Abstract
Bis-GMA-containing resin composites and adhesives undergo biodegradation by human-saliva-derived esterases, yielding Bis-hydroxy-propoxy-phenyl-propane (Bis-HPPP). The hypothesis of this study is that the exposure of dental restorations to saliva-like esterase activities accelerates marginal bacterial microleakage. Resin composites (Scotchbond, Z250, 3M) bonded to human dentin were incubated in either buffer or dual-esterase media (pseudocholinesterase/cholesterol-esterase; PCE+CE), with activity levels simulating those of human saliva, for up to 90 days. Incubation solutions were analyzed for Bis-HPPP by high-performance liquid chromatography. Post-incubation, specimens were suspended in a chemostat-based biofilm fermentor cultivating Streptococcus mutans NG8, a primary species associated with dental caries, for 7 days. Bacterial microleakage was assessed by confocal laser scanning microscopy. Bis-HPPP production and depth and spatial volume of bacterial cell penetration within the interface increased with incubation time and were higher for 30- and 90-day PCE+CE vs. buffer-incubated groups, suggesting that biodegradation can contribute to the formation of recurrent decay.
Keywords: biodegradation, bacterial microleakage, resin-dentin interface
Introduction
Resin-composite matrices based on 2,2-Bis[4-(2-hydroxy-3-methacryloyloxy-propoxy)phenyl]propane (Bis-GMA) undergo significant chemical biodegradation when challenged by esterase activities (Jaffer et al., 2002; Finer and Santerre, 2004a) contained within human saliva (Lin et al., 2005). Hydrolytic cleavage of unhindered ester bonds at both ends of a Bis-GMA unit results in chemical breakdown, releasing bis-hydroxy-propoxy-phenyl-propane (Bis-HPPP), a marker of resin-matrix breakdown (Finer and Santerre, 2003). However, much of the current knowledge of resin biodegradation stems from observations of external surfaces of composite restorations interfacing with fluids from the oral cavity or simulated aging solutions (Santerre et al., 2001).
Bacterial microleakage is the most frequently cited post-operative complication among dentin-bonded composite restorations (Murray et al., 2002), and secondary caries is the principal cause of failure (Hickel and Manhart, 2001; Hicks et al., 2003). In vivo biodegradation has been suggested as a potential contributor to secondary loss of adhesion, microleakage, and caries (Hickel and Manhart, 2001; Hashimoto et al., 2003; Donmez et al., 2005). Therefore, the physical and chemical integrity of a composite restoration’s adhesive bond layer—the interface between the restoration and the tooth—is the most significant factor determining long-term clinical restoration success (Donmez et al., 2005). Very little research has focused on the impact of biodegradation along the tooth-resin interface. Of particular concern are proximal and cervical restorations where materials come into contact with wet dentinal substrate (Bouillaguet, 2004). Our hypothesis was that exposure of resin-composite restorations to saliva-like esterase activities accelerates marginal bacterial microleakage.
Materials & Methods
Preparation of Resin-Dentin Specimens
Dentin blocks cut from fully intact sterilized human third molars (University of Toronto Human Ethics protocol #15482) were bonded (Scotchbond MP, 3M, St. Paul, MN, USA) to composite resin (Z250, 3M) under sterile conditions, according to the manufacturer’s instructions. We used a low-speed water-cooled rotary saw with a thin wafering blade (Isomet, Buehler, Lake Bluff, IL, USA) to prepare standardized (3 x 3 x 6 mm) resin-dentin specimens with cross-sectional areas of 3 mm2. All regions of exposed dentin directly adjacent to the marginal interface were sealed with nail varnish to prevent access to the resin-dentin interface through cut dentinal tubules.
