Abstract
Evidence from epidemiologic studies suggests that periodontal infections are independently associated with subclinical and clinical atherosclerotic vascular disease. Although the strength of the reported associations is modest, the consistency of the data across diverse populations and a variety of exposure and outcome variables suggests that the findings are not spurious or attributable only to the effects of confounders. Analysis of limited data from interventional studies suggests that periodontal treatment generally results in favorable effects on subclinical markers of atherosclerosis, although such analysis also indicates considerable heterogeneity in responses. Experimental mechanistic in vitro and in vivo studies have established the plausibility of a link between periodontal infections and atherogenesis, and have identified biological pathways by which these effects may be mediated. However, the utilized models are mostly mono-infections of host cells by a limited number of ‘model’ periodontal pathogens, and therefore may not adequately portray human periodontitis as a polymicrobial, biofilm-mediated disease. Future research must identify in vivo pathways in humans that may (i) lead to periodontitis-induced atherogenesis, or (ii) result in treatment-induced reduction of atherosclerosis risk. Data from these studies will be essential for determining whether periodontal interventions have a role in the primary or secondary prevention of atherosclerosis.
Keywords: periodontal, infection, atherosclerosis, epidemiology, mechanisms
Introduction
In 2005, Robin Warren and Barry Marshall won the Nobel Prize in Physiology or Medicine “for their discovery of the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer disease” (Pincock, 2005). Although the award was granted fairly recently, their original research dates back to the early 1980s (Warren and Marshall, 1983) and was becoming widely accepted by the mid-1990s (Thagard, 1998). On the heels of this novel hypothesis, research studies began to explore the possible causal role of infections in the pathophysiology of other chronic diseases. In 1989, two studies were published, almost simultaneously, that posited oral infection to have an etiologic role in cardiovascular disease (Mattila et al., 1989; Syrjanen et al., 1989). Since that time, a substantial body of literature has developed, the majority of which supports the oral infection hypothesis. However, several authors have appropriately highlighted the fact that while this research discipline has generated a large number of publications, the majority of these tend to be either original studies with low levels of evidence or reviews (Dietrich and Garcia, 2005). Our purpose with this review is to summarize the state of the science with special emphasis on recent findings from epidemiologic and basic science articles, to highlight areas where corroborating findings from both disciplines strengthen various mechanistic hypotheses, and to identify areas that need to be substantiated further by new research data. In so doing, we will primarily focus on studies related to atherosclerotic vascular disease (AVD) and particularly on coronary heart disease (CHD) and ischemic stroke, as well as on subclinical assessments of atherosclerosis and endothelial function.
Observational Studies
Several earlier reviews have discussed the findings from the major epidemiologic studies (Meurman et al., 2004; Behle and Papapanou, 2006; Demmer and Desvarieux, 2006), and at least three meta-analyses have been published summarizing the association between periodontal disease and clinical cardiovascular outcomes (Janket et al., 2003; Mustapha et al., 2007; Humphrey et al., 2008), consistently concluding that the available evidence suggests a moderate, positive association between periodontal diseases and AVD. Several recent publications support these conclusions and have extended previous research in important ways. Key findings from selected observational studies published between 2006 and 2009 exploring associations between periodontal disease and CHD or stroke are summarized in Table 1. Among these, two publications from the Normative Aging Study (NAS) cohort report positive associations between periodontal disease and both incident CHD (Dietrich et al., 2008) and stroke (Jimenez et al., 2010). Importantly, these studies also had the ability to test two previously described trends as a priori hypotheses. Specifically, these studies reported stronger associations among younger vs. older participants as well as for stroke vs. CHD outcomes (see discussion below). In addition, recently reported data from Korea (Sim et al., 2008; Choe et al., 2009) and India (Pradeep et al., 2010) are, to our knowledge, among the first publications reporting on associations between periodontal disease and clinical AVD events that arise from Asian populations. Therefore, these data enhance the consistency of previous findings that predominantly stem from European and North American cohorts. The growing diversity of study populations from which results have been reported helps assuage concerns about spurious findings related to health behaviors (smoking, dietary patterns, physical activity), health care systems (access to care, clinical guidelines, availability of pharmaceuticals), or environmental risk factors (environmental tobacco smoke, pollution, diet).
Table 1.
Summary of Selected Epidemiologic Observational Studies Exploring Associations between Periodontal Disease and Clinical CHD or Stroke between 2006 and 2009
| Study | N | Country | Age Range | Design | Exposure | Outcome | Adjustments | Measure of Association (95% Confidence Interval) |
|---|---|---|---|---|---|---|---|---|
| Geismar et al., 2006 | 250 | Denmark | NA | Case-control | Radiographic | CHD | 1,2,5,6 | OR 2.0 (0.77, 5.08) |
| Briggs et al., 2006 | 171 | Ireland | 40+ | Case-control | PD | CHD | 1–6,10 | OR 3.06 (1.02–9.17) |
| Holmlund et al., 2006 | 4254 | Sweden | 20–70 | Cross-sectional | Tooth # Radiographic | Self-reported CHD | 1,3,5 | OR 0.80 (0.64, 0.96) 2.69 (1.12, 6.46) |
| Rech et al., 2007 | 114 | Brazil | NR | Case-control | Periodontitis (clinical) | ACS | 1,3,6 | OR 4.5 (1.3, 15.6) |
| Rubenfire et al., 2007 | 440 | USA | NR | Case-control | Positive BANA test | ACS | 1,3,5 | OR In BANA+ participants 3.95 (1.61, 9.71) |
| Andriankaja et al., 2007 | 1461 | USA | 35–69 | Case-control | CAL | Non-fatal MI | 1,3,5–8 | OR 1.46 (1.36, 1.69) |
| Gotsman et al., 2007 | 201 | Israel | NR | Cross-sectional | CAL (% of teeth with CAL ≥ 5) | ACS | 1,5,7,8 | OR 1.03 (1.01, 1.04) |
| Nonnenmacher et al., 2007 | 90 | Germany | 40–80 | Case-control | CAL | CHD | 1–3,5,9 | OR 3.2 (1.2, 9.0) |
| Dietrich et al., 2008* | 1203 | USA (NAS) | 21–59 | Cohort | Radiographic | CHD | 1–10 | HR 2.12 (1.26, 3.60) |
| USA (NAS) | 60–84 | Cohort | Radiographic | CHD | 1–10 | HR 1.81 (NA) | ||
| Senba et al., 2008 | 6816 | Japan | < 66 | Cross-sectional | Self-report periodontitis or tooth loss | CHD men | 1–3,5,6,8,9 | OR 1.51 (0.90, 2.52) 1.54 (0.90, 2.62) |
| Senba et al., 2008 | 23,088 | Japan | < 66 | Cross-sectional | Self-report periodontitis | CHD women | 1–3,6,8,9 | OR 1.48 (0.95, 2.32) 1.68 (1.08, 2.61) |
| Lund Håheim et al., 2008 | 1173 | Norway | 48–77 | Case-control | IgG (Aa, Pg, Td, or Tf) | CHD | 1–3,5–9 | OR 1.31 (1.01, 1.69) |
| Pussinen et al., 2007b | 505 | Finland | 25–64 | Nested case-control | IgA Aa IgG Aa IgA Pg IgG Pg |
CHD & stroke combined | 1–9 | HR 1.43 (0.88, 2.31) 1.64 (1.00, 2.69) 1.53 (0.95, 2.44) 1.53 (0.93, 2.50) |
| Tu et al., 2007 | 12,223 | Scotland | ≤ 30 | Cohort | Tooth loss | Fatal CVD Fatal CHD Fatal stroke |
1,3–5,8,9 | HR 1.35 (1.03, 1.77) 1.19 (0.84, 1.69) 1.64 (0.96, 2.80) |
| Syrjala et al., 2009 | 392 | Finland | 75+ | Cross-sectional | Tooth loss | Stroke & CHD combined | 1–10 | CPR (dendate vs. edentulous)*** 0.9 (0.5,1.8) |
| Pussinen et al., 2007a | 893 | Finland | 30–59 | Nested case-control | IgA Aa IgG Aa IgA Pg IgG Pg |
Stroke | 1,2,4–10 | OR 0.83 (0.62–1.10) 0.93 (0.66–1.32) 1.22 (0.91–1.65) 1.31 (0.97–1.76) |
| Lee et al., 2006 | 5123 | USA (NHANES) | 60+ | Cross-sectional | Periodontal Health Status (PHS; a composite index of periodontitis and tooth loss) | Self-reported stroke | 1,5,6,8,10,14 | OR for PHS Class 2-5 vs. 1 1.34 (0.75, 2.38) 1.97 (0.85, 4.56) 1.99 (1.06, 3.71) 1.56 (0.95, 2.57) |
| Sim et al., 2008 | 479 | Korea | 40–79 | Case-control | CAL | Stroke** | 1–6,8–10 | OR 4.30 (2.27, 8.16) |
| Jimenez et al., 2009 | 1137 | USA (NAS) | 27–84 | Cohort | Radiographic | Stroke | 1–10 | HR 3.52 (1.59, 7.81) |
| Pradeep et al., 2010 | 200 | India | 33–68 | Case-control | PD | Stroke | 1–3 | OR 8.5 (1.1, 68.2) |
| You et al., 2009 | 22,862 | USA | 45+ | Cross-sectional | Tooth loss | Stroke** | 1–8 | OR 1.27 (1.09, 1.49) |
| Choe et al., 2009 | 867,256 | Korea | 30+ | Cohort | Tooth loss | Stroke** | 1–3,5–10 | HR 1.3 (1.2, 1.4) |
Adjustments: 1, age; 2, race; 3, sex; 4, SES (income and/or education); 5, smoking status; 6, diabetes; 7, hyperlipidemia (or continuous LDL-cholesterol and/or HDL-cholesterol); 8, hypertension (or diastolic and/or systolic blood pressure); 9, obesity; 10, alcohol consumption. Abbreviations: PD, probing depth; CAL, clinical attachment loss; CHD, coronary heart disease; ACS, acute coronary syndrome; OR, odds ratio; HR, hazard ratio; CPR, cumulative prevalence ratio; NA, not available; NAS, Normative Aging Study, Boston, MA; IgG, immunoglobulin G; IgA, immunoglobulin A; Pg, Porphyromonas gingivalis; Aa, Aggregatibacter actinomycetemcomitans. *Dietrich et al. (2008) reported results only in age subgroups, so the hazard ratio for the association between radiographic periodontal disease and incident stroke among the full cohort is not available. **Hemorrhagic strokes included. ***The study by Syrjala et al. (2009) computed a cumulative prevalence ratio by comparing risk of prevalent stroke or CHD among dentate participants in the numerator with that of edentulous participants in the denominator; therefore, it can be inferred that there was a modest, non-significant increased prevalence of stroke or CHD among edentulous participants relative to dentate participants.
Another important development is the publication of at least three studies using direct assessments of periodontal bacterial colonization (Renvert et al., 2006; Spahr et al., 2006; Nonnenmacher et al., 2007), all of which report positive associations between specific oral bacterial colonization levels and CVD outcomes. These findings corroborate and extend the earlier reported observations from the Oral Infections and Vascular Disease Epidemiology Study (INVEST): that high colonization by a cluster of species assumed to be either causative for periodontal disease or strong correlates of underlying causative species (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) was associated with significantly increased intima-media thickness in the carotid artery (Desvarieux et al., 2005).
In summary, at least 13 observational studies have been published in the last four years, all of which report at least marginal evidence of a positive association between periodontal disease and clinical CVD. These recent findings support and extend earlier observations, and have directly addressed several key themes relevant to the hypothesized role of periodontal infection/inflammation as an independent contributor to the pathogenesis of atherosclerotic vascular disease, which are discussed below in greater detail.
The Role of Confounding
A prominent and persistent concern regarding the positive associations between periodontitis and AVD is the potential for spurious findings induced by known correlations between established risk factors for AVD and poor periodontal health, or, in epidemiologic terms, confounding, which occurs when a variable is both associated with the exposure (i.e., periodontitis) and is an independent cause of outcome (i.e., AVD) (Hernan et al., 2002). For example, smoking is an established risk factor for both periodontal disease and AVD, which raises the possibility that the increased risk of AVD commonly observed among groups with periodontal disease is actually due to a higher rate of smoking in these groups. Strong arguments have been made to demonstrate the importance of smoking-related confounding, particularly in the context of periodontal infection and cancer (Hujoel et al., 2003), but the conclusions apply to AVD research as well (Hujoel et al., 2002). In response, epidemiologic approaches have been sharpened by many investigators. For example, it is now common practice to carry out multivariable adjustments that include not only smoking status (current, former, never) but also pack-years of smoking and years since cessation. Alternatively, stratified analyses conducted among never-smokers are also common; this ensures that the association exists among participants in whom smoking-related confounding is theoretically impossible (barring second-hand smoking and information bias). In addition to initial key studies that performed these stratified analyses, several recent studies have consistently reported positive associations between periodontal infections and AVD. For example, a Korean case-control study reported an odds ratio (OR) of 3.3 (95%CI: 1.7,6.7) for non-fatal stroke among never-smokers (Sim et al., 2008). In Finland, a nested case-control study observed an OR for incident stroke among male never-smokers of 3.31 (95%CI: 1.31-8.40), while the OR among female never-smokers was 2.36 (95%CI: 1.44-3.88) (Pussinen et al., 2007a). In another publication from Finland (Ylöstalo et al., 2006), an OR of 1.62 (95%CI: 0.70, 3.74) was reported for prevalent angina pectoris associated with gingivitis. Analysis of US data obtained from the Behavioral Risk Factor Surveillance Survey, including 41,891 participants from 22 states, showed that among never-smokers, the respective ORs for CHD among participants missing 1-5 or 6-31 teeth were 1.39 (95%CI: 1.05-1.85) and 1.76 (95%CI: 1.26-2.45) (Okoro et al., 2005).
Despite these advances, smoking remains a critical threat to the validity of findings concerning periodontal infection and AVD. Nevertheless, it is clear that the preponderance of evidence to date in support of a positive association between periodontal infection and CVD cannot be completely explained by smoking, and therefore exists independent of smoking behaviors. With an example from the cancer epidemiology literature, it has been convincingly demonstrated that it is unrealistic to conclude that smoking alone, particularly residual confounding by smoking or environmental tobacco smoke, can completely explain the reported associations in observational epidemiological studies (Michaud et al., 2007; Taguchi, 2007). Nevertheless, future research can benefit from careful methodological work with specific focus on confounding using modern epidemiological tools such as Directed Acyclic Graphs (DAGs) (Merchant and Pitiphat, 2002), which provide a quick visual method for the selection of potential confounders and minimization of bias in the design and analysis of epidemiological studies.
Of equal importance to the problems posed by known confounders is the potential for unknown confounders to underlie the consistent findings in the literature. For example, a recent candidate-gene association study identified a common genetic susceptibility locus, shared by both coronary heart disease and aggressive periodontitis, which may partly account for the observed associations (Schaefer et al., 2009). Unfortunately, very little, if anything, can be done in observational studies to account for this possibility. The remedy for this problem lies in randomization, a key feature required of intervention studies testing the oral infection-AVD hypothesis.
The Role of Effect Modification
Effect modification (also referred to as interaction) is a phenomenon that occurs when “two or more risk factors modify the effect of each other with regard to the occurrence or level of a given outcome” (Szklo et al., 2000). In the context of this review, we are referring to a situation where the effects of periodontal infections on AVD are either stronger or weaker across levels of an additional factor (e.g., age, smoking status, or a polymorphism conferring genetic susceptibility). Two earlier publications have indeed discussed the concept of effect modification in the context of periodontal infection-AVD association (Hyman, 2006; Ylöstalo and Knuuttila, 2006).
From a statistical standpoint, assessing the evidence for effect modification is difficult because it generally requires much larger sample sizes than traditional analyses. Nevertheless, many publications often include subgroup results to inform the potential for effect modification, despite the fact that there is usually poor statistical power for meaningful null hypothesis testing in regard to interaction. This practice is still quite helpful, since it provides alternative views of the data to help assuage concerns regarding confounding—which is a phenomenon distinct from effect modification—and also allows for qualitative assessments regarding effect modification. However, it is important that these subgroup analyses not be over-interpreted in the absence of formal statistical testing for interaction with statistically significant p-values, as well as a clear rationale and a priori hypotheses.
A good example of potential effect modification in the context of periodontal infections and CVD is in regard to the influence of age. A pattern emerged in earlier studies in which the analyzed periodontal disease-AVD associations were consistently stronger among younger individuals (DeStefano et al., 1993; Morrison et al., 1999; Hujoel et al., 2000; Joshipura et al., 2003; Desvarieux et al., 2004; Demmer and Desvarieux, 2006). In terms of clinical CVD, the meta-analysis conducted by Janket and colleagues (Janket et al., 2003) reported that the overall CVD risk associated with periodontal disease was 1.19 (95%CI: 1.08, 1.32), while the CVD risk among individuals under 65 years of age was 1.44 (95%CI: 1.2, 1.7).
