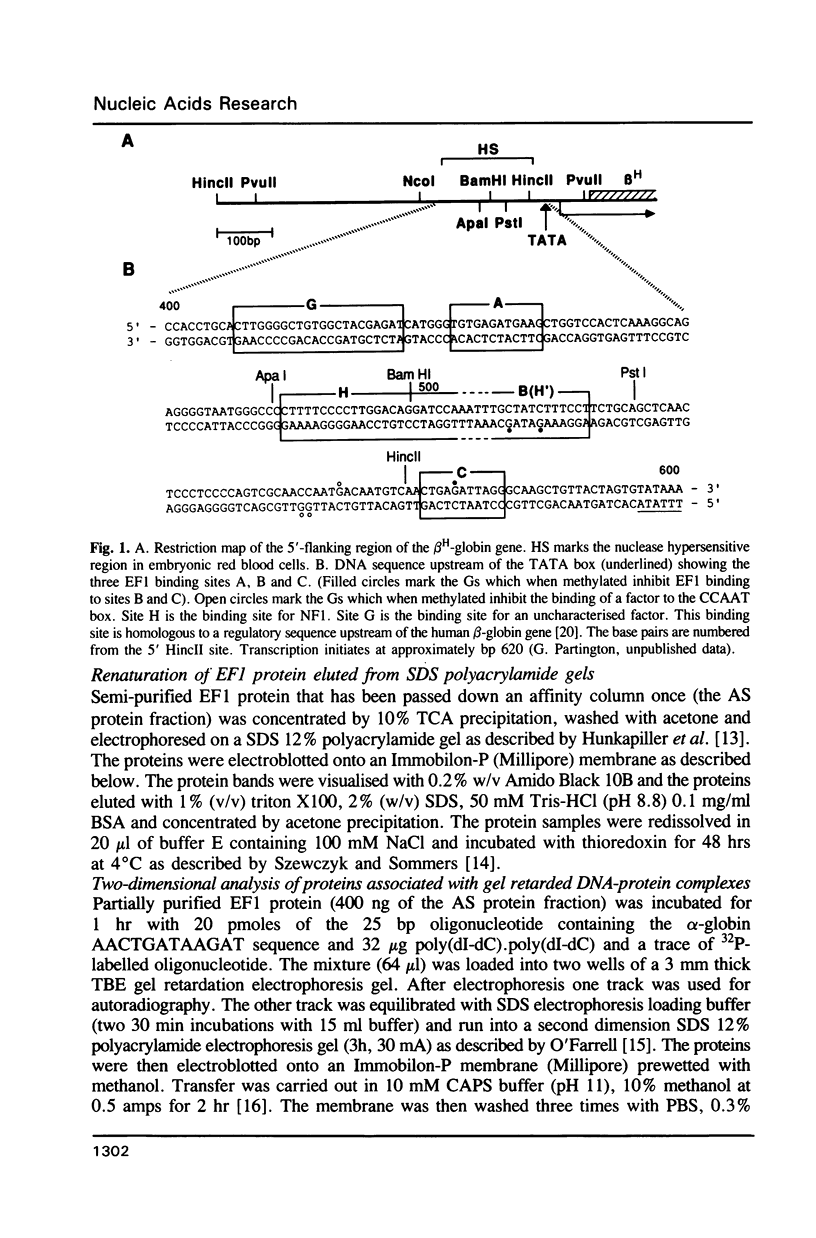
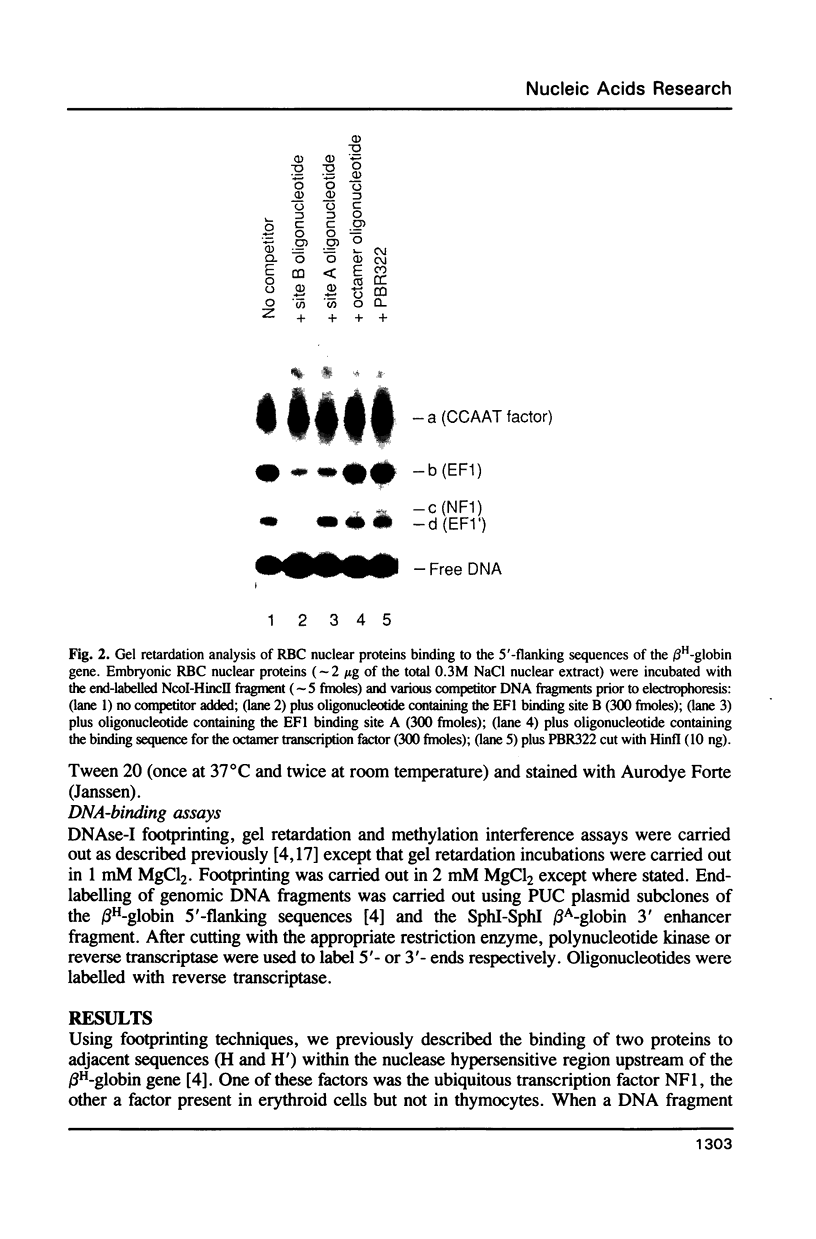
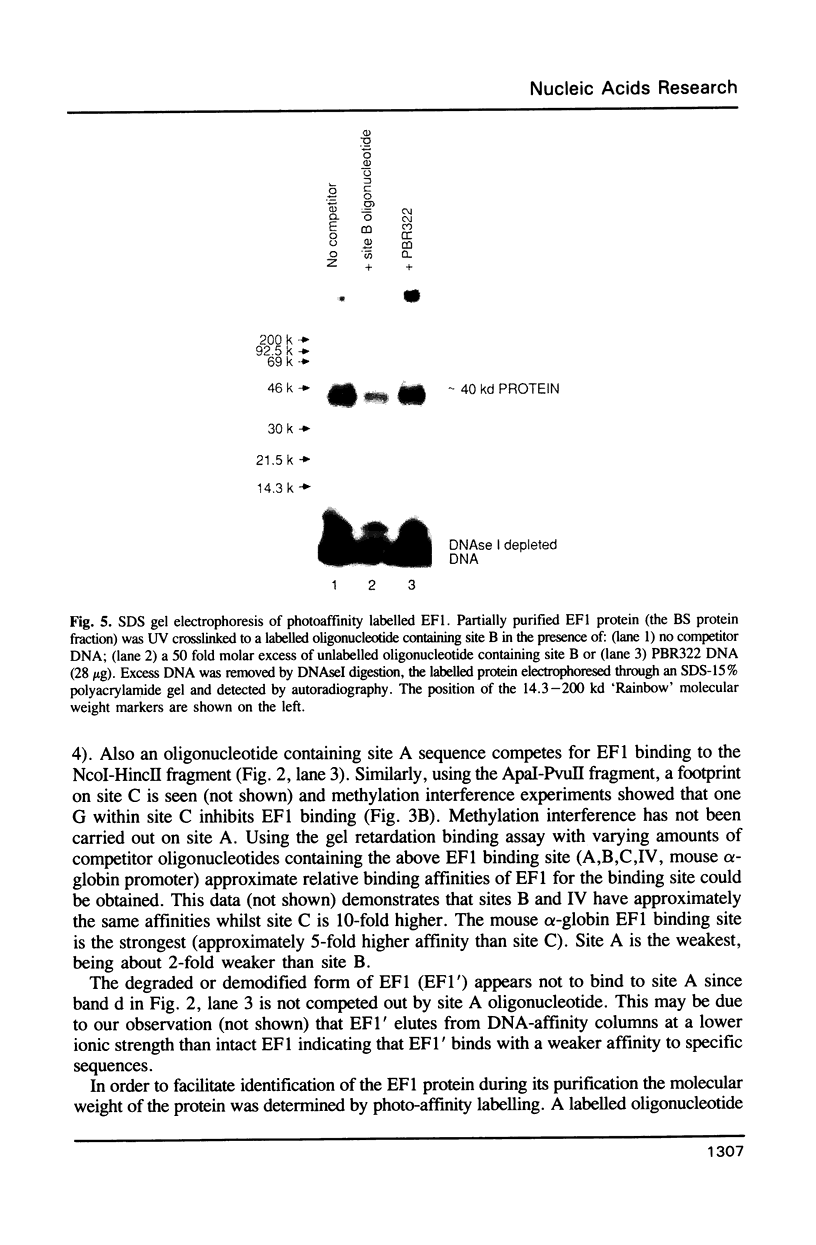
Abstract
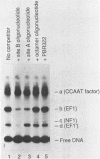
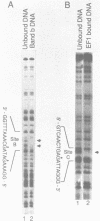
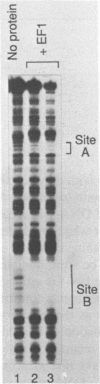
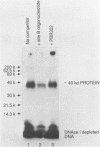
An erythroid nuclear protein (EF1), originally detected as a protein binding within the nuclease hypersensitive site upstream of the chicken beta H-globin gene, has been purified. This protein of 37,000-39,000 molecular weight binds to three sites within the hypersensitive region: one between the CCAAT and TATA boxes, the second (further upstream) next to a NF1 binding site, and the third adjacent to a regulatory element found in a number of beta-globin genes. The EF1 protein also binds to an erythroid-specific promoter element of the mouse alpha-globin gene and to two sites within the chicken beta A-globin enhancer. These six EF1-binding sites are related by the consensus sequence A/TGATAA/GG/C. A minor protein of molecular weight 72,000 which co-purifies with EF1 also binds to the same sequences.