Degradation Media Incubation of Resin-Dentin Specimens
Specimens were randomly assigned to the following sterile incubation conditions: 7-, 30-, or 90-day incubation in phosphate buffer solution (PBS) or esterase solution (PCE+CE). We prepared PCE+CE by dissolving cholesterol esterase (CE) (Genzyme, Cambridge, MA, USA) and pseudocholinesterase (PCE) (Sigma, St. Louis, MO, USA) in PBS (Gibco, Grand Island, NY, USA) to match relevant esterase levels in human saliva (Finer and Santerre, 2004a; Lin et al., 2005). The media were replaced every 48 hrs. Media from defined incubation periods were pooled for high-performance liquid chromatography (HPLC) analysis.
Bis-HPPP By-product Isolation
We used a WatersTM HPLC system to isolate and quantify Bis-HPPP (Lin et al., 2005). Product identification was confirmed by mass spectrometry (QStar-XL, Applied Biosystems/MDS Sciex, Foster City, CA, USA).
Incubation of Resin-Dentin Specimens in Chemostat-based Biofilm Fermentor (CBBF)
Following assigned incubation periods, specimens were suspended within a closed-system biofilm fermentor designed to cultivate steady-state monoclonal biofilms of Streptococcus mutans NG8 over interfacial margins (Cvitkovitch et al., 2003). Fresh medium (Todd Hewitt yeast extract supplemented with 10 mM sucrose and 0.01% hog gastric mucin, 4X diluted) was pumped into the vessel at a flow rate of 0.72 L/day at a dilution rate of D = 0.075/hr, mimicking the resting flow rate of human saliva (Pratten et al., 2000). Daily maintenance of the CBBF included optical density readings, viable cell count, and pH adjustments. Specimens were aseptically removed after 7 days, rinsed with sterile water, and stained by means of a Live/Dead Baclight Bacterial Viability Kit (Molecular Probes, Eugene, OR, USA).
Confocal Laser Scanning Microscopy (CLSM) Analysis
Stained specimens were assessed individually for bacterial penetration by CLSM (Zeiss LSM 510 META NLO, Carl Zeiss MicroImaging Inc., Toronto, ON, Canada). Six equidistant Z-stack series were captured along one side of each resin-dentin interface through a C-Apochromat 63x/1.2W (water-immersion) objective lens, zoom 2X. All 6 regions of interest (ROI) were standardized for orientation by the positioning of each specimen beneath the objective such that the composite-resin region was located at the top of the image screen, while the bottom of the image screen aligned with the dentinal region; the marginal interface was defined as that which occurred between the composite-resin and dentinal regions. CLSM Z-stack images were processed (ImageJ software) (Hope et al., 2002) to remove background fluorescence and allow for quantification of cells.
Statistical Analysis
We performed two-way ANOVA and conducted Scheffé’s post hoc analysis (p < 0.05) to determine the effects of incubation time and condition on the amount of Bis-HPPP and total levels and depths of bacterial cells within the resin-dentin interface. All study groups were run in parallel, with 3 independent samples in each group. Each experiment was conducted 3 separate times.
Results
Biodegradation
Levels of Bis-HPPP released from specimens incubated in PCE+CE media were significantly higher (p < 0.0005) than those from PBS-incubated specimens for all incubation times (Fig. 1A), with highest amounts measured at 90 days (297 ± 62 µg/cm2). The highest rate of Bis-HPPP daily production occurred within the first 7 days (Fig. 1B). In comparison, total Bis-HPPP accumulation for the 90-day PBS-incubated specimens reached 9.68 ± 0.55 µg/cm2, with no Bis-HPPP detected prior to 30 days (Fig. 1B).
Figure 1.
