Abstract
Tooth regeneration by cell delivery encounters translational hurdles. We hypothesized that anatomically correct teeth can regenerate in scaffolds without cell transplantation. Novel, anatomically shaped human molar scaffolds and rat incisor scaffolds were fabricated by 3D bioprinting from a hybrid of poly-ε-caprolactone and hydroxyapatite with 200-µm-diameter interconnecting microchannels. In each of 22 rats, an incisor scaffold was implanted orthotopically following mandibular incisor extraction, whereas a human molar scaffold was implanted ectopically into the dorsum. Stromal-derived factor-1 (SDF1) and bone morphogenetic protein-7 (BMP7) were delivered in scaffold microchannels. After 9 weeks, a putative periodontal ligament and new bone regenerated at the interface of rat incisor scaffold with native alveolar bone. SDF1 and BMP7 delivery not only recruited significantly more endogenous cells, but also elaborated greater angiogenesis than growth-factor-free control scaffolds. Regeneration of tooth-like structures and periodontal integration by cell homing provide an alternative to cell delivery, and may accelerate clinical applications.
Keywords: tooth regeneration, cell homing, stem cells, bioprinting, periodontal
Introduction
A tooth is a major organ consisting of biological viable pulp encased in mineralized dentin that may be covered with cementum and enamel ontogenetically in various species (Poole, 1967). Life ends in wildlife species after complete tooth loss. In humans, tooth loss can lead to physical and mental suffering that compromises self-esteem and quality of life (Pihlstrom et al., 2005; USDHHS, 2005). Contemporary dentistry restores missing teeth with dental implants or dentures. Dental implants, despite being the preferred treatment modality, can fail and have no ability to remodel with surrounding bone, which undergoes physiologically necessary remodeling throughout life (Ferreira et al., 2007). Accordingly, there has been intensifying interest in the regeneration of orofacial tissues, including teeth (Modino and Sharpe, 2005; Young et al., 2005; Mao et al., 2006).
Cell delivery has been the predominant approach in tooth regeneration. Disassociated cells of porcine or rat tooth buds in biomaterials yielded putative dentin and enamel organ (Young et al., 2002; Duailibi et al., 2004). Tooth bud cells and bone marrow osteoprogenitor cells in collagen, PLGA, or silk-protein scaffolds induced putative tooth-like tissues, alveolar bone, and periodontal ligament (Young et al., 2005; Duailibi et al., 2008; Kuo et al., 2008). Embryonic oral epithelium and adult mesenchyme together up-regulate odontogenesis genes upon mutual induction, and yielded dental structures upon transplantation into adult renal capsules or jaw bone (Ohazama et al., 2004). Similarly, implantation of E14.5 rat molar rudiments into adult mouse maxilla produced tooth-like structures with surrounding bone (Modino and Sharpe, 2005; Mantesso and Sharpe, 2009). Multipotent cells of the tooth apical papilla in tricalcium phosphate in swine incisor extraction sockets generated soft and mineralized tissues resembling the periodontal ligament (Sonoyama et al., 2006). Mouse E14.5 oral epithelium and dental mesenchyme were reconstituted in collagen gel and cultured ex vivo (Nakao et al., 2007), and, when they were implanted into the maxillary molar extraction sockets in 5-week-old mice, tooth morphogenesis took place and was followed by eruption into occlusion (Ikeda et al., 2009). Several studies have begun to tackle an obligatory task of scale-up toward human tooth size (Xu et al., 2008; Abukawa et al., 2009).
