Abstract
The subgingival microbiome is largely uncultivated, and therefore, cultivation-based and targeted molecular approaches have limited value in examining the effect of smoking on this community. We tested the hypothesis that the subgingival biofilm is compositionally different in current and never-smokers by using an open-ended molecular approach for bacterial identification. Subgingival plaque from deep sites of current and never-smokers matched for disease was analyzed by 16S sequencing. Smokers demonstrated greater abundance of Parvimonas, Fusobacterium, Campylobacter, Bacteroides, and Treponema and lower levels of Veillonella, Neisseria, and Streptococcus. Several uncultivated Peptostreptococci, Parvimonas micra, Campy-lobacter gracilis, Treponema socranskii, Dialister pneumosintes, and Tannerella forsythia were elevated in this group, while Veillonella sp. oral clone B2, Neisseria sp. oral clone 2.24, Streptococcus sanguinis, and Capnocytophaga sp. clone AH015 were at lower levels. The microbial profile of smoking-associated periodontitis is distinct from that of non-smokers, with significant differences in the prevalence and abundance of disease-associated and health-compatible organisms.
Keywords: bacteria, subgingival, molecular, DNA, smoking, periodontitis
Introduction
Evidence indicates that chronic periodontitis results from the presence of complex microbial communities in the subgingival sulcus (Socransky and Haffajee, 2005), and that smoking significantly increases the risk for the development of extensive and severe disease (Tomar and Asma, 2000; Johnson and Hill, 2004). Although both bacterial plaque and smoking play important roles in the pathogenesis of periodontitis, associations between smoking and subgingival bacterial species or consortia have not been well-elucidated.
Earlier investigations have used traditional methods for bacterial identification—for example, culturing or targeted DNA-based assays (PCR, real-time PCR, DNA-DNA hybridization) (Stoltenberg et al., 1993; Darby et al., 2000; Bostrom et al., 2001; Haffajee and Socransky, 2001; van Winkelhoff et al., 2001; Van der Velden et al., 2003; Apatzidou et al., 2005; Natto et al., 2005). However, recent investigations have revealed that the subgingival microbiome is largely uncultivated, and that several uncultivated phylotypes may be important in disease etiology (Kumar et al., 2006). Because culturing selects for organisms that grow under certain conditions and targeted molecular approaches are limited to the examination of only previously known species, these tools are inadequate for a comprehensive examination of this complex ecosystem. An exploration of this microbiome by a comprehensive, quantitative, open-ended molecular approach will advance our understanding of the effect of smoking on the composition of the subgingival biofilm.
Researchers have extensively used 16S cloning and sequencing to examine several host-associated ecosystems (Bittar et al., 2008). The presence of large tracts of variable sequences within the 16S rRNA gene provides unique bacterial ‘signatures’ that allow for accurate bacterial identification, while sequencing a finite number of clones from each sample provides quantitative information on the relative abundance of each organism within a community. We have previously used this assay to provide representational information on previously unknown and unsuspected bacteria associated with periodontal health and disease in non-smokers (Kumar et al., 2005, 2006).
The purpose of the present investigation was to explore the subgingival microbiome of current and never-smokers with periodontitis using 16S cloning and sequencing for bacterial identification.
Methods
Participant Selection
Approval for this study was obtained from the Office of Responsible Research Practices at The Ohio State University. Fifteen current smokers (tobacco exposure of 10 pack-years or more) and 15 age- and sex-matched never-smokers with generalized moderate to severe chronic periodontitis (attachment loss ≥ 6 mm and probe depths ≥ 5 mm in 30% or more of sites) were identified following clinical and radiographic examination and after informed consent had been obtained. Exclusion criteria for both groups included diabetes, HIV infection, use of immunosuppressant medications, bisphosphonates, or steroids, antibiotic therapy, or oral prophylactic procedures within the preceding 3 mos, and fewer than 20 teeth in the dentition.
