Abstract
Periodontal infections have been associated with a state of chronic inflammation. To ascertain whether severe periodontitis and its treatment are associated with oxidative stress, we recruited 145 cases (periodontitis) and 56 controls in a case-control study. A further pilot intervention study of 14 cases (periodontal therapy) was performed. Blood samples were taken at baseline (case-control) and 1, 3, 5, 7, and 30 days after treatment (intervention). Diacron-reactive oxygen metabolites (D-ROM), anti-oxidant potential, C-reactive protein (CRP), interleukin-6, and lipid profiles were determined with high-sensitivity assays in serum. Patients with severe periodontitis exhibited higher D-ROM levels (P < 0.001) and lower total anti-oxidant capacity (P < 0.001) compared with healthy control individuals. These findings were independent of age, gender, smoking habits, ethnicity, and standard lipids differences. D-ROM levels were positively correlated with CRP (R = 0.4, P < 0.001) and clinical periodontal parameters (R = 0.20, P < 0.05). Acute increases of D-ROM (P < 0.01) were observed following periodontal therapy. Analysis of these data suggests a positive association between severe periodontitis and oxidative stress.
Keywords: periodontitis, Oxidative Stress, antioxidant, CRP, inflammation
Introduction
Reactive oxygen species (ROS) have emerged as important signaling molecules in various cellular processes. These molecules originate from molecular oxygen and predominantly produce cellular damage (proteins, lipids, and DNA) if not neutralized by anti-oxidant substances. Their production is an essential component of the host response to a variety of insults, including bacteria (Fialkow et al., 2007) and traumas/burns (Parihar et al., 2008). Major producers of ROS are mitochondria, cytochrome P-450 reactions, peroxisomal fatty acid metabolism, and NADPH oxidase activity (Hyslop et al., 1988; Downey et al., 1995). Oxidative stress is induced by an imbalance between excessive ROS production and anti-oxidant mechanisms. Increased levels of ROS leading to a state of Oxidative Stress have been implicated in the pathogenesis of a large number of diseases, including cardiovascular diseases and diabetes (Camera et al., 2007; Di Filippo et al., 2007; Castelao and Gago-Dominguez, 2008).
More recently, evidence has also emerged for a crucial role of ROS in periodontal tissue destruction (Chapple and Matthews, 2007). Taking into consideration that individuals suffering from periodontitis might be at higher risk of developing other chronic systemic inflammatory diseases, like cardiovascular diseases and diabetes (Pihlstrom et al., 2005), the potential role of ROS as one of the mechanisms of these association is worth exploring in more detail.
There is overwhelming evidence that chronic periodontitis induces a state of systemic inflammation, as assessed by serum levels of C-reactive protein (CRP) (Paraskevas et al., 2008). Individuals suffering from aggressive periodontitis present with an enhanced host response and metabolic changes (Salzberg et al., 2006). Successful periodontal therapy also reduces systemic markers of inflammation (D’Aiuto et al., 2004, 2005a; Paraskevas et al., 2008). Periodontal therapy, however, presents itself as a moderate inflammatory stimulus. Indeed, we reported that a single-sitting session of periodontal therapy (IPT) triggered an acute inflammatory response of one week’s duration (D’Aiuto et al., 2005b). This is also associated with vascular impairment (Tonetti et al., 2007). Nevertheless, the exact pathways involved in such responses are still under investigation.
Oxidative Stress has been linked with both onset of periodontal tissue destruction (Chapple and Matthews, 2007) and systemic inflammation (Basu et al., 2009). We therefore hypothesized an association between Oxidative Stress and systemic inflammation in people with severe periodontitis. The primary aim of this study is to compare measures of Oxidative Stress (D-ROM and BAP tests) between individuals suffering from severe periodontitis and control individuals. Our secondary aims include evaluating Oxidative Stress markers according to diagnosis of periodontitis, and characterizing their changes after IPT.