Tooth regeneration by cell transplantation is a meritorious approach. However, there are hurdles in the translation of cell-delivery-based tooth regeneration into therapeutics. Autologous embryonic tooth germ cells are inaccessible for human applications (Modino and Sharpe, 2005; Nakao et al., 2007; Ikeda et al., 2009). Xenogenic embryonic tooth germ cells (from non-human species) may elicit immunorejection and tooth dysmorphogenesis. Autologous post-natal tooth germ cells (e.g., third molars) or autologous dental pulp stem cells are of limited availability. Regardless of the cell source, cell delivery for tooth regeneration, similar to cell-based therapies for other tissues, encounters translational barriers (Ahsan et al., 2007; Evaluation criteria for musculoskeletal and craniofacial tissue engineering constructs, 2008). To date, excessive cost of commercialization and difficulties in regulatory approval have precluded any significant clinical translation of tooth regeneration. As a first step to addressing the limitations of cell delivery, we devised a cell-homing approach for tooth regeneration. A novel anatomically shaped scaffold was fabricated with interconnecting microchannels (diam., 200 µm) as conduits for the homing of host endogenous cells and angiogenesis. Remarkably, a putative periodontal ligament and de novo alveolar bone regenerated at the scaffold’s interface with native alveolar bone upon 9-week in vivo implantation. Cell homing by stromal-derived factor-1 (SDF1) and bone morphogenetic protein-7 (BMP7) not only recruited endogenous cells, but also induced angiogenesis. These findings represent the first demonstration of de novo formation of anatomically shaped tooth-like structures and periodontal integration in vivo, and may provide a clinically translatable approach.
Materials & Methods
Design and 3D Bioprinting of Anatomically Shaped Tooth Scaffolds
Anatomic shape and dimensions of the rat mandibular central incisor were derived from multiple slices of 2D laser scanning of extracted rat incisor per our prior methods (Lee et al., 2009; Stosich et al., 2009). The dimensions of the permanent mandibular first molar were derived from textbook averages and therefore were exempt from institutional review board approval. Scaffolds with the shape of the rat mandibular central incisor (Fig. 1A) and human mandibular first molar (Fig. 1B) were fabricated via 3D layer-by-layer apposition (Lee et al., 2009; Stosich et al., 2009). The composite consisted of 80 wt% polycaprolactone (PCL) and 20 wt% of hydroxyapatite (HA) (Sigma, St. Louis, MO, USA). PCL-HA was co-molten at 120°C and dispensed through a 27-gauge metal nozzle to create repeating 3D microstrands (200-µm wall thickness) and interconnecting microchannels (diam., 200 µm) (Figs. 1C, 1D).
Figure 1.
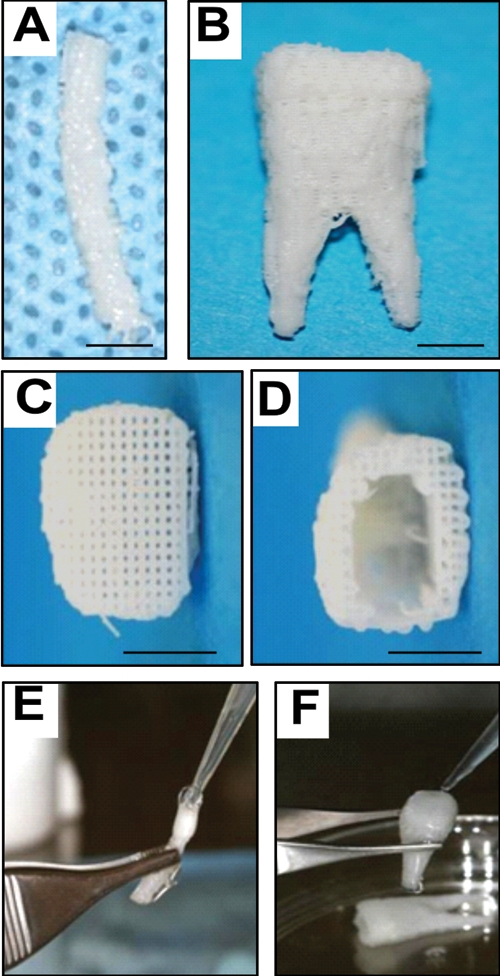
Design and fabrication of anatomically shaped human and rat tooth scaffolds by 3D bioprinting. Anatomic shape of the rat mandibular central incisor (A) and human mandibular first molar (B) were used for 3D reconstruction and bioprinting of a hybrid scaffold of poly-ϵ-caprolactone and hydroxyapatite, with 200-µm microstrands and interconnecting microchannels (diam., 200 µm), which serve as conduits for cell homing and angiogenesis (C,D). A blended cocktail of stromal-derived factor-1 (100 ng/mL) and bone morphogenetic protein-7 (100 ng/mL) was delivered in 2 mg/mL neutralized type I collagen solution and infused in scaffold microchannels for rat incisor scaffold (E) and human molar scaffold (F), followed by gelation.