Data Collection
Smoking status and tobacco exposure were assessed by questionnaire. All participants were examined by a single periodontist. Probe depths and attachment levels were recorded throughout the mouth on 6 sites per tooth by means of a PCP-UNC 15 probe. Bleeding on probing and plaque levels were recorded on a binary scale (presence/absence) for each surface.
Sample Collection and DNA Isolation
Subgingival plaque samples were collected and pooled from 4 non-adjacent proximal sites demonstrating at least 6 mm of attachment loss and 5 mm of probe depths. We collected samples by inserting 15 sterile endodontic paper-points (Caulk-Dentsply, Milford, DE, USA) into the pockets for 10 sec, following isolation and supragingival plaque removal. Samples were placed in 1.5-mL microcentrifuge tubes and frozen until further analysis. Bacteria were separated from the paper-points by the addition of 200 µL of phosphate-buffered saline (PBS) to the tubes, followed by vortexing. The points were then removed, and DNA was isolated with a Qiagen DNA MiniAmp kit (Qiagen, Valencia, CA, USA) using the tissue protocol according to the manufacturer’s instructions.
Amplification of 16S rDNA
Bacterial 16S rRNA genes were amplified from the community DNA with universal bacterial primers A17 (5′-GTT TGA TCC TGG CTC AG- 3′) and 317 (5′AAG GAG GTG ATC CAG GC 3′) (Biosynthesis, Lewisville, TX, USA). PCR was performed as previously described (Kumar et al., 2005). The PCR products were purified with the Qiaquick PCR purification kit (Qiagen, Valencia, CA, USA).
Cloning and Sequencing
The 16S amplicons generated by PCR were cloned into E. coli with the use of a commercially available kit (TOPO TA cloning kit, Invitrogen, San Diego, CA, USA). Inserts of the correct molecular size (≅ 1500 bp) were confirmed by PCR amplification. The products were purified with a Millipore kit (Millipore, Billerica, MA, USA) and sequenced with an ABI Prism cycle sequencing kit (BigDye Terminator Cycle Sequencing kit) with an ABI 3730 instrument.
Sequence Analysis
Partial sequences of 1100 to 1300 bp were obtained from each amplicon. The sequences generated were compared with the GenBank database for genus-level identification. Sequences belonging to the same genus were then aligned to each other, and a similarity matrix was constructed from the alignments by the method of Jukes and Cantor (1969). Phylogenetic trees were constructed by the ‘neighbor joining’ method. Sequence similarity of 95% was used to delineate genera, and sequences with 98% or greater similarity within a genus were considered the same species. MacVector software (MacVector, Cary, NC, USA) was used to generate alignments and similarity matrices, and in phylogenetic tree construction.
Statistical Analysis
A minimum of 100 clones were identified from each sample. Statistical analysis was carried out with JMP (SAS Institute Inc., Cary, NC, USA). A variance-stabilizing transformation was used to create normal distribution of the data. The proportion (p) of each species in the community of each subject was expressed as:
We used a two-sample t test to compare the means of this transformed variable X between current and never-smokers. Fisher’s exact test was used to test for the presence or absence of species and genera in smokers and non-smokers. Regression analysis with an interaction term was used to examine the effect of smoking on inter-bacterial relationships. Reported p-values correspond to two-tailed tests.
Results
Fifteen current smokers and 15 never-smokers with generalized moderate to severe chronic periodontitis were recruited. There were no differences in clinical and demographic characteristics between the two groups except for tobacco exposure and bleeding on probing (Table 1).
Table 1.
