Abstract
The development of new medical formulations (NMF) for reconstructive therapies has considerably improved the available treatment options for individuals requiring periodontal repair or oral implant rehabilitation. Progress in tissue engineering and regenerative medicine modalities strongly depends on validated pre-clinical research. Pre-clinical testing has contributed to the recent approval of NMF such as GEM 21S® and INFUSE® bone grafts for periodontal and oral regenerative therapies. However, the selection of a suitable pre-clinical model for evaluation of the safety and efficacy of a NMF remains a challenge. This review is designed to serve as a primer to choose the appropriate pre-clinical models for the evaluation of NMF in situations requiring periodontal or oral reconstruction. Here, we summarize commonly used pre-clinical models and provide examples of screening and functional studies of NMF that can be translated into clinical use.
Keywords: regenerative medicine, medical devices, periodontal regeneration, oral implants, bone regeneration, tissue engineering
Introduction
Periodontal and peri-implant tissue destruction mainly occur as a consequence of a chronic inflammatory reaction that can culminate in tooth or implant loss. Tooth loss, in turn, favors bone atrophy, making stable placement of dental implants a challenge. Periodontal diseases and the demand for fixed prostheses increase with the aging of the population, clearly indicating the growing future demand for innovative reconstructive therapies (Kaigler et al., 2006). The development of new medical formulations (NMF) is based on the understanding of the etiology and pathogenesis of the disease, its progression, and the general principles of tissue repair. However, knowing all these aspects does not necessarily lead to conclusions about the safety and efficacy of NMF for the reconstruction of oral and periodontal defects. Unfortunately, the use of in vitro studies alone for the simulation of natural disease in humans is inadequate for direct entry into clinical trials, underscoring the strong need for robust pre-clinical modeling. The requirements of thorough pre-clinical evaluation are reflected by the regulatory approval agencies such as the U.S. Food and Drug Administration and European Medicines Agency (EMEA), which encourage a sequence or targeted pre-clinical evaluations before human trials are initiated. Based on these demands, pre-clinical studies have paved the way for clinical studies that led to the recent approval of NMF such as GEM 21S® (Osteohealth Co., Shirley, NY, USA) (containing recombinant PDGF-BB; for the treatment of intrabony and furcation defects as well as gingival recession in periodontal disease) and INFUSE® bone graft (Medtronic, Minneapolis, MN, USA) (containing recombinant BMP-2; for sinus floor augmentation and for localized alveolar ridge augmentation following tooth extraction) in the US.
Planning of a pre-clinical study must be fitted for the purpose. It does not necessarily mean that a NMF that successfully improves oral bone regeneration is also appropriate for the treatment of periodontal defects and vice versa. Moreover, the objectives of a pre-clinical study must relate to the efficient and effective design of the reconstructive therapy. The choice of the potential endpoints is therefore a critical issue in the study design. Moreover, endpoints need to be defined to estimate the sample size necessary to achieve the desired power. Consideration of the morphological changes related to the anatomical defect that can occur over time can help in the estimation of the appropriate study duration. The selection of pre-clinical models often takes the phylogenetic tree into consideration; however, this can be hampered by the different anatomical and healing characteristics of rodents and larger animals. Overall, the planning of a pre-clinical study to test a NMF requires decisions about animal species, the defect type, study endpoint, and study duration. Furthermore, many pre-clinical studies for NMF testing may use a battery of histological endpoints or those that involve a series of more invasive measures not amenable to human trials. Much information can be gleaned in an animal model that cannot be ethically performed in a human investigation. Thus, the pre-clinical testing can guide the researcher in the rational choice of appropriate surrogate measures of osseous or soft-tissue regeneration. It is the aim of this review to provide a timely summary of commonly used pre-clinical models to evaluate periodontal and oral reconstructive therapies, highlighting those related to devices, drugs, and biologics. We briefly describe the surgical protocols and study duration of each approach. The commonly used measures and morphological characteristics of bone and soft-tissue healing are summarized. This information should serve as a primer for those planning to choose the appropriate pre-clinical models to help the translation of NMF into the clinical arena. The advantages and disadvantages of each of the regenerative pre-clinical models are highlighted in Tables 1 and 2.
Table 1.
Advantages and Disadvantages of Pre-clinical Rodent Models for Oral Reconstruction
| Defect Type | Advantages | Disadvantages |
|---|---|---|
| Fenestration (periodontal) | • Gives a proof-of-concept in a short time frame
• Well-contained defects • No gingival tissue ingrowth |
• Narrow healing time window
• Small size, surgical microscopes required; technically challenging • Rapid repair as kinetic healing model • Cannot measure healing at junctional epithelial-connective tissue interface • Not a “natural disease” model with microbial influence |
| Capsule (vertical ridge) | • Standardized shape-dimension
• Well-contained defect from the local environment • Contained regenerative response with cells and tissue emanating from the residual bone |
• Not applicable to alveolar bone
• Isolation from oral environment • Ectopic bone healing model (bone repair beyond normal bony envelope) |
| Alveolar Socket (tooth extraction) | • Easy and fast to perform
• Well reproduces the events occurring in bone healing |
• Small size
• Rapid bone repair compared to human • Non-critical size (kinetic) defect • Mandibular extractions technically demanding |
| Infrabony Peri-implant Defect | Surgically created
• Short time-frame needed for defect generation • Standard morphological dimension |
• Rapid bone repair
• Narrow evaluation time window (kinetic defect) • Surgical defect without microbial influence |
Table 2.
Advantages and Disadvantages of Pre-clinical Large-animal Defect Models for Oral Reconstruction
| Defect Type | Animal Model | Advantages | Disadvantages |
|---|---|---|---|
| Furcation/Infrabony Periodontal Defect | Canine &Non-human Primate | Surgical Acute-Chronic
• Short time period to develop defects; more cost-effective • Standardized morphological characteristics • Chronic disease model • Class II-III furcations can be created in a standardized fashion • Class III defects in Canine are of “critical size” • Bilateral symmetrical defects • Horizontal defect allows for reproducible landmarks • Well-studied model used for pre-clinical • investigation prior to human studies (canine) • Minimal palatal recession (non-human primate) |
Surgical (Acute)
• Do not reproduce inflammatory/infective conditions • Spontaneous partial repair (non-human primate) Surgical (Chronic) • Soft tissues compromised • Variable amount of connective tissue repair • More time-consuming than acute • Technically challenging |
| Ligature-induced
• Microbiological features similar to humans • Morphological features similar to humans • Minimal spontaneous repair • Reasonable consistency in defect severity |
Ligature-induced
• Disease development can be variable, depending on ligature placement and stability at the tooth sites • Non-standardized defect morphology (canine) • Require time to be created; expensive |
||
| Alveolar Socket | Canine & Non-human Primate | • Easy and fast to perform
• Well reproduce the events occurring in bone healing |
• Rapid bone repair compared with human (canine)
• Kinetic (non-critical-size) defect |
| Infrabony Peri-implant Defect | Canine & Non-human Primate | Surgically Created
• Short time needed to generate defect • Standard morphology-dimension • Ligature-induced • Morphological and microbiological similarities to humans |
Surgically Created
• Spontaneous repair • Ligature-induced • Spontaneous repair • Significant time required to generate defects |
| Supra-alveolar Peri-implant Defect | Canine | • Limited spontaneous regeneration
• Reproducibly created |
• Requires space-providing devices
• Wound dehiscences |
Rat as a Candidate Small-Defect Model
Rodents (mice and rats) and rabbits are the most commonly used animal models in biomedical research. Rats are cost-effective, easy to handle, and allow for the standardization of experimental conditions in genetically similar individuals (summarized in Table 1). Rats are suitable for study of the effects of systemic diseases and pharmacological therapies on tissue destruction and regeneration (Graves et al., 2008), and for evaluation of physiological alterations related to aging (Benatti et al., 2006). In addition, tissue destruction and regeneration in an immunodeficient background can be investigated in this model (Klausen, 1991). Surgery and the evaluation of study endpoints are challenging, however, because of the animals’ small size. Moreover, the rodent dentition undergoes continuous tooth eruption, including bone and cementum apposition, which has to be considered when a study is planned (Belting et al., 1953).
