Abstract
The inflammatory response, which has both genetic and environmental components, is a central mechanism linking oral and systemic diseases. We hypothesized that dental plaque accumulation over 21 days in the experimental gingivitis model would elicit systemic inflammatory responses [change in white blood cell (WBC) count and neutrophil activity], and that these responses would differ by gender/race. We recruited 156 healthy young adults, including black and white males and females. Plaque Index (PI), Gingival Index (GI), systemic WBC counts, and peripheral neutrophil oxidative activity were recorded. Overall, 128 participants completed the study. During the experimental phase, the correlation between PI and GI was 0.79. Total WBC and neutrophil counts did not change. Neutrophil activity increased in blacks but not whites, suggesting that there may be racial differences in the inflammatory response to dental plaque accumulation.
Keywords: dental plaque, experimental gingivitis, neutrophil
Introduction
Over the past decade, there has been a resurgence of interest in the connection between oral and systemic diseases. Recent studies support an association between periodontal infection and several systemic diseases (Beck and Offenbacher, 2005; Lin et al., 2007). The central mechanism that has been suggested to mediate this association is the series of host inflammatory reactions to oral infection.
The link between oral infection, with particular emphasis on chronic periodontitis, and systemic diseases has been addressed in many studies. However, while some of these have been case-controlled and cohort studies, the majority have been cross-sectional in design. While results of these studies have generally supported an association, it has not been possible to elucidate whether oral infection plays a causal role in the systemic diseases. Longitudinal investigations are thus essential, but are challenging logistically in the case of periodontitis, which has a long time-course.
Gingivitis is an inflammatory response to dental plaque, elicited in the superficial periodontal tissues. Dental plaque accumulation and gingivitis are universal; thus, their potential contributions to systemic diseases could be immense. Because gingivitis is reversible and rapidly inducible, it could be an appropriate model for longitudinal investigation of the role of oral infection in systemic diseases. It can be readily induced experimentally according to the “experimental gingivitis model” (Löe et al., 1965).
Gender and racial differences exist in the incidence, morbidity, and mortality associated with complex diseases that possess an inflammatory component, including rheumatoid arthritis, diabetes mellitus, and cardiovascular disease (Cooper and Stroehla, 2003; Albert, 2007). The influence of gender and race on the systemic inflammatory response to dental plaque has not been previously addressed and can be examined according to the experimental gingivitis model. Identification of individuals exhibiting an exaggerated inflammatory response to dental plaque, whether due to gender, race, or other factors, is important in the design of preventive oral health care programs and could provide information on their susceptibility to inflammatory diseases.
Peripheral blood neutrophils comprise the primary cells of the acute inflammatory response. In addition to their role as a first line of defense against pathogens, neutrophils can also mediate tissue destruction in inflammatory diseases (Hansen, 1995). Periodontal disease, including gingivitis, has been associated with an increase in the number and activation state of circulating neutrophils (Loos et al., 2000; Kowolik et al., 2001; Matthews et al., 2007). Activated neutrophils release highly cytotoxic oxidants, which can be assessed by luminol-enhanced chemiluminescence, as well as proteolytic enzymes, both of which have the potential for tissue damage.
The objective of this study was to use the experimental gingivitis model to examine the systemic neutrophil responses to dental plaque accumulation over time in a large sample (N = 156) of young, healthy, male and female, white and black adults. Based on earlier findings (Kowoliket al., 2001), we hypothesized that plaque accumulation in healthy adults would elicit systemic neutrophil responses. We also hypothesized that these responses would vary according to gender and race.
Materials & Methods
Study Design
The study protocol was approved by the Institutional Review Board of Indiana University Purdue University Indianapolis/Clarian Health (approval number 0405-50). All participants provided written informed consent for study participation. The study design is summarized in Table 1. Following a screening visit, participants received a professional oral prophylaxis and oral hygiene (OH) instructions (cleaning visit). They then entered the three study phases in chronological order. In the Control Phase, participants performed optimal OH for 21 days. In the Experimental Phase, they refrained from OH measures, including brushing, flossing, mouthrinsing, and gum chewing, for 21 days. Finally, in the Recovery Phase, participants received a professional oral prophylaxis and OH instructions and resumed normal OH practices for 21 days. Participants visited the clinic weekly. During each visit, oral examinations were conducted, and peripheral blood samples were collected.
Table 1.