Full text
PDF








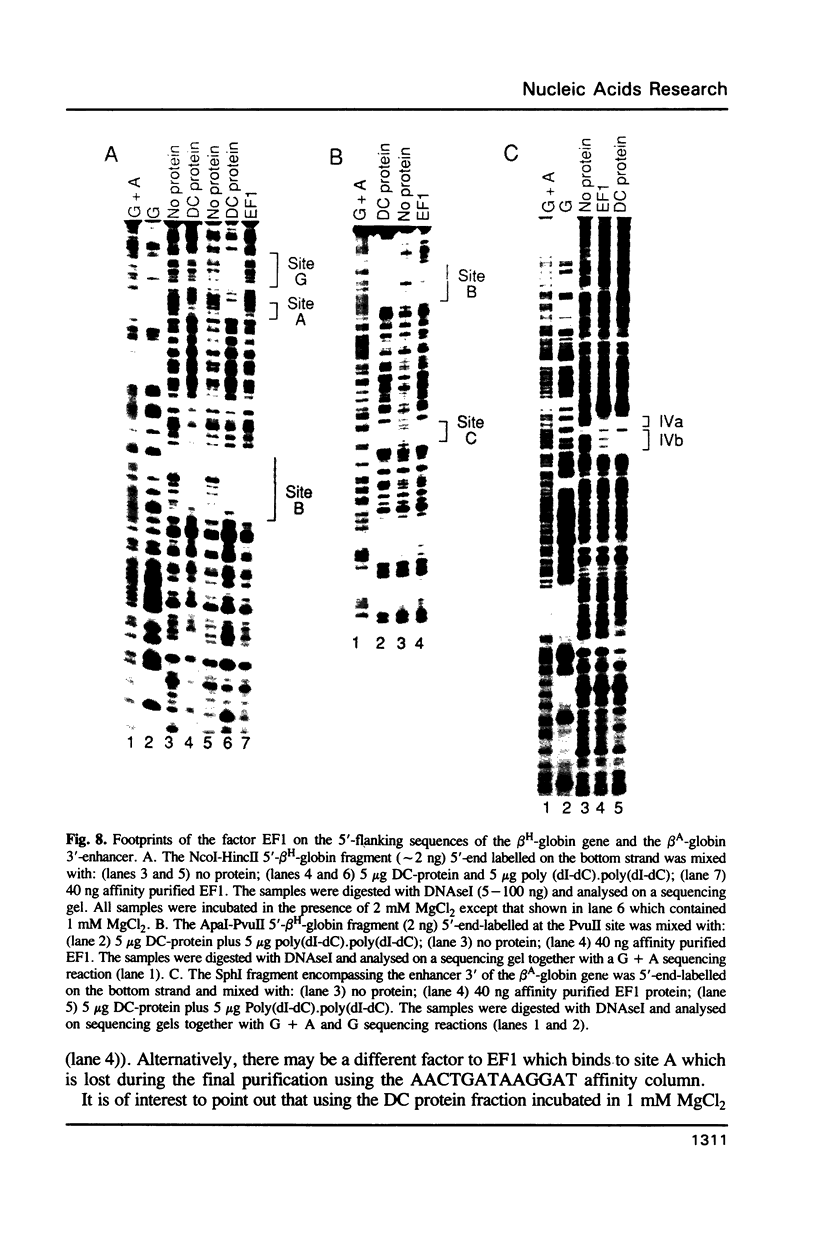



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniou M., deBoer E., Habets G., Grosveld F. The human beta-globin gene contains multiple regulatory regions: identification of one promoter and two downstream enhancers. EMBO J. 1988 Feb;7(2):377–384. doi: 10.1002/j.1460-2075.1988.tb02824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Palmieri S., Freudenstein C., Zentgraf H., Graf T. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell. 1982 Apr;28(4):907–919. doi: 10.1016/0092-8674(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. A 3' enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult beta-globin gene. Nature. 1986 Oct 23;323(6090):731–734. doi: 10.1038/323731a0. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Lewis C. D., Felsenfeld G. Interaction of specific nuclear factors with the nuclease-hypersensitive region of the chicken adult beta-globin gene: nature of the binding domain. Cell. 1985 May;41(1):21–30. doi: 10.1016/0092-8674(85)90057-1. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Jackson P. D., Felsenfeld G. Analysis of the tissue-specific enhancer at the 3' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4786–4790. doi: 10.1073/pnas.84.14.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., DeChiara T., Efstratiadis A. A promoter of the rat insulin-like growth factor II gene consists of minimal control elements. J Mol Biol. 1988 Jan 5;199(1):61–81. doi: 10.1016/0022-2836(88)90379-8. [DOI] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Mocikat R., Zachau H. G. Sequences closely related to an immunoglobulin gene promoter/enhancer element occur also upstream of other eukaryotic and of prokaryotic genes. Nucleic Acids Res. 1986 Nov 25;14(22):8819–8827. doi: 10.1093/nar/14.22.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galson D. L., Housman D. E. Detection of two tissue-specific DNA-binding proteins with affinity for sites in the mouse beta-globin intervening sequence 2. Mol Cell Biol. 1988 Jan;8(1):381–392. doi: 10.1128/mcb.8.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H. Identification of three sequence-specific DNA-binding proteins which interact with the Rous sarcoma virus enhancer and upstream promoter elements. J Virol. 1988 Jun;62(6):2186–2190. doi: 10.1128/jvi.62.6.2186-2190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Nickol J. M., Lieber M. R., Felsenfeld G. Regulated gene expression in transfected primary chicken erythrocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4312–4316. doi: 10.1073/pnas.83.12.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Jackson P. D., Felsenfeld G. Protein-binding sites within the 5' DNase I-hypersensitive region of the chicken alpha D-globin gene. Mol Cell Biol. 1987 Jun;7(6):2059–2069. doi: 10.1128/mcb.7.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsovali A., Müller M. M., Weber F., Marcaud L., Farache G., Schreiber E., Schaffner W., Scherrer K. A transcriptional enhancer located between adult beta-globin and embryonic epsilon-globin genes in chicken and duck. Gene. 1987;58(2-3):167–175. doi: 10.1016/0378-1119(87)90373-8. [DOI] [PubMed] [Google Scholar]