Recent reports confirm these trends (Table 2). Accordingly, Dietrich et al. (2008) reported a two-fold increase in the risk of incident CHD among male participants with vs. those with little or no evidence of clinically and radiographically defined periodontal disease at baseline. These positive findings from the Veterans Affairs Normative Aging Study (NAS) were limited to participants under age 60, while the association was not evident among older individuals. Similar findings for stroke were recently reported from the same study (Jimenez et al., 2009). These results are important and convincing, since: (i) they stem from a well-conducted population-based study that was initiated in the 1960s (i.e., 20 years prior to the publication of the first reports of a periodontal-CVD association), which minimizes the potential for bias; (ii) they represent both fatal and non-fatal CVD events occurring during 35 years of longitudinal follow-up; and (iii) the periodontal disease/age interaction hypothesis was specified a priori based on the previously reported observations of increased risk among younger participants mentioned above (DeStefano et al., 1993; Mattila et al., 2000; Joshipura et al., 2003; Grau et al., 2004) and is likely not a spurious post hoc finding.
Table 2.
A Subset of the Observational Studies Reported in Table 1 that Provides Information on Age and CVD Outcome Trends (Data are presented separately for individuals younger than 68 yrs or over 65 yrs of age.)
| Study | N | Country | Age Range | Design | Exposure | Outcome | Adjustments | Measure of Association (95% Confidence Interval) |
|---|---|---|---|---|---|---|---|---|
| Geismar et al., 2006 | 250* | Denmark | < 60 | Case-control | Radiographic | CHD | 1,2,5,6 | OR 6.6 (1.69, 25.6) |
| Tu et al., 2007 | 12,223 | Scotland | ≤ 30 | Cohort | Tooth loss | Fatal CHD | 1,3–5,8,9 | HR 1.19 (0.84, 1.69) |
| Dietrich et al., 2008 | 1203* | USA (NAS) | 21–59 | Cohort | Radiographic | CHD | 1–10 | HR 2.12 (1.26, 3.60) |
| Pussinen et al., 2007b | 505 | Finland | 25–64 | Nested case-control | IgA Aa IgG Aa IgA Pg IgG Pg |
CHD & stroke combined | 1–9 | HR 1.43 (0.88, 2.31) 1.64 (1.00, 2.69) 1.53 (0.95, 2.44) 1.53 (0.93, 2.50) |
| Pussinen et al., 2007a | 893 | Finland | 30–59 | Nested case-control | IgA Aa IgG Aa IgA Pg IgG Pg |
Stroke | 1,2,4–10 | OR 0.83 (0.62–1.10) 0.93 (0.66–1.32) 1.22 (0.91–1.65) 1.31 (0.97–1.76) |
| Tu et al., 2007 | 12,223 | Scotland | ≤ 30 | Cohort | Tooth loss | Fatal stroke | 1,3–5,8,9 | HR 1.64 (0.96, 2.80) |
| Sim et al., 2008 | 160 | Korea | 40–59 | Case-control | CAL | Stroke | 1–6,8–10 | OR 25.9 (5.77, 117) |
| Jimenez et al., 2009 | NA | USA (NAS) | 27–64 | Cohort | Radiographic | Stroke | 1–10 | HR 5.81 (1.63,20.68) |
| Pradeep et al., 2010 | 200 | India | 33–68 | Case-control | PD > 4.5 mm | Stroke | 1–3 | OR 8.5 (1.1, 68.2) |
| Geismar et al., 2006 | 250* | Denmark | 65+ | Case-control | Radiographic | CHD | 1,2,5,6 | OR 0.8(0.26, 2.69) |
| Dietrich et al., 2008 | 1203* | USA (NAS) | 60–84 | Cohort | Radiographic | CHD | 1–10 | HR 1.81 (NA) |
| Sim et al., 2008 | 197 | Korea | 60–79 | Case-control | CAL | Stroke | 1–6,8–10 | OR 2.45 (1.06, 5.67) |
| Jimenez et al., 2009 | NA | USA (NAS) | 65–84 | Cohort | Radiographic | Stroke | 1–10 | HR 2.39 (0.91, 6.25) |
Adjustments: 1, age; 2, race; 3, sex; 4, SES (income and/or education); 5, smoking status; 6, diabetes; 7, hyperlipidemia (or continuous LDL-cholesterol and/or HDL-cholesterol); 8, hypertension (or diastolic and/or systolic blood pressure); 9, obesity; 10, alcohol consumption. Abbreviations: PD, probing depth; CAL, clinical attachment level; CHD, coronary heart disease; OR, odds ratio; HR, hazard ratio; NA, not available; NAS, Normative Aging Study, Boston, MA; IgG, immunoglobulin G; IgA, immunoglobulin A; Pg, Porphyromonas gingivalis; Aa, Aggregatibacter actinomycetemcomitans. *Represents total cohort—sample size in age subgroups not specified.
In addition, analysis of case-control data from a Danish population showed an odds ratio of 6.6 (95%CI: 1.69, 25.6) for the association between periodontal disease and CHD among participants < 60 years of age, while there was no association observed among participants 60+ years (Geismar et al., 2006). Even more dramatic were the reported case-control data from Korea, with a 25-fold increase in the odds of ischemic stroke among participants with vs. those without periodontitis in ages 40-59 years, as compared with a 2.5-fold increase among participants in ages 60-79 years (Sim et al., 2008).
These age-associated trends were not corroborated by data from Finland (Pussinen et al., 2007a,b) that reported moderate or no associations between serum antibody levels to specific periodontal pathogens and both CHD and stroke among participants under the age of 65 years. However, it must be realized that the exposure definition in this study was limited to serum antibody titers to two periodontal pathogens (A. actinomycetemcomitans and P. gingivalis). In addition, the prevalence of edentulism in the study population was not accounted for, which is problematic given its impact on antibody levels discussed above. Therefore, while these findings add counter-balance to the otherwise convincing trends of stronger risk among younger individuals, they should be viewed in the context of their epidemiological limitations.
The age trends reported in studies with clinical AVD outcomes have been extended to studies of subclinical AVD among young participants in at least two separate studies. Analysis of data from the population-based Study of Health in Pomerania (SHIP) demonstrated that, when comparing participants with high vs. those with low levels of periodontal disease, the prevalence of carotid artery plaque increased by approximately 15% among men under the age 59 years, while among participants aged 59 years and older, the increase was only approximately 5% (Desvarieux et al., 2004). Likewise, the odds of having increased carotid artery intima-media thickness (IMT) among periodontitis patients under 40 years of age was increased over eight-fold when compared with odds in periodontally healthy control individuals matched on age, gender, obesity, and smoking behaviors (Cairo et al., 2008).
Analyses of some data have also reported smoking (Hyman et al., 2002) and gender (Desvarieux et al., 2004; Grau et al., 2004; Volzke et al., 2006; Desvarieux et al., 2010) to be potential effect modifiers of the periodontal infection/AVD association. However, we are unaware of any studies that have reported statistically significant interactions between periodontal infections and either smoking status or gender for the prediction of incident clinical cardiovascular disease. As stated above, conducting the appropriate analyses for these tests generally requires large sample sizes and reasonable AVD event rates. Thus, additional research is required to determine whether smoking and gender are true effect modifiers.
The Appropriate Exposure Measure
Relative to the large body of literature that has accumulated on the topic of periodontitis-AVD associations, there has been very little methodological work to help inform and optimize the appropriate case definition of periodontitis that best reflects the infectious etiological agents that are hypothesized as the primary exposure of interest in this context (Fong, 2002).
The currently accepted paradigm on the pathogenesis of periodontal disease is based on the notion that bacterial colonization elicits an immune response by the host that, under additional conditions, may result in the manifestation of clinical disease. From an epidemiologic standpoint, there is a need for strong methodological research in this area so that bias related to misclassification of exposure status can be better understood and minimized. Bias of this nature is insidious but critically important. As has been demonstrated in simulation studies, misclassification of periodontal infection exposure status can yield substantially biased parameter estimates of periodontal infection-AVD associations (Dietrich and Garcia, 2005).
A majority of studies reporting on this field have used either clinical or radiographic measures of periodontal disease as a surrogate for periodontal infection. Clinical measures have included assessments of attachment loss, pocket depth, bleeding on probing, and even tooth loss, while radiographic measures have included linear or proportional assessments of alveolar bone loss. However, the definition of a “periodontitis case” has varied greatly across studies. Moreover, when oral infection is considered as a risk factor for AVD, restricting the exposure to a distinct clinical phenotype may be inappropriate. For example, attachment loss is not always the result of an infectious process, since, in certain instances, it may be due to trauma. Therefore, careful consideration of appropriate exposure definitions based on clinical periodontal measures is required.
Beck and Offenbacher (2002) were the first to publish data regarding appropriate exposure definitions in this context. Their approach helped to introduce two important concepts: (i) periodontal measures that are relevant in the context of exposure for AVD are possibly different from definitions used to classify the various clinical periodontal disease entities and periodontitis in particular; and (ii) when oral infection models are used to study infectious etiologies for AVD, clinical exposure definitions should be “predicated upon those clinical signs that best represent the underlying mechanisms and temporal sequence that may affect that systemic outcome.” Illustrating this point, they reported that attachment loss showed a weaker association with acute-phase systemic biomarkers of AVD risk than probing depth or bleeding on probing. Subsequent research has extended these initial findings by performing robust sensitivity analyses allowing for a side-by-side comparison of the association between systemic AVD risk markers and periodontal exposure definitions (Demmer et al., 2008a), and demonstrated that optimal definitions varied according to whether the particular marker represented an acute or chronic condition. For example, pocket depth was more strongly correlated with the acute-phase marker fibrinogen, while attachment and tooth loss tended to be more strongly correlated with the chronic marker hemoglobin A1c or carotid artery atherosclerosis.
Going beyond clinical definitions, several studies described above have used assessments of periodontal bacterial colonization as exposures associated with subclinical AVD (Desvarieux et al., 2005) or coronary heart disease outcomes (Renvert et al., 2006; Spahr et al., 2006; Nonnenmacher et al., 2007). No data are so far available addressing the association between colonization levels and stroke outcomes. Alternatively, at least 11 studies have now been published using serum antibody responses to periodontal bacteria as the main exposure of interest (Pussinen et al., 2003, 2004a,b, 2005, 2007a,b; Taniguchi et al., 2003; Beck et al., 2005a,b; Johansson et al., 2005; Lund Håheim et al., 2008). A meta-analysis including several of these studies (Mustapha et al., 2007) reported the overall trend to suggest a 36% increased risk for CVD outcomes associated with elevated systemic antibody responses, although the summary measure was not statistically significant (p = 0.09). When only CHD outcomes were considered, the findings were stronger and statistically significant: odds ratio 1.75 (95% CI, 1.32 to 2.34; p < 0.001).
While the aforementioned studies have attempted to relate CVD outcomes to bacterial exposures and systemic immune responses, there are persisting limitations in the exposure assessments used. For example, despite a substantial body of literature regarding the microbial etiology of periodontal diseases and the several hundreds of bacterial species that colonize the periodontal niche (Paster et al., 2001; Socransky and Haffajee, 2005), only a handful have been recognized as etiologic agents (Proceedings of the 1996 World Workshop in Periodontics), while the subset of microbiota investigated in most studies to date is largely biased toward cultivable species. Another important limitation in regard to bacterial exposure assessments is the high resource burden it puts on research studies. The development of high-throughput molecular techniques such as bacterial microarray platforms (Colombo et al., 2009) is likely to facilitate large-scale “periodontal microbiome” assessments in the future, but these data will be subject to analytical and statistical limitations, including issues related to multiple comparisons. Techniques that utilize knowledge about bacterial clusters (Socransky et al., 1998), as well as analytical techniques to combine key species across clusters, are likely to be part of the solution (Desvarieux et al., 2005; Demmer et al., 2008b), but more research in this area is necessary.
Likewise, the utilization of systemic antibody titers for measuring the degree of infectious exposure is also subject to limitations. Recent studies have demonstrated complex, species-specific associations between serum antibody to periodontal bacteria and clinical periodontal conditions, with certain high titers likely suggesting the presence of a protective adaptive response, with others being positively correlated with the severity of periodontitis (Dye et al., 2009). In turn, these observations complicate the interpretation of findings from studies using systemic antibody levels to define periodontitis-associated infectious exposure for AVD outcomes. Thus, it is unclear whether the few studies reporting inverse associations between antibody titer levels and AVD (Mustapha et al., 2007) are due to bias, chance findings, or true associations, the latter possibility suggesting that a robust antibody response to periodontal pathogens is protective against AVD. This hypothesis is intriguing when one considers the fact that among studies included in recent meta-analyses (Janket et al., 2003; Humphrey et al., 2008) utilizing mainly clinical measures of periodontal disease and clinical CVD outcomes, only one study has reported an inverse association (Tuominen et al., 2003). Furthermore, results from studies using exposure definitions based on antibody titers also need to consider the influence of partial or complete edentulism, since edentulous participants, despite a likely history of periodontal infection, tend to display lower antibody titers than their dentate counterparts (Vlachojannis et al., 2010). Beyond these issues, additional problems include the potential cross-reactivity among antibodies and the differential correlation of different immunoglobulin subclasses with chronic, cumulative bacterial stimuli (typically IgG responses) or more recent, transient exposures (better represented by IgA responses).
To date, no large-scale methodological studies have systematically assessed inter-relationships among clinical, microbial, and antibody population-based data to examine whether combinations of these measures may enhance the precision of exposure definitions and enhance the prediction of AVD-related outcomes.
Significance of AVD Outcomes
Evidence suggests that periodontal infections may be a stronger risk factor for ischemic stroke outcomes as compared with coronary outcomes. The most compelling data supporting this notion originate from three separate studies that have reported associations between periodontal disease and both coronary and stroke outcomes in the same populations, using the same exposure definitions. Specifically, Wu et al. (2000) reported strong associations between periodontal disease and stroke in NHANES I and its follow-up study, while Hujoel et al. (2000) reported weak/null associations for CHD. Accordingly, analysis of data published by Joshipura et al. from the Health Professionals Follow-Up Study reported positive associations for stroke (Joshipura et al., 2003), but weak/null findings for CHD (Joshipura et al., 1996). More recently, two publications mentioned above from the Normative Aging Study (NAS; Table 1) also corroborate that clinical periodontal disease may be more strongly associated with stroke (Jimenez et al., 2009) than CHD (Dietrich et al., 2008; Jimenez et al., 2009). Interestingly, analysis of the NAS data regarding periodontal disease and CHD did demonstrate a statistically significant association [HR(95%CI) = 2.12(1.26, 3.60)] among younger individuals (aged 21-59 years, Table 1). Accordingly, the NHANES I results reported by Hujoel et al. (2000) also indicated a positive statistically significant association between periodontal index and incident CHD [HR(95%CI) = 1.80 (1.04-3.10)] among the subgroup of participants aged 35-44 years. While these subgroup findings were not particularly relevant at the time of publication in 2000, they have become noteworthy in light of more recent trends suggesting age interactions as discussed above.
However, these trends were not confirmed by a recent meta-analysis (Mustapha et al., 2007) that included only studies that used exposure definitions based on systemic antibody responses to two periodontal bacteria (P. gingivalis and A. actinomycetemcomitans) and reported stronger associations between periodontal infection and CHD as opposed to stroke outcomes. In addition to the limitation of serological exposure definitions discussed above, this meta-analysis included only two studies reporting stroke outcomes, both of which also included hemorrhagic stroke (Pussinen et al., 2004a; Johansson et al., 2005). This is important, since the currently considered plausible association between periodontal infection and cerebrovascular disease is limited to atherogenic stroke. Future studies that can analyze results for stroke and CHD outcomes using multiple exposure definitions in the same population sample will be informative in this context.
Intervention Studies
Treatment studies of patients with periodontitis have contributed with additional insights into the potential link between periodontal infections and AVD, and may serve as an intermediate, translational link between the observational studies referenced above and the mechanistic studies reviewed below. Typically, these studies have examined whether periodontal therapy may induce favorable changes in markers of systemic inflammation or on surrogate markers of subclinical AVD, and point to potential biological pathways that can be modulated in vivo by periodontal therapy and may contribute to the promotion of an anti-atherogenic phenotype.
It has been well-established that multiple cytokines and inflammatory markers, including IL-1, IL-6, IL-8 and TNF, are abundantly produced locally in pathological periodontal tissues and can be recovered in gingival crevicular fluid (GCF) samples obtained from periodontally involved tooth sites (Ebersole, 2003; Lamster and Ahlo, 2007). It has been postulated that these locally produced inflammatory mediators are introduced into the bloodstream, although periodontitis has not been shown to induce a sustained elevation of plasma IL-1 beta (Mengel et al., 2002) or TNF-alpha (Meyle, 1993). Nevertheless, parallel to the induction of short-lived bacteremias, described under the review of potential mechanisms below, chronic periodontal infection has been shown to contribute to a state of systemic inflammation characterized by plasma elevation of acute-phase proteins such as CRP (Loos et al., 2000; Noack et al., 2001; Ebersole et al., 2002; Loos, 2005), inflammatory cytokines such as IL-6 (Loos et al., 2000), and coagulation factors such as fibrinogen (Loos, 2005).