Overview of Clinical and Laboratory Procedures Performed During Each Study Visit
| Screening Visit | Dental Cleaning | Control Phase | Experimental Phase | Recovery Phase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day of phase | 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21 | ||
| Day of study | 0 | 7 | 14 | 21 | 28 | 35a | 35a | 42 | 49 | 56b | 56b | 63 | 70 | 77 |
| Visit number | 1 | 2 | 3 | 4 | 5 | 6 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Blood draw | X | X | X | X | X | X | X | X | X | X | ||||
| Plaque and gingivitis assessment | X | X | X | X | X | X | X | X | X | X | ||||
| Dental prophylaxis | X | Xc | ||||||||||||
| Oral hygiene instruction | X | X | X | X | X | |||||||||
Same day of study.
Same day of study.
Dental prophylaxis scheduled no more than 2 days after Day 21 of the experimental phase.
Study Population
The study enrolled healthy adults (N = 156), aged 18 to 31 yrs, with approximately equal numbers of men and women, and blacks and whites. Individuals were excluded if they used tobacco, medications known to affect the oral soft tissues or local/systemic inflammatory responses, or antimicrobial drugs within the preceding 3 mos, or if they had periodontitis (periodontal probing depths ≥ 4 mm), or gross dental caries.
Clinical Procedures
Clinical examinations and blood draws were conducted at the General Clinical Research Center (GCRC), Indiana University School of Medicine. Oral examinations and assessments of plaque and gingivitis were performed by a single trained and calibrated examiner using the Plaque Index (PI) (Silness and Löe, 1964) and Gingival Index (GI) (Löe and Silness, 1963), respectively. Measurements were made on 6 sites per tooth on all teeth present, with the exception of third molars. Venous blood samples were collected for laboratory analyses.
Laboratory Analyses
Total and differential white blood cell (WBC) counts were measured in an automated cell counter (Coulter Stack S, Coulter Electronics Inc., Hialeah, FL, USA) in the laboratories of Clarian Health Partners Inc.
Neutrophils were isolated from heparinized peripheral blood samples by density gradient centrifugation on Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) (Sabroe et al., 2003) at 400 g for 25 min at 25°C. Neutrophils were washed twice with Isotonic Phosphate-buffered Saline solution (Sigma) and once with RPMI-1640 (Sigma) by centrifugation at 250 g for 10 min. Isolated cells were suspended in RPMI-1640. Neutrophils were counted in a hemacytometer, and viability was assessed by trypan blue exclusion. Cells were primed for 30 min at 37°C with 10−10 M formyl-methionyl-leucyl-phenylalanine (fMLP) (Sigma) and re-suspended to a concentration of 1 x 106 cells/mL in RPMI-1640.
Luminol-enhanced chemiluminescence was measured with the use of a 1251 Bio-Orbit Luminometer (Bio-Orbit, Turku, Finland) for 90 min. Into each cuvette, a 100-µL quantity of 10−5 M Luminol (Sigma) was dispensed at baseline, followed by cell activation with 100 µL of 10−5 M fMLP at 30 min. Chemiluminescence values were expressed as peak and total integrated energy output. Samples were run in triplicate, and means were measured. Negative controls included the reaction solution without cells.
Statistical Analysis
Natural logarithms of the measurements in this per-protocol analysis were used for all analyses, because the distributions of all outcomes (PI, GI, WBC counts, neutrophil counts, and chemiluminescence) were non-normal. Means and confidence intervals (CI) reported were transformed to the original measurement scale, to aid in interpretation of the results. Correlation coefficients were calculated for assessment of the association between PI and GI to validate the experimental gingivitis model. Repeated-measures analysis of variance (ANOVA) was used for each outcome, to test for changes between the ends of the control, experimental, and recovery phases, and to compare genders and races. ANOVAs included gender, race, and time effects, as well as interactions of time with gender and race. Interactions between gender and race were examined, but were not significant, and so were not included in the final analysis model. The ANOVAs allowed each phase to have a different variance and allowed for different correlations between phases. Intermediate time-points during each phase were included in the figures to show the full time-course of the outcomes, but were not included in the analyses. Age and body mass index (BMI) were examined as covariates, but were not significantly associated with changes occurring during the study, and thus were not included in the final analysis models.