Delivery of Bioactive Cues in Microchannels
All scaffolds were sterilized in ethylene oxide for 24 hrs. A blended cocktail of SDF1 (100 ng/mL) and BMP7 (100 ng/mL) was adsorbed in 2 mg/mL neutralized type I collagen solution (all from R&D, Minneapolis, MN, USA). SDF1 was selected for its effects to bind to CXCR4 receptors of multiple cell lineages, including mesenchymal stem/progenitor cells (Belema-Bedada et al., 2008; Kitaori et al., 2009). BMP7 was selected for its effects on dental pulp cells, fibroblasts, and osteoblasts in elaborating mineralization (Goldberg et al., 2001; Rutherford, 2001). SDF1 and BMP7 doses were chosen from in vivo work (Vaccaro et al., 2008; Kitaori et al., 2009). SDF1- and BMP7-loaded collagen solution was infused in scaffold microchannels by micropipettes (N = 11 for rat incisor scaffolds; N = 11 for human molar scaffolds) (Figs. 1E, 1F), and crosslinked at 37°C for 1 hr. Control scaffolds were infused with the same collagen gel, but without growth-factor delivery (N = 11 for rat incisor scaffolds; N = 11 for human molar scaffolds).
In vivo Tooth Regeneration Models
Following IACUC approval, 22 male (12-week-old) Sprague-Dawley rats were randomly divided equally into treatment and control groups (Charles River, NY, USA). All rats were anesthetized by i.p. administration of ketamine (80 mg/kg) and xylazine (5 mg/kg). A 2-cm incision was made in the dorsum. Human mandibular molar scaffolds were implanted into surgically created subcutaneous pouches (Fig. 2A), followed by wound closure. The rat right mandibular central incisor was then extracted with periotome (Figs. 2C, 2D), followed by implantation of the anatomically shaped mandibular incisor scaffold (Fig. 2E) into the extraction socket. The tooth was carefully luxated with the smallest possible periotome and Allen’s microsurgical instruments, to minimize root fractures. Practice was needed to minimize root fracture when extracting rat lower incisors. Upon the completion of pilot experiments, we were able to perform atraumatic extractions without fracturing the root (Fig. 2D). In rare cases of root fracture, the animals were excluded. The mandibular incisor scaffold protruded 3 mm from the alveolar edge. The flap was advanced for primary closure around the scaffold. Buprenorphine (0.05 mg/kg) was administered i.p. post-operatively for analgesia.
Figure 2.
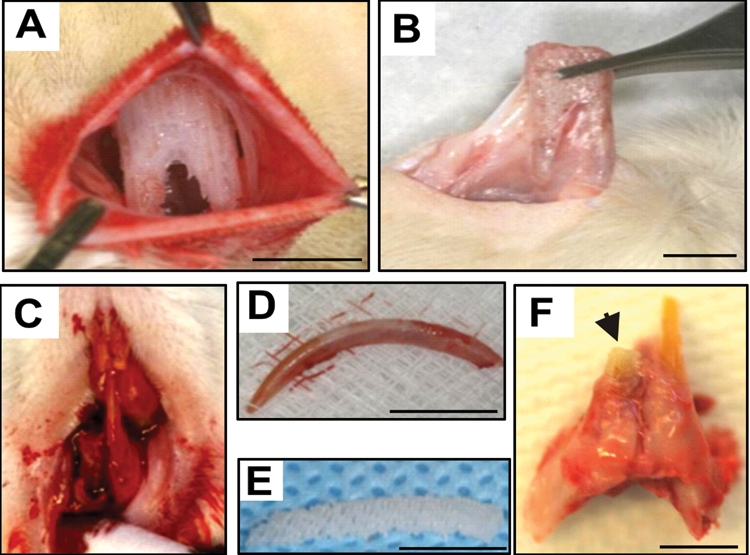
In vivo orthotopic and ectopic implantation of anatomically shaped tooth scaffolds. (A) In vivo implantation of human mandibular molar scaffold into rat’s dorsum constitutes an ectopic model for tooth regeneration. (B) Harvest of human molar scaffold showing integration and tissue ingrowth. (C) Extraction of the right rat mandibular central incisor. (D) The extracted rat mandibular central incisor. (E) The fabricated rat mandibular central incisor scaffold. (F) Harvest of in vivo-implanted rat mandibular central incisor scaffold orthotopically in the extraction socket showing integration of the implanted scaffold. Scale: 5 mm.