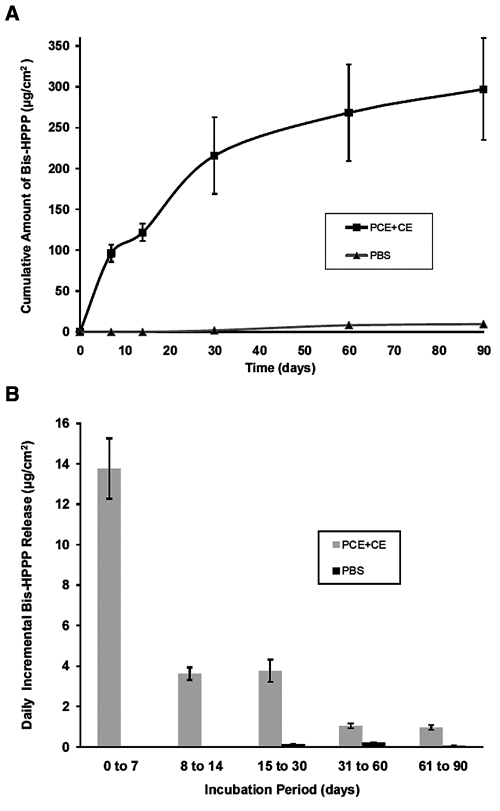
Bis-HPPP release from resin-dentin specimens. (A) Cumulative amount of Bis-HPPP produced from resin-dentin specimens incubated in PCE+CE or PBS buffer for 7, 14, 30, and 90 days (pH 7, 37°C). (B) Incremental amount of Bis-HPPP produced from resin-dentin specimens incubated in PCE+CE or PBS buffer. All data are reported with standard error of the mean (n = 3).
Bacterial Microleakage
Specimens incubated for 90 days demonstrated significantly higher levels of interfacial cellular penetration (p < 0.001) than those incubated under the same culture conditions for shorter periods of time (Fig. 2A). Furthermore, after the 30-day incubation timepoint, the cumulative numbers of bacterial cells found penetrating the marginal interface were found to be significantly higher among PCE+CE-incubated specimens (p < 0.005) as compared with PBS-incubated specimens (Fig. 2A).
Figure 2.
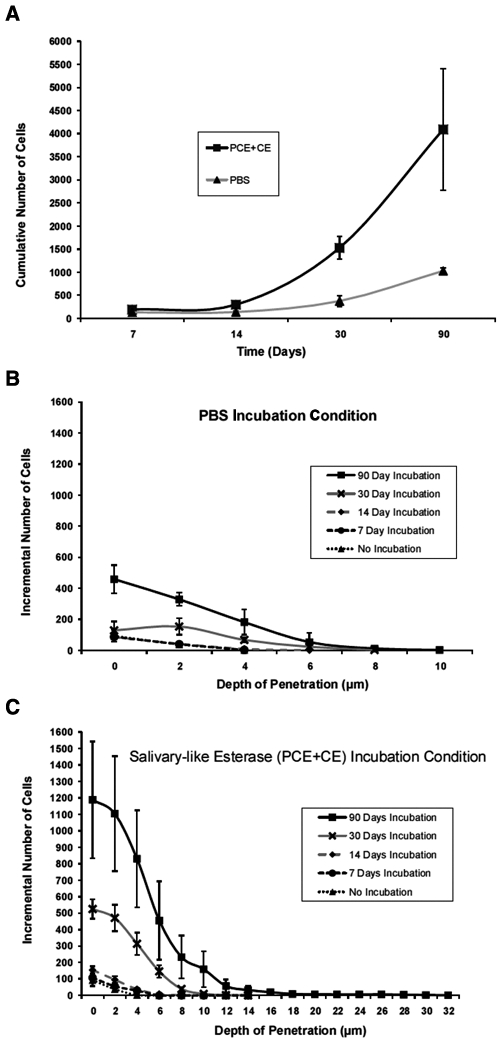
Bacterial penetration along the resin-dentin marginal interface. (A) Cumulative numbers of bacterial cells found penetrating the marginal interface for PBS controls and PCE+CE-incubated specimens over time. Number of cells vs. depth of penetration at interfacial ROIs of (B) PBS-incubated and (C) PCE+CE-incubated specimens for 0, 7, 14, 30, and 90 days (pH 7, 3°C). All data are reported with standard error of the mean (n = 3).
Within the 90-day incubation condition, specimens in PCE+CE demonstrated nearly 4 times as many (p < 0.005) bacteria than those in the PBS condition (Fig. 2B compared with 2C). Maximum interfacial depths of penetration were also nearly 4 times deeper among PCE+CE-incubated specimens than in their PBS counterparts (p < 0.05) (Fig. 2B compared with 2C).