Results
Of the 156 individuals enrolled, only the 128 who completed the study were included in the analyses. Of the 28 individuals not included in the analyses, one participant was lost because of non-compliance, and 27 were lost because of protocol deviation (e.g., antimicrobial use, missed prophylaxis, failure to follow up, relocation, or pregnancy). The cohort was comprised of healthy young adults (mean age, 24 yrs), with a relatively high mean BMI of 30 (range, 18-54).
Plaque and Gingivitis
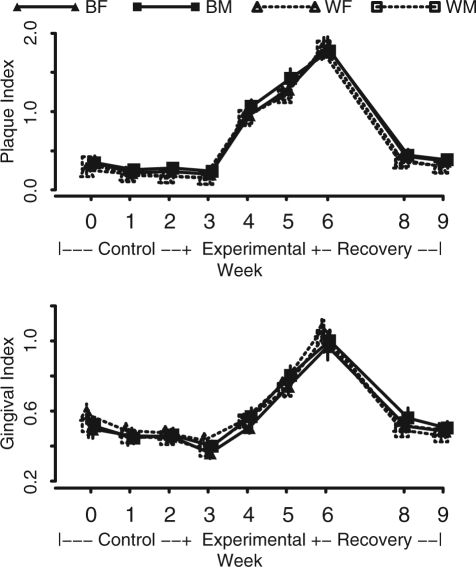
Baseline values and changes in PI and GI are shown in Table 2 and Fig. 1. PI was marginally higher in blacks vs. whites (0.35 vs. 0.29, p = 0.06) and in men vs. women (0.35 vs. 0.29, p = 0.08), whereas GI was not significantly different by race (p = 0.12) or gender (p = 0.40).
Table 2.
Mean and 95% Confidence Interval for Study Outcomes at Baseline and Changes During the Experimental Phase of the Study by Race and Gender
| White (N = 66) | Black (N = 62) | p-valuea | Female (N = 65) | Male (N = 63) | p-valuea | ||
|---|---|---|---|---|---|---|---|
| Plaque Index | Baseline | 0.25 (0.21, 0.31) | 0.34 (0.29, 0.41) | 0.0604 | 0.35 (0.30, 0.41) | 0.35 (0.28, 0.43) | 0.0810 |
| Change | 1.64 (1.43, 1.89) | 1.58 (1.36, 1.83) | 0.0003 | 1.62 (1.4, 1.86) | 1.61 (1.39, 1.85) | 0.4874 | |
| pb < 0.0001 | pb < 0.0001 | pb < 0.0001 | pb < 0.0001 | ||||
| Gingival Index | Baseline | 0.58 (0.53, 0.64) | 0.52 (0.45, 0.60) | 0.1159 | 0.50 (0.45, 0.56) | 0.51 (0.47, 0.56) | 0.4004 |
| Change | 0.64 (0.58, 0.70) | 0.61 (0.55, 0.67) | 0.8230 | 0.62 (0.56, 0.68) | 0.62 (0.57, 0.68) | 0.6684 | |
| pb < 0.0001 | pb < 0.0001 | pb < 0.0001 | pb < 0.0001 | ||||
| White blood cells (x 109 cells/L) | Baseline | 6.6 (6.1, 7.3) | 5.4 (5.1, 5.8) | 0.0094 | 5.4 (4.8, 6.2) | 5.2 (4.7, 5.7) | 0.0090 |
| Change | −0.1 (−0.3, 0.2) | 0.1 (−0.2, 0.3) | 0.4673 | −0.1 (−0.3, 0.2) | 0.1 (−0.2, 0.3) | 0.5451 | |
| pb = 0.6115 | pb = 0.6022 | pb = 0.6837 | pb = 0.6533 | ||||
| Neutrophils (x 109 cells/L) | Baseline | 4.0 (3.5, 4.5) | 3.0 (2.8, 3.3) | 0.0021 | 2.9 (2.4, 3.5) | 2.7 (2.3, 3.2) | 0.0111 |
| Change | −0.1 (−0.3, 0.2) | 0.1 (−0.1, 0.3) | 0.2724 | −0.1 (−0.3, 0.2) | 0.1 (−0.1, 0.3) | 0.3894 | |
| pb = 0.5317 | pb = 0.3558 | pb = 0.6599 | pb = 0.4352 | ||||
| Total CLc (millivolt.min) | Baseline | 7.8 (6.0, 10.1) | 5.4 (3.9, 7.5) | 0.9952 | 7.1 (5.3, 9.5) | 6.2 (4.3, 9.0) | 0.1211 |
| Change | 0.1 (−1.4, 2.0) | 1.7 (0.2, 3.7) | 0.1250 | 1.8 (−0.1, 4.2) | 0.3 (−0.9, 1.9) | 0.3212 | |
| pb = 0.9414 | pb = 0.0292 | pb = 0.0678 | pb = 0.6410 | ||||
| Peak CLc (millivolt) | Baseline | 14.8 (11.3, 19.3) | 10.1 (6.9, 14.8) | 0.2820 | 10.8 (7.6, 15.4) | 10.0 (6.8, 14.7) | 0.1640 |
| Change | 0.5 (−2.4, 4.1) | 2.9 (0.2, 6.5) | 0.1877 | 2.5 (−0.9, 6.9) | 1.3 (−1.0, 4.3) | 0.7665 | |
| pb = 0.7520 | pb = 0.0349 | pb = 0.1541 | pb = 0.2943 |
p-value for comparisons of white vs. black or women vs. men.