Sample Harvesting, Tissue Analysis, and Statistics
Nine weeks post-surgery, all rats were killed by pentobarbital overdose. The dorsum scaffolds were retrieved with surrounding fascia (Fig. 2B). The rat incisor scaffolds were harvested with surrounding bone and native tooth structures (Fig. 2F). All samples were fixed in 10% formalin, embedded in poly(methyl methacrylate) (PMMA), and sectioned at 5-µm thickness for hematoxylin and eosin (H&E) and von Kossa (VK) staining (HSRL, Mount Jackson, VA, USA). PMMA was used because PCL-HA scaffolds cannot be de-mineralized for paraffin embedding. The average areal cell density and blood vessel numbers were quantified from the coronal, middle, and apical thirds of the rat incisor scaffolds (Fig. 3J) and similarly of the human molar scaffolds (Fig. 4G) by a blinded and calibrated examiner. Upon confirmation of normal data distribution, Students’ t tests were used to compare the treated and control groups, with alpha at 0.05.
Figure 3.
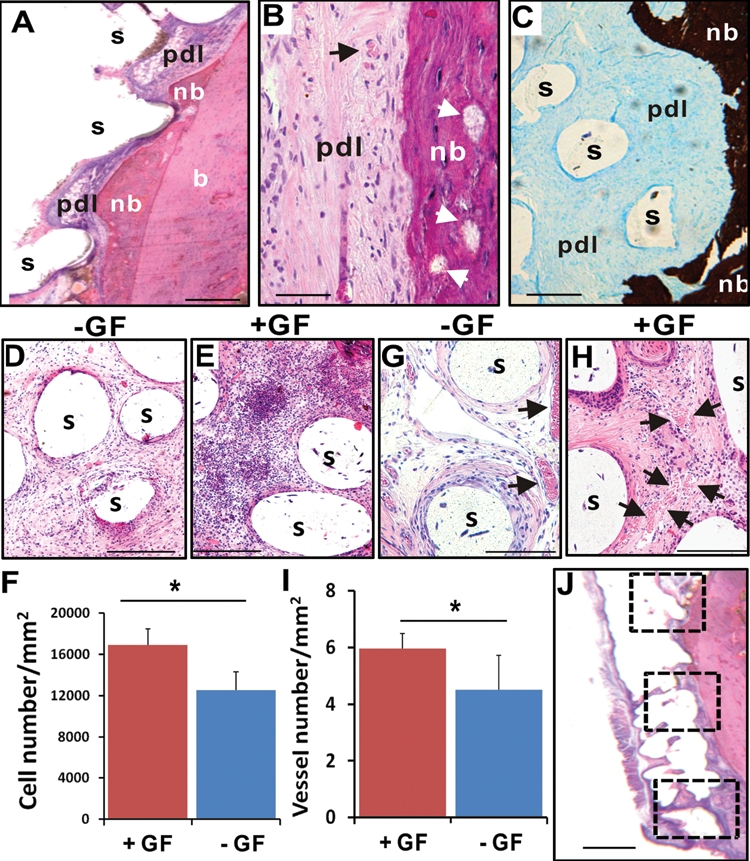
Orthotopic tooth regeneration. (A) The rat mandibular incisor scaffold integrated with surrounding tissue, showing tissue ingrowth into scaffold microchannels and multiple tissue phenotypes, including the native alveolar bone (b), newly formed bone (nb), and a fibrous tissue interface reminiscent of the periodontal ligament (pdl). The newly formed bone (nb) showed ingrowth into microchannel openings and inter-staggered with scaffold microstrands (s). (B) Newly formed bone (nb) has bone trabeculae-like structures (arrows) and embedded osteocyte-like cells, immediately adjacent to a putative periodontal ligament (pdl) consisting of fibroblast-like cells and collagen buddle-like structures. (C) Newly formed bone (nb) is well-mineralized (von Kossa preparation), in contrast to the adjacent unmineralized, putative periodontal ligament (pdl). (D) Cells populated the scaffold’s microchannels even without growth-factor delivery. Remarkably, SDF1 and BMP7 delivery yielded substantial cell homing in microchannels (E). (F) Combined SDF1 and BMP7 delivery homed significantly more cells into microchannels than without growth-factor delivery (p < 0.01; N = 11). Angiogenesis took place in scaffolds’ microchannels without growth-factor delivery (G), but was more substantial with growth-factor delivery (H). (I) Combined SDF1 and BMP7 delivery elaborated significantly more blood vessels than without growth-factor delivery (p < 0.05; N = 11). (J) The numbers of recruited cells and blood vessels were quantified from 3 different locations along the entire root length of the rat mandibular incisor scaffold: the superior region of alveolar ridge and the inferior region of root apex, with a midpoint in between. s, scaffold; GF, growth factor(s). Scale: 100 µm.