Clinical and Demographic Characteristics of Participants (mean ± standard deviation)
| Current Smokers (N = 15) | Never-smokers (N = 15) | p-value | |
|---|---|---|---|
| Mean age (yrs) | 52.3 ± 2.1 | 50.2 ± 3.3 | 0.53 |
| Gender (male) | 11 | 11 | |
| Tobacco exposure (mean pack-yrs ± SD) | 18.3 ± 2.7 | 0 | 0.007 |
| Clinical parameters at sampled sites | |||
| Mean plaque index (± SD) | 2.3 ± 0.6 | 2.6 ± 0.4 | 0.32 |
| Mean bleeding on probing (% ± SD) | 37.0 ± 2.8 | 52.8 ± 1.2 | 0.043 |
| Mean clinical attachment levels (mm ± SD) | 7.0 ± 0.6 | 6.8 ± 0.8 | 0.56 |
| Mean probing depths (mm ± SD) | 6.7 ± 0.3 | 7.1 ± 0.5 | 0.72 |
Significant differences in tobacco exposure and bleeding on probing were seen between the two groups (two-sample t test) (AYS, HNN, PSK).
In total, 3157 clones were identified, representing 197 species/phylotypes in current smokers and 176 in never-smokers. The mean number of species in individuals who smoked was 37.1 ± 10.5, while never-smokers demonstrated 36.8 ± 9.1 species (p = 0.67, t test). There was no difference in the number of as-yet-uncultivated phylotypes between current and never-smokers (38.8% and 44.5%, respectively, p = 0.09, two-sample t test on transformed clone numbers). In both current and never-smokers, the taxa Synergistes, Lachnospira, and Desulfobulbus were composed entirely of as-yet-uncultivated phylotypes, and Selenomonas, Neisseria, Veillonella, Eubacterium, and Catonella were predominantly uncultivated (Fig. 1).
Figure 1.
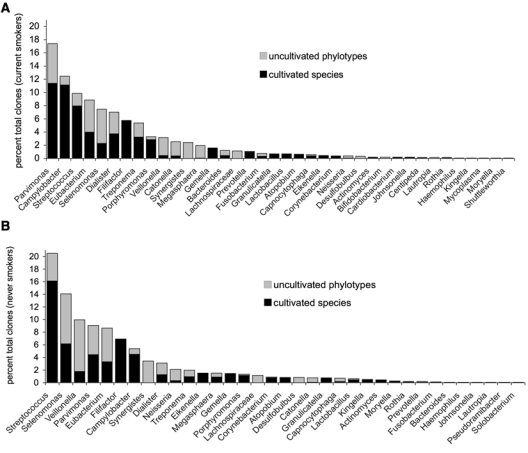
Distribution of cultivated species and uncultivated phylotypes by genus in 15 current smokers (A) and 15 never-smokers (B). There was no difference in the number of cultivated or as-yet-uncultivated organisms between the two groups (p > 0.05, two-sample t test on transformed data) (AYS, HNN, PSK).
Current smokers demonstrated significantly greater levels of the genera Parvimonas, Campylobacter, Treponema, Bacteroides, and Fusobacterium, while never-smokers demonstrated higher levels of Streptococcus, Veillonella, and Neisseria (p < 0.05, two-sample t test) (Fig. 2A). The genus Neisseria was detected more frequently in never-smokers (p = 0.01, Fisher exact test), while Bacteroides were detected more frequently in current smokers (p < 0.05) (Fig. 2B).
Figure 2.