Fenestration Defect Model for Periodontal Regeneration in the Rat
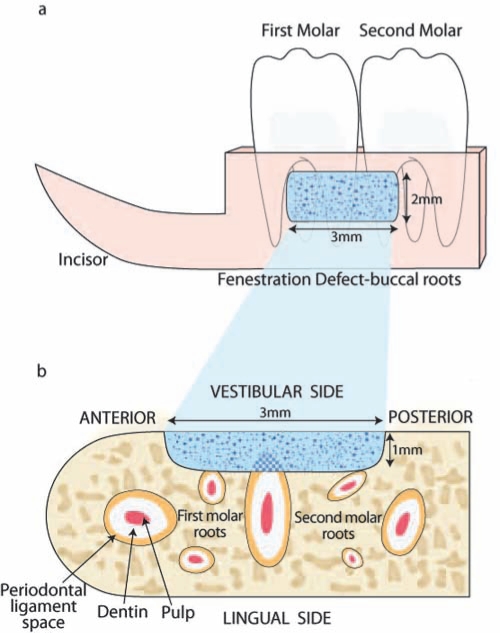
Rats are not susceptible to the development of natural periodontitis. Chronic inflammation leads to periodontal destruction that can be induced by the placement of cotton or silk ligatures into the sulci around molar teeth (Jin et al., 2007). Chronic inflammation can also be achieved by repeated intra-gingival injection of bacterial lipopolysaccharide (LPS), eliciting the release of host-derived pro-inflammatory cytokines (Park et al., 2007; Rogers et al., 2007). Both models are suitable for evaluating the pathogenesis of periodontitis and studying therapeutic strategies to modulate disease progression (Graves et al., 2008). However, rodent studies addressing reconstructive therapies typically require surgically created periodontal defects (Melcher, 1970; Klausen, 1991). After a flap is raised by extra-oral access to expose the mandibular alveolus, the distal and buccal roots of the first molar and the mesial root of the second molar are denuded, including the superficial dentin (Figs. 1a, 1b). NMF can be delivered to the standardized fenestration defects (3 x 2 x 1 mm) and secured by flap repositioning. The consistency of defect location is determined by the targeted location to the bony supportive housing of the first and second molar roots. The early healing process follows the conserved sequence of wound healing that is initiated by blood coagulation and immigration of neutrophils and monocytes for wound debridement and bone resorption. This microenvironment favors the proliferation and migration of mesenchymal progen-itors which can originate from the periodontal ligament (PDL) and bone (Lekic et al., 1996a,b). After 10 days, a thin cementum layer with a connective tissue attachment can be observed, particularly on the apical side of the teeth, where the cementum is thicker compared with the narrow coronal region (King et al., 1997). Bone formation is typically initiated from the bony margins of the lesions (Rajshankar et al., 1998). In young rats (King et al., 1997), periodontal regeneration is complete after 1 mo, while geriatric animals at 18 mos of age show a delayed healing capacity (Benatti et al., 2006). It is therefore crucial that investigators select the appropriate time-point(s) to determine the therapeutic efficacy “window” of a candidate NMF. The distal root of the first molar is the main target for histologic and histomorphometric evaluation. Even though the fenestration defect is not of critical size, this model provides a reasonable proof-of-concept kinetic approach over a confined time interval. Moreover, isolation from the oral environment excludes the negative variables resulting from bacterial and foreign body contamination of wounds that occurs with rodents in caged housing. Based on this model, cementum and bone regeneration has been evaluated following the delivery of BMPs (King et al., 1997; King and Hughes, 1999, 2001; Talwar et al., 2001) and PDGF (Jin et al., 2004), as well as other growth factors, genes, and cells (Jin et al., 2003; Zhao et al., 2004; Huang et al., 2005). Potential endpoints include the dimension and extension of new cementum, PDL, and bone in the defect region. The length of new cementum and PDL extension can be measured from the histologic notch. The length of new bone, cementum, new bone area, and osseous defect fill are assessed through histomorphometric analysis.
Figure 1.
Rat periodontal fenestration defect. (a) The fenestration defect was created on the buccal surface of the mandibular alveolar bone. Distal and buccal roots of the first molar and the buccal root of the second molar were exposed. Novel medical formulations (NMF in sky-blue) were delivered into the defect. (b) The coronal section of the alveolar bone shows the defect outline. The cemental layer, superficial dentin, and the periodontal ligament were removed. At 3, 10, 21, and 35 days post-surgery, tissue samples were harvested, and the regeneration of bone and cementum was evaluated. Adapted from King et al., 1997.
Alveolar Extraction Socket Model in the Rat
Reconstructive therapies following tooth extraction aim to enhance the process of bone regeneration and to preserve the alveolar dimension, both being critical determinants for the success of subsequent oral implant placement. Tooth extractions are typically performed on incisors (Balabanian et al., 2006) and molars (Hahn et al., 1988) after dissection of the PDL by sharp instruments. Extracted teeth require evaluation for completeness, since root fragments are frequently left behind and can impair complete bone regeneration. Healing of the extraction socket follows a conserved sequence of events: Within one week, the blood coagulum is replaced by a highly vascularized connective tissue, before woven bone formation is initiated at the alveolar walls. Trabecular bone volume increases over time, and within 4 wks, a keratinized epithelium covers the mineralized area. Bone remodeling continues until mature bone encompasses the extraction socket (Guglielmotti and Cabrini, 1985; Pereira et al., 2007). The small size of the extraction socket and the strong innate healing capacity, however, make this model less suitable for the screening of NMF. Thus, most studies conducted with this model focus on the impact of systemic variables on the repair of extraction sites (Pereira et al., 2007; Karalis et al., 2008; Wu et al., 2008). Potential endpoints include the rate and amount of new bone formation, resorption of the alveolar ridge, and consolidation of implanted biomaterials (Hahn et al., 1988; Hile et al., 2005; Balabanian et al., 2006). The resorption of the alveolar ridge can be measured as area, percentage, or height change compared with control. The consolidation of biomaterials can be measured by thickness of the fibrous capsule surrounding the implant.
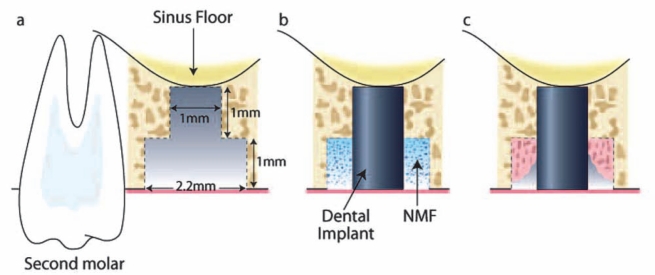
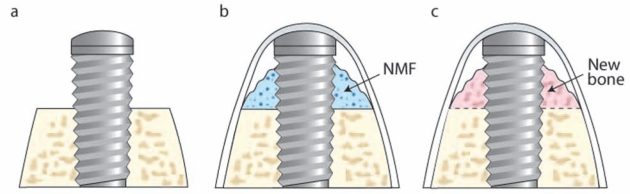
Infrabony Peri-implant Defect Model in the Rat
Reconstructive therapies targeting peri-implant bone become necessary to provide sufficient mechanical support for the stable placement, loading, and maintenance of dental implants. The rat maxilla can be used as an orthotopic region for study of the process of osseointegration. One month after tooth extraction, the maxillary bone is typically healed to allow oral implants to be installed (Karimbux et al., 1995). After the alveolar ridge is exposed, the implant bed is prepared by a custom-made drill. The apical diameter of 1 mm allows for the fixation of cylindrical titanium implants by press-fit into the prepared osteotomy. The coronal part of the drill is 2 mm and creates a circumferential rim between the alveolar bone and the implant surface. A NMF can be placed into the mouth of the defect prior to flap closure (Dunn et al., 2005) (Figs. 2b, 2c). The potent regenerative capacity of rodents requires critical selection of suitable time-points, which are usually between 1 and 4 wks. This pre-clinical model has been used for the determination of peri-implant bone regeneration in the maxilla, but can be adapted to the mandible. However, evaluation time-points should be determined through pilot studies, since mandibular bone is generally denser than maxillary bone, leading to significant tooth fracture during the extraction process of the mandible (Seol et al., 2010). Based on this pre-clinical model of maxillary alveolar bone, the effects of BMP gene delivery at peri-implant sites have been evaluated (Karimbux et al., 1995; Dunn et al., 2005). Potential endpoints are based on sagittal sections prepared along the implant axis, including the histomorphometric parameters of osseointegration (e.g., bone-to-implant contact [BIC], new bone area, new bone height, and osseous defect fill).
Figure 2.
Infrabony peri-implant defect in the rat. (a) After extraction of the maxillary first molar, the socket was allowed heal for ~ 4 wks. (b) During the second surgery, a bone defect was created by means of an osteotomy in the location of the former tooth. An implant (1 mm x 2 mm) was press-fit into position, and the NMF was delivered to the peri-implant bone defect, followed by soft-tissue wound closure. (c) At multiple time-points (10, 14, and 21 days), tissue samples were harvested, and bone healing was evaluated.