Figure 4.
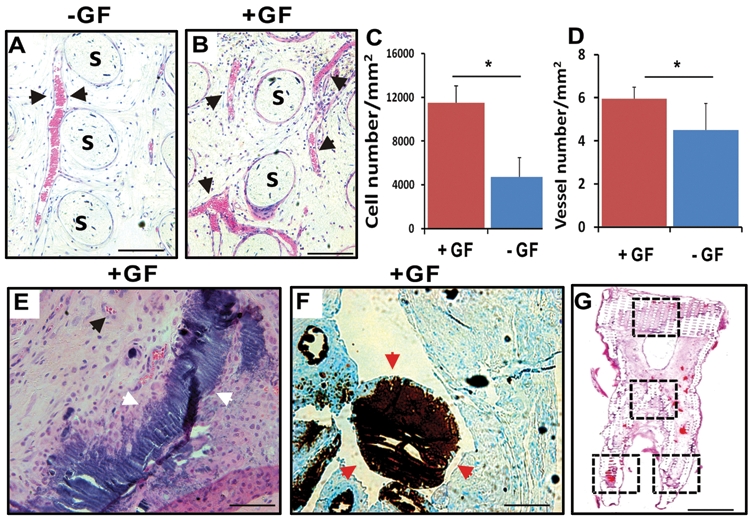
Ectopic tooth regeneration. (A) In human mandibular molar scaffolds, cells populated scaffold microchannels without growth-factor delivery. (B) Combined SDF1 and BMP7 delivery induced substantial cell homing into microchannels. (C) Combined SDF1 and BMP7 delivery homed significantly more cells into the microchannels than without growth-factor delivery (p < 0.01; N = 11). (D) Combined SDF1 and BMP7 delivery elaborated significantly more blood vessels than without growth-factor delivery (p < 0.05; N = 11). (E,F) Mineral tissue in isolated areas in microchannels adjacent to blood vessels and abundant cells, and confirmed by von Kossa staining. (G) Tissue sections from coronal, middle, and two root portions of human molar scaffolds were quantified for cell density and angiogenesis. s, scaffold; GF, growth factor(s). Scale: 100 µm.
Results
Orthotopic Tooth Regeneration without Cell Transplantation
The mandibular incisor extraction socket represents an orthotopic location for tooth regeneration. Scaffolds in the shape of the rat mandibular incisor integrated with surrounding tissue, showing tissue ingrowth into scaffold microchannels (Fig. 3A). It was not possible to separate the implanted scaffolds without physical damage to surrounding tissue. Microscopically, the scaffolds within the extraction sockets clearly showed multiple tissue phenotypes, including the native alveolar bone (b), newly formed bone (nb), and a fibrous tissue interface reminiscent of the periodontal ligament (pdl) (Fig. 3A). The newly formed bone (nb) showed ingrowth into microchannel openings and inter-staggered with scaffold microstrands (s) (Fig. 3A). Higher magnification showed newly formed bone (nb) with bone trabeculae-like structures (arrows in Fig. 3B) and embedded cells resembling osteocytes. Immediately adjacent is a structure reminiscent of the periodontal ligament consisting of fibroblast-like cells and collagen-like structures (pdl in Fig. 3B). Von Kossa preparation showed that the newly formed bone (nb) was well-mineralized, in contrast to adjacent unmineralized, putative periodontal ligament (pdl) (Fig. 3C). Although host cells populated the microchannels of growth-factor-free control scaffolds (Fig. 3D), combined SDF1 and BMP7 delivery (Fig. 3E) homed significantly more cells into the microchannels of the rat incisor scaffolds (p < 0.01) (Fig. 3F). Angiogenesis took place in scaffolds’ microchannels with or without growth-factor delivery (Figs. 3G, 3H). Quantitatively, combined SDF1 and BMP7 delivery elaborated significantly more blood vessels than the growth-factor-free group (p < 0.05) (Fig. 3I). The numbers of recruited cells and blood vessels were quantified from 3 different locations along the entire root length of the rat mandibular incisor scaffold: the superior region of the alveolar ridge, and the midpoint and the inferior region of the root apex (Fig. 3J).