Selected CLSM Z-stack images of samples are shown in Fig. 3. Among all 7-day-incubated specimens incubated in PBS or PCE+CE media, resin-dentin interfacial structural morphology resembled that of controls, which had been either non-incubated (3A) or incubated in either PBS or PCE+CE media, but not suspended within the CBBF (Figs. 3B, 3C; data for inoculated controls not shown). Limited amounts of adherence and penetration of S. mutans biofilm cells were observed among interfacial surface micro-porosities for 7-day-incubated specimens, up to a maximum depth of 4 µm (Figs. 2B, 3B). All component layers of the resin-dentin margin appeared linearly oriented to one another, and well-infiltrated by the priming resin. In 30-day samples, morphological changes to the resin-dentin interfacial margin emerged, albeit to various extents, depending on the incubation medium (Figs. 3D, 3E). For PBS samples, dentinal tubules of the hybrid layer commonly experienced finite structural degradation, particularly near the composite resin-resin adhesive region of the marginal interface. These changes were more extensive for the PCE+CE specimens (compare Figs. 3D and 3E).
Figure 3.
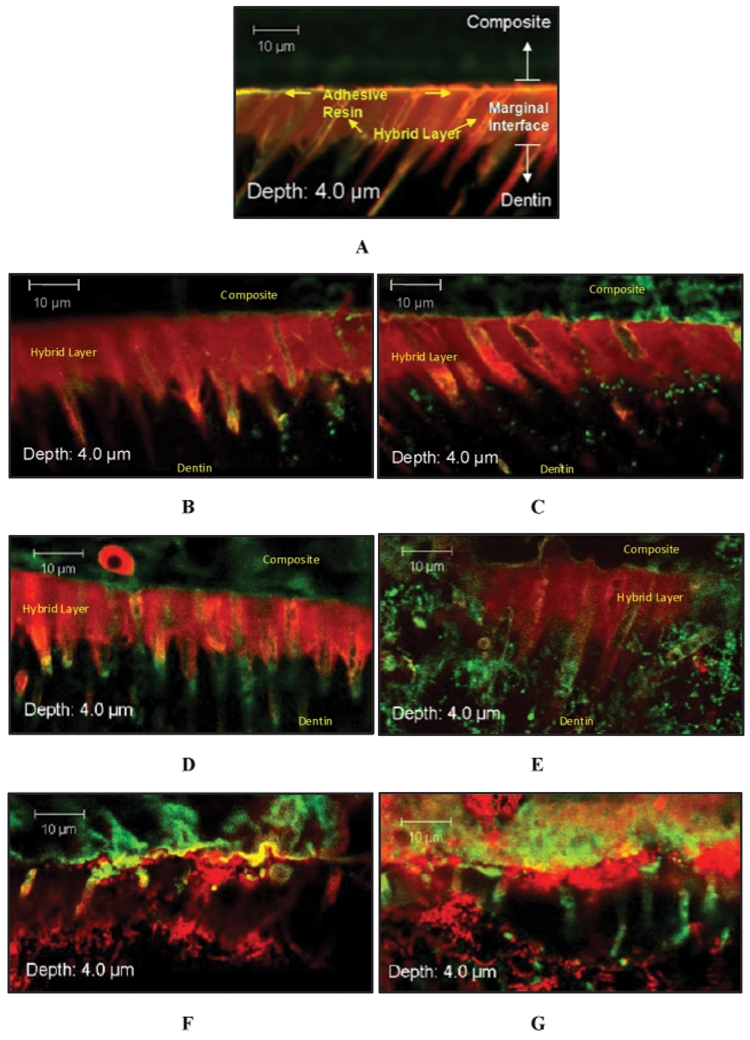
Selected Z-stack image series captured from interfacial margins of resin-dentin specimens assigned to either (A) non-incubated, (B) 7-day PBS incubation, (C) 7-day PCE+CE incubation, (D) 30-day PBS incubation, (E) 30-day PCE+CE incubation, (F) 90-day PBS incubation, or (G) 90-day PCE+CE incubation. Interfacial zones (composite, adhesive, hybrid layer, and dentin) are distinguishable in A and, to a lesser extent, in B-E; however, in F and G, the organization of these marginal components is disrupted. Resin impregnation of dentinal tubules in the hybrid layer is disrupted (F and G). Specimens were stained by means of a Live/Dead Baclight Viability Kit (magnification X62, 2X zoom). Live cells indicated by green fluorescence through interaction with Syto9; dead cells indicated by red fluorescence through interaction with propidium iodide.