A review of the intervention studies investigating the effect of periodontal therapy on plasma levels of inflammatory mediators reveals somewhat inconsistent findings. Patients treated by non-surgical periodontal therapy were shown to display a significant increase in plasma TNF-alpha, CRP, and IL-6 levels immediately after intervention, suggesting a systemic acute-phase response, possibly due to a massive bacterial inoculation in conjunction with the mechanical instrumentation of the periodontal tissues (D’Aiuto et al., 2004a; Ide et al., 2004; D’Aiuto et al., 2005b; Tonetti et al., 2007). Two small-scale studies (Ide et al., 2003; Yamazaki et al., 2005), the latter also involving surgical periodontal therapy followed by a short course of systemic antibiotics, reported no significant changes of serum levels of CRP, IL-6, or TNF-alpha 3 months after the completion of therapy. In contrast, a 6-month post-treatment follow-up of individuals enrolled in a single-arm intervention study (D’Aiuto et al., 2004b) involving conventional periodontal therapy demonstrated significant reductions in serum IL-6 (median decrease 0.2 ng/L, 95% CI 0.1-0.4 ng/L) and CRP (median decrease 0.5 mg/L, 95% CI 0.4-0.7). A subsequent pilot randomized control trial that compared mechanical periodontal therapy alone vs. identical therapy supplemented by local adjunctive antibiotics (D’Aiuto et al., 2005a) reported significant reductions in serum CRP and IL-6 in both treatment arms, and a significant reduction in total and low-density lipoprotein (LDL) cholesterol in the group that received adjunctive antibiotics. Yet, a larger randomized controlled trial by the same research group (Tonetti et al., 2007) reported no significant differences in post-treatment plasma levels of CRP, IL-6, and plasminogen activator inhibitor-1 (PAI-1) levels between the treatment and control groups at 6 months, although the treatment group experienced lower levels of serum-soluble E-selectin and lower neutrophil counts. The most recent available systematic review of six treatment studies investigating the effects of periodontal therapy (scaling and root planing, with or without adjunctive local or systemic antibiotics) on serum CRP levels (Paraskevas et al., 2008) concluded that there is modest evidence for a treatment-induced reduction (weighted mean difference of reductions of 0.50 mg/L (95% CI 0.08-0.93). Exploring further the apparent heterogeneity in the short-term post-treatment responses in serological inflammatory marker levels, Behle et al. (2009) used a composite score (Summary Inflammatory Score) to represent the aggregate post-treatment response to a panel of 19 individual biomarkers. These investigators demonstrated that approximately one-third and one-fourth, respectively, of the treated patients showed a marked reduction or a pronounced increase in systemic inflammation, while the remainder remained seemingly unchanged. Interestingly, periodontal therapy resulted in significant differential regulation of multiple genes expressed in peripheral blood monocytes, especially in genes related to innate immunity, apoptosis, and cell signaling, in a manner compatible with the promotion of an anti-atherogenic phenotype (Papapanou et al., 2007). Thus, although it appears that the above studies indicate a general trend toward a treatment-induced suppression of systemic inflammation, the effects of therapy on specific markers are not entirely consistent across studies, and their sustainability over time has not been convincingly established.
Another group of studies has addressed the effect of periodontal therapy on endothelial dysfunction, which is characterized by reduced vasodilator capability of peripheral blood vessels (Verma et al., 2003) and is assessed by measurement of the difference in diameter of a peripheral artery prior to and after reactive hyperemia induced through occlusion of blood flow (Celermajer et al., 1992). Endothelial dysfunction has been shown to be associated with adverse long-term outcomes of coronary artery disease in at-risk patients or patients with early stages of AVD (Schachinger et al., 2000; Suwaidi et al., 2000; Perticone et al., 2001). Association studies have established that endothelial dysfunction is more pronounced in patients with periodontitis when compared with periodontally healthy control individuals (Amar et al., 2003; Mercanoglu et al., 2004). This is thought to occur due to “endothelial stunning”, i.e., a transient reduction in endothelium-dependent dilatation (EDD) when endothelial cells are challenged by bacterial products such as endotoxins (Bhagat et al., 1996). Numerous periodontal treatment modalities have been shown to improve EDD in small-sized (N range, 22-30) single-arm intervention studies involving mechanical (non-surgical and surgical) periodontal therapy alone (Mercanoglu et al., 2004; Elter et al., 2006) or supplemented by systemic antibiotics (Seinost et al., 2005). Recently, a randomized controlled trial involving a total of 120 patients with severe periodontitis, 61 of whom received full-mouth subgingival debridement completed within a single session and accompanied by extensive application of local antibiotics in all deep periodontal pockets (Tonetti et al., 2007), demonstrated a significant improvement in EDD in the treatment group at a 6-month follow-up examination. Notably, this intense intervention resulted in a transient deterioration of EDD and a significant increase in multiple inflammatory mediators in the plasma immediately after the intervention.
Extending the observations of several association studies demonstrating that severe periodontitis (Beck et al., 2001), high subgingival colonization levels by specific periodontal pathogens (Desvarieux et al., 2005), and high serum IgG titers against individual periodontal bacteria (Beck et al., 2005b) are significantly related with increased carotid artery IMT in adjusted analyses, a recent pilot study suggested that mechanical treatment of mild to moderate periodontitis in otherwise healthy individuals may result in a significant reduction of carotid artery IMT 12 months after completion of treatment (Piconi et al., 2009).
To date, only a single, multi-center pilot study has examined the effects of periodontal therapy on the secondary prevention of cardiac events. The Periodontitis and Vascular Events (PAVE) study (Beck et al., 2008; Offenbacher et al., 2009) randomized patients with periodontitis and a history of severe CVD to either community care or a study protocol that consisted of oral hygiene instruction and mechanical periodontal therapy. Over a 25-month follow-up period, cardiovascular adverse events occurred with similar frequency in the community control and the periodontal treatment groups, but the periodontal therapy administered resulted in a rather limited improvement of periodontal status at 6 months after the intervention, and these positive effects were not sustainable at the one-year follow-up. What further complicates the interpretation of the findings of this study is the fact that a substantial proportion of the individuals randomized in the community care group did receive some form of preventive or periodontal care outside the study. Last, obesity appeared to nullify the effects of periodontal treatment on a reduction in serum CRP levels. Important lessons were thus learned by this pilot trial that will inform the design of future randomized controlled trials with respect to: (i) the required intensity of the protocol-provided periodontal intervention to result in clinically and biologically meaningful and sustainable positive effects on the periodontal status; (ii) the role of co-existing risk factors for AVD that may negate the treatment-induced positive modulation of systemic inflammation; and (iii) the overall feasibility of the study design.
Potential Mechanisms Linking Periodontal Infections and Atherosclerosis
In this section, we will review recent publications on the potential pathogenic mechanisms mediating the direct or indirect effects of periodontal infections on the initiation and perpetuation of AVD, as well as the plausible biological pathways that may account for the observed systemic effects of periodontal therapy. Delineation of the mechanisms and pathways that link oral infections to atherogenesis is critical for establishing causality, for the identification of patient populations that may benefit from periodontal intervention in the context of prevention/arrest of AVD, and for the design of relevant treatment strategies.
To facilitate an understanding of the various potential mechanisms involved in different stages of atherogenesis, we have summarized the most relevant pathways in three schematic figures that depict critical events in the development of AVD: Fig. 1 illustrates the potential role of periodontal pathogens and their products in the development of endothelial dysfunction; Fig. 2 summarizes their potential contributions to the formation of fatty streaks and atherosclerotic plaques; and finally, Fig. 3 illustrates potential pathways that modulate the maturation of atheromatic plaques and facilitate their rupture and vascular thrombosis. For a detailed review of the pathogenesis of AVD, the reader is referred to the article by Libby (2002).
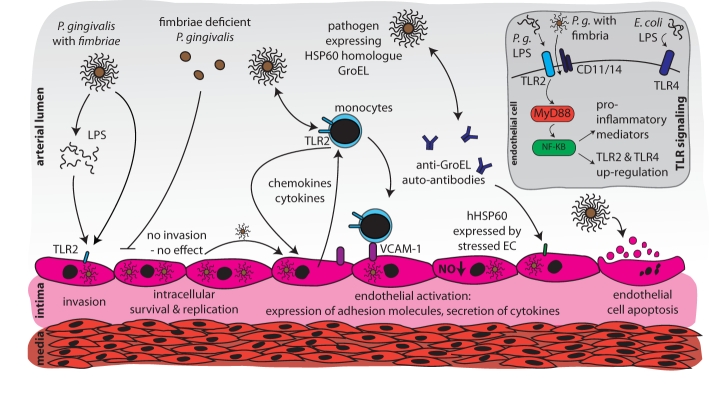
Figure 1.
Schematic overview of potential mechanisms linking periodontal infections and endothelial dysfunction/incipient atherosclerosis. Vascular endothelial cells are invaded by fimbriated pathogens, e.g., P. gingivalis. These pathogens can persist and multiply intracellularly. Activation of Toll-like receptor 2 (TLR2) by fimbriated bacteria or LPS results in release of pro-inflammatory mediators and up-regulation of cell adhesion molecules. Monocytes are recruited by a gradient of chemotactic cytokines, such as MCP-1. Antibodies against bacterial heat-shock proteins, such as HSP60-related GroEL, auto-react with mammalian HSP60 expressed by activated endothelium, resulting in cell destruction. Further, P. gingivalis induces apoptosis of endothelial cells.
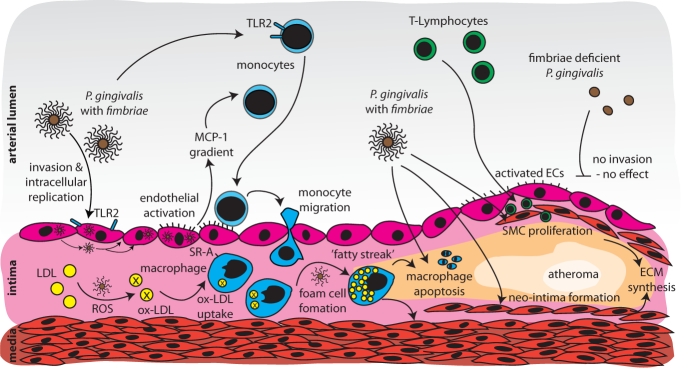
Figure 2.
Potential mechanisms linking periodontal infections and fatty-streak formation/plaque maturation. Monocytes activated by periodontal pathogens chemotactically migrate into the sub-endothelial space, and transform into macrophages and, subsequently, into foam cells after uptake of oxidized LDL. Apoptosis of LDL-laden macrophages results in accumulation of lipids in the sub-endothelial space. Furthermore, periodontal pathogens induce smooth-muscle-cell proliferation in the intima and neo-intima formation. Extracellular matrix build-up and extravasation of T-cells consummate the formation of a fibrous cap covering the plaque.
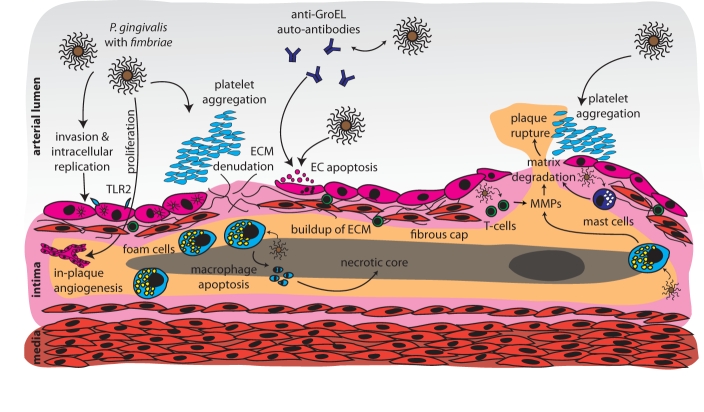
Figure 3.
Potential mechanisms linking periodontal infections to mature atherosclerotic plaques and plaque rupture. Pathogen-mediated in-plaque angiogenesis is a hallmark of plaque organization. Denudation of the fibrous cap and its pro-thrombotic components occurs after endothelial cell apoptosis mediated by whole periodontal pathogens, or anti-endothelial auto-antibodies. Plaque rupture is induced by pathogen-mediated extracellular matrix degradation by endothelial cells, plaque macrophages, T-cells, and plasma cells, leading to exposure of pro-thrombotic plaque components, and subsequent vessel occlusion.
The Role of Bacteremias
Entry of oral bacteria and/or bacterial products into the bloodstream [recently reviewed by Iwai (2009)] is thought to be one of the key initiators of biological events that link oral infections to AVD. Transient bacteremias are common after dental procedures, regardless of periodontal status (Olsen, 2008), occurring frequently after mastication or after personal oral hygiene (Lockhart et al., 2008; Crasta et al., 2009). The incidence and intensity of these bacteremias correlate positively with the extent and severity of periodontitis (Kinane et al., 2005; Forner et al., 2006) and are in line with histopathologic observations demonstrating disruption of the epithelial integrity of the periodontal pocket, a sizeable ulcerated surface amounting to up to 8 to 20 cm2 (Hujoel et al., 2001), and the proximity of highly vascularized tissue to the subgingival biofilm (Nanci and Bosshardt, 2006).
Oral and periodontal bacteria have been occasionally incriminated as causative for infections at distant organs, including the lung (De Soyza et al., 2000), the central nervous system (Ewald et al., 2006; Mueller et al., 2009), or endovascular protheses (Grace et al., 1988), suggesting that they are able to establish themselves at extra-oral locations. A great many studies have thus evaluated whether bacteria of oral or periodontal origin are detectable, retrievable, and cultivable from atherothrombotic plaques or vascular biopsies. Bacterial DNA from several periodontal pathogens has been detected in human endarterectomy specimens by PCR (Haraszthy et al., 2000; Stelzel et al., 2002; Fiehn et al., 2005; Ford et al., 2005; Kozarov et al., 2006; Padilla et al., 2006; Nakano et al., 2007; Pucar et al., 2007; Nakano et al., 2008; Gaetti-Jardim et al., 2009), by a combination of anaerobic culture and subsequent PCR identification (Padilla et al., 2006), by checkerboard DNA-DNA hybridizations (Zaremba et al., 2007), or by fluorescence in situ hybridizations (FISH) (Cavrini et al., 2005). Furthermore, viable A. actinomycetemcomitans and P. gingivalis were recovered and cultured from human atheromatous plaques originating from a patient with periodontal disease (Kozarov et al., 2005). Two studies have so far failed to detect periodontal pathogen DNA in atheromatous plaques by PCR (Cairo et al., 2004; Romano et al., 2007), but given the majority of positive studies, it appears that the assumption that periodontal pathogens may disseminate through the circulation and localize within atheromatic lesions is likely correct. It must be recognized, however, that this is likely not an exclusive property of periodontal pathogens. For example, Nakano et al. (2006) used PCR and subsequent sequencing, and identified Streptococcus mutans DNA in 74% of the investigated atheromatous plaque samples, while DNA from other species, including periodontal pathogens, was detected at far lower frequencies and levels. Thus, it appears that the disruption of the pocket epithelial integrity that occurs in periodontitis may also provide a point of entry for non-periodontal pathogens, such as the highly prevalent S. mutans in caries-affected dentitions. A recent study utilizing 16S rRNA sequencing identified 98 different bacterial species in the peripheral blood from 151 individuals with bacteremia; of these, 19 species were novel (Bahrani-Mougeot et al., 2008). Given that the oral microbiome is comprised of approximately 700 species (Parahitiyawa et al., 2009), it is highly unlikely that the effects of periodontal infections on AVD are mediated by the limited number of periodontal pathogens studied so far.
Bacteria/Bacterial Products and Key Atherogenesis-promoting Processes
In the following text, we briefly summarize recent findings describing interactions of oral bacteria and bacterial products with specific host cells involved in the atherosclerotic process. The majority of the reviewed studies are primarily in vitro investigations of the effects of specific mono-infections on cultured host cells. Experimental animal studies and relevant data from human studies are presented whenever available. A critical appraisal of the limitations of these studies is offered at the end of this section.
Vascular Endothelial Activation
Upon entering the bloodstream, bacteria are rapidly cleared by host immune cells. To survive and elicit effects at distant sites, they have evolved several host-evasion strategies that have been investigated in multiple in vitro studies with P. gingivalis as a model periodontal pathogen. One such strategy is the ability of this micro-organism to invade vascular endothelial cells (Deshpande et al., 1998; Dorn et al., 1999; Progulske-Fox et al., 1999). Within the endothelial cell, the survival of P. gingivalis depends on the concurrent activation of autophagy and suppression of apoptosis, which provides an intracellular niche where the pathogen can replicate unobstructed by host immune responses. In fact, repression of autophagy by chemical inhibitors, such as wortmannin, results in transition of the bacteria to the phagolysosome and subsequent degradation (Bélanger et al., 2006).
P. gingivalis’ invasion of endothelial cells is dependent on fimbriae and a specific hemagglutinin (Takahashi et al., 2006). Infection of human aortic endothelial cells by the invasive P. gingivalis strain 381 results in up-regulation of the chemokine IL-8, adhesion molecules such as ICAM-1, VCAM-1, and E-selectin, and cyclo-oxygenase-2 (COX-2). In contrast, corresponding expression profiles in cells infected with a non-invasive fimA mutant remain largely unchanged (Chou et al., 2005). Thus, fimbriae appear to be critical for both the invasive and pro-atherogenic properties of P. gingivalis. Induction of IL-6 in vascular endothelial cells has also been shown to be a process dependent on fimbriae, nuclear factor kappa B (NF-κB), and meiosis-specific kinase 1, which is regulated by the autocrine IL-6 signal transducer gp130 (Ho et al., 2009). Again, it should be noted that in vitro invasion of endothelial cells is not an exclusive feature of specific periodontal pathogens, since S. mutans has also been shown to invade human aortic endothelial cells and to persist intracellularly over prolonged periods of time (Abranches et al., 2009).