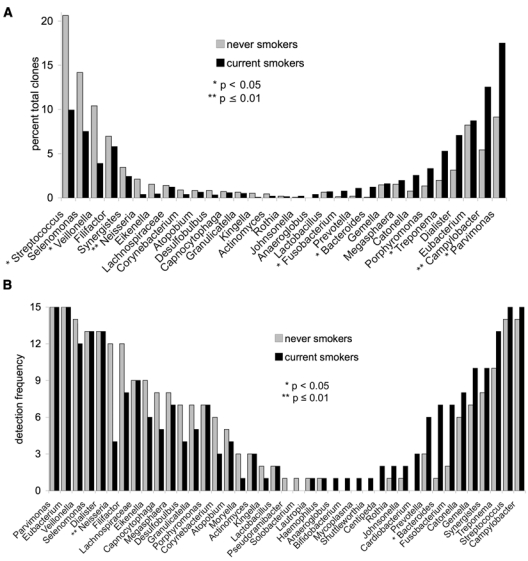
Distribution of 3157 clones by genus in 15 current and 15 never-smokers. Levels of genera are shown in (A) and detection frequency in (B). Genera predominant in never-smokers are arranged on the left, and those predominant in current smokers are arranged on the right. Significant differences in abundance (p < 0.05, two-sample t test on transformed variable) and prevalence (p < 0.05, Fisher’s exact test) of certain genera were observed between the two groups (AYS, HNN, PSK).
Significant differences were observed in both the prevalence and abundance of certain species between current and never-smokers (Table 2). Parvimonas micra and Campylobacter gracilis formed a large fraction of the subgingival microbial community in smokers, but were not as abundant in never-smokers (p < 0.05 and p < 0.01, respectively; two-sample t test). Current smokers also demonstrated higher levels of Treponema socranskii (p < 0.05), Dialister pneumosintes (p < 0.01), and Peptostreptococcus sp. oral clones BS044, FG014, AP24, and 2002–69396 97 (p < 0.05). Never-smokers demonstrated greater levels of Veillonella sp. oral clone B2 (p < 0.05), Neisseria sp. oral clone 2.24 (p < 0.05), and Streptococcus sanguinis (p < 0.01). Tannerella forsythia and Parvimonas sp. oral clone FG014 were detected only in current smokers, while Capno-cytophaga sp. oral clone AH015 was found only in never-smokers (p < 0.05, Fisher’s exact test).
Table 2.
Species and Phylotypes Demonstrating Significant Differences in Detection Frequency and Levels between 15 Current and 15 Never-smokers, Arranged in Order of Decreasing Overall Prevalence
| Detection Frequency |
Mean, Median (range) |
||||
|---|---|---|---|---|---|
| Species/Phylotype(s) | Percent Total Clones N = 3157 | Current Smokers N = 15 | Never-smokers N = 15 | Current Smokers | Never-smokers |
| Parvimonas micra* | 7.3 | 13 | 12 | 10.4, 5.6 (0, 35.9) | 3.8, 1.7 (0, 15.7) |
| Campylobacter gracilis** | 3.4 | 12 | 7 | 5.5, 5.5 (0, 13.7) | 1.5, 0 (0, 5.1) |
| Veillonella sp. oral clone VeillB2* | 2.3 | 9 | 12 | 1.3, 1 (0, 4.7) | 3.3, 2.6 (0, 9.6) |
| Treponema socranskii* | 1.7 | 10 | 7 | 2.7, 2 (0, 7.0) | 0.6, 0 (0, 1.9) |
| Dialister pneumosintes** | 1.5 | 10 | 4 | 2.5, 2 (0, 7.8) | 0.5, 0 (0, 4.3) |
| Peptostreptococcus sp. oral clone BS044* | 1.3 | 7 | 3 | 2.4, 0 (0, 11.6) | 0.3, 0 (0, 2.9) |
| Peptostreptococcus sp. oral clone AP24* | 1.2 | 1 | 6 | 0.1, 0 (0, 1.9) | 2.2, 0 (0, 13.2) |
| Streptococcus sanguinis##** | 1.1 | 2 | 9 | 0.1, 0 (0, 1.0) | 2.2, 0 (0, 9.5) |
| Peptostreptococcus sp. 2002–69396 97#* | 0.9 | 9 | 2 | 0.9, 0 (0, 5.3) | 0.8, 0 (0, 9.7) |
| Neisseria sp. clone 2.24* | 0.8 | 2 | 7 | 0.1, 0 (0, 1.0) | 1.3, 0 (0, 8.6) |
| Peptostreptococcus sp. oral clone FG014#* | 0.5 | 5 | 0 | 1.1, 0 (0, 7.3) | 0, 0 (0, 0) |
| Tannerella forsythia#* | 0.2 | 4 | 0 | 0.5, 0 (0, 2.9) | 0, 0 (0, 0) |
| Capnocytophaga sp. oral clone AH015#* | 0.2 | 0 | 4 | 0, 0 (0, 0) | 0.3, 0 (0, 2.3) |
Mean, median, and range are shown; p-values for prevalence are indicated by pound signs (# p < 0.05, and ## p < 0.01), and significant differences in levels are indicated by asterisks (* p < 0.01, ** p ≤ 0.01) (t test and Fisher’s exact test) (AYS, HNN, PSK).