Materials & Methods
In total, 201 consenting individuals, selected from among patients referred to the UCL Eastman Dental Institute and Hospital, were included in this analysis. Cases (n = 145) were selected as suffering from severe generalized periodontitis if presenting with periodontal pockets with probing pocket depths of > 6 mm and marginal alveolar bone loss of > 30% in 50% or more of their teeth (D’Aiuto et al., 2005a). Fifty-six control individuals were recruited from among patients referred to other departments of the dental hospital and with no history of periodontal disease, and no tooth loss due to, or clinical signs of, periodontitis. We matched control individuals to cases by gender and age within 5 yrs, based on their medical records. Individuals (cases or controls) with any medical disorder, including cancer, type 2 diabetes, hypertension, and major cardiovascular/endocrine diseases, were excluded from the study. Smoking habits and ethnic origin were confirmed by a questionnaire. Clinical periodontal parameters were recorded by calibrated examiners as previously reported (D’Aiuto et al., 2005a). Cases included 118 individuals suffering from chronic and 27 from aggressive periodontitis. Diagnosis of aggressive periodontitis was made based on the 1999 Consensus Classification of Periodontal Diseases (Lang et al., 1999). The study was reviewed and approved by the local ethics committee.
A small subgroup of 14 consecutive cases (included with the above-mentioned criteria) was recruited into a small intervention trial of periodontal therapy (IPT). Therapy consisted of an intensive session of full-mouth subgingival root debridement delivered within a four-hour period under local anesthesia as previously described (D’Aiuto et al., 2005b). Serial blood samples were taken at baseline and 1, 3, 5, 7, and 30 days after treatment and processed in a fashion similar to that described above. Clinical periodontal parameters were recorded at baseline and 30 days after periodontal therapy.
Laboratory Measurements
Blood samples were collected in plain vacutainer tubes (Becton Dickinson, Plymouth, England), immediately processed (centrifuged at 2000 rpm for 15 min), and stored in several aliquots in a -70°C freezer. Serum concentrations of CRP (Tina Quant immunoturbidimetric assay, Roche, Mannheim, Germany) and IL-6 (Quantikine ELISA, R&D Systems, Minneapolis, MN, USA) were quantified with high-sensitivity assays according to manufacturer instructions. Further standard lipid profiles (total, HDL, and LDL cholesterol, triglycerides) were determined by routine biochemistry. Photometric quantification of both reactive oxygen metabolites (D-ROM test expressed in relative units, one Carratelli unit (UCarr) equivalent to 0.08 mg/dL of a hydrogen peroxide water solution) (Vassalle et al., 2007; Vassalle, 2008) and plasma biological anti-oxidant potential (BAP test of agents able to reduce the iron from its ferric Fe3+ to ferrous form Fe2+) (Komatsu et al., 2009) was performed on all samples with a multiple analyzer according to manufacturer instructions (Diacron International, Grosseto, Italy). Briefly, all samples were diluted 1:100 with distilled water. For the D-ROM test, 10 µL of sample and 1 mL of acetate buffer (pH 4.8) were mixed with 20 mL chromogenic substrate (N,N-diethylparaphenylendiamine). After incubation in an automated analyzer (Free Radical Elective Evaluator, Diacron International) for 5 min at 37°C, the 505-nm absorbance was recorded. Similarly for the BAP test, a 10-µL quantity of sample was dissolved with a solution of ferric ions (ferric chloride, FeC13) and a chromogen (ammonium thiocyanate, NH4SCN). After a five-minute incubation period at 37°C, the entity of decoloration was detected photometrically on the same analyzer as absorbance change at 505 nm. Inter-assay coefficients of variation for both assays were < 5%.