Nevertheless, the infection-induced effects on endothelial cells also show species-specific variation. For example, expression of the chemokine monocyte chemoattractant protein-1 (MCP-1), an important regulator of monocyte migration from the vessel lumen to the sub-endothelial space, was strongly induced in human umbilical vein endothelial cells after P. gingivalis infection, while it was minimally up-regulated after infection with T. forsythia and virtually unaffected after infection by T. denticola (Niu and Kolattukudy, 2009). Induction of MCP-1 expression was attenuated by inhibition of several pathways, including the mitogen-activated (MAP) kinase, NF-κB, c-Jun N-terminal-kinase (JNK), and activator-protein 1 (AP-1) pathways (Choi et al., 2005). P. gingivalis infection resulted in activation of NF-κB and AP-1.
In addition to whole bacteria-endothelial cell interactions, studies have examined the effects of specific bacterial products such as microbial proteases and lipopolysaccharide (LPS). Arginine-specific gingipain, a P. gingivalis-specific protease (Fitzpatrick et al., 2009), increased the responsiveness of endothelial cells to live P. gingivalis and P. gingivalis LPS, by inducing Weibel-Palade body exocytosis through activation of protease-activated receptors (PARs). Weibel-Palade bodies are vesicles in endothelial cells that store vaso-active substances, such as angiopoietin-2, which may enhance IL-8 production by LPS-stimulated cells (Inomata et al., 2007). Another class of biologically active bacterial products investigated in this context is outer membrane vesicles (OMVs), i.e., vesicles ‘budding off’ from growing bacterial cells and comprising a protein fraction and LPS. P. gingivalis OMVs were found to impair growth and tube formation in human umbilical vein endothelial cells, an effect mediated by the protein fraction of OMVs, since it was effectively inhibited by heat inactivation (Bartruff et al., 2005). In addition, a free-soluble surface material, released by A. actinomycetemcomitans grown either in a biofilm or in a planktonic form, was found to induce production of several pro-inflammatory cytokines in human whole blood. Interestingly, the effect was only partially LPS-dependent, as shown in LPS blocking experiments with polymyxin B, and did not depend on the presence of A. actinomycetemcomitans toxins, including the cytolethal distending toxin, leukotoxin, or peptidoglycan-associated lipoprotein (Oscarsson et al., 2008). Since both OMVs and free-soluble surface material are abundantly produced locally in the plaque biofilm, their potential entry into the circulation may constitute a significant source of inflammatory stimulants along with the planktonic bacteria in the bloodstream.
Induction of apoptosis in vascular endothelial cells, a hallmark of developing endothelial dysfunction (Hotchkiss et al., 2009; Pober et al., 2009), is another bacterial strategy of critical importance in atherogenesis. P. gingivalis gingipains were shown to induce cell adhesion molecule cleavage, detachment, and apoptotic cell death in bovine coronary artery endothelial cells (Sheets et al., 2005, 2006). Given that the effector enzymes in apoptosis are caspases, a family of cysteine proteases (Pop and Salvesen, 2009), these effects could efficiently be blocked by pre-incubation with cysteine-protease inhibitors. Interestingly, both gingipains (Sheets et al., 2005, 2006) and whole P. gingivalis (Desta and Graves, 2007) were also able to induce caspase-independent programmed cell death, as shown with the irreversible caspase inhibitor z-VAD-fmk. However, apoptosis in endothelial cells was strongly dependent on the relative proportion of planktonic pathogens to cultured cells, i.e., the multiplicity-of-infection (MOI), with invasive P. gingivalis consistently inducing apoptosis at MOI ranging between 500 and 1000, but not at MOI between 50 and 100 (Roth et al., 2007a).
Conversely, the potential ability of periodontal bacteria or their products to induce vascular cell proliferation is also relevant in the context of atherogenesis, since smooth-muscle-cell proliferation results in thickening of the vessel media, and endothelial proliferation is needed for local angiogenesis within atheromatous plaques. Interestingly, only cell-free products of P. gingivalis, but not whole bacteria, induced proliferation of aortic smooth-muscle cells in vitro after pre-incubation with human plasma (Inaba et al., 2009). Proliferation could not be induced with live P. gingivalis in the absence of plasma, and was shown to be dependent on the up-regulation of S100 calcium-binding protein A9, a hitherto-unrecognized proliferation factor. These findings are important, since they indicate that the presence of live bacteria is seemingly not required for the induction of these proliferative effects that may be mediated by OMVs or free-soluble surface material. P. gingivalis-mediated proliferation in human endothelial cells, including tube formation, and angiogenesis in matrigel plugs was found to be dependent on activation of the mitogen-activated extracellular signal-regulated kinase-1 and -2 (ERK1/2) (Koo et al., 2007). Similarly, E. corrodens was found to possess angiogenic, proliferative, and pro-inflammatory effects on endothelial cells utilizing a MAPK-dependent mechanism (Yumoto et al., 2007).
Interactions with Monocytes/Tissue Macrophages
Interactions of periodontal bacteria with other host cells that participate in atherogenesis have included studies involving monocytes, which are central to the formation of fatty streaks (see Fig. 2) (Webb and Moore, 2007). Roth et al. (2007b) observed an increased adhesion of monocytes to human aortic endothelial cells infected with invasive P. gingivalis when compared with adhesion to non-infected controls, or to cells infected with a fimbriae-deficient P. gingivalis mutant, mediated by elevated expression of adhesion molecules and chemotactic cytokines in the endothelial cells. Complementing these observations, infection of monocytes with invasive strains of P. gingivalis enhanced migration and elicited the expression of the pro-inflammatory cytokines TNF-alpha and IL-6, whereas infection by the fimbriae-deficient mutant had virtually no effect (Pollreisz et al., 2010). Likewise, monocyte infection with invasive P. gingivalis strains promoted enhanced LDL-uptake and foam cell formation to a greater extent than infection with a non-invasive fimbriae-deficient mutant (Giacona et al., 2004). Interestingly, similar experiments with A. actinomycetemcomitans LPS suggest that the presence of whole bacterial cells is not necessary for these effects. Indeed, LPS-challenged monocyte-derived macrophages showed enhanced secretion of TNF-alpha and interleukin-1beta and induction of foam cell formation and accumulation of LDL. LPS stimulation also decreased mRNA levels of scavenger receptor B, and ATP-binding cassette transporter-1, i.e., of two receptors that mediate the efflux of cholesterol from macrophages (Lakio et al., 2006). These results are in agreement with findings from a recent in vivo study that demonstrated that sub-acute endotoxinemia resulted in a significantly impaired reverse cholesterol transport independent of plasma HDL levels (McGillicuddy et al., 2009).
Pro-thrombotic and Pro-coagulant Effects
Platelets can be activated either by direct interaction of pathogens or their products, or indirectly via the vascular endothelium (Jennings, 2009). Several recent in vitro studies addressed direct and indirect effects of the model organism P. gingivalis on platelet aggregation.
P. gingivalis induces platelet aggregation via a TLR2-dependent mechanism, since its pro-coagulant properties were effectively blocked by pre-treatment with a TLR2-blocking antibody, or by inhibition of the downstream phosphoinositide 3-kinase (PI3-K)/Akt signaling pathway activated by TLR2 (Blair et al., 2009). Platelet aggregation in plasma was shown to depend on the adhesion molecule Hgp44 and the P. gingivalis protease Lys-gingipain (Kgp), but not on active Arg-gingipain (Rgp) (Naito et al., 2006). Importantly, in experiments with an MOI below the required threshold to activate platelet aggregation, P. gingivalis had a sensitizing effect on human platelets, enhancing epinephrine-induced aggregation. This effect was attributed to a limited activation of protease-activated receptors (PARs) on the platelet surface by gingipains, with subsequent mobilization of Ca2+, leading to a marked coagulant response to epinephrine binding to the alpha(2) adrenergic receptor (Nylander et al., 2008).
Evidence of indirect pathogen-induced promotion of a pro-thrombotic state has been provided in studies of the interactions of P. gingivalis and vascular endothelial cells and smooth-muscle cells. P. gingivalis gingipains induced hydrolysis of platelet endothelial cell adhesion molecule 1 (PECAM-1/CD31), suggesting ability to enhance vascular permeability (Yun et al., 2005). Infection of endothelial cells with invasive P. gingivalis strains resulted in enhanced tissue factor expression and activity, suppressed levels of tissue factor inhibitor, decreased levels and activity of tissue plasminogen activator, and increased plasminogen activator inhibitor-1 antigen levels (Roth et al., 2006). Interestingly, these effects were most prominent at later time-points, suggesting that they are due to downstream intracellular pathways triggering pro-coagulant mechanisms. In aortic smooth-muscle cells, whole P. gingivalis, but not P. gingivalis LPS, induced a pro-thrombotic phenotype by down-regulation of tissue factor pathway inhibitor (Roth et al., 2009). P. gingivalis arginine- and lysine-specific gingipains induced degradation of vascular endothelial cell thrombomodulin in vitro, an observation corroborated by the reduced expression of thrombomodulin in the gingival microvascular endothelia of patients with periodontitis (Inomata et al., 2009).
Recent human in vivo studies support and extend these observations. A comparison of platelet-activating factor (PAF) levels in sera and GCF from patients with periodontitis, patients with coronary heart disease (CHD) without periodontitis, patients with periodontitis and CHD, and healthy control individuals showed significantly higher serum and GCF levels in all patient groups when compared with levels in control individuals (Chen et al., 2009). In another case-control study (Papapanagiotou et al., 2009), significantly elevated soluble P-selectin, a marker of platelet activation, was documented in the plasma of patients when compared with periodontitis-free control individuals. Furthermore, platelets from periodontitis patients showed an increased binding of the glycoprotein IIb-IIIa complex, a direct measure of platelet activation, which correlated positively with the extent and severity of periodontitis of the donor. The same research group (Nicu et al., 2009) stimulated platelets and leukocytes from periodontitis patients and periodontally healthy control individuals with four oral bacteria (A. actinomycetemcomitans, P. gingivalis, Tannerella forsythia, and Streptococcus sanguis) and reported higher platelet expression of P-selectin, and increased formation of platelet-monocyte complexes in periodontitis donors. Furthermore, platelet/monocyte complexes displayed a better ability to bind and phagocytose A. actinomycetemcomitans, suggesting that increased atherothrombosis was paralleled by enhanced bacterial clearance.
It thus appears that: (i) there is cross-sectional evidence of increased platelet activation in periodontitis patients; (ii) this activation can be attributed to either direct effects of a periodontal “model” organisms on platelets, or indirect, pro-thrombotic effects on vascular endothelial and smooth-muscle cells; and (iii) the pro-thrombotic/pro-coagulant state also appears to serve as an antimicrobial defense.
Finally, a conceivable alternative pathway by which periodontal infections may exert pro-thrombotic effects is by means of pathogen-mediated apoptosis of vascular endothelial cells, a key event in the development of endothelial dysfunction, which exposes the basement membrane and allows for interaction of platelets with collagen, resulting in local thrombosis (Bombeli et al., 1997). Activated endothelium by either direct interaction with periodontal pathogens, or via systemic inflammatory molecules, is known to express tissue factor (thrombokinase), an important mediator of thrombin formation (Pober et al., 2009). However, tissue factor expressed on vascular endothelium is often ‘encrypted’, i.e., is rendered ineffective by post-translational modification and, thus, is unable to exert its pro-coagulant function (Bach, 2006). Apoptosis of endothelial cells can ‘decrypt’ tissue factor by increasing calcium concentrations and proteolytic cleavage and thereby can trigger thrombosis, even when the basement membrane is not uncovered by endothelial desquamation (Greeno et al., 1996).
It is important to note that thrombosis induction is not an exclusive feature of periodontal pathogens. In fact, it has been well-established that other oral bacteria, including Streptococcus species (Herzberg et al., 2005), can also induce platelet aggregation.
Oral Bacteria and Atheromatic Plaque Disruption
A role of bacteria and bacterial products is also conceivable in plaque disruption, one of the final and critically important events in atherosclerosis that is caused either by rupture of the fibrous cap of an unstable plaque, leading to exposure of the pro-thrombotic contents of the plaque, or through plaque erosion by apoptosis, triggering local thrombosis (Virmani et al., 2006; Libby, 2009; Ward et al., 2009) (see Fig. 3.). These events result in the clinical presentation of atherosclerotic vascular disease in the form of a myocardial or cerebrovascular infarction. Degradation of fibrous caps is mediated by matrix metalloproteinases (MMPs) produced within the plaques by macrophages (Galis et al., 1994; Sukhova et al., 1999). P. gingivalis and other periodontal bacteria (Ding et al., 1995), including P. intermedia (Guan et al., 2009), have been reported to induce production of several MMPs in different cell types, including macrophages and endothelial cells, while at the same time reducing the expression of the MMP antagonist tissue inhibitor of MMPs (TIMPs) (Sato et al., 2009). In vitro, P. gingivalis was shown to degrade fibrous cap material isolated from human autopsy plaque samples (Kuramitsu et al., 2001). The pro-apoptotic effects of periodontal pathogens and their products were discussed above and can also contribute to plaque erosion.
Last, the reported pro-inflammatory effects of A. actinomycetemcomitans in human mast cells (Oksaharju et al., 2009) may also be relevant in this context. Although mast cells are uncommon in vascular tissues, they do localize in atherosclerotic plaques and particularly in shoulder regions of rupture-prone plaques (Lindstedt et al., 2007). Activation of this cell population and subsequent production of levels of proteases capable of destabilization of atheromatic plaques correlate with intra-plaque hemorrhage, endothelial cell and macrophage apoptosis, and vascular leakage (Bot et al., 2007). In vivo, mast-cell-deficient mice showed increased collagen content and fibrous cap development, and decreased atherosclerosis (Sun et al., 2007). Activation of mast cells may thus be a possible route of bacterially triggered plaque rupture, although the available data are sparse.
Activation of Innate Immune Signaling Associated with Atherosclerosis by Periodontal Bacteria
Accumulating evidence suggests that periodontal pathogens and their bacterial products may exert pro-atherogenic effects in vascular endothelial cells via Toll-like receptors (TLRs) and other pattern recognition receptors (PRRs). These primary receptors of the innate immune system recognize highly conserved pathogen-associated molecular patterns (PAMPs). Activation of TLRs and their downstream signaling pathways leads to cellular activation and a specific response to microbial infection. Expression of TLRs is strongly induced in endothelial cells and macrophages in atherosclerotic lesions (Xu et al., 2001; Edfeldt et al., 2002), but exposure of in vitro cultured endothelial cells to laminar flow down-regulated TLR2 (Dunzendorfer et al., 2004). Patients suffering from chronic inflammatory diseases show higher B-cell expression of TLR2 and TLR4 (Jagannathan et al., 2009), while these receptors are also induced on monocytes in diabetes (Devaraj et al., 2008). Deficiency in MyD88, a central downstream signaling molecule for most TLRs, was shown to result in decreased atherosclerosis in vivo (Bjorkbacka et al., 2004). Similarly, deletion of TLR2 (Mullick et al., 2005) and TLR4 (Michelsen et al., 2004) in mice had an atheroprotective effect, suggesting that agonists of these receptors play a role in advancing atherosclerosis (Erridge, 2008). However, a recent study utilizing an in vitro model of atherosclerosis found that only blockade of TLR2, but not of TLR4, resulted in significantly attenuated levels of inflammatory mediators and tissue-degrading MMPs (Monaco et al., 2009).
Interestingly, P. gingivalis LPS appears to interact with different TLRs in a cell-type-dependent manner (Kocgozlu et al., 2009). For example, activation of human vascular endothelial cells, monocytes, and mouse macrophages by P. gingivalis was not mediated by TLR4, the typical receptor interacting with LPS from Gram-negative bacteria, but by TLR2 (Triantafilou et al., 2007; Hajishengallis et al., 2008). As discussed above, P. gingivalis mediates its own uptake as a host defense evasion strategy, by interacting with TLR2 and the integrins CD11b/CD18 (Harokopakis and Hajishengallis, 2005). In fact, P. gingivalis fimbriae interacting with human monocytes and mouse macrophages induce CXCR4/TLR2 co-association in lipid rafts, i.e., cholesterol-enriched micro-domains involved membrane fluidity and cellular trafficking, which in turn inhibit TLR2-mediated pro-inflammatory and antimicrobial responses. Cholesterol depletion inhibits pathogen uptake and attenuates pathogen-induced signaling, suggesting that hypercholesterolemia may result in accelerated TLR2-mediated atherosclerosis (Wang and Hajishengallis, 2008). TLR2 deletion in a mouse model of periodontitis resulted in a significant attenuation of periodontitis-aggravated atherosclerosis (Liu et al., 2008), supporting a predominant role for this receptor in atherogenesis.
Stimulation of human aortic endothelial cells by P. gingivalis fimbriae resulted in increased expression of both TLR2 and TLR4, resulting in sensitization of these cells to enterococcal LPS that acts via TLR4 (Yumoto et al., 2005). These observations suggest the synergistic potential of multiple bacterial stimuli in eliciting enhanced pro-atherogenic responses in vascular endothelial cells. P. gingivalis LPS stimulation of human coronary-artery-derived endothelial cells, but not of venous endothelial cells, resulted in increased expression of TLR2, and an induction of IL-8 secretion, E-selectin expression, and increased monocyte adhesion (Erridge et al., 2007; Kocgozlu et al., 2009). Thus, the selective pathogen-induced expression of TLR2 in arterial endothelial cells supports its involvement in atherogenesis.