Significantly opposing patterns of co-colonization were observed between Parvimonas and 3 other genera in current and never-smokers (Fig. 3). In both groups, these interactions were significant (p < 0.05). In smokers, a positive co-association was observed between Parvimonas and Streptococcus in each individual, while a negative co-association was observed among Parvimonas, Synergistes, and Porphyromonas.
Figure 3.
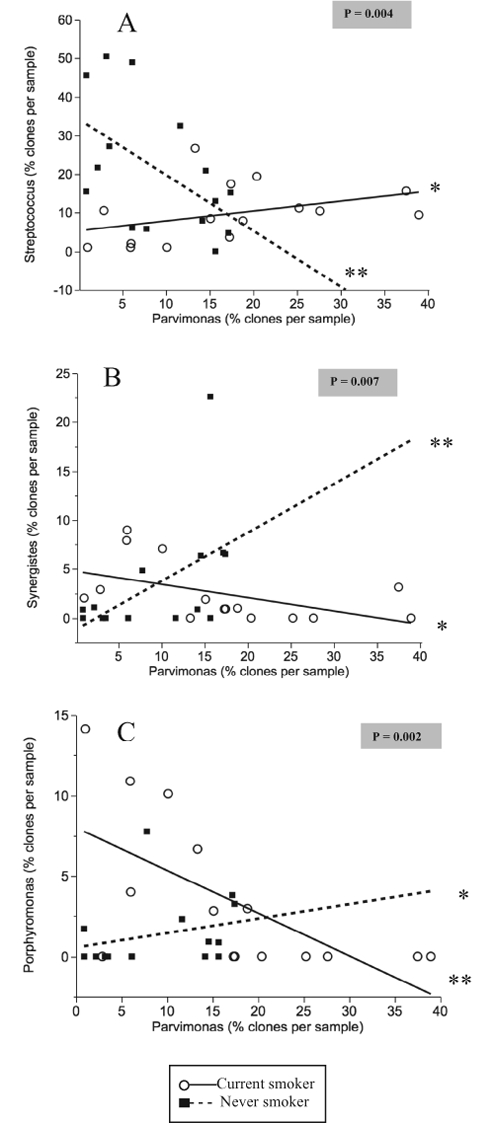
Best linear fits for co-association between genera in current and never-smokers. P-values highlighted in grey correspond to the null hypothesis that the two slopes are the same. P-values corresponding to the null hypothesis that each slope is zero are also shown (*p < 0.05, **p < 0.01) (AYS, HNN, PSK).
Discussion
The subgingival microbiome of smokers has previously been examined by either culturing or molecular methods targeting bacterial species identified by cultivation (Zambon et al., 1996; Shiloah et al., 2000; Haffajee and Socransky, 2001; van Winkelhoff et al., 2001; Van der Velden et al., 2003; Darby et al., 2005). These closed-ended or selective approaches showed no clear association between smoking and changes in the subgingival microbiome. In the present study, we used cloning and sequencing of bacterial 16S rRNA genes, allowing for the identification and enumeration of as-yet-uncultivated organisms as well as novel and previously unsuspected species.