Statistical Analysis
A sample size of a minimum of 141 cases and 47 controls (ratio 3:1) was sufficient to detect a 20% difference in Oxidative Stress between study groups, with a standard deviation of 105 UCarr 95% power, and alpha set at 0.05. Data are reported as means ± SD unless specified. Because matching was performed as the most convenient means for selecting control individuals comparable with cases in age and gender, and not as the principal means for confounding, matching was not preserved in the analysis. Comparisons of continuous variables of Oxidative Stress between cases and control individuals were performed with analysis of variance (ANOVA). Univariate general linear models with D-ROM and BAP (dependent variables) were also performed, accounting for smoking habits, ethnicity, and standard lipids differences (covariates). We then used unconditional logistic regression to explore the association between diagnosis of periodontitis and higher Oxidative Stress (defined as UCarr units greater than 400) (Tamaki et al., 2008, 2009), adjusting for the matching variables age (continuous) and gender (male, female), and for other variables linked to Oxidative Stress such as smoking habits, ethnicity, and lipids. Correlation analyses were performed with the Spearman test. In the substudy of periodontal therapy, we performed repeated-measures ANOVA to compare changes in Oxidative Stress over time, using a conservative F-test for time-by-treatment interaction. Logarithmic transformation of the data was performed where appropriate, and post hoc Fisher paired and unpaired least-significant-difference tests were performed and interpreted with the Bonferroni–Holm adjustment for multiple comparisons. The alpha value was set at 0.05 with SPSS software (version 17, Chicago, IL, USA).
Results
Case-Control
The Table summarizes both study groups in terms of common general characteristics. No statistically significant differences were found for all demographic parameters and standard lipid profiles. Nevertheless, cases exhibited a statistically significant increase in serum levels of CRP compared with control individuals (mean difference between groups of 1.6 ± 0.8 mg/L, P = 0.036).
Table.
Study Sample Characteristics
| Groups (Mean ± SD) |
|||
|---|---|---|---|
| Characteristic | Periodontitis (n = 145) | Controls (n = 56) | P Value |
| Age, yrs | 47.3 ± 8.3 | 46.4 ± 7.4 | 0.704 |
| Gender, Males, n (%) | 70 (48.4) | 28 (49.7) | 0.923* |
| Smokers, Never, n (%) | 101 (69.3) | 38 (68.2) | 0.828* |
| Ethnicity, Caucasians, n (%) | 102 (70.6) | 42 (74.2) | 0.523* |
| IL-6, pg/mL | 2.3 ± 2.3 | 2.1 ± 4.4 | 0.891 |
| CRP, mg/L | 3.0 ± 4.1 | 1.4 ± 1.2 | 0.036 |
| Cholesterol, mmol/L | 5.2 ± 1.1 | 5.2 ± 0.9 | 0.922 |
| HDL, mmol/L | 1.5 ± 0.4 | 1.6 ± 0.4 | 0.083 |
| LDL, mmol/L | 3.1 ± 1.0 | 2.8 ± 0.8 | 0.241 |
| Triglycerides, mmol/L | 1.5 ± 1.3 | 1.5 ± 1.0 | 0.898 |
| D-ROM (UCarr) | 423.5 ± 114.0 | 308.1 ± 135.1 | < 0.001 |
| BAP (mmol/L) | 2511.9 ± 254.4 | 5117.7 ± 822.3 | < 0.001 |
P values are calculated from comparisons between test and control individuals with ANOVA, with the exception of categorical variables compared with Chi-square test (asterisks).
Patients with severe periodontitis exhibited higher serum Oxidative Stress D-ROM (mean difference ± SE of 115.4 ± 23 UCarr P < 0.001) and lower total anti-oxidant capacity BAP (mean difference ± SE of 2605.9 ± 176.5 mmol/L, P < 0.001) when compared with control individuals (Fig. 1). These findings were independent of matching variables (age and gender) and additional confounders, including ethnicity, smoking habits, and standard lipid profiles. Diagnosis of periodontitis was associated with 3.6 odds of having higher levels of Oxidative Stress (95% CI 1.2–10.5) independently of matching variables, smoking habits, and lipids differences. Further, there was a not-statistically-significant association between diagnosis of aggressive periodontitis and higher levels of Oxidative Stress (Fig. 1C) and lower anti-oxidant capacity (Fig. 1D) resulting from both logistic and multiple regression analyses.