Autoimmune Responses to Periodontal Bacteria
Molecular mimicry is a term used to describe the possibility that antibody responses targeted against bacterial antigens can essentially function as autoimmune responses, due to the high degree of homology between specific bacterial antigenic peptides and mammalian proteins, and has been considered as a biologically plausible mechanism linking infection and atherosclerotic vascular disease (Wick et al., 1999; Epstein et al., 2000; Lamb et al., 2003; Rajaiah and Moudgil, 2009). Central to this notion is a family of highly conserved heat-shock proteins (HSPs) (Fink, 1999), which is expressed on certain bacterial membranes, as well as by eukaryotic cells when exposed to stress (Polla, 1988). Bacterial HSPs are considered major antigenic determinants (Kaufmann, 1990) that elicit antibodies and specific reactive T-cells (Young and Elliott, 1989) that can cross-react with host cells expressing homologous molecules, resulting in auto-aggressive destruction (Mayr et al., 1999).
The mammalian host-protective heat-shock protein 60 (hHSP60) can be induced in vascular endothelial cells, macrophages, and smooth-muscle cells by several pro-atherogenic stimuli, including bacterial endotoxin, oxidated lipids, inflammatory mediators, hypertension, or mechanical shear stress (Seymour et al., 2007; Galkina and Ley, 2009). Although hHSP60 is not a trans-membrane protein and is thus seemingly unreachable by circulating antibodies, it synergizes with mitochondrial HSP70 (mtHSP70), which is expressed on the surfaces of stressed cells. hHSP60 and mtHSP70 associate within lipid rafts, resulting in endothelial activation with up-regulation of adhesion molecules and pro-inflammatory mediators, or in programmed cell death in endothelial cells (Alard et al., 2009), and in cytokine production by macrophages (Van Eden et al., 2007).
A high degree of homology between HSP from C. pneumoniae, a pathogen implicated in AVD, and that of human HSPs has been demonstrated (Kol et al., 1998; Huittinen et al., 2002). Likewise, high homology was found between the P. gingivalis HSP60—termed GroEL—and mammalian HSP60 family members (Maeda et al., 1994). P. gingivalis GroEL was shown to be highly immunogenic, and was recognized by serum antibodies isolated from patients suffering from periodontal disease (Ford et al., 2005, 2006).
In comparisons of antibody titers to hHSP60 and GroEL in patients with atherosclerosis and periodontal disease, systemically healthy periodontitis patients, and healthy control individuals, the highest titers for both human and bacterial HSP60 were found in patients with both conditions, followed by the systemically healthy group with periodontitis, while the control group showed the lowest antibody levels. In addition, clonal analysis of the T-cells found both hHSP60- and GroEL-reactive populations in the circulation of atherosclerosis patients. These T-cell populations were also found in atherosclerotic plaques in several patients (Yamazaki et al., 2004).
The effects of auto-antibodies to pathogens were evaluated in a series of in vivo studies in mice. In ApoE-deficient mice immunized with intra-peritoneal injections of live P. gingivalis and/or C. pneumoniae, only P. gingivalis inoculations had a pro-atherogenic effect. A positive correlation of atherosclerotic lesion development and anti-GroEL titers was found, as well as hHSP60 in the lesions themselves (Ford et al., 2007). The presence of circulating anti-HSP60 auto-antibodies resulted in an increased expression of P-selectin and von-Willebrand factor (vWF), with an altered morphology of endothelial cells in uninjured carotid arteries. In a model of injured carotid arteries, auto-antibodies promoted thrombosis and recruitment of inflammatory cells (Dieude et al., 2009).
Interestingly, H. pylori, a gastrointestinal tract pathogen that expresses HpHSP60, a HSP60 family member similar to GroEL, was recently found to induce atherosclerosis in mice. Subcutaneous immunization with HpHSP60, or eradication of H. pylori infection by the use of antibiotics, resulted in significantly reduced atherosclerosis, decline of Th1-immune response, and reduction of T-cell chemotaxis beyond the endothelium (Ayada et al., 2009).
Induction of Oxidative Stress by Periodontal Pathogens—Role of ox-LDL
A potential pathway through which periodontitis may contribute to atherogenesis is through induction of oxidative stress. Oxidation of LDL via reactive oxygen species (ROS) is a prerequisite for cholesterol uptake by macrophages and the formation of foam cells (Stoll and Bendszus, 2006; Itabe, 2009), but also results in several additional pro-atherogenic effects (Verhoye and Langlois, 2009). Thus, apart from its involvement in the formation of fatty streaks, ox-LDL also affects the vascular endothelium both directly and indirectly. Direct effects include the induction of cellular activation and apoptosis by interaction with lectin-like oxidized low-density lipoprotein receptor (LOX-1) (D Li et al., 2002; Ma et al., 2006). Indirect effects are exerted through down-regulation of the expression of endothelial nitric oxide synthase (eNOS), which results in increased production of ROS, ongoing LDL oxidation, and endothelial dysfunction (Victor et al., 2009). Ox-LDL also inhibits differentiation and induces apoptosis (D Li et al., 2002; Ma et al., 2006) of endothelial progenitor cells (EPCs), a subpopulation of bone-marrow-derived stem cells that participates in vascular repair (Friedrich et al., 2006; Wassmann et al., 2006; Zenovich and Taylor, 2008). Low levels of EPCs in the peripheral blood were found in states of poor vascular health (Werner et al., 2007), and were predictive of adverse cardiovascular outcomes (Werner et al., 2005). Last, it has been established that ox-LDL up-regulates pro-atherogenic chemokines and adhesion molecules via the CD40/CD40L pathway (Li et al., 2003) and triggers IL-6, TNF-alpha, and CRP secretion (Hulthe and Fagerberg, 2002).
In in vitro studies with P. gingivalis as a model organism, incubation of whole bacteria with blood resulted in higher levels of oxidized LDL, increased proportions of apolipoprotein M, and cleavage of apolipoprotein B-100, a part of the LDL core, by arginine-specific gingipains (Bengtsson et al., 2008). In addition, P. gingivalis-modified ox-LDL induced vascular smooth-muscle-cell proliferation in vitro, suggesting a potential role in intima-media thickening. Anti-oxidant treatment of endothelial cells infected with P. gingivalis resulted in an attenuated production of MCP-1 (Choi et al., 2005), suggesting an inhibition of monocyte migration into subendothelial spaces, the site where oxidation of LDL primarily takes place, likely due to the limited activity of anti-oxidants outside the vessel lumen (Verhoye and Langlois, 2009).
In an in vivo setting, higher levels of ROS and oxidative stress-related genes were found in the aortas of rats with experimental periodontitis (Ekuni et al., 2009a). An intervention with vitamin C, a potent anti-oxidative agent, resulted in attenuated oxidative stress, and decreased lipid deposition in the aorta (Ekuni et al., 2009b).
Periodontal Pathogens in Animal Models of Atherogenesis
Several studies conducted in ApoE-deficient mice, a mouse model prone to accelerated atherosclerosis, evaluated the effect of P. gingivalis infection on atherogenesis. Thus, intravenous injection of P. gingivalis (L Li et al., 2002), P. gingivalis LPS (Gitlin and Loftin, 2009), or repeated oral/anal bacterial applications (Lalla et al., 2003) resulted in enhanced atherosclerosis in infected animals when compared with uninfected controls. Periodontal tissue destruction correlated positively with atherosclerotic lesion formation, serum levels of IL-6 expression, and VCAM-1 expression in the aorta (Lalla et al., 2003). Consistent with the in vitro data discussed above, only invasive P. gingivalis, as compared with a fimbriae-deficient mutant, was able to induce periodontitis, increase atherogenesis, and trigger up-regulation of Toll-like receptors TLR2 and TLR4 (Gibson et al., 2004).
P. gingivalis-mediated atherosclerosis was prevented by prior immunization with P. gingivalis (Miyamoto et al., 2006; Koizumi et al., 2008, 2009). Interestingly, it appeared that pro-atherogenic changes, i.e., development of atheromatous plaques in the aortic sinus, were dependent on innate immune responses occurring early during the course of the infection, rather than on the establishment of chronic systemic inflammation (Miyamoto et al., 2006). It must be realized that the above studies tested the preventive effects of immunization prior to the induction of periodontitis, and can thus not be extrapolated to represent also the effects of periodontal therapy on atherogenesis-related events. In an experimental study that modeled the effects of periodontal therapy on systemic inflammation and atherosclerosis—bearing in mind that mechanical periodontal therapy is not feasible in mice—systemic doxycycline was found to reduce atherosclerotic lesion size and pro-atherogenic cytokine production in ApoE heterozygotic mice (Madan et al., 2007). In this context, it is important to note that human periodontal disease is a chronic infection that is mediated by a biofilm rather than by planktonic bacteria. Pathogens in a biofilm are largely protected from antibiotics (del Pozo and Patel, 2007), and disruption of the dental plaque by mechanical means is considered essential for the successful management of periodontal disease, and for any downstream systemic effects (Schaudinn et al., 2009). Therefore, the reported lack of efficacy of systemic antibiotic therapy in the secondary prevention of cardiac events (O’Connor et al., 2003; Cannon et al., 2005; Grayston et al., 2005; Stassen et al., 2008) should not be interpreted to demonstrate the inability of periodontal therapy to reduce the incidence of atherosclerosis-related events, since concomitant mechanical periodontal therapy was not administered in these studies. In contrast, the pro-atherogenic changes induced in the mouse model studies above (Madan et al., 2007) were mediated by repeated injections of planktonic P. gingivalis, without formation of a dental plaque biofilm. Therefore, the observed anti-atherogenic effects of antibiotic monotherapy cannot be readily extrapolated to the setting of human periodontitis. In addition, the study did not control for the inherent anti-inflammatory and MMP-inhibiting (Gu et al., 2010) effects of doxycycline.
Interestingly, treatment of P. gingivalis LPS induced atherosclerosis in ApoE-/- mice with a COX-2 inhibitor increased the extent of atherosclerotic lesions. This effect could be mediated by an inhibition of COX-2-dependent prostaglandin E2 expression by the drug, leading to increased TNF-alpha production and subsequent increased atherosclerosis (Gitlin and Loftin, 2009). Similarly unanticipated was the observation that deletion of IL-6, a major pro-atherogenic cytokine (Schuett et al., 2009), resulted in a significant increase in lesion size and alterations in atheromatic plaque composition toward an unstable phenotype in mice (Madan et al., 2008). These findings underscore the complexity of the process of atherogenesis and the apparent redundancy of the atherosclerosis-promoting pathways that complicate the interpretation of experimental mechanistic studies.
It should be emphasized that the above studies modeling the periodontitis/atherosclerosis associations used genetically modified ApoE-deficient mice on a high-fat, high-cholesterol diet (‘Western diet’), and the obtained findings may have limited relevance to the human situation. In contrast, wild-type mice were shown to be relatively resistant to a plethora of atherogenic stimuli, and did not develop atherosclerosis within reasonable time periods (Graves et al., 2008). However, in other animal models involving pigs, P. gingivalis was shown to induce rapid atherogenesis, even in normo-cholesterolemic situations (Brodala et al., 2005). Nevertheless, cost-related issues and, more importantly, the possibility to generate knock-out or knock-in animals make mouse models extremely useful in the study of specific molecular targets of atherogenesis.
The effects of periodontal pathogens were further studied in the liver, an organ of central importance for lipid metabolism, and for the production of acute-phase proteins, such as CRP. Repeated intravenous injections of live A. actinomycetemcomitans in mice resulted in detection of the pathogen in liver tissue, along with pronounced inflammatory cell infiltration, elevated mRNA levels for pro-inflammatory genes, and elevated serum amyloid A and LPS (Hyvarinen et al., 2009). Thus, induction of hepatic inflammation is conceivably another route by which periodontal pathogens may contribute to atherogenesis by interfering with lipid metabolism and promoting systemic inflammation.
Limitations of Available Mechanistic Studies
The majority of the mechanistic studies reviewed above used a ‘model’ periodontal pathogen, in most instances P. gingivalis, to dissect pathways relevant to the course of a poly-microbial infection such as periodontitis. Studies that utilized multiple bacterial stimuli simultaneously, e.g., in a co-infection model of human aortic endothelial cells with both P. gingivalis and F. nucleatum (Metzger et al., 2009; Polak et al., 2009), demonstrated a two- to 20-fold increased invasive capacity of P. gingivalis (Saito et al., 2008), suggesting that inferences based on mono-infections may not adequately portray the in vivo capabilities of the investigated pathogens. Furthermore, the selection of the particular strain of the model organism to be used in experimentation is of importance. For example, in the case of P. gingivalis, its virulence and potency to induce a pro-inflammatory host response depend on the types of fimbriae expressed, with Type II strains such as those frequently isolated from clinical lesions eliciting significantly stronger responses than Type I strains, which are most often used as model organisms (Wang et al., 2009). In future studies, the use of artificial biofilms (Thurnheer et al., 2004) incorporating multiple species rather than of planktonic bacterial cells will provide the possibility to account for properties ascribed to biofilm-derived bacterial products such as outer membrane vesicles, and may further enhance the ability of in vitro systems to model the in vivo situation. Similar issues arise from the selection of specific cell lines to be used as the effector targets, the properties of which may vary widely (Bouis et al., 2001; Staton et al., 2009). Last, the MOIs used in in vitro studies are likely much higher than a reasonable pathogen/host cell ratio that may plausibly be encountered in an in vivo setting. For example, the capacity of low P. gingivalis inocula to induce pro-inflammatory responses in human endothelial cells was rather weak in comparison with the effects of TNF-alpha or interleukin-1 beta at concentrations commonly measured in serum from periodontal patients (Honda et al., 2005). Likewise, Roth et al. (2007a) reported a lack of pro-apoptotic capability of invasive P. gingivalis on vascular endothelial cells in MOIs lower than 500. However, intracellular replication has recently been considered as a mechanism through which a pathogen may reach sufficiently high MOIs in vivo to exert biologic effects. In fact, P. gingivalis was recently shown to exploit its ability for intercellular transmission and conversion from a latent state of dormancy to a viable state, and thereby to be able to multiply and persist in vascular tissues (Li et al., 2008).
Concluding Remarks
Evidence from observational epidemiologic studies that has accumulated over the past few years has extended earlier observations and suggests that periodontal infections are independently associated with cardiovascular and cerebrovascular clinical outcomes. Although the strength of the reported associations is generally modest, the consistency of the data that have emerged from geographically and ethnically diverse populations across a variety of exposure and outcome variables suggests that the findings cannot be ascribed solely to the effects of confounders. Analysis of limited data from interventional epidemiologic studies suggests that treatment of periodontal infections results in lower levels of systemic inflammation and favorable effects on subclinical markers of atherosclerosis, although analysis of the data suggests substantial heterogeneity in responses. However, there are no data available to date suggesting that the prevention or amelioration of periodontal infections will result in reduced incidence of cardiovascular or cerebrovascular clinical events. A great many experimental mechanistic in vitro and in vivo studies have established the plausibility of a link between periodontal infections and atherogenesis, and have identified biological pathways by which these effects are mediated. Future research must expand into the identification of in vivo pathways in humans that lead to periodontitis-mediated atherogenesis or result in treatment-induced reduction in atherosclerosis risk. Ultimately, these findings will pave the way for the conduction of appropriately designed clinical trials that will determine if periodontal interventions have a role in the primary or secondary prevention of AVD.
Acknowledgments
Dr. Kebschull was supported by the German Research Council/Deutsche Forschungsgemeinschaft (Clinical Research Unit 208, TP6 & TP9), Dr. Demmer by NIH grant # K99 DE-018739, and Dr. Papapanou by NIH grants #DE-015649 and CTSA Award #RR-025158, and by a grant from Colgate-Palmolive, USA.