Ectopic Tooth Regeneration without Cell Transplantation
Human mandibular molar scaffolds implanted into the dorsum represent an ectopic location for tooth regeneration. Microscopically, host cells populated scaffold microchannels without growth-factor delivery (Fig. 4A). Quantitatively, combined SDF1 and BMP7 delivery (Fig. 4B) homed significantly more cells into the microchannels of the human molar scaffolds than without growth-factor delivery (p < 0.01) (Fig. 4C). Angiogenesis took place in microchannels with or without growth-factor delivery (Figs. 4A, 4B). However, combined SDF1 and BMP7 delivery elaborated significantly more blood vessels than without growth-factor delivery (p < 0.05) (Fig. 4D). Mineral tissue was present in isolated areas in microchannels adjacent to blood vessels and abundant cells (Fig. 4E). Von Kossa staining confirmed ectopic mineralization (Fig. 4F), likely owing to BMP7 delivery. Tissue sections from coronal, middle, and two root portions of human molar scaffolds were quantified for cell density and angiogenesis (Fig. 4G).
Discussion
These findings represent the first report of regeneration of anatomically shaped tooth-like structures in vivo, and by cell homing without cell delivery. The potency of cell homing is substantiated not only by cell recruitment into scaffold microchannels, but also by regeneration of a putative periodontal ligament and newly formed alveolar bone. Tooth regeneration requires condensation of sufficient cells of multiple lineages (Modino and Sharpe, 2005; Yelick and Vacanti, 2006). The observed putative periodontal ligament and newly formed alveolar bone suggest the ability of SDF1 and/or BMP7 to recruit multiple cell lineages. SDF1 is chemotactic for bone marrow stem/progenitor cells and endothelial cells, both of which are critical for angiogenesis (Herodin et al., 2003; Belema-Bedada et al., 2008; Nait Lechguer et al., 2008). SDF1 binds to CXCR4, a chemokine receptor for endothelial cells and bone marrow stem/progenitor cells (Belema-Bedada et al., 2008; Kitaori et al., 2009). Here, SDF1 likely has homed mesenchymal and endothelial stem/progenitor cells in native alveolar bone into porous tooth scaffolds that were implanted in rat jaw bone, and connective tissue progenitor cells in dorsal subcutaneous tissue into human molar scaffold (Alhadlaq and Mao, 2004; Steinhardt et al., 2008; Crisan et al., 2009). BMP7 plays important roles in osteoblast differentiation and phosphorylation via SMAD pathways, which induces transcription of multiple osteogenic/odontogenic genes (Hahn et al., 1992; Itoh et al., 2001). Here, BMP7 likely is responsible for newly formed, mineralized alveolar bone in rat extraction socket and ectopic mineralization in human tooth scaffold implanted into the dorsum. Our ongoing work has identified additional growth factors that may constitute an optimal conglomerate for tooth regeneration. Cell homing is an under-recognized approach in tissue regeneration (Mao et al., 2010), and offers an alternative to cell-delivery-based tooth regeneration. Omission of cell isolation and ex vivo cell manipulation may accelerate regulatory, commercial, and clinical processes. The cost of tooth regeneration by cell homing is not anticipated to be nearly as excessive as for cell delivery.
The present scaffold design represents a variation from previous approaches in tooth regeneration by relying primarily on soft materials, including collagen gel, silk, or PLGA (e.g., Young et al., 2002; Modino and Sharpe, 2005; Ikeda et al., 2009). Mechanical stiffness of PCL-HA hybrid is suitable for load-bearing (Woodfield et al., 2005). Among rapid prototyping methods, 3D bioprinting offers the advantage of precise control of pore size, porosity, stiffness, and interconnectivity as well as anatomic dimensions (Woodfield et al., 2005; Lee et al., 2009). Clinically, the patient’s healthy, contralateral tooth form can be imaged by CT or MR, and then fed into a computer-aided design and a bioprinter to generate 3D scaffolds. Anatomically shaped scaffolds can either be patient-specific or of generic sizes, and made available as off-the-shelf implants in dental offices.