Figure 1.
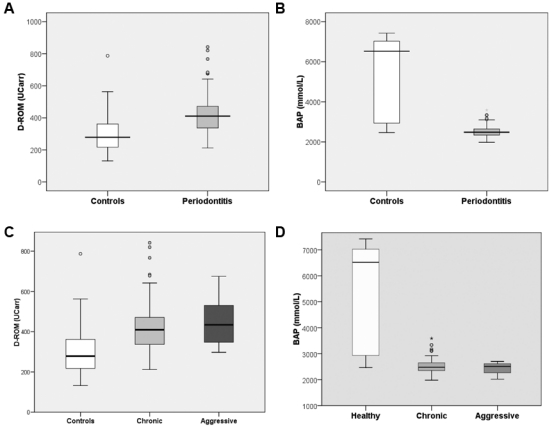
Box and whiskers plots of Oxidative Stress. (A) D-ROM (UCarr units) and anti-oxidant (B) BAP (mmol/L) measurements between individuals with periodontitis compared with control individuals. (C) D-ROM (UCarr units) and anti-oxidant (D) BAP (mmol/L) measurements among individuals with diagnosis of chronic (n = 122) or aggressive (n = 23) periodontitis compared with control individuals (n = 56). Boxes refer to the 25th (bottom) and 75th (up) percentiles, and the median is the large horizontal line; fences refer to the 10th (lower) and 90th (upper) percentiles, respectively. Open circles represent outliers.
Multiple regression analysis resulted in a statistically significant association between probing depths and serum D-ROM values (corrected model P < 0.0001, F = 10, β ± SE 30.0 ± 11.2, P = 0.009). In addition, D-ROM levels were also associated with gender (β ± SE 103.9 ± 17, P < 0.0001). When CRP serum levels (β ± SE 12.3 ± 1.9, P < 0.001) were included as covariates in the model (corrected model P < 0.0001, F = 16.1), probing pocket depths were no longer statistically significantly associated with D-ROM levels (β ± SE, 15.8 ± 10.0, P = 0.115). In the same model, age (β ± SE 3.0 ± 1.2, P = 0.01) and gender (β ± SE 84.4 ± 15.0, P < 0.001) were associated with D-ROM levels. No statistically significant associations were found with BAP values (data not shown).
BAP and D-ROM levels were negatively correlated (r = -0.4, P < 0.001). A positive linear correlation between D-ROM levels and markers of systemic inflammation (CRP, r = 0.4, P < 0.001) was observed in all individuals (Fig. 2A). Among cases, however, the magnitude of this association increased (CRP, r = 0.5, P < 0.001). Further whole-mouth probing pocket depths were weakly positively correlated with D-ROM serum levels (R = 0.20, P = 0.03) (Fig. 2B), and there was a not-statistically-significant negative correlation with BAP results (R = -0.2, P = 0.075).
Figure 2.
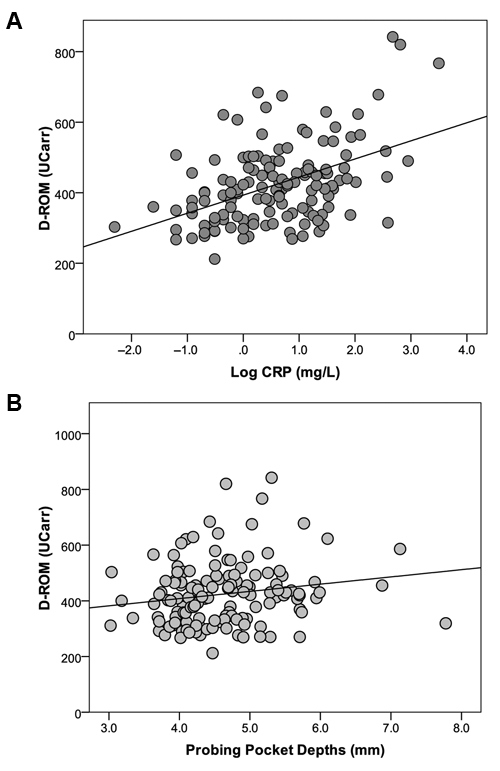
Scatter plot of serum D-ROM (UCarr) against (A) CRP levels (R = 0.4, P < 0.001), and (B) severity of periodontitis (Probing pocket depths, mm) (R = 0.2, P = 0.04) by Spearman’s rank correlation test.