- Landes G. M., Villeponteau B., Pribyl T. M., Martinson H. G. Hemoglobin switching in chickens. Is the switch initiated post-transcriptionally? J Biol Chem. 1982 Sep 25;257(18):11008–11014. [PubMed] [Google Scholar]
- Larsen A., Weintraub H. An altered DNA conformation detected by S1 nuclease occurs at specific regions in active chick globin chromatin. Cell. 1982 Jun;29(2):609–622. doi: 10.1016/0092-8674(82)90177-5. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nickol J. M., Felsenfeld G. Bidirectional control of the chicken beta- and epsilon-globin genes by a shared enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2548–2552. doi: 10.1073/pnas.85.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Plumb M. A., Lobanenkov V. V., Nicolas R. H., Wright C. A., Zavou S., Goodwin G. H. Characterisation of chicken erythroid nuclear proteins which bind to the nuclease hypersensitive regions upstream of the beta A- and beta H-globin genes. Nucleic Acids Res. 1986 Oct 10;14(19):7675–7693. doi: 10.1093/nar/14.19.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M., Felsenfeld G. Mutational analysis of the chicken beta-globin enhancer reveals two positive-acting domains. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6267–6271. doi: 10.1073/pnas.85.17.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson I. B., Ingram V. M. Expression and partial DNA sequence of the chicken beta H-globin gene. J Biol Chem. 1983 Jan 25;258(2):802–809. [PubMed] [Google Scholar]
- Rupp R. A., Nicolas R. H., Borgmeyer U., Lobanenkov V. V., Plumb M. A., Sippel A. E., Goodwin G. H. TGGCA protein is present in erythroid nuclei and binds within the nuclease-hypersensitive sites 5' of the chicken beta H- and beta A-globin genes. Eur J Biochem. 1988 Nov 15;177(3):505–511. doi: 10.1111/j.1432-1033.1988.tb14401.x. [DOI] [PubMed] [Google Scholar]
- Rupp R. A., Sippel A. E. Chicken liver TGGCA protein purified by preparative mobility shift electrophoresis (PMSE) shows a 36.8 to 29.8 kd microheterogeneity. Nucleic Acids Res. 1987 Dec 10;15(23):9707–9726. doi: 10.1093/nar/15.23.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B., Cohen R. B., Garfinkel S., Thompson J. A. DNA affinity labeling of adenovirus type 2 upstream promoter sequence-binding factors identifies two distinct proteins. Mol Cell Biol. 1988 Jan;8(1):105–113. doi: 10.1128/mcb.8.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B., Summers D. F. Preparative elution of proteins blotted to Immobilon membranes. Anal Biochem. 1988 Jan;168(1):48–53. doi: 10.1016/0003-2697(88)90008-5. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Beug H., Groudine M., Graf T. Temperature-sensitive changes in the structure of globin chromatin in lines of red cell precursors transformed by ts-AEV. Cell. 1982 Apr;28(4):931–940. doi: 10.1016/0092-8674(82)90072-1. [DOI] [PubMed] [Google Scholar]