Epidemiological evidence indicates that current smokers have greater extent and severity of disease as compared with never-smokers (Tomar and Asma, 2000). It has been suggested that the greater severity of periodontitis in smokers as compared with non-smokers may account for differences in subgingival bacterial profiles (Haffajee and Socransky, 2001). Therefore, in the present investigation, current and never-smokers with similar severity and extent of attachment loss and probing depths were selected. Comparing bacterial profiles of the deep sites from these disease-matched groups allowed us to explore the association between smoking and the disease-associated biofilm.
A large fraction of the subgingival microbiome in both smokers and non-smokers was uncultivated; however, the numbers of uncultivated organisms were significantly lower in the present study as compared with those reported in earlier investigations (Kumar et al., 2006). Improvements in high-throughput sequencing have made it consistently possible to obtain high-quality sequences that encompass nearly the entire 16S rRNA gene. Because the gene is a mosaic of conserved and variable sequence tracts, longer sequences allow for highly stringent classifications of species and phylotypes. Thus, analysis of the data supports the importance of using longer sequences for phylogenetic analysis.
Uncultivated phylotypes of Peptostreptococci clustered with P. micra within the same genus; similarly, Bacteroides and Tannerella formed a genus-level cluster. The deep sites of current smokers exhibited significantly lower levels of Veillonella, Strepto-coccus, and Neisseria than non-smokers. These genera are reported to be abundant in health-associated biofilms, and their levels decrease in disease (Paster et al., 2001; Kumar et al., 2005, 2006), suggesting that in smokers periodontitis is associated with a greater depletion of beneficial bacteria than in non-smokers. Smokers also demonstrated a greater abundance of Parvimonas, Campylobacter, Treponema, Bacteroides, and Fusobacterium—genera that are consistently associated with disease (Socransky et al., 1998; Kumar et al., 2005). Analysis of the data, taken together, suggests that periodontitis in smokers is associated with a microbial community that is preferentially enriched for disease-associated pathogens. We have also reported that these disease-associated organisms decrease in levels following smoking cessation (Delima et al., 2010). Thus, pathogen enrichment may be a mechanism by which smoking increases the risk for severe and extensive periodontitis, and this requires further study with a longitudinal study design to examine disease onset and progression in current and never-smokers.
Several opposing patterns of bacterial co-colonization were observed in smokers and non-smokers. The most robust of these associations was between Streptococci and Parvimonas, two abundant genera in both current and never-smokers. Smokers with high levels of Streptococci also exhibited high levels of Parvimonas, in contrast to non-smokers. Streptococci normally form a large fraction of a health-associated microbiome (Aas et al., 2005), and recent evidence suggests that they play a critical role in preventing colonization of this niche by pathogens (Stingu et al., 2008; Van Hoogmoed et al., 2008). It is possible that this protective function of Streptococci is impaired in smokers, leading to an altered pattern of co-colonization. In the present study, higher levels of Parvimonas also correlated with lower levels of Synergistes and Porphyromonas in current smokers, but not in never-smokers. Parvimonas normally co-aggregates with Porphyromonas (Kremer and van Steenbergen, 2000), and in vitro investigations indicate that it is essential for growth of Synergistes (Downes et al., 2009). Analysis of the data, taken together, suggests that smoking may alter normal inter-bacterial relationships, thus contributing to preferential colonization by certain species. Longitudinal studies that examine the acquisition and colonization of bacteria in smokers are warranted for better elucidation of the effect of smoking on subgingival bacterial inter-relationships.