Intervention Study
Following IPT, a marked increase in D-ROM levels (P < 0.01) was found over the first week after periodontal therapy. In particular, statistically significant increases were noted after 1 day (mean increase ± SE of 105.2 ± 12.0 UCarr, P = 0.001), 3 days (mean increase ± SE of 124.0 ± 56.5 UCarr, P = 0.045), and 5 days (mean increase ± SE of 166.3 ± 48.3 UCarr, P = 0.026) compared with baseline (Fig. 3A). This acute rise in Oxidative Stress was independent of age, gender, ethnicity, smoking habits, and standard lipid profiles differences. Treatment time recorded in minutes was positively correlated with the changes in Oxidative Stress (P = 0.023). No major changes were observed in BAP except on day 1 after therapy (Fig. 3B).
Figure 3.
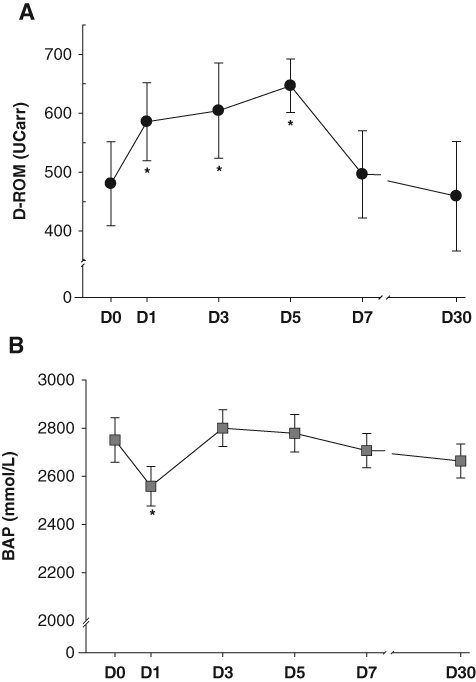
Mean values (standard errors) of serum (A) D-ROM (UCarr) and (B) BAP (mmol/L) before (D0), 1 (D1), 3 (D3), 5 (D5), 7 (D7), and 30 (D30) days after intensive periodontal therapy. Asterisks refer to statistically significant difference (P < 0.05) between visits as computed with analysis of variance for repeated measures (post hoc comparison with Bonferroni corrections).
Discussion
This study provides evidence that periodontitis, a common potential source of low-grade inflammation, is associated with a systemic Oxidative Stress state and reduced global anti-oxidant capacity. A direct, albeit weak, linear association among clinical measures of periodontitis, systemic inflammation, and systemic Oxidative Stress may exist. We did not observe a difference in terms of inflammation or Oxidative Stress based on diagnosis of chronic or aggressive periodontitis. Further, we report that IPT produces short-lived acute changes in Oxidative Stress, resembling those of an acute-phase response.
These results are in broad agreement with the published reports on gingival and saliva levels of ROS and anti-oxidant molecules in people with periodontitis (Chapple and Matthews, 2007a). However, inconsistent evidence of increased serum levels of markers of Oxidative Stress—including D-ROM (Tamaki et al., 2008), protein carbonyl (Baltacioglu et al., 2008), and ROOH (Akalin et al., 2007)—between individuals with periodontitis and control individuals has been published in the last 5 years. Smaller sample sizes, cross-sectional design, and highly variable and non-comparable measures of Oxidative Stress might explain this discrepancy.