References
- Abranches J, Zeng L, Bélanger M, Rodrigues PH, Simpson-Haidaris PJ, Akin D, et al. (2009). Invasion of human coronary artery endothelial cells by Streptococcus mutans OMZ175. Oral Microbiol Immunol 24:141-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alard JE, Dueymes M, Mageed RA, Saraux A, Youinou P, Jamin C. (2009). Mitochondrial heat shock protein (HSP) 70 synergizes with HSP60 in transducing endothelial cell apoptosis induced by anti-HSP60 autoantibody. FASEB J 23:2772-2779 [DOI] [PubMed] [Google Scholar]
- Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. (2003). Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol 23:1245-1249 [DOI] [PubMed] [Google Scholar]
- Andriankaja OM, Genco RJ, Dorn J, Dmochowski J, Hovey K, Falkner KL, et al. (2007). Periodontal disease and risk of myocardial infarction: the role of gender and smoking. Eur J Epidemiol 22:699-705 [DOI] [PubMed] [Google Scholar]
- Ayada K, Yokota K, Hirai K, Fujimoto K, Kobayashi K, Ogawa H, et al. (2009). Regulation of cellular immunity prevents Helicobacter pylori-induced atherosclerosis. Lupus 18:1154-1168 [DOI] [PubMed] [Google Scholar]
- Bach RR. (2006). Tissue factor encryption. Arterioscler Thromb Vasc Biol 26:456-461 [DOI] [PubMed] [Google Scholar]
- Bahrani-Mougeot FK, Paster BJ, Coleman S, Ashar J, Barbuto S, Lockhart PB. (2008). Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol 46:2129-2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartruff JB, Yukna RA, Layman DL. (2005). Outer membrane vesicles from Porphyromonas gingivalis affect the growth and function of cultured human gingival fibroblasts and umbilical vein endothelial cells. J Periodontol 76:972-979 [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. (2002). Relationships among clinical measures of periodontal disease and their associations with systemic markers. Ann Periodontol 7:79-89 [DOI] [PubMed] [Google Scholar]
- Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. (2001). Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol 21:1816-1822 [DOI] [PubMed] [Google Scholar]
- Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, et al. (2005a). Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation 112:19-24 [DOI] [PubMed] [Google Scholar]
- Beck JD, Eke P, Lin D, Madianos P, Couper D, Moss K, et al. (2005b). Associations between IgG antibody to oral organisms and carotid intima-medial thickness in community-dwelling adults. Atherosclerosis 183:342-348 [DOI] [PubMed] [Google Scholar]
- Beck JD, Couper DJ, Falkner KL, Graham SP, Grossi SG, Gunsolley JC, et al. (2008). The Periodontitis and Vascular Events (PAVE) pilot study: adverse events. J Periodontol 79:90-96 [DOI] [PubMed] [Google Scholar]
- Behle JH, Papapanou PN. (2006). Periodontal infections and atherosclerotic vascular disease: an update. Int Dent J 56(4 Suppl 1):S256-S262 [DOI] [PubMed] [Google Scholar]
- Behle JH, Sedaghatfar MH, Demmer RT, Wolf DL, Celenti R, Kebschull M, et al. (2009). Heterogeneity of systemic inflammatory responses to periodontal therapy. J Clin Periodontol 36:287-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M, Rodrigues PH, Dunn WA, Progulske-Fox A. (2006). Autophagy: a highway for Porphyromonas gingivalis in endothelial cells. Autophagy 2:165-170 [DOI] [PubMed] [Google Scholar]
- Bengtsson T, Karlsson H, Gunnarsson P, Skoglund C, Elison C, Leanderson P, et al. (2008). The periodontal pathogen Porphyromonas gingivalis cleaves apoB-100 and increases the expression of apoM in LDL in whole blood leading to cell proliferation. J Intern Med 263:558-571 [DOI] [PubMed] [Google Scholar]
- Bhagat K, Moss R, Collier J, Vallance P. (1996). Endothelial “stunning” following a brief exposure to endotoxin: a mechanism to link infection and infarction? Cardiovasc Res 32:822-829 [PubMed] [Google Scholar]
- Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, et al. (2004). Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 10:416-421 [DOI] [PubMed] [Google Scholar]
- Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, et al. (2009). Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res 104:346-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombeli T, Karsan A, Tait JF, Harlan JM. (1997). Apoptotic vascular endothelial cells become procoagulant. Blood 89:2429-2442 [PubMed] [Google Scholar]
- Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, et al. (2007). Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation 115:2516-2525 [DOI] [PubMed] [Google Scholar]
- Bouis D, Hospers GA, Meijer C, Molema G, Mulder NH. (2001). Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 4:91-102 [DOI] [PubMed] [Google Scholar]
- Briggs JE, McKeown PP, Crawford VL, Woodside JV, Stout RW, Evans A, et al. (2006). Angiographically confirmed coronary heart disease and periodontal disease in middle-aged males. J Periodontol 77:95-102 [DOI] [PubMed] [Google Scholar]
- Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, et al. (2005). Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol 25:1446-1451 [DOI] [PubMed] [Google Scholar]
- Cairo F, Gaeta C, Dorigo W, Oggioni MR, Pratesi C, Pini Prato GP, et al. (2004). Periodontal pathogens in atheromatous plaques. A controlled clinical and laboratory trial. J Periodontal Res 39:442-446 [DOI] [PubMed] [Google Scholar]
- Cairo F, Castellani S, Gori AM, Nieri M, Baldelli G, Abbate R, et al. (2008). Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J Clin Periodontol 35:465-472 [DOI] [PubMed] [Google Scholar]
- Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, et al. (2005). Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med 352:1646-1654 [DOI] [PubMed] [Google Scholar]
- Cavrini F, Sambri V, Moter A, Servidio D, Marangoni A, Montebugnoli L, et al. (2005). Molecular detection of Treponema denticola and Porphyromonas gingivalis in carotid and aortic atheromatous plaques by FISH: report of two cases. J Med Microbiol 54(Pt 1):93-96 [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. (1992). Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340:1111-1115 [DOI] [PubMed] [Google Scholar]
- Chen H, Zheng P, Zhu H, Zhu J, Zhao L, El Mokhtari N, et al. (2009). Platelet-activating factor levels of serum and gingival crevicular fluid in nonsmoking patients with periodontitis and/or coronary heart disease. Clin Oral Investig DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Kim YH, Park JW, Kim SY, Lee SY, Jee SH. (2009). Tooth loss, hypertension and risk for stroke in a Korean population. Atherosclerosis 203:550-556 [DOI] [PubMed] [Google Scholar]
- Choi E-K, Park S-A, Oh W-M, Kang H-C, Kuramitsu HK, Kim B-G, et al. (2005). Mechanisms of Porphyromonas gingivalis-induced monocyte chemoattractant protein-1 expression in endothelial cells. FEMS Immunol Med Microbiol 44:51-58 [DOI] [PubMed] [Google Scholar]
- Chou H-H, Yumoto H, Davey M, Takahashi Y, Miyamoto T, Gibson FC, et al. (2005). Porphyromonas gingivalis fimbria-dependent activation of inflammatory genes in human aortic endothelial cells. Infect Immun 73:5367-5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. (2009). Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol 80:1421-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crasta K, Daly CG, Mitchell D, Curtis B, Stewart D, Heitz-Mayfield LJ. (2009). Bacteraemia due to dental flossing. J Clin Periodontol 36:323-332 [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Nibali L, Mohamed-Ali V, Vallance P, Tonetti MS. (2004a). Periodontal therapy: a novel non-drug-induced experimental model to study human inflammation. J Periodontal Res 39:294-299 [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, et al. (2004b). Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res 83:156-160 [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. (2005a). Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res 84:269-273 [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Parkar M, Tonetti MS. (2005b). Periodontal therapy: a novel acute inflammatory model. Inflamm Res 54:412-414 [DOI] [PubMed] [Google Scholar]
- De Soyza A, Higgins B, Gould K. (2000). An unusual case of pulmonary abscess. J Infect 41:114. [DOI] [PubMed] [Google Scholar]
- del Pozo JL, Patel R. (2007). The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther 82:204-209 [DOI] [PubMed] [Google Scholar]
- Demmer RT, Desvarieux M. (2006). Periodontal infections and cardiovascular disease: the heart of the matter. J Am Dent Assoc 137(Suppl):14S-20S [DOI] [PubMed] [Google Scholar]
- Demmer RT, Kocher T, Schwahn C, Volzke H, Jacobs DR, Jr, Desvarieux M. (2008a). Refining exposure definitions for studies of periodontal disease and systemic disease associations. Community Dent Oral Epidemiol 36:493-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Papapanou PN, Jacobs DR, Jr, Desvarieux M. (2008b). Bleeding on probing differentially relates to bacterial profiles: the Oral Infections and Vascular Disease Epidemiology Study. J Clin Periodontol 35:479-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande RG, Khan MB, Genco CA. (1998). Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun 66:5337-5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desta T, Graves DT. (2007). Fibroblast apoptosis induced by Porphyromonas gingivalis is stimulated by a gingipain and caspase-independent pathway that involves apoptosis-inducing factor. Cell Microbiol 9:2667-2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. (1993). Dental disease and risk of coronary heart disease and mortality. BMJ 306:688-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Schwahn C, Volzke H, Demmer RT, Ludemann J, Kessler C, et al. (2004). Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke 35:2029-2035 [DOI] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Sacco RL, et al. (2005). Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation 111:576-582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Jacobs DR, Jr, Rundek T, Boden-Albala B, Sacco RL, et al. (2010). Periodontal bacteria and hypertension. J Hypertens 28:1413-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. (2008). Increased Toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 93:578-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich T, Garcia RI. (2005). Associations between periodontal disease and systemic disease: evaluating the strength of the evidence. J Periodontol 76(11 Suppl):S2175-S2184 [DOI] [PubMed] [Google Scholar]
- Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. (2008). Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation 117:1668-1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieude M, Gillis MA, Theoret JF, Thorin E, Lajoie G, Levine JS, et al. (2009). Autoantibodies to heat shock protein 60 promote thrombus formation in a murine model of arterial thrombosis. J Thromb Haemost 7:710-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Uitto VJ, Firth J, Salo T, Haapasalo M, Konttinen YT, et al. (1995). Modulation of host matrix metalloproteinases by bacterial virulence factors relevant in human periodontal diseases. Oral Dis 1:279-286 [DOI] [PubMed] [Google Scholar]
- Dorn BR, Dunn WA, Jr, Progulske-Fox A. (1999). Invasion of human coronary artery cells by periodontal pathogens. Infect Immun 67:5792-5798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunzendorfer S, Lee HK, Tobias PS. (2004). Flow-dependent regulation of endothelial Toll-like receptor 2 expression through inhibition of SP1 activity. Circ Res 95:684-691 [DOI] [PubMed] [Google Scholar]
- Dye BA, Herrera-Abreu M, Lerche-Sehm J, Vlachojannis C, Pikdoken L, Pretzl B, et al. (2009). Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol 80:634-647 [DOI] [PubMed] [Google Scholar]
- Ebersole JL. (2003). Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000 31:135-166 [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Cappelli D, Mathys EC, Steffen MJ, Singer RE, Montgomery M, et al. (2002). Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Ann Periodontol 7:102-111 [DOI] [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. (2002). Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105:1158-1161 [PubMed] [Google Scholar]
- Ekuni D, Tomofuji T, Sanbe T, Irie K, Azuma T, Maruyama T, et al. (2009a). Periodontitis-induced lipid peroxidation in rat descending aorta is involved in the initiation of atherosclerosis. J Periodontal Res 44:434-442 [DOI] [PubMed] [Google Scholar]
- Ekuni D, Tomofuji T, Sanbe T, Irie K, Azuma T, Maruyama T, et al. (2009b). Vitamin C intake attenuates the degree of experimental atherosclerosis induced by periodontitis in the rat by decreasing oxidative stress. Arch Oral Biol 54:495-502 [DOI] [PubMed] [Google Scholar]
- Elter JR, Hinderliter AL, Offenbacher S, Beck JD, Caughey M, Brodala N, et al. (2006). The effects of periodontal therapy on vascular endothelial function: a pilot trial. Am Heart J 151:47. [DOI] [PubMed] [Google Scholar]
- Epstein SE, Zhu J, Burnett MS, Zhou YF, Vercellotti G, Hajjar D. (2000). Infection and atherosclerosis: potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb Vasc Biol 20:1417-1420 [DOI] [PubMed] [Google Scholar]
- Erridge C. (2008). The roles of pathogen-associated molecular patterns in atherosclerosis. Trends Cardiovasc Med 18:52-56 [DOI] [PubMed] [Google Scholar]
- Erridge C, Spickett CM, Webb DJ. (2007). Non-enterobacterial endotoxins stimulate human coronary artery but not venous endothelial cell activation via Toll-like receptor 2. Cardiovasc Res 73:181-189 [DOI] [PubMed] [Google Scholar]
- Ewald C, Kuhn S, Kalff R. (2006). Pyogenic infections of the central nervous system secondary to dental affections—a report of six cases. Neurosurg Rev 29:163-166 [DOI] [PubMed] [Google Scholar]
- Fiehn N-E, Larsen T, Christiansen N, Holmstrup P, Schroeder TV. (2005). Identification of periodontal pathogens in atherosclerotic vessels. J Periodontol 76:731-736 [DOI] [PubMed] [Google Scholar]
- Fink AL. (1999). Chaperone-mediated protein folding. Physiol Rev 79:425-449 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RE, Wijeyewickrema LC, Pike RN. (2009). The gingipains: scissors and glue of the periodontal pathogen, Porphyromonas gingivalis. Future Microbiol 4:471-487 [DOI] [PubMed] [Google Scholar]
- Fong IW. (2002). Infections and their role in atherosclerotic vascular disease. J Am Dent Assoc 133(Suppl):7S-13S [DOI] [PubMed] [Google Scholar]
- Ford PJ, Gemmell E, Hamlet SM, Hasan A, Walker PJ, West MJ, et al. (2005). Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral Microbiol Immunol 20:296-302 [DOI] [PubMed] [Google Scholar]
- Ford PJ, Gemmell E, Chan A, Carter CL, Walker PJ, Bird PS, et al. (2006). Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol Immunol 21:206-211 [DOI] [PubMed] [Google Scholar]
- Ford PJ, Gemmell E, Timms P, Chan A, Preston FM, Seymour GJ. (2007). Anti-P. gingivalis response correlates with atherosclerosis. J Dent Res 86:35-40 [DOI] [PubMed] [Google Scholar]
- Forner L, Larsen T, Kilian M, Holmstrup P. (2006). Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 33:401-407 [DOI] [PubMed] [Google Scholar]
- Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. (2006). CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res 98:e20-e25 [DOI] [PubMed] [Google Scholar]
- Gaetti-Jardim E, Marcelino SL, Feitosa ACR, Romito GA, Avila-Campos MJ. (2009). Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol 58 (Pt 12):1568-1575 [DOI] [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, Libby P. (1994). Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 94:2493-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E, Ley K. (2009). Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol 27:165-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geismar K, Stoltze K, Sigurd B, Gyntelberg F, Holmstrup P. (2006). Periodontal disease and coronary heart disease. J Periodontol 77:1547-1554 [DOI] [PubMed] [Google Scholar]
- Giacona MB, Papapanou PN, Lamster IB, Rong LL, D’Agati VD, Schmidt AM, et al. (2004). Porphyromonas gingivalis induces its uptake by human macrophages and promotes foam cell formation in vitro. FEMS Microbiol Lett 241:95-101 [DOI] [PubMed] [Google Scholar]
- Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, et al. (2004). Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 109:2801-2806 [DOI] [PubMed] [Google Scholar]
- Gitlin JM, Loftin CD. (2009). Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice. Cardiovasc Res 81:400-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotsman I, Lotan C, Soskolne WA, Rassovsky S, Pugatsch T, Lapidus L, et al. (2007). Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J Periodontol 78:849-558 [DOI] [PubMed] [Google Scholar]
- Grace CJ, Levitz RE, Katz-Pollak H, Brettman LR. (1988). Actinobacillus actinomycetemcomitans prosthetic valve endocarditis. Rev Infect Dis 10:922-929 [DOI] [PubMed] [Google Scholar]
- Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, et al. (2004). Periodontal disease as a risk factor for ischemic stroke. Stroke 35:496-501 [DOI] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng Y-T, Van Dyke TE, Hajishengallis G. (2008). The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol 35:89-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, et al. (2005). Azithromycin for the secondary prevention of coronary events. N Engl J Med 352:1637-1645 [DOI] [PubMed] [Google Scholar]
- Greeno EW, Bach RR, Moldow CF. (1996). Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Invest 75:281-289 [PubMed] [Google Scholar]
- Gu Y, Lee HM, Sorsa T, Simon SR, Golub LM. (2010). Doxycycline inhibits mononuclear cell-mediated connective tissue breakdown. FEMS Immunol Med Microbiol 58:218-225 [DOI] [PubMed] [Google Scholar]
- Guan SM, Shu L, Fu SM, Liu B, Xu XL, Wu JZ. (2009). Prevotella intermedia upregulates MMP-1 and MMP-8 expression in human periodontal ligament cells. FEMS Microbiol Lett 299:214-222 [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. (2008). Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci U S A 105:13532-13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. (2000). Identification of periodontal pathogens in atheromatous plaques. J Periodontol 71:1554-1560 [DOI] [PubMed] [Google Scholar]