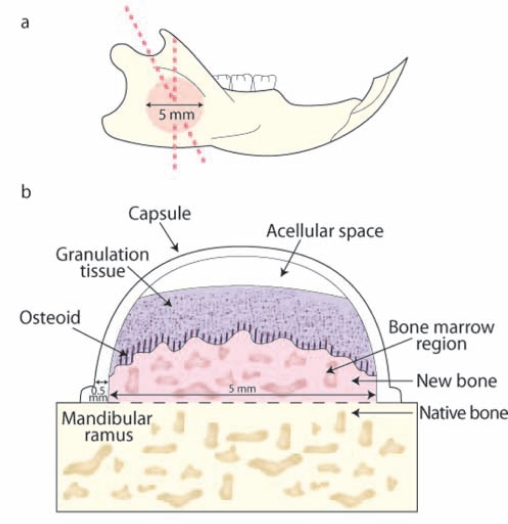
Vertical Bone Augmentation (Capsule Model) in the Rat
Bone augmentation is a reconstructive therapy to provide sufficient bone volume for dental implant installation and oral rehabilitation. A pre-clinical model for bone augmentation has been developed based on the principles of guided bone regeneration (GBR) (Kostopoulos and Karring, 1994). The approach consists of a space-providing Teflon capsule with an internal radius of approximately 5 mm being placed on the vestibular side of the mandibular ramus (Fig. 3). Teflon capsules filled with NMF can be secured on the ramus by resorbable sutures or screws (Mardas et al., 2003). Within 2 wks, the space beneath the capsule is partially filled with a highly vascularized connective tissue. At 4 mos, from 40 to 70% of the space is filled with bone, showing a gradient of maturation toward the top of the capsule. Within 1 yr, the space is completely filled with mature lamellar bone (Mardas et al., 2003; Stavropoulos et al., 2003). To determine the impact of NMF on bone formation, one can obtain histologic sections perpendicular to the surface of the ramus in the transverse plane. It should be considered, however, that bone formation is evaluated outside the envelope of the native ramus, which is typically under physiological biomechanical loading. This model has been used for study of the consolidation of biomaterials, either alone or in the presence of growth factor biologics (Lioubavina-Hack et al., 2005). Potential study endpoints include histomorphometric parameters such as the cross-sectional areas of: (1) the space created by the capsule, (2) newly formed bone, (3) biomaterial remnants, (4) loose connective tissue, (5) height of the capsules, and (6) height of the newly formed bone (Mardas et al., 2003; Stavropoulos et al., 2003).
Figure 3.
Vertical bone augmentation in rat (capsule model). (a,b) The Teflon capsule was positioned on the vestibular surface of the mandibular ramus and stabilized by means of sutures. (c) The pattern of bone neogenesis included the deposition of loose granulation tissue, subsequently replaced by osteoid and new bone. The newly formed mineralized tissue was in close contact with the original bone of the mandibular ramus. An acellular region was noted superior to the surface of the granulation tissue. Tissue samples were harvested from 2 to 6 mos, and bone neogenesis was evaluated.
Large Animal Models
Once NMF have been demonstrated to be efficacious in rodent models, pre-clinical research is often expanded to large animals prior to human studies, although this sequence is not an FDA regulatory requirement. Given that most rodent osseous defect models do not represent critical-size defects or a well-characterized compromised wound-healing situation, large animals usually validate a move to consideration of clinical applications. These large models include dogs, sheep, pigs, and non-human primates. Large animals allow for the study of NMF in critical-size defects that, by definition, do not heal spontaneously during the lifetime of the animal (Hollinger and Kleinschmidt, 1990). Another advantage of large animal models is that higher-order animals generally more closely simulate the anatomical, physiological, and pathological conditions found in humans (Schectman et al., 1972; Attström et al., 1975; Brecx et al., 1985; Hollinger and Kleinschmidt, 1990). Consequently, pre-clinical studies involving dogs and non-human primates are generally preferred by regulatory bodies for demonstration of the safety and efficacy of candidate NMF (Kaigler et al., 2010). It is recognized that different rates of bone healing occur when large and small animal models are compared (Frost, 1964; Roberts et al., 1987). Thus, critical-size defects of the periodontium or alveolar ridge are generally challenging to create in rodents (much more rapid and complete repair) vs. large animals (slower repair and not as complete). Furthermore, the anatomical similarities (especially for non-human primates) and defect size characteristics favor the consideration of large animals over small animals prior to more definitive studies in humans.
Canine as Large Animal Defect Model
Natural Periodontal Lesions
Most (although not all) canines spontaneously develop periodontitis following accumulation of bacterial plaque biofilm and calculus (Lindhe et al., 1975). Similar to humans, elevated levels of B. asaccharolyticus and spirochetes and an increased proportion of Gram-negative anaerobes are found in the developing plaque biofilms of beagle dogs (Syed et al., 1981). Spontaneous periodontal lesions typically become manifest after 5 or more yrs. For periodontal tissue destruction to be achieved within 2 yrs, canines are typically fed a soft diet to promote plaque accumulation. The clinical hallmarks of the disease are increasing clinical attachment levels, marginal alveolar bone loss, and apical shift of the gingival margin. The severity of periodontal lesions generally decreases from the first to the fourth premolars, followed by the first molars (Page and Schroeder, 1981). NMF can be delivered to these natural lesions that display Class II-III furcation defects. Following surgical debridement of natural periodontal lesions, 2 wks after flap surgery, formation of a long junctional epithelium lining the root extending to the base of the instrumented root surface is detectable (Lynch et al., 1989). Connective tissue with randomly oriented fibers fills the majority of the defect volume. At 5 wks, 15% of new bone and 9% of new attachment can be observed. However, in naturally occurring periodontal disease, it is difficult to predict the shapes and extensions of the lesions, as well as the onset of tissue destruction (Lindhe et al., 1975; Wikesjö and Nilvéus, 1991). These characteristics limit the use of natural periodontal lesion models for pre-clinical studies (Haney et al., 1995). In contrast, the pathogenesis of natural periodontal lesions in canines closely resembles the human situation and represents the animal disease model closest to humans in terms of healing and pathogen-associated microbiota (Syed et al., 1981; Giannobile et al., 1994; Madianos et al., 1994). Natural periodontal lesions have been used to test the effects of biologics such as growth factors and barrier membranes on periodontal regeneration (Lynch et al., 1991; Giannobile et al., 1994; Lekovic et al., 1998). Histomorphometric endpoints include the area or height of newly formed bone, cementum, complete new attachment formation (new bone cementum and PDL; CNAA), osseous defect fill, root resorption, and ankylosis.
Surgically Created Periodontal Defects
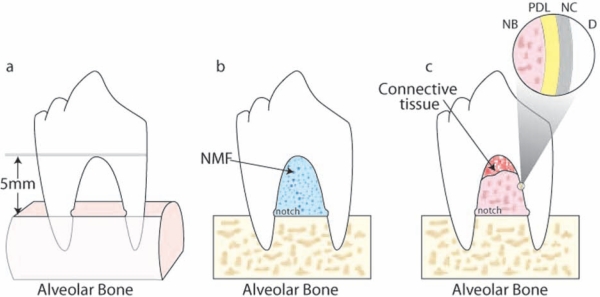
Supra-alveolar critical-size periodontal defects are produced by the resection of buccal and lingual/palatal bone of premolar teeth. Osseous resection can be restricted to the interdental area, which measures ~ 4-5 mm (height) and 3 mm (width) (Araujo et al., 1998, 1999; Chen et al., 2006), or extended to create a horizontal circumferential defect up to 5-6 mm below the fornix of furcation (Fig. 4) (Wikesjö and Nilvéus, 1991; Giannobile et al., 1998; Wikesjo et al., 2003c). The cementum is removed, and notches are placed to mark the bone level at the time of surgical resection. Reducing the crowns of teeth allows for a submerged repositioning of the flap for situations where the oral environmental factors can affect flap dehiscence (Wikesjö et al., 2003c). In the acute defect model, lesions are immediately treated with NMF before wound closure. In the chronic defect model, non-resorbable material is positioned in the furcation area, or the root surface is exposed to the oral environment to inhibit tissue regeneration (Wikesjö et al., 1994). NMF are then positioned in and around the chronic perio-dontal defects. According to the conserved process of wound healing, the clot is replaced by granulation tissue. At the end of the first wk, cellular and fibrous-rich connective tissue gradually replaces the granulation tissue, progressing along the root surface to the center of the defect. Woven bone formation starts at 2 wks and later undergoes remodeling. Cementum formation also initiates at about 2 wks after surgery, characterized by organized collagen fibers adjacent and perpendicular to the root. Concomitant with the formation of cementum, the formation of a PDL, both originating from the bottom of the furcation, is also observed (Matsuura et al., 1995; Araujo et al., 1997, 1999). After 12 to 20 wks, maturation of the periodontium is indicated by intrinsic and extrinsic fibers. Acute periodontal defects heal with a substantial connective tissue repair, a short junctional epithelium, and a minimal amount of new bone, cementum, and attachment, limited at the apical region. Chronic periodontal defects heal spontaneously, with a longer junctional epithelium and little connective tissue repair, with limited bone and cementum formation. Surgical periodontal defects are rapidly created, possess standardized anatomical characteristics and morphological similarity to the challenging human class III furcation defects, and do not heal spontaneously. However, acute periodontal defects fail to reproduce the microbiological and inflammatory conditions present in periodontal disease. Surgical periodontal defects have been largely used to evaluate periodontal regeneration following placement of barrier membranes (Wikesjö et al., 2003b), biologics such as BMPs (Giannobile et al., 1998; Wikesjö et al., 2003c, 2004), PDGF (Park et al., 1995), and other biomaterials (Sorensen et al., 2004; Chen et al., 2006). Histomorphometric endpoint analyses quantify the length of the regenerated cementum, bone, PDL, and junctional epithelium (Wikesjö et al., 2003a; Murano et al., 2006).