In PBS and PCE+CE specimens at 90 days, junctions among the resin restorations, the adhesive layer, and the hybrid layer were interrupted and with an undulating pattern (Figs. 3F, 3G).
Two of the numerous examples of gross interface deformation found in the PCE+CE samples are shown in Fig. 4. A distinctive interfacial void at the top of the hybrid layer, where the S. mutans biofilm adhered to the top and bottom of the axial walls, is shown in Fig. 4A. At sample depths of 6 µm and over, biofilm growth extended to span the entire width of the void. An interfacial gap spanning more than 20 µm is shown in Fig. 4B. Sagittal sections through this particular ROI reveal a characteristically distinct three-dimensional mushroom-shaped pattern of S. mutans biofilm growth (Hope et al., 2002) extending up to 34 µm beneath the surface of the interface (images not shown).
Figure 4.
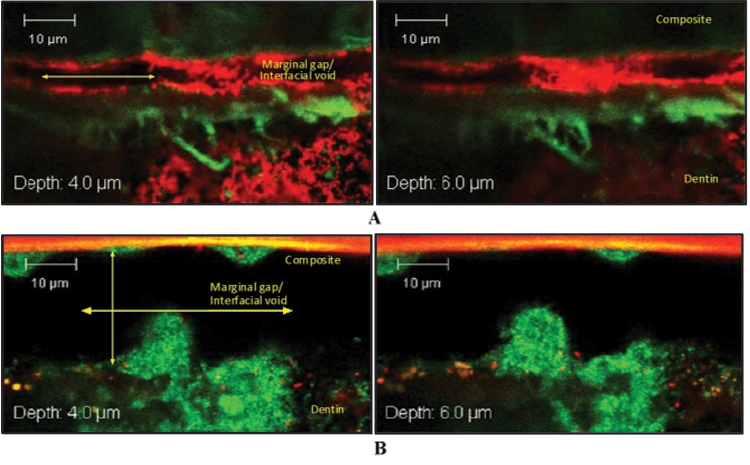
Selected Z-stack image series captured at interfacial ROIs of 2 90-day PCE+CE-incubated resin-dentin specimens. (A) Interfacial void spanning approximately 4-5 µm in height. (B) Interfacial void spanning over 20 µm in height. Characteristic of three-dimensional biofilm growth are interstitial voids that can be seen among fluorescently stained S. mutans microcolonies. In (B), large mushroom-shaped biofilm structures are found colonizing both the top and bottom axial walls. Specimens were stained by means of a Live/Dead Baclight Viability Kit (magnification X62, 2X zoom). Live cells indicated by green fluorescence through interaction with Syto9; dead cells indicated by red fluorescence through interaction with propidium iodide.
Discussion
Relative to PBS controls, PCE+CE-incubated resin-dentin specimens generated significantly higher amounts of Bis-HPPP (p < 0.0001) at all incubation timepoints and resulted in greater bacterial surface adherence and penetration along the resin-dentin marginal interface with time. Therefore, the hypothesis that exposure of resin-composite restorations to saliva-like esterase activities accelerates marginal bacterial microleakage was shown to be true. Analysis of the current data demonstrated that biodegradation of resin-adhesives and composites is a time-dependent process that progressively compromised the clinical value of resin materials with increased incubation time.