- Harokopakis E, Hajishengallis G. (2005). Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol 35:1201-1210 [DOI] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. (2002). Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155:176-184 [DOI] [PubMed] [Google Scholar]
- Herzberg MC, Nobbs A, Tao L, Kilic A, Beckman E, Khammanivong A, et al. (2005). Oral streptococci and cardiovascular disease: searching for the platelet aggregation-associated protein gene and mechanisms of Streptococcus sanguis-induced thrombosis. J Periodontol 76(11 Suppl):S2101-S2105 [DOI] [PubMed] [Google Scholar]
- Ho Y-S, Lai M-T, Liu S-J, Lin C-T, Naruishi K, Takashiba S, et al. (2009). Porphyromonas gingivalis fimbriae-dependent interleukin-6 autocrine regulation by increase of gp130 in endothelial cells. J Periodontal Res 44:550-556 [DOI] [PubMed] [Google Scholar]
- Holmlund A, Holm G, Lind L. (2006). Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J Periodontol 77:1173-1178 [DOI] [PubMed] [Google Scholar]
- Honda T, Oda T, Yoshie H, Yamazaki K. (2005). Effects of Porphyromonas gingivalis antigens and proinflammatory cytokines on human coronary artery endothelial cells. Oral Microbiol Immunol 20:82-88 [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. (2009). Cell death. N Engl J Med 361:1570-1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huittinen T, Leinonen M, Tenkanen L, Manttari M, Virkkunen H, Pitkanen T, et al. (2002). Autoimmunity to human heat shock protein 60, Chlamydia pneumoniae infection, and inflammation in predicting coronary risk. Arterioscler Thromb Vasc Biol 22:431-437 [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. (2000). Periodontal disease and coronary heart disease risk. JAMA 284:1406-1410 [DOI] [PubMed] [Google Scholar]
- Hujoel PP, White BA, Garcia RI, Listgarten MA. (2001). The dentogingival epithelial surface area revisited. J Periodontal Res 36:48-55 [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. (2002). Periodontitis-systemic disease associations in the presence of smoking—causal or coincidental? Periodontol 2000 30:51-60 [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. (2003). An exploration of the periodontitis-cancer association. Ann Epidemiol 13:312-316 [DOI] [PubMed] [Google Scholar]
- Hulthe J, Fagerberg B. (2002). Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arterioscler Thromb Vasc Biol 22:1162-1167 [DOI] [PubMed] [Google Scholar]
- Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. (2008). Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med 23:2079-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman J. (2006). The importance of assessing confounding and effect modification in research involving periodontal disease and systemic diseases. J Clin Periodontol 33:102-103 [DOI] [PubMed] [Google Scholar]
- Hyman JJ, Winn DM, Reid BC. (2002). The role of cigarette smoking in the association between periodontal disease and coronary heart disease. J Periodontol 73:988-994 [DOI] [PubMed] [Google Scholar]
- Hyvarinen K, Tuomainen AM, Laitinen S, Bykov IL, Tormakangas L, Lindros K, et al. (2009). Chlamydial and periodontal pathogens induce hepatic inflammation and fatty acid imbalance in apolipoprotein E-deficient mice. Infect Immun 77:3442-3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. (2003). Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol 30:334-340 [DOI] [PubMed] [Google Scholar]
- Ide M, Jagdev D, Coward PY, Crook M, Barclay GR, Wilson RF. (2004). The short-term effects of treatment of chronic periodontitis on circulating levels of endotoxin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6. J Periodontol 75:420-428 [DOI] [PubMed] [Google Scholar]
- Inaba H, Hokamura K, Nakano K, Nomura R, Katayama K, Nakajima A, et al. (2009). Upregulation of S100 calcium-binding protein A9 is required for induction of smooth muscle cell proliferation by a periodontal pathogen. FEBS Lett 583:128-134 [DOI] [PubMed] [Google Scholar]
- Inomata M, Into T, Ishihara Y, Nakashima M, Noguchi T, Matsushita K. (2007). Arginine-specific gingipain A from Porphyromonas gingivalis induces Weibel-Palade body exocytosis and enhanced activation of vascular endothelial cells through protease-activated receptors. Microbes Infect 9:1500-1506 [DOI] [PubMed] [Google Scholar]
- Inomata M, Ishihara Y, Matsuyama T, Imamura T, Maruyama I, Noguchi T, et al. (2009). Degradation of vascular endothelial thrombomodulin by arginine- and lysine-specific cysteine proteases from Porphyromonas gingivalis. J Periodontol 80:1511-1517 [DOI] [PubMed] [Google Scholar]
- Itabe H. (2009). Oxidative modification of LDL: its pathological role in atherosclerosis. Clin Rev Allergy Immunol 37:4-11 [DOI] [PubMed] [Google Scholar]
- Iwai T. (2009). Periodontal bacteremia and various vascular diseases. J Periodontal Res 44:689-694 [DOI] [PubMed] [Google Scholar]
- Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, et al. (2009). TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol 183:7461-7470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janket SJ, Baird AE, Chuang SK, Jones JA. (2003). Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95:559-569 [DOI] [PubMed] [Google Scholar]
- Jennings LK. (2009). Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost 102:248-257 [DOI] [PubMed] [Google Scholar]
- Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. (2009). Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol 66:505-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Johansson I, Eriksson M, Ahren AM, Hallmans G, Stegmayr B. (2005). Systemic antibodies to the leukotoxin of the oral pathogen Actinobacillus actinomycetemcomitans correlate negatively with stroke in women. Cerebrovasc Dis 20:226-232 [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. (1996). Poor oral health and coronary heart disease. J Dent Res 75:1631-1636 [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Hung H-C, Rimm EB, Willett WC, Ascherio A. (2003). Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke 34:47-52 [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. (1990). Heat shock proteins and the immune response. Immunol Today 11:129-136 [DOI] [PubMed] [Google Scholar]
- Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. (2005). Bacteraemia following periodontal procedures. J Clin Periodontol 32:708-713 [DOI] [PubMed] [Google Scholar]
- Kocgozlu L, Elkaim R, Tenenbaum H, Werner S. (2009). Variable cell responses to P. gingivalis lipopolysaccharide. J Dent Res 88:741-745 [DOI] [PubMed] [Google Scholar]
- Koizumi Y, Kurita-Ochiai T, Oguchi S, Yamamoto M. (2008). Nasal immunization with Porphyromonas gingivalis outer membrane protein decreases P. gingivalis-induced atherosclerosis and inflammation in spontaneously hyperlipidemic mice. Infect Immun 76:2958-2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi Y, Kurita-Ochiai T, Oguchi S, Yamamoto M. (2009). Intranasal immunization with Porphyromonas gingivalis and atherosclerosis. Immunopharmacol Immunotoxicol 31:352-357 [DOI] [PubMed] [Google Scholar]
- Kol A, Sukhova GK, Lichtman AH, Libby P. (1998). Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 98:300-307 [DOI] [PubMed] [Google Scholar]
- Koo TH, Jun HO, Bae S-K, Kim S-R, Moon C-P, Jeong S-K, et al. (2007). Porphyromonas gingivalis, periodontal pathogen, lipopolysaccharide induces angiogenesis via extracellular signal-regulated kinase 1/2 activation in human vascular endothelial cells. Arch Pharm Res 30:34-42 [DOI] [PubMed] [Google Scholar]
- Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr, Progulske-Fox A. (2005). Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol 25:e17-e18 [DOI] [PubMed] [Google Scholar]
- Kozarov E, Sweier D, Shelburne C, Progulske-Fox A, Lopatin D. (2006). Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect 8:687-693 [DOI] [PubMed] [Google Scholar]
- Kuramitsu HK, Qi M, Kang IC, Chen W. (2001). Role for periodontal bacteria in cardiovascular diseases. Ann Periodontol 6:41-47 [DOI] [PubMed] [Google Scholar]
- Lakio L, Lehto M, Tuomainen AM, Jauhiainen M, Malle E, Asikainen S, et al. (2006). Pro-atherogenic properties of lipopolysaccharide from the periodontal pathogen Actinobacillus actinomycetemcomitans. J Endotoxin Res 12:57-64 [DOI] [PubMed] [Google Scholar]
- Lalla E, Lamster IB, Hofmann MA, Bucciarelli L, Jerud AP, Tucker S, et al. (2003). Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 23:1405-1411 [DOI] [PubMed] [Google Scholar]
- Lamb DJ, El-Sankary W, Ferns GA. (2003). Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis 167:177-185 [DOI] [PubMed] [Google Scholar]
- Lamster IB, Ahlo JK. (2007). Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann NY Acad Sci 1098:216-229 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Garcia RI, Janket SJ, Jones JA, Mascarenhas AK, Scott TE, et al. (2006). The association between cumulative periodontal disease and stroke history in older adults. J Periodontol 77:1744-1754 [DOI] [PubMed] [Google Scholar]
- Li D, Chen H, Romeo F, Sawamura T, Saldeen T, Mehta JL. (2002). Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. J Pharmacol Exp Ther 302:601-605 [DOI] [PubMed] [Google Scholar]
- Li D, Liu L, Chen H, Sawamura T, Mehta JL. (2003). LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol 23:816-821 [DOI] [PubMed] [Google Scholar]
- Li L, Messas E, Batista EL, Jr, Levine RA, Amar S. (2002). Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation 105:861-867 [DOI] [PubMed] [Google Scholar]
- Li L, Michel R, Cohen J, Decarlo A, Kozarov E. (2008). Intracellular survival and vascular cell-to-cell transmission of Porphyromonas gingivalis. BMC Microbiol 8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. (2002). Inflammation in atherosclerosis. Nature 420:868-874 [DOI] [PubMed] [Google Scholar]
- Libby P. (2009). Molecular and cellular mechanisms of the thrombotic complications of atherosclerosis. J Lipid Res 50(Suppl):S352-S357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt KA, Mayranpaa MI, Kovanen PT. (2007). Mast cells in vulnerable atherosclerotic plaques—a view to a kill. J Cell Mol Med 11:739-758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, et al. (2008). Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis 196:146-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. (2008). Bacteremia associated with toothbrushing and dental extraction. Circulation 117:3118-3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos BG. (2005). Systemic markers of inflammation in periodontitis. J Periodontol 76(11 Suppl):S2106-S2115 [DOI] [PubMed] [Google Scholar]
- Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. (2000). Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol 71:1528-1534 [DOI] [PubMed] [Google Scholar]
- Lund Håheim L, Olsen I, Nafstad P, Schwarze P, Ronningen KS. (2008). Antibody levels to single bacteria or in combination evaluated against myocardial infarction. J Clin Periodontol 35:473-478 [DOI] [PubMed] [Google Scholar]
- Ma FX, Zhou B, Chen Z, Ren Q, Lu SH, Sawamura T, et al. (2006). Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J Lipid Res 47:1227-1237 [DOI] [PubMed] [Google Scholar]
- Madan M, Bishayi B, Hoge M, Messas E, Amar S. (2007). Doxycycline affects diet- and bacteria-associated atherosclerosis in an ApoE heterozygote murine model: cytokine profiling implications. Atherosclerosis 190:62-72 [DOI] [PubMed] [Google Scholar]
- Madan M, Bishayi B, Hoge M, Amar S. (2008). Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis 197:504-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Miyamoto M, Hongyo H, Nagai A, Kurihara H, Murayama Y. (1994). Heat shock protein 60 (GroEL) from Porphyromonas gingivalis: molecular cloning and sequence analysis of its gene and purification of the recombinant protein. FEMS Microbiol Lett 119:129-135 [DOI] [PubMed] [Google Scholar]
- Mattila KJ, Nieminen MS, Valtonen VV, Rasi VP, Kesaniemi YA, Syrjala SL, et al. (1989). Association between dental health and acute myocardial infarction. BMJ 298:779-781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila KJ, Asikainen S, Wolf J, Jousimies-Somer H, Valtonen V, Nieminen M. (2000). Age, dental infections, and coronary heart disease. J Dent Res 79:756-760 [DOI] [PubMed] [Google Scholar]
- Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, et al. (1999). Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 99:1560-1566 [DOI] [PubMed] [Google Scholar]
- McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, et al. (2009). Inflammation impairs reverse cholesterol transport in vivo. Circulation 119:1135-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel R, Bacher M, Flores-De-Jacoby L. (2002). Interactions between stress, interleukin-1beta, interleukin-6 and cortisol in periodontally diseased patients. J Clin Periodontol 29:1012-1022 [DOI] [PubMed] [Google Scholar]
- Mercanoglu F, Oflaz H, Oz O, Gokbuget AY, Genchellac H, Sezer M, et al. (2004). Endothelial dysfunction in patients with chronic periodontitis and its improvement after initial periodontal therapy. J Periodontol 75:1694-1700 [DOI] [PubMed] [Google Scholar]
- Merchant AT, Pitiphat W. (2002). Directed acyclic graphs (DAGs): an aid to assess confounding in dental research. Community Dent Oral Epidemiol 30:399-404 [DOI] [PubMed] [Google Scholar]
- Metzger Z, Lin YY, Dimeo F, Ambrose WW, Trope M, Arnold RR. (2009). Synergistic pathogenicity of Porphyromonas gingivalis and Fusobacterium nucleatum in the mouse subcutaneous chamber model. J Endod 35:86-94 [DOI] [PubMed] [Google Scholar]
- Meurman JH, Sanz M, Janket SJ. (2004). Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med 15:403-413 [DOI] [PubMed] [Google Scholar]
- Meyle J. (1993). Neutrophil chemotaxis and serum concentration of tumor-necrosis-factor-alpha (TNFA). J Periodontal Res 28(6 Pt 2):491-493 [DOI] [PubMed] [Google Scholar]
- Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. (2007). A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst 99:171-175 [DOI] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. (2004). Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101:10679-10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Yumoto H, Takahashi Y, Davey M, Gibson FC, 3rd, Genco CA. (2006). Pathogen-accelerated atherosclerosis occurs early after exposure and can be prevented via immunization. Infect Immun 74:1376-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco C, Gregan SM, Navin TJ, Foxwell BM, Davies AH, Feldmann M. (2009). Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation 120:2462-2469 [DOI] [PubMed] [Google Scholar]
- Morrison HI, Ellison LF, Taylor GW. (1999). Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk 6:7-11 [DOI] [PubMed] [Google Scholar]
- Mueller AA, Saldamli B, Stubinger S, Walter C, Fluckiger U, Merlo A, et al. (2009). Oral bacterial cultures in nontraumatic brain abscesses: results of a first-line study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:469-476 [DOI] [PubMed] [Google Scholar]
- Mullick AE, Tobias PS, Curtiss LK. (2005). Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest 115:3149-3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapha IZ, Debrey S, Oladubu M, Ugarte R. (2007). Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: a systematic review and meta-analysis. J Periodontol 78:2289-1302 [DOI] [PubMed] [Google Scholar]
- Naito M, Sakai E, Shi Y, Ideguchi H, Shoji M, Ohara N, et al. (2006). Porphyromonas gingivalis-induced platelet aggregation in plasma depends on Hgp44 adhesin but not Rgp proteinase. Mol Microbiol 59:152-167 [DOI] [PubMed] [Google Scholar]
- Nakano K, Inaba H, Nomura R, Nemoto H, Takeda M, Yoshioka H, et al. (2006). Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol 44:3313-3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Inaba H, Nomura R, Nemoto H, Tamura K, Miyamoto E, et al. (2007). Detection and serotype distribution of Actinobacillus actinomycetemcomitans in cardiovascular specimens from Japanese patients. Oral Microbiol Immunol 22:136-139 [DOI] [PubMed] [Google Scholar]
- Nakano K, Inaba H, Nomura R, Nemoto H, Takeuchi H, Yoshioka H, et al. (2008). Distribution of Porphyromonas gingivalis fimA genotypes in cardiovascular specimens from Japanese patients. Oral Microbiol Immunol 23:170-172 [DOI] [PubMed] [Google Scholar]
- Nanci A, Bosshardt DD. (2006). Structure of periodontal tissues in health and disease. Periodontol 2000 40:11-28 [DOI] [PubMed] [Google Scholar]
- Nicu EA, Van der Velden U, Nieuwland R, Everts V, Loos BG. (2009). Elevated platelet and leukocyte response to oral bacteria in periodontitis. J Thromb Haemost 7:162-170 [DOI] [PubMed] [Google Scholar]
- Niu J, Kolattukudy PE. (2009). Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 117:95-109 [DOI] [PubMed] [Google Scholar]
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. (2001). Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol 72:1221-1227 [DOI] [PubMed] [Google Scholar]
- Nonnenmacher C, Stelzel M, Susin C, Sattler AM, Schaefer JR, Maisch B, et al. (2007). Periodontal microbiota in patients with coronary artery disease measured by real-time polymerase chain reaction: a case-control study. J Periodontol 78:1724-1730 [DOI] [PubMed] [Google Scholar]
- Nylander M, Lindahl TL, Bengtsson T, Grenegård M. (2008). The periodontal pathogen Porphyromonas gingivalis sensitises human blood platelets to epinephrine. Platelets 19:352-358 [DOI] [PubMed] [Google Scholar]
- O’Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, et al. (2003). Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA 290:1459-1466 [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Beck JD, Moss K, Mendoza L, Paquette DW, Barrow DA, et al. (2009). Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol 80:190-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro CA, Balluz LS, Eke PI, Ajani UA, Strine TW, Town M, et al. (2005). Tooth loss and heart disease: findings from the Behavioral Risk Factor Surveillance System. Am J Prev Med 29(5 Suppl 1):S50-S56 [DOI] [PubMed] [Google Scholar]
- Oksaharju A, Lappalainen J, Tuomainen AM, Pussinen PJ, Puolakkainen M, Kovanen PT, et al. (2009). Pro-atherogenic lung and oral pathogens induce an inflammatory response in human and mouse mast cells. J Cell Mol Med 13:103-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. (2008). Update on bacteraemia related to dental procedures. Transfus Apher Sci 39:173-178 [DOI] [PubMed] [Google Scholar]