A large body of evidence implicates Oxidative Stress in the pathogenesis of periodontal tissue destruction. Numerous studies indicated that an excess of ROS and depletion of anti-oxidant levels in gingival crevicular fluid (Tsai et al., 2005; Chapple and Matthews, 2007; Konopka et al., 2007) are responsible for the chronic local activation of periodontal inflammation and tissue destruction. Recruitment of neutrophils at the gingival site and release of proteolytic enzymes and ROS are today considered the two main aspects of the host response upon bacterial antigen stimulation in periodontitis-susceptible individuals. This process might also result in a systemic inflammatory response (Matthews et al., 2007). Further evidence from animal models confirms higher levels of lipid peroxidation, hydrogen peroxides, and oxidative DNA damage with experimental periodontitis (Yamamoto et al., 2010).
Some previous studies have also demonstrated that anti-oxidant levels and potential are reduced in both gingival and serum of individuals suffering from periodontitis (Chapple et al., 2007; Konopka et al., 2007). This has been replicated in experimental models of periodontitis demonstrating a reduction in vitamin C (Sanbe et al., 2009) and overall decreased gingival oxidant defenses (Tomofuji et al., 2006).
Fewer studies have examined the relationship between Oxidative Stress inflammation and periodontitis (Chapple et al., 2007a). Our results tend to suggest that Oxidative Stress observed in patients with periodontitis could be closely linked to biomarkers of inflammation, including CRP. Within the limitations of our analysis, the inclusion of CRP levels in a linear regression model attenuated the magnitude of association between clinical measures of periodontitis (probing pocket depth) and D-ROM levels. We could therefore speculate that the observed association between probing depths and Oxidative Stress could be mediated by CRP levels. An alternative mechanism could be the association between measures of periodontitis (i.e., probing depths), systemic dissemination of bacteria (low-grade bacteremia) and subsequent oxidative damage at various sites in the host, resulting in higher oxidative stress (Lockhart et al., 2008). Further research will be needed to elucidate their temporal association and the mechanisms involved.
Diagnosis of aggressive vs. chronic periodontitis has been associated with an enhanced host response as defined by higher serum levels of inflammatory markers (CRP) (Salzberg et al., 2006). However, this seems not to be associated with different levels of Oxidative Stress. Our data are in agreement with some previous reports that failed to find a statistically significant difference between individuals with aggressive and those with chronic periodontitis (Konopka et al., 2007).
After IPT, we have previously shown a one-week inflammatory acute response and acute perturbation of the coagulation and endothelial cell activation pathways (D’Aiuto et al., 2005b). In this study, we also observed some marked changes in Oxidative Stress, but weaker in total anti-oxidant capacity. Sustained increases in Oxidative Stress were observed up to 5 days from a single session of IPT. These findings, if confirmed, would suggest that periodontal therapy could not only alter the local ROS production and anti-oxidant state (Chapple and Matthews, 2007), but also the host systemic oxidative state. In turn, this host perturbation could represent one of the mechanisms behind the association between periodontal therapy and vascular function changes (Tonetti et al., 2007). Further research on the correlation of vascular measures with Oxidative Stress after periodontal therapy could help answer some of these questions.
We wish to highlight, however, several limitations of our experiments that would urge caution in generalizing these data to all individuals suffering from periodontitis. Indeed, the nature of the studies presented does not allow us to infer whether the Oxidative Stress state preceded periodontitis development or vice versa. A trend of reduction of Oxidative Stress (D-ROM) measured 1 month after therapy, as well as recent evidence (Tamaki et al., 2008, 2009) would suggest, though, that successful periodontal therapy could reduce Oxidative Stress. Further, we have studied a population with severe generalized periodontitis, and therefore our findings could not be extrapolated to all individuals suffering from milder forms of periodontitis. Both D-ROM and BAP test are global measures of Oxidative Stress and therefore lack specificity as to which exact ROS or anti-oxidants are affected by the presence and treatment of periodontitis. Several factors, including lipid levels, could have affected our findings, even if the statistical analyses adjusted for these factors. This is why properly sized trials should be conducted to provide further evidence on this association.