P. micra (formerly Peptostreptococcus micros) was the most abundant species overall, and was detected in both smokers and non-smokers with equal frequency. However, the levels of this species were significantly greater in the deep sites of smokers. This Gram-positive, micro-aerophilic organism is found in polymicrobial infections such as intracranial abscesses, sinus infections, and periodontitis (Murdoch et al., 1988; Brook, 2006). P. micra possesses several virulence factors that contribute to its pathogenic potential. The cell wall of P. micra has been shown to induce a potent inflammatory response in macrophages (Tanabe et al., 2007), and it produces proteases that enable it to penetrate the basement membrane (Grenier and Bouclin, 2006). Further, P. micra demonstrates a carbohydrate- mediated co-aggregation with Fusobacterium nucleatum and Porphyromonas gingivalis (Kremer and van Steenbergen, 2000).
C. gracilis is a non-motile, micro-aerophilic, Gram-negative organism that has been consistently found in polymicrobial anaerobic infections such as liver, foot, and lung abscesses (Johnson et al., 1985), as well as in periodontal and endodontic infections (Sassone et al., 2008). Evidence regarding the pathogenic mechanisms of these organisms is limited; however, they demonstrate a high level of resistance to several antibiotics, including penicillins and cephalosporins (Johnson et al., 1985), which may contribute to their persistence in chronic infections. Further studies are needed to explore the role played by these organisms in smoking-related periodontitis.
In summary, the microbial profile of smoking-related periodontitis is distinct from that of non-smokers. More differences were observed in abundance than in prevalence of species, suggesting that smoking preferentially enriches an indigenous microbial community for pathogens, while depleting it of health-compatible commensals. Opposing patterns of bacterial co-colonization were observed in smokers as compared with non-smokers, providing evidence for altered inter-bacterial interactions within this community.
Footnotes
The study was supported by a seed grant from the College of Dentistry, The Ohio State University, to Purnima Kumar and by an NIH CTSA grant (UL1RR025755) to Haikady Nagaraja.
References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. (2005). Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43:5721-5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apatzidou DA, Riggio MP, Kinane DF. (2005). Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J Clin Periodontol 32:973-983 [DOI] [PubMed] [Google Scholar]
- Bittar F, Richet H, Dubus JC, Reynaud-Gaubert M, Stremler N, Sarles J, et al. (2008). Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One 3:e2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom L, Bergström J, Dahlén G, Linder LE. (2001). Smoking and subgingival microflora in periodontal disease. J Clin Periodontol 28:212-219 [DOI] [PubMed] [Google Scholar]
- Brook I. (2006). Microbiology of intracranial abscesses associated with sinusitis of odontogenic origin. Ann Otol Rhinol Laryngol 115:917-920 [DOI] [PubMed] [Google Scholar]
- Darby IB, Hodge PJ, Riggio MP, Kinane DF. (2000). Microbial comparison of smoker and non-smoker adult and early-onset periodontitis patients by polymerase chain reaction. J Clin Periodontol 27:417-424 [DOI] [PubMed] [Google Scholar]
- Darby IB, Hodge PJ, Riggio MP, Kinane DF. (2005). Clinical and microbiological effect of scaling and root planing in smoker and non-smoker chronic and aggressive periodontitis patients. J Clin Periodontol 32:200-206 [DOI] [PubMed] [Google Scholar]
- Delima SL, McBride RK, Preshaw PM, Heasman PA, Kumar PS. (2010). Response of subgingival bacteria to smoking cessation. J Clin Microbiol 48:2344-2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes J, Vartoukian SR, Dewhirst FE, Izard J, Chen T, Yu WH, et al. (2009). Pyramidobacter piscolens gen. nov., sp. nov., a member of the phylum ‘Synergistetes’ isolated from the human oral cavity. Int J Syst Evol Microbiol 59(Pt 5):972-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D, Bouclin R. (2006). Contribution of proteases and plasmin-acquired activity in migration of Peptostreptococcus micros through a reconstituted basement membrane. Oral Microbiol Immunol 21:319-325 [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS. (2001). Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol 28:377-388 [DOI] [PubMed] [Google Scholar]