Figure 4.
Supra-alveolar periodontal defect in the canine. (a) Alveolar bone was removed around the 3rd and 4th premolar teeth to create a horizontal defect 4-6 mm from reduced bone to the fornix of the furcation. Notches on the internal root surfaces were created at the level of the reduced bone. (b) The furcation lesion was filled with the NMF, and the soft tissues were coronally advanced to cover the defect. Tissue samples were harvested at 4, 8, and 12 wks after surgery. (c) Formation of new periodontal ligament (PDL), new cementum (NC), and new bone (NB) was evaluated in the furcation region. D: dentin.
Implant Placement in Extraction Sockets Immediately or in a Staged Approach
To evaluate the process of osseointegration, investigators have explored studies considering dental implants positioned in fresh extraction sockets in canines. NMF can be filled into freshly debrided alveolar sockets before positioning of the titanium implants. Peri-implant bone regeneration follows the same pattern as the empty alveolar socket, where the blood clot is replaced by granulation tissue and bone. After either extraction or the placement of dental implants, the alveolar ridge typically undergoes resorption, being more pronounced on the buccal than on the lingual side (Araujo and Lindhe, 2005). Resorption is most prominent within the bundle bone, which represents the majority of the crestal portion of the buccal wall. At 4 wks, most of the defect is filled with newly formed bone. Lingual and buccal alveolar crestal resorption continues, involving buccal bone and resulting in buccal dehiscence on the order of 2 mm or more. For these pre-clinical investigations, histomorphometric evaluations are generally performed between 2 and 6 mos post-regenerative therapy. A staged implant placement approach can be performed (i.e., allowing the extraction socket to heal, to allow for a more ideally placed implant fixture). This model has been utilized to study the effects of rhBMP-2 in alveolar bone healing. Mandibular premolar teeth are extracted, followed by a healing interval of 12 wks and subsequent implant installation after 12 wks in both canines and non-human primates (Wikesjö et al., 2008a,b). Potential endpoints include measures of osseointegration (BIC or stiffness [from Young’s Modulus]), new bone height, area, and defect fill. The ridge dimensional changes are determined by measurement of the lingual and buccal bone height, alveolar wall, and total bone width and can be expressed as length or ratio.
Ligature-induced Peri-implant Defects
Once implants are osseointegrated, peri-implantitis can be induced similar to the periodontitis models in rodents, canines, and non-human primates (reviewed in Baron et al., 2000). Cotton or silk ligatures are positioned around the necks of fixture abutments in the submarginal position, and animals are fed a soft diet to allow for plaque biofilm accumulation. Ligatures are typically removed after 2-3 mos, when approximately 30-50% of bone support is lost. Peri-implant bone defects are circumferential and wide, but rarely result in shallow craters (Lindhe et al., 1992; Grunder et al., 1993; Nociti et al., 2001a; Schwarz et al., 2007). The microflora associated with experimental peri-implantitis demonstrates many similarities to natural peri-implantitis lesions (Nociti et al., 2001b; Shibli et al., 2003). Reconstructive therapy includes removal of granulation tissue, treatment of the implant surfaces with abrasive instruments, and potential application of NMF as a regenerative therapy. Peri-implantitis defects treated with open-flap debridement alone show limited new bone formation and repair of about 15% of the vertical component of the defect (Grunder et al., 1993; Hürzeler et al., 1997; Nociti et al., 2001a). Histomorphometric analyses are usually performed on tissue samples harvested at 5 mos after reconstructive surgery. However, bone regeneration occurs in cases of flap debridement alone; thus, the model is not particular stringent for the testing of NMF as a critical-size defect model. Moreover, it requires months to create the lesions. In contrast, the microflora and the morphology are similar to the human situation, making this model suitable for studying the effects of NMF on the treatment of peri-implantitis. Consideration of biomaterials, biologics, and GBR techniques have been preferentially evaluated in these models (Nociti et al., 2001a; You et al., 2007). Potential endpoint measurements include percentage of vertical bone fill, height of new bone, and BIC (mm or %), as well as other parameters of osseointegration.
Supra-alveolar Peri-implant Defect
The supra-alveolar peri-implant defect model has been developed in canines (Wikesjö et al., 1994). After extraction of premolar teeth, the alveolar process is flattened, and titanium implants are positioned into the residual extraction sites. Titanium implants are exposed out of the alveolar process (Figs. 5a, 5b). Incisions of flaps are performed to submerge the fixtures without tension. When the defect is left untreated, only a marginal regeneration of the alveolus is observed, even after prolonged healing periods (Fig. 5c). Under these rigorous model conditions, NMF can be positioned around the implant fixtures and also covering the coronal portions of the implants. However, for space maintenance, GBR strategies that prevent collapse of the soft tissues into the lesions require space provision by the barriers or the underlying implanted NMF. This defect model has been used for evaluating alveolar bone augmentation and dental implant osseointegration following placement of biologics including BMP-2 and other biomaterials (Wikesjö et al., 2004, 2008c). Potential study endpoints include the new bone defect height, new bone height and area, and the parameters of osseointegration (BIC or stiffness).
Figure 5.
Supra-alveolar peri-implant defect in the canine. Approximately 6 mm of alveolar bone was removed around mandibular premolar teeth as measured from the CEJ. (a) After extraction of the premolar teeth, implants were positioned in the osteotomies prepared in the extraction site. Implants were primarily stably contained within 5 mm of native alveolar bone. (b) NMF was delivered around the exposed implants, and the soft tissues were positioned to cover the implant fixtures. (c) Tissue samples were harvested, and the regenerated bone was evaluated at 2, 4, and 6 mos post-surgery.
Infrabony Peri-implant Defect
Similar to the rat model, alveolar extraction sockets are allowed to heal before implant placement in a staged approach. NMF are delivered in an artificial circumferential space created around the oral implant fixtures. The dimensions of the circumferential defects measure approximately 1.5 mm in width and 5 mm in height. Defects can be covered by a GBR membrane before the flap is replaced to submerge the implant fixtures (Cochran et al., 1999). At 1 mo, woven bone typically fills about one-third of the defect. Highly vascularized connective tissue occupies the remaining area. Osseointegration is observed at the apical portion of the defect and implant surface. At 2 mos, new bone encompasses approximately half of the defect showing signs of remodeling, deriving from the host bone margins, a process that continues over time. Compared with implant placement in the extraction socket, this model allows for a more precise evaluation of bone regeneration in a surgically created defect with standardized dimension and morphology. Circumferential peri-implant defects have been used to evaluate the effects of biomaterials and NMF (Polyzois et al., 2007; Abushahba et al., 2008). Potential histomorphometric endpoints include the parameters of osseointegration (BIC or stiffness), height, and area of new bone regeneration.
Non-human Primates
Non-human primates possess anatomic and biologic features that closely resemble those of humans. These characteristics make the model valuable for evaluation of the safety and efficacy of NMF for oral and periodontal regenerative therapies. However, the expense and demanding maintenance, as well as regulatory requirements, limit the use of non-human primates on a broad scale. Different strains of non-human primates have been involved in biomedical research, including macaque, baboon, squirrel monkey, cynomolgus macaque, and chimpanzee. Non-human primates possess both deciduous and permanent dentitions highly similar to those of humans. These animals also form microbial plaque and calculus in the periodontium; however, they rarely exhibit spontaneous progression of gingival inflammation to periodontal disease (Schou et al., 1993). Various approaches have been designed for the predictable induction of periodontal defects (Kornman et al., 1981; Brecx et al., 1985). Thus, given the great similarities in relation to tooth size, anatomy, and healing characteristic, non-human primates make for an appropriate model system for the evaluation of NMF.