The degradation by-product of choice for this study, Bis-HPPP, is a derivative of Bis-GMA, a common monomer used in both adhesive and composite materials investigated in the current study. Bis-HPPP production was shown to be a good indicator of overall resin matrix degradation (Finer and Santerre, 2007; Shokati et al., 2010).
Periodic daily incremental release rates of Bis-HPPP within the 90-day period for PCE+CE-incubated specimens varied over time. The highest rate of accumulation occurred within the first 7 days of PCE+CE incubation (13.8 ± 1.49 µm/cm2 per day), decreased by a factor of 4 between 8 and 14 days of PCE+CE incubation, and reached a plateau at a constant release rate of 1 ± 0.1 µm/cm2 per day between days 30 and 90. Theoretically, for the first 7 days of incubation, hydrolytic reaction rates are defined primarily by the catalytic activity of the esterases. By the 8th day of incubation, the number of readily accessible ester linkages within the Bis-GMA-based resin matrix gradually declines. The rate-controlling factor becomes the physical access of esterases to the un-reacted ester substrate, slowing the hydrolytic process. After 30 days of incubation, the most readily accessible ester linkages within the resin matrix have become hydrolyzed. At this point, longer-term biochemical breakdown of the resin matrix depends on rates at which previously inaccessible ester linkages become unmasked by the elution of degraded oligomers (Finer and Santerre, 2004b, 2007).
Bacterial adherence and penetration among the superficial sub-layers (0-4 µm) of minimally compromised interfacial margins were mainly localized to the top and bottom of the hybrid layer in samples incubated for 7 days with either PBS or PCE+CE. Intrinsic interfacial porosities are often formed during bond application, potentially generated by polymerization shrinkage at the top of the hybrid layer, or incomplete resin impregnation of demineralization dentin occurs at the bottom (Suppa et al., 2005). Both phenomena may also account for the superficial bacterial microleakage observed at the interfacial sub-layers of control resin-dentin specimens un-incubated with degradation media (data not shown).
Discrepancy between the depth of the demineralization and resin infiltration is common among commercial three-step “etch-and-rinse” adhesives such as Scotch Bond Multi-Purpose (Spencer and Wang, 2002). Oral streptococcal cells span approximately 0.5 to 0.7 µm in diameter (Love and Jenkinson, 2002). Given access, they can penetrate nanometer-sized voids at the bottom of the hybrid layer and directly bind to intra-tubular collagen type I components of the dentinal tubules (Love and Jenkinson, 2002). Furthermore, given the widely reported effects of water sorption at the top and bottom of the restorative interface (Sauro et al., 2009), as well as associated elution of adhesive resin components over time in both in vivo (Pashley et al., 2004) and in vitro conditions (Hebling et al., 2005; Shokati et al., 2010), nanometer-sized interfacial voids can expand with prolonged incubation in media (Suppa et al., 2005). The results from this investigation corroborated such findings, since localization of bacterial microleakage among resin-dentin specimens incubated for 7- and 30-day time periods was centralized near the top and base of the hybrid layer. In light of significant interfacial disruptions found among specimens incubated for 90 days, though, bacterial microleakage for all samples at this time period was non-specific and occurred across the entire interfacial span.
Qualitative assessments of interfacial structural integrity among resin-dentin specimens also led to the conclusion that while incubation in PBS altered the morphology of the marginal interface over time (Pashley et al., 2004), exposure to salivary-like esterase activities of PCE+CE media greatly amplified the intrinsic effects of hydrolytic processes. The reduction of signal scatter in the inter-tubular layer of the hybrid zone suggests a loss of resin and/or mineral content, a morphological change consistent with that of carious dentin (Zavgorodniy et al., 2008). While an overall reduction in red fluorescence signal scatter was observed at inter-tubular regions of the hybrid layer in 90-day PBS-incubated specimens, it was almost entirely absent among those of 90-day PCE+CE-incubated specimens.
The presence of blister-like voids and the observed undulating pattern of the interface layer may be a consequence of interfacial water sorption (Sauro et al., 2008) causing swelling and plasticization of resin polymers (Ferracane, 2006). Given that ongoing hydrolytic processes can propagate marginal gap formation over time (Hashimoto et al., 2003), it is then not surprising that the largest marginal gaps were found exclusively among 90-day PCE+CE-incubated specimens, which generated the greatest amounts of Bis-HPPP.