The present study, being the first of its kind for de novo formation of tooth-like tissues by cell homing, is not without limitations. All in vivo harvested samples were embedded in PMMA, because PCL-HA cannot be decalcified for paraffin embedding. PMMA embedding disallows immunoblotting by certain antibodies (Lee et al., 2009). The regenerated mandibular incisor-like structure was primarily the root with a portion of sub-occlusal crown. Further, no attempt was made to regenerate enamel or dentin. Nonetheless, we suggest that a regenerated tooth is biological primarily because of its root, rather than the crown, which can be readily restored with a clinical crown anchorable to a biologically regenerated root. Regeneration of a putative periodontal ligament and new bone that integrated with native alveolar bone appears to provide the ground for a clinically translatable approach. The present work does not preclude parallel studies of tooth regeneration by cell transplantation. Our recent work continues to explore regeneration of multiple tissues by cell delivery (Lee et al., 2009; Yang et al., 2010). One of the pivotal issues in tooth regeneration is to devise economically viable approaches that are not cost-prohibitive and can translate into therapies for patients who cannot afford or are contra-indicated for dental implants. Cell-homing-based tooth regeneration may provide a tangible pathway toward clinical translation.
Acknowledgments
We thank F. Guo and K. Hua for technical and administrative assistance.
Footnotes
This research was supported by NIH Grant 5RC2 DE020767 from the National Institute of Dental and Craniofacial Research (NIDCR).
References
- Abukawa H, Zhang W, Young CS, Asrican R, Vacanti JP, Kaban LB, et al. (2009). Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J Oral Maxillofac Surg 67:335-347 [DOI] [PubMed] [Google Scholar]
- Ahsan T, Bellamkonda R, Nerem RM. (2007). Tissue engineering and regenerative medicine: advancing toward clinical therapies. In: Translational approaches in tissue engineering and regenerative medicine. Mao JJ, Vunjak-Novakovic G, Mikos AG, editors. Norwood, MA, USA: Artech House, Inc., pp. 3-16 [Google Scholar]
- Alhadlaq A, Mao JJ. (2004). Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev 13:436-448 [DOI] [PubMed] [Google Scholar]
- Belema-Bedada F, Uchida S, Martire A, Kostin S, Braun T. (2008). Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell 2:566-575 [DOI] [PubMed] [Google Scholar]
- Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Peault B. (2009). Perivascular multipotent progenitor cells in human organs. Ann NY Acad Sci 1176:118-123 [DOI] [PubMed] [Google Scholar]
- Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. (2004). Bioengineered teeth from cultured rat tooth bud cells. J Dent Res 83:523-528 [DOI] [PubMed] [Google Scholar]
- Duailibi SE, Duailibi MT, Zhang W, Asrican R, Vacanti JP, Yelick PC. (2008). Bioengineered dental tissues grown in the rat jaw. J Dent Res 87:745-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CF, Magini RS, Sharpe PT. (2007). Biological tooth replacement and repair. J Oral Rehabil 34:933-939 [DOI] [PubMed] [Google Scholar]
- Goldberg M, Six N, Decup F, Buch D, Soheili Majd E, Lasfargues JJ, et al. (2001). Application of bioactive molecules in pulp-capping situations. Adv Dent Res 15:91-95 [DOI] [PubMed] [Google Scholar]
- Hahn GV, Cohen RB, Wozney JM, Levitz CL, Shore EM, Zasloff MA, et al. (1992). A bone morphogenetic protein subfamily: chromosomal localization of human genes for BMP5, BMP6, and BMP7. Genomics 14:759-762 [DOI] [PubMed] [Google Scholar]
- Herodin F, Bourin P, Mayol JF, Lataillade JJ, Drouet M. (2003). Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma-irradiation promotes survival. Blood 101:2609-2616 [DOI] [PubMed] [Google Scholar]
- Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, et al. (2009). Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci USA 106:13475-13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh F, Asao H, Sugamura K, Heldin CH, ten Dijke P, Itoh S. (2001). Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J 20:4132-4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, et al. (2009). Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 60:813-823 [DOI] [PubMed] [Google Scholar]