Surgically Created Periodontal Defects in Non-human Primates
A variety of defect configurations has been surgically created in non-human primate dentitions, including palatal dehiscence (Laurell et al., 2006) and intrabony (Sculean et al., 1997, 2002), Class II-III furcation (Ripamonti et al., 2001; Hovey et al., 2006), and fenestration lesions (Sculean et al., 2000a, 2001). Fenestration defects typically heal spontaneously, due to the residual periodontal tissues whereby mesenchymal progenitor cells can easily repopulate the defect. Thus, the fenestration model is not considered optimal for studying the effects of NMF on periodontal regeneration (Caton et al., 1994; Sculean et al., 2000a). For the acute model, mucoperiosteal flaps are raised, and removal of supporting bone, tooth-associated PDL, and the cementum is performed. Reconstructive therapy can be administered immediately after defect creation (Ripamonti et al., 2001); however, to reduce spontaneous regeneration, the lesions are filled with space-providing mechanical devices (Ramfjord, 1951; Ellegaard et al., 1973, 1974; Wirthlin and Hancock, 1982). Mechanical devices such as metal strips, orthodontic wires and bands, or cotton floss ligatures are positioned in the defects for 1 to 3 mos, capable of provoking a chronic inflammatory response. At surgical re-entry, the mechanical devices are removed, and the lesions are debrided of granulation tissue, plaque biofilm, and calculus. After the root surface is scaled, candidate NMF can be delivered into the defects (Takayama et al., 2001; Graziani et al., 2005; Laurell et al., 2006). During the early stages of repair following guided tissue regeneration (GTR) therapy, an organized blood clot develops, containing inflammatory cells and vascular sprouting from newly forming granulation tissue. By 1 mo, a thin epithelium lines the coronal portion of the root surface, and new cementum extends to the original cementum layer. Collagen fibers appear disorganized in the coronal areas, while in the apical regions collagen fibers anchor in the newly formed cementum (Sander and Karring, 1995). Periodontal regeneration progresses until about 5 mos, when new bone with a mature periodontium is found (Karatzas et al., 1999; Sculean et al., 2000b; Donos et al., 2003; Zhang et al., 2004). Chronic periodontal defects do not regenerate spontaneously, allowing for a greater observation window for the regenerative response to NMF. Moreover, chronic defects closely resemble those in the human situation, with respect to the microbial flora and the inflammatory reaction (Caton et al., 1994; Karatzas et al., 1999). Surgically created periodontal defects have been used to study the impact of GTR or GBR and the application of biologics, including enamel matrix proteins, BMP-2, FGF-2, TGF-β3, and PDGF (Sculean et al., 1997, 2000b; Ripamonti et al., 2001; Takayama et al., 2001; Donos et al., 2003; Teare et al., 2008). Potential endpoint analyses are generally performed between 3 and 6 mos by measurement of the length and/or area of new attachment, cementum, and bone.
Ligature-induced Periodontal Lesions in Non-human Primates
Periodontal lesions are induced by orthodontic elastic ligatures or silk sutures placed around the candidate teeth for 3 to 6 mos. Both devices elicit an ulceration of the junctional epithelia, exposure to the oral environment, and plaque accumulation, together culminating in the initiation of periodontitis. Additionally, inoculation of the ligatures with Porphyromonas gingivalis can accelerate disease progression (Holt et al., 1988; Rutherford et al., 1992). After 3 mos, approximately 50% alveolar bone loss can be noted radiographically (Kostopoulos and Karring, 2004). Bone resorption shows an angular pattern when the ligature is positioned around a single tooth, and has a horizontal pattern when ligatures are positioned around multiple teeth, due to the narrow interdental regions in the non-human primate dentition (Schou et al., 1993). The microflora is taxonomically comparable with that detected in human periodontitis (Brecx et al., 1985), with a shift of subgingival flora from Gram-positive cocci and rods to Gram-negative rods, and finally to Gram-negative anaerobes that are associated with disease progression (Kornman et al., 1981). After conventional periodontal surgery, spontaneous healing of ligature-induced lesions is characterized by formation of a long junctional epithelium extending to the most apical level of the root. Thus, intervals suggested for histomorphometric analysis are 1, 3, and 6 mos (Giannobile et al., 1994). Limitations are that the defects are restricted to the interproximal region, the maintenance of non-human primates is expensive, and the establishment of periodontal lesions requires significant time (Kornman et al., 1981; Caton et al., 1994). This model has been used for evaluation of the efficacy of biologics and GTR barriers (Rutherford et al., 1992; Giannobile et al., 1994, 1996; Kostopoulos and Karring, 2004). Potential endpoints include: the area/length of regenerated bone, cementum, and PDL; CNAA; osseous defect fill and downgrowth of the junctional epithelium; root resorption; and ankylosis.
Application to the Clinical Arena and Future Directions
Pre-clinical animal models remain a critical component in the development of NMF for human clinical investigation. In vivo models provide distinct advantages for better understanding of aspects of the molecular, cellular, tissue, and anatomical processes that occur in response to the delivery of prototype NMF drugs, devices, or biologics. Some pre-clinical studies have failed to be accurately corroborated in human trials, most notably for the use of root-conditioning agents to promote repair of Class II and III furcation defects (reviewed in Mariotti, 2003). However, most large-animal (canine or non-human primate) pre-clinical studies have accurately predicted the outcomes of large Phase II and Phase III randomized controlled human trials for biologics such as FGF-2 (Murakami et al., 2003; Kitamura et al., 2008), PDGF-BB (Giannobile et al., 1996; Howell et al., 1997; Nevins et al., 2005), and enamel matrix proteins (Hammarström et al., 1997; Tonetti et al., 2002). Thus, these models have very strong applicability in the guidance of clinical guidelines and endpoints for the initiation of Phase II and Phase III human clinical trials.
The increased growth in the development of biologics and devices for oral regenerative medicine application requires a thorough examination of when and how the appropriate endpoints can be evaluated prior to entry into human clinical trial testing. With continued innovations in non-invasive biomedical imaging, the need for extensive pre-clinical testing will decrease. Other endpoints that are increasingly being targeted by regulatory agencies for NMF development include the obtaining of quality-of-life measures that can examine patient perception of clinical outcome (Berretin-Felix et al., 2008; Patel et al., 2008; Kaigler et al., 2010). To date, quality-of-life measures have not been explored fully in the pre-clinical arena for clinical trial guidance. This aspect likely can be provided only by human trials.
Advancements are still needed for the better exploitation of pre-clinical animal models for the evaluation of NMF prior to human testing. These include obvious differences in host-microbial interactions, defect morphology, and the implications of long-term (on the order of decades of disease progression, in some cases with humans) disease development (Graves et al., 2008). Furthermore, the sheer size scale of osseous defects found in humans makes assessment of tissue neogenesis and oxygen and nutrient diffusion through prototype scaffold matrices, especially in large defects, challenging (Cancedda et al., 2007). Continued development of disease induction protocols (modulating the host defense, microbial flora) as well as systemic health status (e.g., simulating common disease conditions that alter wound repair, such as diabetes mellitus, cigarette smoking, osteoporosis, etc.) may aid in the continued refinement of pre-clinical animal models to allow for more targeted and refined human clinical investigation. As the clinical practice arena enters the realm of pharmaco-genomics and personalized medicine, host-susceptibility and identification of those individuals who are responders and non-responders to NMF may aid in the improvement of safety and clinical effectiveness. The ultimate result of enhanced pre-clinical testing will greatly advance patient treatment outcomes.
Acknowledgments
The authors thank Mr. Chris Jung for his assistance with the figures.
Footnotes
WVG has a financial interest in Biomimetic Therapeutics.
This research was supported by NIH/NIDCR DE13397 and by the AO Foundation Biotechnology Advisory Council (Davos, Switzerland).