It was also within the expanded marginal gap region of 90-day PCE+CE-incubated specimens that the most extensive colonization of S. mutans biofilms was found. Highly characteristic biofilm structures (Lewandowski et al., 2007) were anchored to the composite resin or dentinal axial walls of marginal gaps spanning 10 µm or more. Recently, it was suggested that larger-sized marginal gaps provide the necessary space and access to the nutrients necessary for successful colonization by larger numbers of micro-organisms (Totiam et al., 2007).
It is the current belief that secondary loss of marginal integrity is primarily attributed to mechanical forces such as occlusal loading (Bouillaguet, 2004) as well as thermal stress and polymerization contraction (van Noort, 1994). Yet, analysis of the increasingly emerging data in the scientific literature has suggested a potential for secondary loss of adhesion due to in vivo chemical attack (Hickel and Manhart, 2001; Hashimoto et al., 2003; Donmez et al., 2005). The current findings make this case abundantly clear. Evidence of increased bacterial penetration coupled with dentin demineralization suggests that the biodegradation process can contribute to the formation of recurrent decay—the most common cause of restoration failure (Hicks et al., 2003).
The results of this study also demonstrated high reproducibility as well as clinical relevance, a factor which is imperative in the evaluation of biomaterials with an in vitro experimental system. As a result, this model shows great potential for further development into a standardized testing system of biochemical stability among various commercial adhesive and composite materials prior to use in clinical settings. Where hybrid layer interruption and marginal gaps do occur, the current system presents a practical non-invasive imaging method for intact biofilms adhering to and proliferating on and within the resin-dentin interface. To the best of our knowledge, the current investigation provides the first physiologically relevant in vitro characterization of bacterial microleakage within the resin-dentin interface.
Acknowledgments
The authors thank the Canadian Institute of Health Research for Grant MOP 68947 and the NIH for grant 5R01DE013230-10. This paper is based on a thesis submitted to the Graduate Department of the Faculty of Dentistry, University of Toronto, in partial fulfillment of the requirements for the MASc degree.
References
- Bouillaguet S. (2004). Biological risks of resin-based materials to the dentin-pulp complex. Crit Rev Oral Biol Med 15:47-60 [DOI] [PubMed] [Google Scholar]
- Cvitkovitch DG, Li YH, Ellen RO. (2003). Quorum sensing and biofilm formation in Streptococcal infections. J Clin Invest 112:1626-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez N, Belli S, Pashley DH, Tay FR. (2005). Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res 84:355-359 [DOI] [PubMed] [Google Scholar]
- Ferracane JL. (2006). Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater 22:221-222 [DOI] [PubMed] [Google Scholar]
- Finer Y, Santerre JP. (2003). Biodegradation of a dental composite by esterases: dependence on enzyme concentration and specificity. J Biomater Sci Polym Ed 14:837-849 [DOI] [PubMed] [Google Scholar]
- Finer Y, Santerre JP. (2004a). Salivary esterase activity and its association with the biodegradation of dental composites. J Dent Res 83:22-26 [DOI] [PubMed] [Google Scholar]
- Finer Y, Santerre JP. (2004b). The influence of resin chemistry on a dental composite’s biodegradation. J Biomed Mater Res A 69:233-246 [DOI] [PubMed] [Google Scholar]
- Finer Y, Santerre JP. (2007). Influence of silanated filler content on the biodegradation of bisGMA/TEGDMA dental composite resins. J Biomed Mater Res A 81:75-84 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Tay FR, Ohno H, Sano H, Kaga M, Yiu C, et al. (2003). SEM and TEM analysis of water degradation of human dentinal collagen. J Biomed Mater Res Part B Appl Biomater 66:287-298 [DOI] [PubMed] [Google Scholar]