- Kuo TF, Huang AT, Chang HH, Lin FH, Chen ST, Chen RS, et al. (2008). Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitinhyaluronan tri-copolymer scaffold in swine. J Biomed Mater Res A 86:1062-1068 [DOI] [PubMed] [Google Scholar]
- Lee CH, Marion NW, Scott HJ, Mao J. (2009). Tissue formation and vascularization of anatomically shaped human tibial condyle in vivo. Tissue Eng Part A 15:3923-3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantesso A, Sharpe P. (2009). Dental stem cells for tooth regeneration and repair. Expert Opin Biol Ther 9:1143-1154 [DOI] [PubMed] [Google Scholar]
- Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. (2006). Craniofacial tissue engineering by stem cells. J Dent Res 85:966-979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JJ, Stosich MS, Moioli E, Lee CH, Fu S, Bastian B, et al. (2010). Facial reconstruction by biosurgery: cell transplantation vs. cell homing. Tissue Eng Part B Rev [Epub ahead of print, March 8, 2010] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modino SA, Sharpe PT. (2005). Tissue engineering of teeth using adult stem cells. Arch Oral Biol 50:255-258 [DOI] [PubMed] [Google Scholar]
- Nait Lechguer A, Kuchler-Bopp S, Hu B, Haikel Y, Lesot H. (2008). Vascularization of engineered teeth. J Dent Res 87:1138-1143 [DOI] [PubMed] [Google Scholar]
- Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. (2007). The development of a bioengineered organ germ method. Nat Methods 4:227-230 [DOI] [PubMed] [Google Scholar]
- No Authors Given (2008). Evaluation criteria for musculoskeletal and craniofacial tissue engineering constructs: a conference report. Tissue Eng Part A 14:2089-2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohazama A, Modino SA, Miletich I, Sharpe PT. (2004). Stem-cell-based tissue engineering of murine teeth. J Dent Res 83:518-522 [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. (2005). Periodontal diseases. Lancet 366:1809-1820 [DOI] [PubMed] [Google Scholar]
- Poole DFGI. (1967). Structural and chemical organization of teeth. Vol. I Miles AEW, editor. New York, USA: Academic Press, pp. 111-149 [Google Scholar]
- Rutherford B. (2001). BMP-7 gene transfer to inflamed feret dental pulps. Eur J Oral Sci 109:422-424 [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. (2006). Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt Y, Aslan H, Regev E, Zilberman Y, Kallai I, Gazit D, et al. (2008). Maxillofacial-derived stem cells regenerate critical mandibular bone defect. Tissue Eng Part A 14:1763-1773 [DOI] [PubMed] [Google Scholar]
- Stosich MS, Moioli EK, Wu JK, Lee CH, Rohde C, Yoursef AM, et al. (2009). Bioengineering strategies to generate vascularized soft tissue grafts with sustained shape. Methods 47:116-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS (2005). Oral health in America: a report of the Surgeon General. Rockville, MD: USDHHS, University Press of the Pacific [Google Scholar]
- Woodfield TB, Van Blitterswijk CA, De Wijn J, Sims TJ, Hollander AP, Riesle J. (2005). Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng 11:1297-1311 [DOI] [PubMed] [Google Scholar]
- Xu WP, Zhang W, Asrican R, Kim HJ, Kaplan DL, Yelick PC. (2008). Accurately shaped tooth bud cell-derived mineralized tissue formation on silk scaffolds. Tissue Eng Part A 14:549-557 [DOI] [PubMed] [Google Scholar]
- Yang R, Chen M, Lee CH, Yoon R, Lal S, Mao JJ. (2010). Clones of ectopic stem cells in the regeneration of muscle defects in vivo. PloS One (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelick PC, Vacanti JP. (2006). Bioengineered teeth from tooth bud cells. Dent Clin North Am 50:191-203, viii [DOI] [PubMed] [Google Scholar]
- Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. (2002). Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res 81:695-700 [DOI] [PubMed] [Google Scholar]
- Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, et al. (2005). Tissue-engineered hybrid tooth and bone. Tissue Eng 11:1599-1610 [DOI] [PubMed] [Google Scholar]