References
- Abushahba F, Renvert S, Polyzois I, Claffey N. (2008). Effect of grafting materials on osseointegration of dental implants surrounded by circumferential bone defects. An experimental study in the dog. Clin Oral Implants Res 19:329-334 [DOI] [PubMed] [Google Scholar]
- Araujo MG, Lindhe J. (2005). Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol 32:212-218 [DOI] [PubMed] [Google Scholar]
- Araujo MG, Berglundh T, Lindhe J. (1997). On the dynamics of periodontal tissue formation in degree III furcation defects. An experimental study in dogs. J Clin Periodontol 24:738-746 [DOI] [PubMed] [Google Scholar]
- Araujo MG, Berglundh T, Lindhe J. (1998). GTR treatment of degree III furcation defects with 2 different resorbable barriers. An experimental study in dogs. J Clin Periodontol 25:253-259 [DOI] [PubMed] [Google Scholar]
- Araujo MG, Berglundh T, Albrektsson T, Lindhe J. (1999). Bone formation in furcation defects. An experimental study in the dog. J Clin Periodontol 26:643-652 [DOI] [PubMed] [Google Scholar]
- Attström R, Graf-de Beer M, Schroeder HE. (1975). Clinical and histologic characteristics of normal gingiva in dogs. J Periodontal Res 10:115-127 [DOI] [PubMed] [Google Scholar]
- Balabanian CA, Coutinho-Netto J, Lamano-Carvalho TL, Lacerda SA, Brentegani LG. (2006). Biocompatibility of natural latex implanted into dental alveolus of rats. J Oral Sci 48:201-205 [DOI] [PubMed] [Google Scholar]
- Baron M, Haas R, Dörtbudak O, Watzek G. (2000). Experimentally induced peri-implantitis: a review of different treatment methods described in the literature. Int J Oral Maxillofac Implants 15:533-544 [PubMed] [Google Scholar]
- Belting CM, Schour I, Weinmann JP, Shepro MJ. (1953). Age changes in the periodontal tissues of the rat molar. J Dent Res 32:332-353 [DOI] [PubMed] [Google Scholar]
- Benatti BB, Neto JB, Casati MZ, Sallum EA, Sallum AW, Nociti FH., Jr (2006). Periodontal healing may be affected by aging: a histologic study in rats. J Periodontal Res 41:329-333 [DOI] [PubMed] [Google Scholar]
- Berretin-Felix G, Nary Filho H, Padovani CR, Machado WM. (2008). A longitudinal study of quality of life of elderly with mandibular implant-supported fixed prostheses. Clin Oral Implants Res 19:704-708 [DOI] [PubMed] [Google Scholar]
- Brecx MC, Nalbandian J, Ooya K, Kornman KS, Robertson PB. (1985). Morphological studies on periodontal disease in the cynomolgus monkey. II. Light microscopic observations on ligature-induced periodontitis. J Periodontal Res 20:165-175 [DOI] [PubMed] [Google Scholar]
- Cancedda R, Giannoni P, Mastrogiacomo M. (2007). A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28:4240-4250 [DOI] [PubMed] [Google Scholar]
- Caton J, Mota L, Gandini L, Laskaris B. (1994). Non-human primate models for testing the efficacy and safety of periodontal regeneration procedures. J Periodontol 65:1143-1150 [DOI] [PubMed] [Google Scholar]
- Chen FM, Zhao YM, Wu H, Deng ZH, Wang QT, Zhou W, et al. (2006). Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin-like growth factor-I from dextran-co-gelatin microspheres. J Control Release 114:209-222 [DOI] [PubMed] [Google Scholar]
- Cochran DL, Schenk R, Buser D, Wozney JM, Jones AA. (1999). Recombinant human bone morphogenetic protein-2 stimulation of bone formation around endosseous dental implants. J Periodontol 70:139-150 [DOI] [PubMed] [Google Scholar]
- Donos N, Sculean A, Glavind L, Reich E, Karring T. (2003). Wound healing of degree III furcation involvements following guided tissue regeneration and/or Emdogain. A histologic study. J Clin Periodontol 30:1061-1068 [DOI] [PubMed] [Google Scholar]
- Dunn CA, Jin Q, Taba M, Jr, Franceschi RT, Rutherford RB, Giannobile WV. (2005). BMP gene delivery for alveolar bone engineering at dental implant defects. Mol Ther 11:294-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegaard B, Karring T, Listgarten M, Löe H. (1973). New attachment after treatment of interradicular lesions. J Periodontol 44:209-217 [DOI] [PubMed] [Google Scholar]
- Ellegaard B, Karring T, Davies R, Löe H. (1974). New attachment after treatment of intrabony defects in monkeys. J Periodontol 45:368-377 [DOI] [PubMed] [Google Scholar]
- Frost H. (1964). The laws of bone structure. Springfield, IL: Charles C. Thomas [Google Scholar]
- Giannobile WV, Finkelman RD, Lynch SE. (1994). Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J Periodontol 65:1158-1168 [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D’Andrea M, et al. (1996). Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res 31:301-312 [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Ryan S, Shih MS, Su DL, Kaplan PL, Chan TC. (1998). Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J Periodontol 69:129-137 [DOI] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. (2008). The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol 35:89-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani F, Laurell L, Tonetti M, Gottlow J, Berglundh T. (2005). Periodontal wound healing following GTR therapy of dehiscence-type defects in the monkey: short-, medium- and long-term healing. J Clin Periodontol 32:905-914 [DOI] [PubMed] [Google Scholar]
- Grunder U, Hurzeler MB, Schüpbach P, Strub JR. (1993). Treatment of ligature-induced peri-implantitis using guided tissue regeneration: a clinical and histologic study in the beagle dog. Int J Oral Maxillofac Implants 8:282-293 [PubMed] [Google Scholar]
- Guglielmotti MB, Cabrini RL. (1985). Alveolar wound healing and ridge remodeling after tooth extraction in the rat: a histologic, radiographic, and histometric study. J Oral Maxillofac Surg 43:359-364 [DOI] [PubMed] [Google Scholar]
- Hahn E, Sonis S, Gallagher G, Atwood D. (1988). Preservation of the alveolar ridge with hydroxyapatite-collagen implants in rats. J Prosthet Dent 60:729-734 [DOI] [PubMed] [Google Scholar]
- Hammarström L, Heijl L, Gestrelius S. (1997). Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J Clin Periodontol 24(9 Pt 2):669-677 [DOI] [PubMed] [Google Scholar]
- Haney JM, Zimmerman GJ, Wikesjö UM. (1995). Periodontal repair in dogs: evaluation of the natural disease model. J Clin Periodontol 22:208-213 [DOI] [PubMed] [Google Scholar]
- Hile DD, Sonis ST, Doherty SA, Tian X, Zhang Q, Jee WS, et al. (2005). Dimensional stability of the alveolar ridge after implantation of a bioabsorbable bone graft substitute: a radiographic and histomorphometric study in rats. J Oral Implantol 31:68-76 [DOI] [PubMed] [Google Scholar]
- Hollinger JO, Kleinschmidt JC. (1990). The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg 1:60-68 [DOI] [PubMed] [Google Scholar]
- Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. (1988). Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 239:55-57 [DOI] [PubMed] [Google Scholar]
- Hovey LR, Jones AA, McGuire M, Mellonig JT, Schoolfield J, Cochran DL. (2006). Application of periodontal tissue engineering using enamel matrix derivative and a human fibroblast-derived dermal substitute to stimulate periodontal wound healing in Class III furcation defects. J Periodontol 77:790-799 [DOI] [PubMed] [Google Scholar]
- Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. (1997). A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol 68:1186-1193 [DOI] [PubMed] [Google Scholar]
- Huang KK, Shen C, Chiang CY, Hsieh YD, Fu E. (2005). Effects of bone morphogenetic protein-6 on periodontal wound healing in a fenestration defect of rats. J Periodontal Res 40:1-10 [DOI] [PubMed] [Google Scholar]
- Hürzeler MB, Quinones CR, Schüpbach P, Morrison EC, Caffesse RG. (1997). Treatment of peri-implantitis using guided bone regeneration and bone grafts, alone or in combination, in beagle dogs. Part 2: Histologic findings. Int J Oral Maxillofac Implants 12:168-175 [PubMed] [Google Scholar]
- Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. (2004). Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther 9:519-526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Cirelli JA, Park CH, Sugai JV, Taba M, Jr, Kostenuik PJ, et al. (2007). RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J Periodontol 78:1300-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. (2003). Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol 74:202-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaigler D, Cirelli JA, Giannobile WV. (2006). Growth factor delivery for oral and periodontal tissue engineering. Exp Opin Drug Deliv 3:647-662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaigler D, Fuller K, Giannobile WV. (2010). Regulatory agencies for the evaluation of dental drugs, devices and biologics. In: Clinical research in oral health. Giannobile WV, Burt B, Genco RJ. editors. Copenhagen: Wiley-Blackwell Publishers [Google Scholar]