In conclusion, analysis of these data indicates that severe periodontitis is independently associated with increased Oxidative Stress and reduced anti-oxidant capacity. In addition, acute inflammation following IPT is also associated with raised systemic levels of reactive oxygen species.
Acknowledgments
We thank Prof. Eugenio Iorio for assistance with the laboratory oxidative stress analyses.
Footnotes
This work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. Dr. D’Aiuto holds a Clinical Senior Lectureship Award supported by the UK Clinical Research Collaboration.
References
- Akalin FA, Baltacioglu E, Alver A, Karabulut E. (2007). Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol 34:558-565 [DOI] [PubMed] [Google Scholar]
- Baltacioglu E, Akalin FA, Alver A, Deger O, Karabulut E. (2008). Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Arch Oral Biol 53:716-722 [DOI] [PubMed] [Google Scholar]
- Basu S, Helmersson J, Jarosinska D, Sallsten G, Mazzolai B, Barregard L. (2009). Regulatory factors of basal F(2)-isoprostane formation: population, age, gender and smoking habits in humans. Free Radic Res 43:85-91 [DOI] [PubMed] [Google Scholar]
- Camera A, Hopps E, Caimi G. (2007). Diabetic microangiopathy: physiopathological, clinical and therapeutic aspects. Minerva Endocrinol 32:209-229 [PubMed] [Google Scholar]
- Castelao JE, Gago-Dominguez M. (2008). Risk factors for cardiovascular disease in women: relationship to lipid peroxidation and oxidative stress. Med Hypotheses 79:31-44 [DOI] [PubMed] [Google Scholar]
- Chapple IL, Matthews JB. (2007). The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000 43:160-232 [DOI] [PubMed] [Google Scholar]
- Chapple IL, Brock GR, Milward MR, Ling N, Matthews JB. (2007a). Compromised GCF total antioxidant capacity in periodontitis: cause or effect? J Clin Periodontol 34:103-110 [DOI] [PubMed] [Google Scholar]
- Chapple IL, Milward MR, Dietrich T. (2007b). The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr 137:657-664 [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, et al. (2004). Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res 83:156-160 [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. (2005a). Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res 84:269-273 [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Parkar M, Tonetti MS. (2005b). Periodontal therapy: a novel acute inflammatory model. Inflamm Res 54:412-414 [DOI] [PubMed] [Google Scholar]
- Di Filippo C, Verza M, Coppola L, Rossi F, D’Amico M, Marfella R. (2007). Insulin resistance and postprandial hyperglycemia the bad companions in natural history of diabetes: effects on health of vascular tree. Curr Diabetes Rev 3:268-273 [DOI] [PubMed] [Google Scholar]
- Downey GP, Fukushima T, Fialkow L. (1995). Signaling mechanisms in human neutrophils. Curr Opin Hematol 2:76-88 [DOI] [PubMed] [Google Scholar]
- Fialkow L, Wang Y, Downey GP. (2007). Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 42:153-164 [DOI] [PubMed] [Google Scholar]
- Hyslop PA, Hinshaw DB, Halsey WA, Jr, Schraufstatter IU, Sauerheber RD, Spragg RG, et al. (1988). Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem 263:1665-1675 [PubMed] [Google Scholar]
- Komatsu F, Kagawa Y, Ishiguro K, Kawabata T, Purvee B, Otgon J, et al. (2009). The association of very high hair manganese accumulation and high oxidative stress in Mongolian people. Curr Aging Sci 2:28-42 [DOI] [PubMed] [Google Scholar]