- Hebling J, Pashley DH, Tjäderhane L, Tay FR. (2005). Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res 84:741-746 [DOI] [PubMed] [Google Scholar]
- Hickel R, Manhart J. (2001). Longevity of restorations in posterior teeth and reasons for failure. J Adhes Dent 3:45-64 [PubMed] [Google Scholar]
- Hicks J, Garcia-Godoy F, Donly K, Flaitz C. (2003). Fluoride-releasing restorative materials and secondary caries. J CA Dent Assoc 31:229-245 [PubMed] [Google Scholar]
- Hope CK, Clements D, Wilson M. (2002). Determining the spatial distribution of viable and nonviable bacteria in hydrated microcosm dental plaques by viability profiling. J Applied Microbiol 93:448-455 [DOI] [PubMed] [Google Scholar]
- Jaffer F, Finer Y, Santerre JP. (2002). Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials 23:1707-1719 [DOI] [PubMed] [Google Scholar]
- Lewandowski Z, Beyenal H, Myers J, Stookey D. (2007). The effect of detachment on biofilm structure and activity: the oscillating pattern of biofilm accumulation. Water Sci Technol 55:429-436 [DOI] [PubMed] [Google Scholar]
- Lin BA, Jaffer F, Duff MD, Tang YW, Santerre JP. (2005). Identifying enzyme activities within human saliva which are relevant to dental resin composite biodegradation. Biomaterials 26:4259-4264 [DOI] [PubMed] [Google Scholar]
- Love RM, Jenkinson HF. (2002). Invasion of dentinal tubules by oral bacteria. Crit Rev Oral Biol Med 13:171-183 [DOI] [PubMed] [Google Scholar]
- Murray PE, Hafez AA, Smith AJ, Cox CF. (2002). Bacterial microleakage and pulp inflammation associated with various restorative materials. Dent Mater 18:470-478 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221 [DOI] [PubMed] [Google Scholar]
- Pratten J, Andrews CS, Craig DQ, Wilson M. (2000). Structural studies of microcosm dental plaques grown under different nutritional conditions. FEMS Microbiol Lett 189:215-218 [DOI] [PubMed] [Google Scholar]
- Santerre JP, Shajii L, Leung BW. (2001). Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med 12:136-151 [DOI] [PubMed] [Google Scholar]
- Sauro S, Pashley DH, Mannocci F, Tay FR, Pilecki P, Sherriff M, et al. (2008). Micropermeability of current self-etching and etch-and-rinse adhesives bonded to deep dentine: a comparison study using double-staining/confocal microscopy technique. Eur J Oral Sci 116:184-193 [DOI] [PubMed] [Google Scholar]
- Sauro S, Watson TF, Mannocci F, Miyake K, Huffman BP, Tay FR, et al. (2009). Two-photon laser confocal microscopy of micropermeability of resin-dentin bonds made with water or ethanol wet bonding. J Biomed Mater Res B Appl Biomater 90:327-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokati B, Tam L, Santerre JP, Finer Y. (2010). Effect of salivary esterase on the integrity and fracture toughness of the resin-dentin interface. J Biomed Mater Res (in press). [DOI] [PubMed] [Google Scholar]
- Spencer P, Wang Y. (2002). Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res 62:447-456 [DOI] [PubMed] [Google Scholar]
- Suppa P, Breschi L, Ruggeri A, Mazzotti G, Prati C, Chersoni S, et al. (2005). Nanoleakage within the hybrid layer: a correlative FEISEM/TEM investigation. J Biomed Mater Res B Appl Biomater 73:7-14 [DOI] [PubMed] [Google Scholar]
- Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. (2007). A new in vitro model to study the relationship of gap size and secondary caries. Caries Res 41:467-473 [DOI] [PubMed] [Google Scholar]
- van Noort R. (1994). Introduction to dental materials. London, UK: Mosby [Google Scholar]
- Zavgorodniy AV, Rohanizadeh R, Swain MV. (2008). Ultrastructure of dentine carious lesions. Arch Oral Biol 53:124-132 [DOI] [PubMed] [Google Scholar]