- Karalis M, Pavlidis TE, Psarras K, Ballas K, Zaraboukas T, Rafailidis S, et al. (2008). Effect of experimentally induced liver cirrhosis on wound healing of the post-extraction tooth socket in rats. Eur Surg Res 40:190-196 [DOI] [PubMed] [Google Scholar]
- Karatzas S, Zavras A, Greenspan D, Amar S. (1999). Histologic observations of periodontal wound healing after treatment with PerioGlas in nonhuman primates. Int J Periodontics Restorative Dent 19:489-499 [PubMed] [Google Scholar]
- Karimbux NY, Sirakian A, Weber HP, Nishimura I. (1995). A new animal model for molecular biological analysis of the implant-tissue interface: spatial expression of type XII collagen mRNA around a titanium oral implant. J Oral Implantol 21:107-113 [PubMed] [Google Scholar]
- King GN, Hughes FJ. (1999). Effects of occlusal loading on ankylosis, bone, and cementum formation during bone morphogenetic protein-2-stimulated periodontal regeneration in vivo. J Periodontol 70:1125-1135 [DOI] [PubMed] [Google Scholar]
- King GN, Hughes FJ. (2001). Bone morphogenetic protein-2 stimulates cell recruitment and cementogenesis during early wound healing. J Clin Periodontol 28:465-475 [DOI] [PubMed] [Google Scholar]
- King GN, King N, Cruchley AT, Wozney JM, Hughes FJ. (1997). Recombinant human bone morphogenetic protein-2 promotes wound healing in rat periodontal fenestration defects. J Dent Res 76:1460-1470 [DOI] [PubMed] [Google Scholar]
- Kitamura M, Nakashima K, Kowashi Y, Fujii T, Shimauchi H, Sasano T, et al. (2008). Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial. PLoS ONE 3(7):e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen B. (1991). Microbiological and immunological aspects of experimental periodontal disease in rats: a review article. J Periodontol 62:59-73 [DOI] [PubMed] [Google Scholar]
- Kornman KS, Holt SC, Robertson PB. (1981). The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J Periodontal Res 16:363-371 [DOI] [PubMed] [Google Scholar]
- Kostopoulos L, Karring T. (1994). Augmentation of the rat mandible using guided tissue regeneration. Clin Oral Implants Res 5:75-82 [DOI] [PubMed] [Google Scholar]
- Kostopoulos L, Karring T. (2004). Susceptibility of GTR-regenerated periodontal attachment to ligature-induced periodontitis. J Clin Periodontol 31:336-340 [DOI] [PubMed] [Google Scholar]
- Laurell L, Bose M, Graziani F, Tonetti M, Berglundh T. (2006). The structure of periodontal tissues formed following guided tissue regeneration therapy of intra-bony defects in the monkey. J Clin Periodontol 33:596-603 [DOI] [PubMed] [Google Scholar]
- Lekic P, Sodek J, McCulloch CA. (1996a). Osteopontin and bone sialoprotein expression in regenerating rat periodontal ligament and alveolar bone. Anat Rec 244:50-58 [DOI] [PubMed] [Google Scholar]
- Lekic P, Sodek J, McCulloch CA. (1996b). Relationship of cellular proliferation to expression of osteopontin and bone sialoprotein in regenerating rat periodontium. Cell Tissue Res 285:491-500 [DOI] [PubMed] [Google Scholar]
- Lekovic V, Klokkevold PR, Kenney EB, Dimitrijelic B, Nedic M, Weinlaender M. (1998). Histologic evaluation of guided tissue regeneration using 4 barrier membranes: a comparative furcation study in dogs. J Periodontol 69:54-61 [DOI] [PubMed] [Google Scholar]
- Lindhe J, Hamp SE, Löe H. (1975). Plaque induced periodontal disease in beagle dogs. A 4-year clinical, roentgenographical and histometrical study. J Periodontal Res 10:243-255 [DOI] [PubMed] [Google Scholar]
- Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C. (1992). Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res 3:9-16 [DOI] [PubMed] [Google Scholar]
- Lioubavina-Hack N, Carmagnola D, Lynch SE, Karring T. (2005). Effect of Bio-Oss with or without platelet-derived growth factor on bone formation by “guided tissue regeneration”: a pilot study in rats. J Clin Periodontol 32:1254-1260 [DOI] [PubMed] [Google Scholar]
- Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, Zappa UE, et al. (1989). A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol 16:545-548 [DOI] [PubMed] [Google Scholar]
- Lynch SE, de Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, et al. (1991). The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol 62:458-467 [DOI] [PubMed] [Google Scholar]
- Madianos PN, Papapanou PN, Socransky SS, Dahlén G, Sandros J. (1994). Host-related genotypic heterogeneity of Porphyromonas gingivalis strains in the beagle dog. Oral Microbiol Immunol 9:241-247 [DOI] [PubMed] [Google Scholar]
- Mardas N, Kostopoulos L, Stavropoulos A, Karring T. (2003). Osteogenesis by guided tissue regeneration and demineralized bone matrix. J Clin Periodontol 30:176-183 [DOI] [PubMed] [Google Scholar]
- Mariotti A. (2003). Efficacy of chemical root surface modifiers in the treatment of periodontal disease. A systematic review. Ann Periodontol 8:205-226 [DOI] [PubMed] [Google Scholar]
- Matsuura M, Herr Y, Han KY, Lin WL, Genco RJ, Cho MI. (1995). Immunohistochemical expression of extracellular matrix components of normal and healing periodontal tissues in the beagle dog. J Periodontol 66:579-593; erratum in J Periodontol 66:905-914, 1995 [DOI] [PubMed] [Google Scholar]
- Melcher AH. (1970). Repair of wounds in the periodontium of the rat. Influence of periodontal ligament on osteogenesis. Arch Oral Biol 15:1183-1204 [DOI] [PubMed] [Google Scholar]
- Murakami S, Takayama S, Kitamura M, Shimabukuro Y, Yanagi K, Ikezawa K, et al. (2003). Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J Periodontal Res 38:97-103 [DOI] [PubMed] [Google Scholar]
- Murano Y, Ota M, Katayama A, Sugito H, Shibukawa Y, Yamada S. (2006). Periodontal regeneration following transplantation of proliferating tissue derived from periodontal ligament into class III furcation defects in dogs. Biomed Res 27:139-147 [DOI] [PubMed] [Google Scholar]
- Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. (2005). Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol 76:2205-2215 [DOI] [PubMed] [Google Scholar]
- Nociti FH, Jr, Machado MA, Stefani CM, Sallum EA, Sallum AW. (2001a). Absorbable versus nonabsorbable membranes and bone grafts in the treatment of ligature-induced peri-implantitis defects in dogs. Part I. A clinical investigation. Clin Oral Implants Res 12:115-120 [DOI] [PubMed] [Google Scholar]
- Nociti FH, Jr, Cesco De, Toledo R, Machado MA, Stefani CM, Line SR, Gonçalves RB. (2001b). Clinical and microbiological evaluation of ligature-induced peri-implantitis and periodontitis in dogs. Clin Oral Implants Res 12:295-300 [DOI] [PubMed] [Google Scholar]
- Page RC, Schroeder HE. (1981). Spontaneous chronic periodontitis in adult dogs. A clinical and histopathological survey. J Periodontol 52:60-73 [DOI] [PubMed] [Google Scholar]
- Park CH, Abramson ZR, Taba M, Jr, Jin Q, Chang J, Kreider JM, et al. (2007). Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol 78:273-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Matsuura M, Han KY, Norderyd O, Lin WL, Genco RJ, et al. (1995). Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J Periodontol 66:462-477 [DOI] [PubMed] [Google Scholar]
- Patel RR, Richards PS, Inglehart MR. (2008). Periodontal health, quality of life, and smiling patterns—an exploration. J Periodontol 79:224-231 [DOI] [PubMed] [Google Scholar]
- Pereira MC, Zecchin KG, Campagnoli EB, Jorge J. (2007). Ovariectomy delays alveolar wound healing after molar extractions in rats. J Oral Maxillofac Surg 65:2248-2253 [DOI] [PubMed] [Google Scholar]
- Polyzois I, Renvert S, Bosshardt DD, Lang NP, Claffey N. (2007). Effect of Bio-Oss on osseointegration of dental implants surrounded by circumferential bone defects of different dimensions: an experimental study in the dog. Clin Oral Implants Res 18:304-310 [DOI] [PubMed] [Google Scholar]
- Rajshankar D, McCulloch CA, Tenenbaum HC, Lekic PC. (1998). Osteogenic inhibition by rat periodontal ligament cells: modulation of bone morphogenic protein-7 activity in vivo. Cell Tissue Res 294:475-483 [DOI] [PubMed] [Google Scholar]