p-value for significance of change from beginning to end of experimental phase.
CL = Chemiluminescence.
Figure 1.
Mean and 95% confidence interval for Plaque Index and Gingival Index over the course of the study by race and gender.BF = black females (N = 30). BM = black males (N = 32). WF = white females (N = 35). WM = white males (N = 31).
PI increased on average by 1.61 (95% CI, 1.46-1.78) during the experimental phase (p < 0.0001). The increase in PI in blacks was significantly less than in whites (p = 0.0003), while there was no significant difference in the increase for males vs. females (p = 0.49). GI also increased during the experimental phase (p < 0.0001), on average by 0.62. GI increases were not significantly different between races (p = 0.82) or genders (p = 0.67). During the experimental phase, there was a strong positive correlation between changes in PI and GI (r = 0.79, p < 0.0001). This correlation was similar across gender/race groups (range for r, 0.77-0.81). PI and GI were still elevated at the end of the recovery phase compared with the end of the control phase (p < 0.001).
White Blood Cell Count
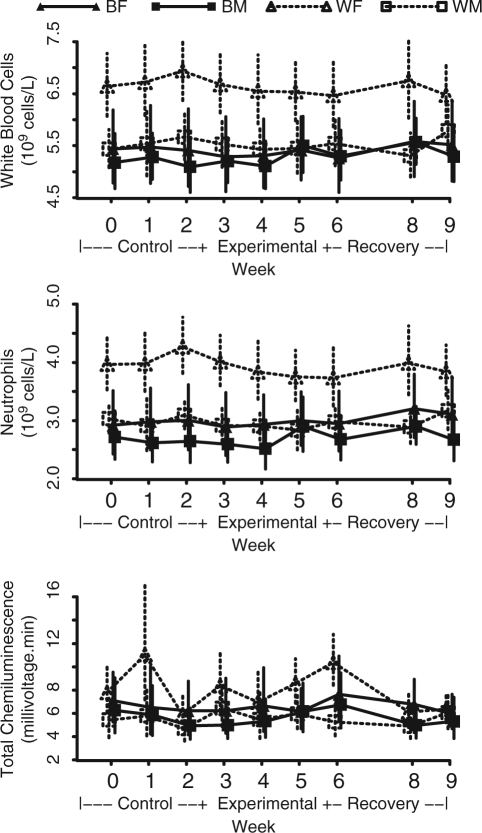
Baseline values and changes in WBC and neutrophil counts are shown in Table 2 and Fig. 2. Baseline WBC counts (x 109/L) were lower in blacks vs. whites (5.3 vs. 6.1, p = 0.0094) and in men vs. women (5.3 vs. 6.1, p = 0.0090). More specifically, neutrophil counts (x 109/L) were lower in blacks vs. whites (2.8 vs. 3.5, p = 0.0021) and in men vs. women (2.9 vs. 3.4, p = 0.0111).
Figure 2.
Means and 95% confidence intervals for white blood cell count, neutrophil count, and total neutrophil chemiluminescenceover the course of the study by race and gender. BF = black females (N = 30). BM = black males (N = 32). WF = white females (N = 35). WM = white males (N = 31).
