] [Google Scholar]
- Arendorf TM, Walker DM. (1987). Denture stomatitis: a review. J Oral Rehabil 14:217-227 [DOI] [PubMed] [Google Scholar]
- Bhalekar MR, Pokharkar V, Madgulkar A, Patil N, Patil N. (2009). Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS PharmSciTech 10:289-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao ZB, Sun XB, Fong H, Sun YY. (2009). Rechargeable antibacterial and antifungal polymeric silver sulfadiazines. J Bioact Compat Polym 24:350-367 [Google Scholar]
- Chow CK, Matear DW, Lawrence HP. (1999). Efficacy of antifungal agents in tissue conditioners in treating candidiasis. Gerodontology 16:110-118 [DOI] [PubMed] [Google Scholar]
- Douglass CW, Shih A, Ostry L. (2002). Will there be a need for complete dentures in the United States in 2020? J Prosthet Dent 87:5-8 [DOI] [PubMed] [Google Scholar]
- Golecka M, Oldakowska-Jedynak U, Mierzwinska-Nastalska E, Adamczyk-Sosinska E. (2006). Candida-associated denture stomatitis in patients after immunosuppression therapy. Transplant Proc 38:155-156 [DOI] [PubMed] [Google Scholar]
- Lefebvre CA, Wataha JC, Cibirka RM, Schuster GS, Parr GR. (2001). Effects of triclosan on the cytotoxicity and fungal growth on a soft denture liner. J Prosthet Dent 85:352-356 [DOI] [PubMed] [Google Scholar]
- Lin DM, Kalachandra S, Valiyaparambil J, Offenbacher S. (2003). A polymeric device for delivery of anti-microbial and anti-fungal drugs in the oral environment: effect of temperature and medium on the rate of drug release. Dent Mater 19:589-596 [DOI] [PubMed] [Google Scholar]
- Matsuura T, Abe Y, Sato Y, Okamoto K, Ueshige M, Akagawa Y. (1997). Prolonged antimicrobial effect of tissue conditioners containing silver-zeolite. J Dent 25:373-377 [DOI] [PubMed] [Google Scholar]
- Nikawa H, Yamamoto T, Hayashi S, Nikawa Y, Hamada T. (1994). Growth and/or acid production of Candida albicans on soft lining materials in vitro. J Oral Rehabil 21:585-594 [DOI] [PubMed] [Google Scholar]
- Perezous LF, Flaitz CM, Goldschmidt ME, Engelmeier RL. (2005). Colonization of Candida species in denture wearers with emphasis on HIV infection: a literature review. J Prosthet Dent 93:288-293 [DOI] [PubMed] [Google Scholar]
- Radford DR, Challacombe SJ, Walter JD. (1999). Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit Rev Oral Biol Med 10:99-116 [DOI] [PubMed] [Google Scholar]
- Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW. (2004). Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98:53-59 [DOI] [PubMed] [Google Scholar]
- Raum K, Kempf K, Hein HJ, Schubert J, Maurer P. (2007). Preservation of microelastic properties of dentin and tooth enamel in vitro—a scanning acoustic microscopy study. Dent Mater 23:1221-1228 [DOI] [PubMed] [Google Scholar]
- Redding S, Bhatt B, Rawls HR, Siegel G, Scott K, Lopez-Ribot J. (2009). Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:669-672 [DOI] [PubMed] [Google Scholar]
- Samaranayake LP, Geddes DA, Weetman DA, MacFarlane TW. (1983). Growth and acid production of Candida albicans in carbohydrate supplemented media. Microbios 37:105-115 [PubMed] [Google Scholar]
- Schneid TR. (1992). An in vitro analysis of sustained release system for the treatment of denture stomatitis. Spec Care Dentist 12:245-250 [DOI] [PubMed] [Google Scholar]
- Senel S, Ikinci G, Kas S, Yousefi-Rad A, Sargon MF, Hincal AA. (2000). Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm 193:197-203 [DOI] [PubMed] [Google Scholar]
- Sun Y. (2010). Rechargeable long-term antifungal denture materials. US provisional patent application # 61306219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. (1998). Candida-associated denture stomatitis. Aetiology and management: a review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust Dent J 43:45-50 [DOI] [PubMed] [Google Scholar]
- Yap AU, Ng BL, Blackwood DJ. (2004). Corrosion behaviour of high copper dental amalgams. J Oral Rehabil 31:595-599 [DOI] [PubMed] [Google Scholar]
- Yoshinari M, Kato T, Matsuzaka K, Hayakawa T, Inoue T, Oda Y, et al. (2006). Adsorption behavior of antimicrobial peptide histatin 5 on PMMA. J Biomed Mater Res Part B Appl Biometer 77:47-54 [DOI] [PubMed] [Google Scholar]