- Ramfjord S. (1951). Experimental periodontal reattachment in rhesus monkeys. J Periodontol 22:67-77 [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Crooks J, Petit JC, Rueger DC. (2001). Periodontal tissue regeneration by combined applications of recombinant human osteogenic protein-1 and bone morphogenetic protein-2. A pilot study in Chacma baboons (Papio ursinus). Eur J Oral Sci 109:241-248 [DOI] [PubMed] [Google Scholar]
- Roberts WE, Turley PK, Brezniak N, Fielder PJ. (1987). Implants: bone physiology and metabolism. CDA J 15:54-61 [PubMed] [Google Scholar]
- Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, et al. (2007). Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol 78:550-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford RB, Niekrash CE, Kennedy JE, Charette MF. (1992). Platelet-derived and insulin-like growth factors stimulate regeneration of periodontal attachment in monkeys. J Periodontal Res 27(4 Pt 1):285-290 [DOI] [PubMed] [Google Scholar]
- Sander L, Karring T. (1995). Healing of periodontal lesions in monkeys following the guided tissue regeneration procedure. A histological study. J Clin Periodontol 22:332-337 [DOI] [PubMed] [Google Scholar]
- Schectman LR, Ammons WF, Simpson DM, Page RC. (1972). Host tissue response in chronic periodontal disease. 2. Histologic features of the normal periodontium, and histologic and ultrastructural manifestations of disease in the marmoset. J Periodontal Res 7:195-212 [DOI] [PubMed] [Google Scholar]
- Schou S, Holmstrup P, Kornman KS. (1993). Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol 64:497-508 [DOI] [PubMed] [Google Scholar]
- Schwarz F, Herten M, Sager M, Bieling K, Sculean A, Becker J. (2007). Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res 18:161-170; erratum in Clin Oral Implants Res 18:397, 2007 [DOI] [PubMed] [Google Scholar]
- Sculean A, Karring T, Theilade J, Lioubavina N. (1997). The regenerative potential of oxytalan fibers. An experimental study in the monkey. J Clin Periodontol 24:932-936 [DOI] [PubMed] [Google Scholar]
- Sculean A, Donos N, Brecx M, Karring T, Reich E. (2000a). Healing of fenestration-type defects following treatment with guided tissue regeneration or enamel matrix proteins. An experimental study in monkeys. Clin Oral Investig 4:50-56 [DOI] [PubMed] [Google Scholar]
- Sculean A, Donos N, Brecx M, Reich E, Karring T. (2000b). Treatment of intrabony defects with guided tissue regeneration and enamel-matrix-proteins. An experimental study in monkeys. J Clin Periodontol 27:466-472 [DOI] [PubMed] [Google Scholar]
- Sculean A, Berakdar M, Pahl S, Windisch P, Brecx M, Reich E, et al. (2001). Patterns of cytokeratin expression in monkey and human periodontium following regenerative and conventional periodontal surgery. J Periodontal Res 36:260-268 [DOI] [PubMed] [Google Scholar]
- Sculean A, Junker R, Donos N, Berakdar M, Brecx M, Dunker N. (2002). Immunohistochemical evaluation of matrix molecules associated with wound healing following regenerative periodontal treatment in monkeys. Clin Oral Investig 6:175-182 [DOI] [PubMed] [Google Scholar]
- Seol Y-J, Pellegrini G, Gruber R, Chang PC, Franco L, Giannobile WV. (2010). Methods for periodontal regeneration procedures. In: Principles of perio-dontal treatment. Seymour G. editor. NY, USA: Humana Press; (in press). [Google Scholar]
- Shibli JA, Martins MC, Lotufo RF, Marcantonio E., Jr (2003). Microbiologic and radiographic analysis of ligature-induced peri-implantitis with different dental implant surfaces. Int J Oral Maxillofac Implants 18:383-390 [PubMed] [Google Scholar]
- Sorensen RG, Wikesjö UM, Kinoshita A, Wozney JM. (2004). Periodontal repair in dogs: evaluation of a bioresorbable calcium phosphate cement (Ceredex) as a carrier for rhBMP-2. J Clin Periodontol 31:796-804 [DOI] [PubMed] [Google Scholar]
- Stavropoulos A, Kostopoulos L, Nyengaard JR, Karring T. (2003). Deproteinized bovine bone (Bio-Oss) and bioactive glass (Biogran) arrest bone formation when used as an adjunct to guided tissue regeneration (GTR): an experimental study in the rat. J Clin Periodontol 30:636-643 [DOI] [PubMed] [Google Scholar]
- Syed SA, Svanberg M, Svanberg G. (1981). The predominant cultivable dental plaque flora of beagle dogs with periodontitis. J Clin Periodontol 8:45-56 [DOI] [PubMed] [Google Scholar]
- Takayama S, Murakami S, Shimabukuro Y, Kitamura M, Okada H. (2001). Periodontal regeneration by FGF-2 (bFGF) in primate models. J Dent Res 80:2075-2079 [DOI] [PubMed] [Google Scholar]
- Talwar R, Di Silvio L, Hughes FJ, King GN. (2001). Effects of carrier release kinetics on bone morphogenetic protein-2-induced periodontal regeneration in vivo. J Clin Periodontol 28:340-347 [DOI] [PubMed] [Google Scholar]
- Teare JA, Ramoshebi LN, Ripamonti U. (2008). Periodontal tissue regeneration by recombinant human transforming growth factor-beta 3 in Papio ursinus. J Periodontal Res 43:1-8 [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Lang NP, Cortellini P, Suvan JE, Adriaens P, Dubravec D, et al. (2002). Enamel matrix proteins in the regenerative therapy of deep intrabony defects. J Clin Periodontol 29:317-325 [DOI] [PubMed] [Google Scholar]
- van der Donk S, Buma P, Aspenberg P, Schreurs BW. (2001). Similarity of bone ingrowth in rats and goats: a bone chamber study. Comp Med 51:336-340 [PubMed] [Google Scholar]
- Wikesjö UM, Nilvéus R. (1991). Periodontal repair in dogs. Healing patterns in large circumferential periodontal defects. J Clin Periodontol 18:49-59 [DOI] [PubMed] [Google Scholar]
- Wikesjö UM, Kean CJ, Zimmerman GJ. (1994). Periodontal repair in dogs: supraalveolar defect models for evaluation of safety and efficacy of periodontal reconstructive therapy. J Periodontol 65:1151-1157 [DOI] [PubMed] [Google Scholar]
- Wikesjö UM, Lim WH, Thomson RC, Cook AD, Wozney JM, Hardwick WR. (2003a). Periodontal repair in dogs: evaluation of a bioabsorbable space-providing macroporous membrane with recombinant human bone morphogenetic protein-2. J Periodontol 74:635-647 [DOI] [PubMed] [Google Scholar]
- Wikesjö UM, Lim WH, Thomson RC, Hardwick WR. (2003b). Periodontal repair in dogs: gingival tissue occlusion, a critical requirement for GTR? J Clin Periodontol 30:655-664 [DOI] [PubMed] [Google Scholar]
- Wikesjö UM, Xiropaidis AV, Thomson RC, Cook AD, Selvig KA, Hardwick WR. (2003c). Periodontal repair in dogs: space-providing ePTFE devices increase rhBMP-2/ACS-induced bone formation. J Clin Periodontol 30:715-725 [DOI] [PubMed] [Google Scholar]
- Wikesjö UM, Sorensen RG, Kinoshita A, Jian Li X, Wozney JM. (2004). Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol 31:662-670 [DOI] [PubMed] [Google Scholar]
- Wikesjö UM, Xiropaidis AV, Qahash M, Lim WH, Sorensen RG, Rohrer MD, et al. (2008a). Bone formation at recombinant human bone morphogenetic protein-2-coated titanium implants in the posterior mandible (Type II bone) in dogs. J Clin Periodontol 35:985-991 [DOI] [PubMed] [Google Scholar]
- Wikesjö UM, Huang YH, Xiropaidis AV, Sorensen RG, Rohrer MD, Prasad HS, et al. (2008b). Bone formation at recombinant human bone morphogenetic protein-2-coated titanium implants in the posterior maxilla (Type IV bone) in non-human primates. J Clin Periodontol 35:992-1000 [DOI] [PubMed] [Google Scholar]
- Wikesjö UM, Qahash M, Polimeni G, Susin C, Shanaman RH, Rohrer MD, et al. (2008c). Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol 35:1001-1010 [DOI] [PubMed] [Google Scholar]
- Wirthlin MR, Hancock EB. (1982). Regeneration and repair after biologic treatment of root surfaces in monkeys. II. Proximal surfaces posterior teeth. J Periodontol 53:302-306 [DOI] [PubMed] [Google Scholar]
- Wu Z, Liu C, Zang G, Sun H. (2008). The effect of simvastatin on remodelling of the alveolar bone following tooth extraction. Int J Oral Maxillofac Surg 37:170-176 [DOI] [PubMed] [Google Scholar]
- You TM, Choi BH, Zhu SJ, Jung JH, Lee SH, Huh JY, et al. (2007). Treatment of experimental peri-implantitis using autogenous bone grafts and platelet-enriched fibrin glue in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:34-37 [DOI] [PubMed] [Google Scholar]
- Zhang X, Kohli M, Zhou Q, Graves DT, Amar S. (2004). Short- and long-term effects of IL-1 and TNF antagonists on periodontal wound healing. J Immunol 173:3514-3523 [DOI] [PubMed] [Google Scholar]
- Zhao M, Jin Q, Berry JE, Nociti FH, Jr, Giannobile WV, Somerman MJ. (2004). Cementoblast delivery for periodontal tissue engineering. J Periodontol 75:154-161 [DOI] [PMC free article] [PubMed] [Google Scholar]