Total WBC counts and neutrophil counts did not change overall from the beginning to the end of the experimental phase (p = 0.98 and p = 0.81, respectively) or within any subgroup. There were no differences between races or genders for change in WBC or neutrophil counts during this time period (p > 0.27). No changes in WBC or neutrophil counts were found between the control and recovery phases (p = 0.29 for WBC counts and p = 0.15 for neutrophil counts).
Neutrophil Activity Assay
Baseline total chemiluminescence (Table 2, Fig. 2) was not significantly different between blacks and whites (p = 0.99) or between men and women (p = 0.12). No significant changes during the experimental phase of the study were observed for total and peak chemiluminescence between races (p = 0.13) and genders (p = 0.32) (Table 2). For blacks, total and peak chemiluminescence increased significantly during the experimental phase, on average, by 1.7 (p = 0.03) and 2.9 (p = 0.04), respectively. In contrast, chemiluminescence did not change significantly for whites from baseline to the end of the experimental period.
Discussion
In this study, dental plaque accumulation did not influence neutrophil counts, but did elicit a systemic peripheral neutrophil response in blacks.
This is the largest of the few studies published that utilized the experimental gingivitis model to induce systemic inflammation (Kowolik et al., 2001). Also, it is the first to use the model to address racial differences in the inflammatory responses to dental plaque. The 21-day experimental gingivitis model can be unpleasant, and thus, retention of and compliance by study participants can be challenging. In this study, the retention rate was high (128 out of 156 individuals completed). In addition, participant compliance was verified by the increase in PI and GI over the experimental phase, with a strong positive correlation between PI and GI (r = 0.8). Based on these findings, use of the experimental gingivitis model to study inflammation in a large cohort is feasible.
The WBC and neturophil counts did not change during this study. In comparison, WBC and neutrophil counts increased during the experimental phase as compared with the control phase in our pilot study, conducted in Mexico and enrolling Hispanic participants (Kowolik et al., 2001). However, in the pilot study, the control phase was 28 days following the experimental phase. Additionally, differences in ethnicity may be responsible for the different findings.
In the present study, although plaque accumulation did not result in a change in WBC counts, there was an increase in the neutrophil activity in blacks, predominantly in black men. While ethnic background was shown to influence the oxidative burst in neutrophils (Siddiqi et al., 2001), in that study, evaluating neutrophil oxidative burst in the absence of induced inflammation, oxidative function was significantly lower in neutrophils from blacks compared with whites.
We postulate that the neutrophil hyperactivity seen in this study in the absence of an increase in WBC counts may be due to the fact that gingivitis is a superficial inflammation of the periodontal tissues. As a mild insult to the host, it may not be strong enough to increase the number of circulating neutrophils, but may be sufficient to hyperactivate the circulating neutrophils. In agreement with this, it has been suggested that neutrophil hyperactivity can be an earlier and more sensitive response by circulating neutrophils to a bacterial challenge than is leukocytosis (Hill et al., 1974).
Within the confines of this study, there were findings of potential clinical relevance. In blacks, there was a heightened neutrophil response to dental plaque accumulation; neutrophil hyperactivity has been shown to play a role in endothelial injury (Lentsch and Ward, 2000) and subsequent tissue damage in several disease processes (Di Filippo et al., 2007; Wittkowskiet al., 2007). It was also suggested that long-term, low-grade infectious challenges may be of greater systemic importance than isolated, clinically obvious events (Roberts, 1999).
Our study has limitations. Although well-controlled, the conditions of the experimental gingivitis model differ from those of clinical gingivitis. A recent trial (Deinzer et al., 2007) revealed that the experimental model may result in local inflammatory differences when compared with persistent gingivitis. It is important to note, however, that in our study, we evaluated the effects of dental plaque on systemic, rather than local, inflammatory markers. Hormonal fluctuations in women, which were not measured, may alter gingival and/or systemic responses to dental plaque (Holm-Pedersen and Löe, 1967; Preshaw et al., 2001). We report on only one aspect of the host systemic response to dental plaque (i.e., peripheral neutrophils); while neutrophils are the primary cells of acute inflammation, other aspects of the inflammatory response should be considered. We are currently investigating the effect of dental plaque accumulation on other parameters of the host response, including serum cytokines, acute phase reactants, and serum lipid profiles, within the same model.