- Konopka T, Krol K, Kopec W, Gerber H. (2007). Total antioxidant status and 8-hydroxy-2′-deoxyguanosine levels in gingival and peripheral blood of periodontitis patients. Arch Immunol Ther Exp (Warsz) 55:417-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang NP, Bartold M, Cullinan M, Jeffcoat M, Mombelli A, Murakami S, et al. (1999). Consensus Report: Aggressive Periodontitis. Annals Periodontol 4:53 [Google Scholar]
- Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. (2008). Bacteremia associated with toothbrushing and dental extraction. Circulation 117:3118-3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JB, Wright HJ, Roberts A, Ling-Mountford N, Cooper PR, Chapple IL. (2007). Neutrophil hyper-responsiveness in periodontitis. J Dent Res 86:718-722 [DOI] [PubMed] [Google Scholar]
- Paraskevas S, Huizinga JD, Loos BG. (2008). A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol 35:277-290 [DOI] [PubMed] [Google Scholar]
- Parihar A, Parihar MS, Milner S, Bhat S. (2008). Oxidative stress and anti-oxidative mobilization in burn injury. Burns 34:6-17 [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. (2005). Periodontal diseases. Lancet 366:1809-1820 [DOI] [PubMed] [Google Scholar]
- Salzberg TN, Overstreet BT, Rogers JD, Califano JV, Best AM, Schenkein HA. (2006). C-reactive protein levels in patients with aggressive periodontitis. J Periodontol 77:933-939 [DOI] [PubMed] [Google Scholar]
- Sanbe T, Tomofuji T, Ekuni D, Azuma T, Irie K, Tamaki N, et al. (2009). Vitamin C intake inhibits serum lipid peroxidation and osteoclast differentiation on alveolar bone in rats fed on a high-cholesterol diet. Arch Oral Biol 54:235-240 [DOI] [PubMed] [Google Scholar]
- Tamaki N, Tomofuji T, Maruyama T, Ekuni D, Yamanaka R, Takeuchi N, et al. (2008). Relationship between periodontal condition and plasma reactive oxygen metabolites in patients in the maintenance phase of periodontal treatment. J Periodontol 79:2136-2142 [DOI] [PubMed] [Google Scholar]
- Tamaki N, Tomofuji T, Ekuni D, Yamanaka R, Yamamoto T, Morita M. (2009). Short-term effects of non-surgical periodontal treatment on plasma level of reactive oxygen metabolites in patients with chronic periodontitis. J Periodontol 80:901-906 [DOI] [PubMed] [Google Scholar]
- Tomofuji T, Azuma T, Kusano H, Sanbe T, Ekuni D, Tamaki N, et al. (2006). Oxidative damage of periodontal tissue in the rat periodontitis model: effects of a high-cholesterol diet. FEBS Lett 580:3601-3604 [DOI] [PubMed] [Google Scholar]
- Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. (2007). Treatment of periodontitis and endothelial function. N Engl J Med 356:911-920 [DOI] [PubMed] [Google Scholar]
- Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, et al. (2005). Lipid peroxidation: a possible role in the induction and progression of chronic periodontitis. J Periodontal Res 40:378-384 [DOI] [PubMed] [Google Scholar]
- Vassalle C. (2008). An easy and reliable automated method to estimate oxidative stress in the clinical setting. Methods Mol Biol 477:31-39 [DOI] [PubMed] [Google Scholar]
- Vassalle C, Maffei S, Boni C, Zucchelli GC. (2008). Gender-related differences in oxidative stress levels among elderly patients with coronary artery disease. Fertil Steril 89:608-613 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Tomofuji T, Tamaki N, Ekuni D, Azuma T, Sanbe T. (2010). Effects of topical application of lipopolysaccharide and proteases on hepatic injury induced by high-cholesterol diet in rats. J Periodontal Res 45:129-135 [DOI] [PubMed] [Google Scholar]