- Johnson CC, Reinhardt JF, Edelstein MA, Mulligan ME, George WL, Finegold SM. (1985). Bacteroides gracilis, an important anaerobic bacterial pathogen. J Clin Microbiol 22:799-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GK, Hill M. (2004). Cigarette smoking and the periodontal patient. J Periodontol 75:196-209 [DOI] [PubMed] [Google Scholar]
- Jukes TH, Cantor CR. (1969). Evolution of protein molecules. In: Mammalian protein metabolism. Munro HN, editor. New York: Academic Press, pp. 21-132 [Google Scholar]
- Kremer BH, van Steenbergen TJ. (2000). Peptostreptococcus micros coaggregates with Fusobacterium nucleatum and non-encapsulated Porphyromonas gingivalis. FEMS Microbiol Lett 182:57-62 [DOI] [PubMed] [Google Scholar]
- Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. (2005). Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol 43:3944-3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. (2006). Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol 44:3665-3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch DA, Mitchelmore IJ, Tabaqchali S. (1988). Peptostreptococcus micros in polymicrobial abscesses. Lancet 1:594. [DOI] [PubMed] [Google Scholar]
- Natto S, Baljoon M, Dahlén G, Bergström J. (2005). Tobacco smoking and periodontal microflora in a Saudi Arabian population. J Clin Periodontol 32:549-555 [DOI] [PubMed] [Google Scholar]
- Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. (2001). Bacterial diversity in human subgingival plaque. J Bacteriol 183:3770-3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone LM, Fidel R, Faveri M, Fidel S, Figueiredo L, Feres M. (2008). Microbiological evaluation of primary endodontic infections in teeth with and without sinus tract. Int Endod J 41:508-515 [DOI] [PubMed] [Google Scholar]
- Shiloah J, Patters MR, Waring MB. (2000). The prevalence of pathogenic periodontal microflora in healthy young adult smokers. J Periodontol 71:562-567 [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. (2005). Periodontal microbial ecology. Periodontol 2000 38:135-187 [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr (1998). Microbial complexes in subgingival plaque. J Clin Periodontol 25:134-144 [DOI] [PubMed] [Google Scholar]
- Stingu CS, Eschrich K, Rodloff AC, Schaumann R, Jentsch H. (2008). Periodontitis is associated with a loss of colonization by Streptococcus sanguinis. J Med Microbiol 57(Pt 4):495-499 [DOI] [PubMed] [Google Scholar]
- Stoltenberg JL, Osborn JB, Pihlstrom BL, Herzberg MC, Aeppli DM, Wolff LF, et al. (1993). Association between cigarette smoking, bacterial pathogens, and periodontal status. J Periodontol 64:1225-1230 [DOI] [PubMed] [Google Scholar]
- Tanabe S, Bodet C, Grenier D. (2007). Peptostreptococcus micros cell wall elicits a pro-inflammatory response in human macrophages. J Endotoxin Res 13:219-226 [DOI] [PubMed] [Google Scholar]
- Tomar SL, Asma S. (2000). Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol 71:743-751 [DOI] [PubMed] [Google Scholar]
- Van der Velden U, Varoufaki A, Hutter JW, Xu L, Timmerman MF, Van Winkelhoff AJ, et al. (2003). Effect of smoking and periodontal treatment on the subgingival microflora. J Clin Periodontol 30:603-610 [DOI] [PubMed] [Google Scholar]
- Van Hoogmoed CG, Geertsema-Doornbusch GI, Teughels W, Quirynen M, Busscher HJ, Van der Mei HC. (2008). Reduction of periodontal pathogens adhesion by antagonistic strains. Oral Microbiol Immunol 23:43-48 [DOI] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Bosch-Tijhof CJ, Winkel EG, van der Reijden WA. (2001). Smoking affects the subgingival microflora in periodontitis. J Periodontol 72:666-671 [DOI] [PubMed] [Google Scholar]
- Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. (1996). Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol 67(10 Suppl):S1050-S1054 [DOI] [PubMed] [Google Scholar]