In conclusion, the results from this study failed to show an obvious systemic response to dental plaque accumulation across all participant groups studied. Interestingly, in a subset of individuals (blacks), dental plaque accumulation and the ensuing gingivitis resulted in hyperactivity of circulating neutrophils. Further studies, including those that use the experimental gingivitis model, could help identify those individuals with an innately increased inflammatory response to a bacterial challenge.
Acknowledgments
We thank Dr. Munro Peacock and the staff of the GCRC, IU School of Medicine, and Dr. Domenick Zero and the staff of the Oral Health Research Institute, IUSD, for their assistance.
Footnotes
This study was supported by NIH # R01DEO15145-01. Dr. Dowsett is a full-time employee at Eli Lilly and Company and holds an adjunct position at Indiana University School of Dentistry.
References
- Albert MA. (2007). Inflammatory biomarkers, race/ethnicity and cardiovascular disease. Nutr Rev 65(12 Pt 2):234-238. [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. (2005). Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol 76 (11 Suppl):2089S-2100S. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Stroehla BC. (2003). The epidemiology of autoimmune diseases. Autoimmun Rev 2:119-125. [DOI] [PubMed] [Google Scholar]
- Deinzer R, Weik U, Kolb-Bachofen V, Herforth A. (2007). Comparison of experimental gingivitis with persistent gingivitis: differences in clinical parameters and cytokine concentrations. J Periodontal Res 42: 318-324. [DOI] [PubMed] [Google Scholar]
- Di Filippo C, Rossi F, D’Amico M. (2007). Targeting polymorphonuclear leukocytes in acute myocardial infarction. ScientificWorld Journal 7:121-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PR. (1995). Role of neutrophils in myocardial ischemia and reperfusion. Circulation 91:1872-1885. [DOI] [PubMed] [Google Scholar]
- Hill HR, Warwick WJ, Dettloff J, Quie PG. (1974). Neutrophil granulocyte function in patients with pulmonary infection. J Pediatr 84:55-58. [DOI] [PubMed] [Google Scholar]
- Holm-Pedersen P, Löe H. (1967).Flow of gingival exudate as related to menstruation and pregnancy. J Periodontal Res 2:13-20. [DOI] [PubMed] [Google Scholar]
- Kowolik MJ, Dowsett SA, Rodriguez J, De La, Rosa RM, Eckert GJ. (2001). Systemic neutrophil response resulting from dental plaque accumulation. J Periodontol 72:146-151. [DOI] [PubMed] [Google Scholar]
- Lentsch AB, Ward PA. (2000). Regulation of inflammatory vascular damage. J Pathol 190:343-348. [DOI] [PubMed] [Google Scholar]
- Lin D, Moss K, Beck JD, Hefti A, Offenbacher S. (2007). Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol 78:833-841. [DOI] [PubMed] [Google Scholar]
- Löe H, Silness J. (1963). Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 21:533-551. [DOI] [PubMed] [Google Scholar]
- Löe H, Theilade E, Jensen SB. (1965). Experimental gingivitis in man. J Periodontol 36:177-187. [DOI] [PubMed] [Google Scholar]
- Loos BG, Craandijk J, Hoek FL, Wertheim-van Dillen PM, van der Velden U. (2000). Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol 71:1528-1534. [DOI] [PubMed] [Google Scholar]
- Matthews JB, Wright HJ, Roberts A, Cooper PR, Chapple IL. (2007). Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin Exp Immunol 147:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preshaw PM, Knutsen MA, Mariotti A. (2001). Experimental gingivitis in women using oral contraceptives. J Dent Res 80:2011-2015. [DOI] [PubMed] [Google Scholar]
- Roberts GJ. (1999). Dentists are innocent! “Everyday” bacteremia is the real culprit: a review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatr Cardiol 20:317-325. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, et al. (2003). Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol 170: 5268-5275. [DOI] [PubMed] [Google Scholar]
- Siddiqi M, Garcia ZC, Stein DS, Denny TN, Spolarics Z. (2001). Relationship between oxidative burst activity and CD11b expression in neutrophils and monocytes from healthy individuals: effects of race and gender. Cytometry 46:243-246. [DOI] [PubMed] [Google Scholar]
- Silness J, Löe H. (1964). Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22:121-135. [DOI] [PubMed] [Google Scholar]
- Wittkowski H, Sturrock A, van Zoelen MA, Viemann D, van der Poll T, Hoidal JR, et al. (2007). Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Crit Care Med 35:1369-1375. [DOI] [PubMed] [Google Scholar]