- Oscarsson J, Karched M, Thay B, Chen C, Asikainen S. (2008). Proinflammatory effect in whole blood by free soluble bacterial components released from planktonic and biofilm cells. BMC Microbiol 8:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla C, Lobos O, Hubert E, Gonzalez C, Matus S, Pereira M, et al. (2006). Periodontal pathogens in atheromatous plaques isolated from patients with chronic periodontitis. J Periodontal Res 41:350-353 [DOI] [PubMed] [Google Scholar]
- Papapanagiotou D, Nicu EA, Bizzarro S, Gerdes VE, Meijers JC, Nieuwland R, et al. (2009). Periodontitis is associated with platelet activation. Atherosclerosis 202:605-611 [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Sedaghatfar MH, Demmer RT, Wolf DL, Yang J, Roth GA, et al. (2007). Periodontal therapy alters gene expression of peripheral blood monocytes. J Clin Periodontol 34:736-747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parahitiyawa NB, Scully C, Leung WK, Yam WC, Jin LJ, Samaranayake LP. (2009). Exploring the oral bacterial flora: current status and future directions. Oral Dis 16:136-145 [DOI] [PubMed] [Google Scholar]
- Paraskevas S, Huizinga JD, Loos BG. (2008). A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol 35:277-290 [DOI] [PubMed] [Google Scholar]
- Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. (2001). Bacterial diversity in human subgingival plaque. J Bacteriol 183:3770-3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, et al. (2001). Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104:191-196 [DOI] [PubMed] [Google Scholar]
- Piconi S, Trabattoni D, Luraghi C, Perilli E, Borelli M, Pacei M, et al. (2009). Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J 23:1196-1204 [DOI] [PubMed] [Google Scholar]
- Pincock S. (2005). Nobel Prize winners Robin Warren and Barry Marshall. Lancet 366:1429. [DOI] [PubMed] [Google Scholar]
- Pober JS, Min W, Bradley JR. (2009). Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol 4:71-95 [DOI] [PubMed] [Google Scholar]
- Polak D, Wilensky A, Shapira L, Halabi A, Goldstein D, Weiss EI, et al. (2009). Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol 36:406-410 [DOI] [PubMed] [Google Scholar]
- Polla BS. (1988). A role for heat shock proteins in inflammation? Immunol Today 9:134-137 [DOI] [PubMed] [Google Scholar]
- Pollreisz A, Huang Y, Roth G, Cheng B, Kebschull M, Papapanou PN, et al. (2010). Enhanced monocyte migration and pro-inflammatory cytokine production by Porphyromonas gingivalis infection. J Periodontal Res 45:239-245 [DOI] [PubMed] [Google Scholar]
- Pop C, Salvesen GS. (2009). Human caspases: activation, specificity, and regulation. J Biol Chem 284:21777-21781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep AR, Hadge P, Arjun Raju P, Shetty SR, Shareef K, Guruprasad CN. (2010). Periodontitis as a risk factor for cerebrovascular accident: a case-control study in the Indian population. J Periodontal Res 45:223-228 [DOI] [PubMed] [Google Scholar]
- Proceedings of the 1996 World Workshop in Periodontics (1996). Consensus report periodontal diseases: pathogenesis and microbial factors. Ann Periodontol 1:926-932 [DOI] [PubMed] [Google Scholar]
- Progulske-Fox A, Kozarov E, Dorn B, Dunn W, Jr, Burks J, Wu Y. (1999). Porphyromonas gingivalis virulence factors and invasion of cells of the cardiovascular system. J Periodontal Res 34:393-399 [DOI] [PubMed] [Google Scholar]
- Pucar A, Milasin J, Lekovic V, Vukadinovic M, Ristic M, Putnik S, et al. (2007). Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary arteries. J Periodontol 78:677-682 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Jousilahti P, Alfthan G, Palosuo T, Asikainen S, Salomaa V. (2003). Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol 23:1250-1254 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Alfthan G, Rissanen H, Reunanen A, Asikainen S, Knekt P. (2004a). Antibodies to periodontal pathogens and stroke risk. Stroke 35:2020-2023 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Alfthan G, Tuomilehto J, Asikainen S, Jousilahti P. (2004b). High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction. Eur J Cardiovasc Prev Rehabil 11:408-411 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Nyyssonen K, Alfthan G, Salonen R, Laukkanen JA, Salonen JT. (2005). Serum antibody levels to Actinobacillus actinomycetemcomitans predict the risk for coronary heart disease. Arterioscler Thromb Vasc Biol 25:833-838 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Alfthan G, Jousilahti P, Paju S, Tuomilehto J. (2007a). Systemic exposure to Porphyromonas gingivalis predicts incident stroke. Atherosclerosis 193:222-228 [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Tuomisto K, Jousilahti P, Havulinna AS, Sundvall J, Salomaa V. (2007b). Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arterioscler Thromb Vasc Biol 27:1433-1439 [DOI] [PubMed] [Google Scholar]
- Rajaiah R, Moudgil KD. (2009). Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev 8:388-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech RL, Nurkin N, da Cruz I, Sostizzo F, Baiao C, Perrone JA, et al. (2007). Association between periodontal disease and acute coronary syndrome. Arq Bras Cardiol 88:185-190 [DOI] [PubMed] [Google Scholar]
- Renvert S, Pettersson T, Ohlsson O, Persson GR. (2006). Bacterial profile and burden of periodontal infection in subjects with a diagnosis of acute coronary syndrome. J Periodontol 77:1110-1119 [DOI] [PubMed] [Google Scholar]
- Romano F, Barbui A, Aimetti M. (2007). Periodontal pathogens in periodontal pockets and in carotid atheromatous plaques. Minerva Stomatol 56:169-179 [PubMed] [Google Scholar]
- Roth GA, Moser B, Huang SJ, Brandt JS, Huang Y, Papapanou PN, et al. (2006). Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J Thromb Haemost 4:2256-2261 [DOI] [PubMed] [Google Scholar]
- Roth GA, Ankersmit HJ, Brown VB, Papapanou PN, Schmidt AM, Lalla E. (2007a). Porphyromonas gingivalis infection and cell death in human aortic endothelial cells. FEMS Microbiol Lett 272:106-117 [DOI] [PubMed] [Google Scholar]
- Roth GA, Moser B, Roth-Walter F, Giacona MB, Harja E, Papapanou PN, et al. (2007b). Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis 190:271-281 [DOI] [PubMed] [Google Scholar]
- Roth GA, Aumayr K, Giacona MB, Papapanou PN, Schmidt AM, Lalla E. (2009). Porphyromonas gingivalis infection and prothrombotic effects in human aortic smooth muscle cells. Thromb Res 123:780-784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenfire M, Grossman NS, Kaciroti N, Apsey DJ, Loesche WJ. (2007). Anaerobic dental flora and the acute coronary syndrome. Coron Artery Dis 18:111-116 [DOI] [PubMed] [Google Scholar]
- Saito A, Inagaki S, Kimizuka R, Okuda K, Hosaka Y, Nakagawa T, et al. (2008). Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol Med Microbiol 54:349-355 [DOI] [PubMed] [Google Scholar]
- Sato Y, Kishi J, Suzuki K, Nakamura H, Hayakawa T. (2009). Sonic extracts from a bacterium related to periapical disease activate gelatinase A and inactivate tissue inhibitor of metalloproteinases TIMP-1 and TIMP-2. Int Endod J 42:1104-1111 [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. (2000). Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101:1899-1906 [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, Nothnagel M, El Mokhtari NE, et al. (2009). Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet 5:e1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. (2009). Periodontitis: an archetypical biofilm disease. J Am Dent Assoc 140:978-986 [DOI] [PubMed] [Google Scholar]
- Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. (2009). How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost 102:215-222 [DOI] [PubMed] [Google Scholar]
- Seinost G, Wimmer G, Skerget M, Thaller E, Brodmann M, Gasser R, et al. (2005). Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J 149:1050-1054 [DOI] [PubMed] [Google Scholar]
- Senba T, Kobayashi Y, Inoue K, Kaneto C, Inoue M, Toyokawa S, et al. (2008). The association between self-reported periodontitis and coronary heart disease—from MY Health Up Study. J Occup Health 50:283-287 [DOI] [PubMed] [Google Scholar]
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. (2007). Relationship between periodontal infections and systemic disease. Clin Microbiol Infect 13(Suppl 4):3-10 [DOI] [PubMed] [Google Scholar]
- Sheets SM, Potempa J, Travis J, Casiano CA, Fletcher HM. (2005). Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect Immun 73:1543-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets SM, Potempa J, Travis J, Fletcher HM, Casiano CA. (2006). Gingipains from Porphyromonas gingivalis W83 synergistically disrupt endothelial cell adhesion and can induce caspase-independent apoptosis. Infect Immun 74:5667-5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim SJ, Kim HD, Moon JY, Zavras AI, Zdanowicz J, Jang SJ, et al. (2008). Periodontitis and the risk for non-fatal stroke in Korean adults. J Periodontol 79:1652-1658 [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. (2005). Periodontal microbial ecology. Periodontol 2000 38:135-187 [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr (1998). Microbial complexes in subgingival plaque. J Clin Periodontol 25:134-144 [DOI] [PubMed] [Google Scholar]
- Spahr A, Klein E, Khuseyinova N, Boeckh C, Muche R, Kunze M, et al. (2006). Periodontal infections and coronary heart disease: role of periodontal bacteria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study. Arch Intern Med 166:554-559 [DOI] [PubMed] [Google Scholar]
- Stassen FR, Vainas T, Bruggeman CA. (2008). Infection and atherosclerosis. An alternative view on an outdated hypothesis. Pharmacol Rep 60:85-92 [PubMed] [Google Scholar]
- Staton CA, Reed MW, Brown NJ. (2009). A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol 90:195-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel M, Conrads G, Pankuweit S, Maisch B, Vogt S, Moosdorf R, et al. (2002). Detection of Porphyromonas gingivalis DNA in aortic tissue by PCR. J Periodontol 73:868-870 [DOI] [PubMed] [Google Scholar]
- Stoll G, Bendszus M. (2006). Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke 37:1923-1932 [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, et al. (1999). Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation 99:2503-2509 [DOI] [PubMed] [Google Scholar]
- Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. (2007). Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med 13:719-724 [DOI] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. (2000). Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101:948-954 [DOI] [PubMed] [Google Scholar]
- Syrjala AM, Ylostalo P, Hartikainen S, Sulkava R, Knuuttila ML. (2009). Number of teeth and myocardial infarction and stroke among elderly never smokers. J Negat Results Biomed 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjanen J, Peltola J, Valtonen V, Iivanainen M, Kaste M, Huttunen JK. (1989). Dental infections in association with cerebral infarction in young and middle-aged men. J Intern Med 225:179-184 [DOI] [PubMed] [Google Scholar]
- Szklo M, Miller D, Nieto FJ. (2000). Epidemiology: beyond the basics. Gaithersburg, MD: Aspen Publishers [Google Scholar]
- Taguchi A. (2007). Re: A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst 99:738-739; author reply 739 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Davey M, Yumoto H, Gibson FC, 3rd, Genco CA. (2006). Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cell Microbiol 8:738-757 [DOI] [PubMed] [Google Scholar]
- Taniguchi A, Nishimura F, Murayama Y, Nagasaka S, Fukushima M, Sakai M, et al. (2003). Porphyromonas gingivalis infection is associated with carotid atherosclerosis in non-obese Japanese type 2 diabetic patients. Metabolism 52:142-145 [DOI] [PubMed] [Google Scholar]
- Thagard P. (1998). Ulcers and bacteria I: discovery and acceptance. Stud Hist Phil Biol Biomed Sci 29:107-136 [Google Scholar]
- Thurnheer T, Gmür R, Guggenheim B. (2004). Multiplex FISH analysis of a six-species bacterial biofilm. J Microbiol Methods 56:37-47 [DOI] [PubMed] [Google Scholar]
- Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. (2007). Treatment of periodontitis and endothelial function. N Engl J Med 356:911-920 [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Gamper FG, Lepper PM, Mouratis MA, Schumann C, Harokopakis E, et al. (2007). Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol 9:2030-2039 [DOI] [PubMed] [Google Scholar]
- Tu YK, Galobardes B, Smith GD, McCarron P, Jeffreys M, Gilthorpe MS. (2007). Associations between tooth loss and mortality patterns in the Glasgow Alumni Cohort. Heart 93:1098-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen R, Reunanen A, Paunio M, Paunio I, Aromaa A. (2003). Oral health indicators poorly predict coronary heart disease deaths. J Dent Res 82:713-718 [DOI] [PubMed] [Google Scholar]
- Van Eden W, Wick G, Albani S, Cohen I. (2007). Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann NY Acad Sci 1113:217-237 [DOI] [PubMed] [Google Scholar]
- Verhoye E, Langlois MR. (2009). Circulating oxidized low-density lipoprotein: a biomarker of atherosclerosis and cardiovascular risk? Clin Chem Lab Med 47:128-137 [DOI] [PubMed] [Google Scholar]
- Verma S, Buchanan MR, Anderson TJ. (2003). Endothelial function testing as a biomarker of vascular disease. Circulation 108:2054-2059 [DOI] [PubMed] [Google Scholar]
- Victor VM, Rocha M, Sola E, Banuls C, Garcia-Malpartida K, Hernandez-Mijares A. (2009). Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des 15:2988-3002 [DOI] [PubMed] [Google Scholar]
- Virmani R, Burke AP, Farb A, Kolodgie FD. (2006). Pathology of the vulnerable plaque. J Am Coll Cardiol 47(8 Suppl):S13-S18 [DOI] [PubMed] [Google Scholar]
- Vlachojannis C, Dye BA, Herrera-Abreu M, Pikdöken L, Lerche-Sehm J, Pretzl B, et al. (2010). Determinants of serum IgG responses to periodontal bacteria in a nationally representative sample of US adults. J Clin Periodontol [Epub ahead of print June 15, 2010] (in press). [DOI] [PubMed] [Google Scholar]
- Volzke H, Schwahn C, Dorr M, Schwarz S, Robinson D, Doren M, et al. (2006). Gender differences in the relation between number of teeth and systolic blood pressure. J Hypertens 24:1257-1263 [DOI] [PubMed] [Google Scholar]
- Wang M, Hajishengallis G. (2008). Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell Microbiol 10:2029-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liang S, Hosur KB, Domon H, Yoshimura F, Amano A, et al. (2009). Differential virulence and innate immune interactions of type I and II fimbrial genotypes of Porphyromonas gingivalis. Oral Microbiol Immunol 24:478-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JR, Wilson HL, Francis SE, Crossman DC, Sabroe I. (2009). Translational mini-review series on immunology of vascular disease: inflammation, infections and Toll-like receptors in cardiovascular disease. Clin Exp Immunol 156:386-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JR, Marshall B. (1983). Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1:1273-1275 [PubMed] [Google Scholar]
- Wassmann S, Werner N, Czech T, Nickenig G. (2006). Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res 99:e74-e83 [DOI] [PubMed] [Google Scholar]
- Webb NR, Moore KJ. (2007). Macrophage-derived foam cells in atherosclerosis: lessons from murine models and implications for therapy. Curr Drug Targets 8:1249-1263 [DOI] [PubMed] [Google Scholar]
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. (2005). Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353:999-1007 [DOI] [PubMed] [Google Scholar]
- Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, et al. (2007). Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol 102:565-571 [DOI] [PubMed] [Google Scholar]
- Wick G, Perschinka H, Xu Q. (1999). Autoimmunity and atherosclerosis. Am Heart J 138(5 Pt 2):S444-S449 [DOI] [PubMed] [Google Scholar]
- Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. (2000). Periodontal disease and risk of cerebrovascular disease: the first National Health and Nutrition Examination Survey and its follow-up study. Arch Intern Med 160:2749-2755 [DOI] [PubMed] [Google Scholar]
- Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, et al. (2001). Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104:3103-3108 [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Ohsawa Y, Itoh H, Ueki K, Tabeta K, Oda T, et al. (2004). T-cell clonality to Porphyromonas gingivalis and human heat shock protein 60s in patients with atherosclerosis and periodontitis. Oral Microbiol Immunol 19:160-167 [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Honda T, Oda T, Ueki-Maruyama K, Nakajima T, Yoshie H, et al. (2005). Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J Periodontal Res 40:53-58 [DOI] [PubMed] [Google Scholar]
- Ylöstalo PV, Knuuttila ML. (2006). Confounding and effect modification: possible explanation for variation in the results on the association between oral and systemic diseases. J Clin Periodontol 33:104-108 [DOI] [PubMed] [Google Scholar]
- Ylöstalo PV, Jarvelin MR, Laitinen J, Knuuttila ML. (2006). Gingivitis, dental caries and tooth loss: risk factors for cardiovascular diseases or indicators of elevated health risks. J Clin Periodontol 33:92-101 [DOI] [PubMed] [Google Scholar]
- You Z, Cushman M, Jenny NS, Howard G. (2009). Tooth loss, systemic inflammation, and prevalent stroke among participants in the reasons for geographic and racial difference in stroke (REGARDS) study. Atherosclerosis 203:615-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RA, Elliott TJ. (1989). Stress proteins, infection, and immune surveillance. Cell 59:5-8 [DOI] [PubMed] [Google Scholar]
- Yumoto H, Chou H-H, Takahashi Y, Davey M, Gibson FC, Genco CA. (2005). Sensitization of human aortic endothelial cells to lipopolysaccharide via regulation of Toll-like receptor 4 by bacterial fimbria-dependent invasion. Infect Immun 73:8050-8059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto H, Yamada M, Shinohara C, Nakae H, Takahashi K, Azakami H, et al. (2007). Soluble products from Eikenella corrodens induce cell proliferation and expression of interleukin-8 and adhesion molecules in endothelial cells via mitogen-activated protein kinase pathways. Oral Microbiol Immunol 22:36-45 [DOI] [PubMed] [Google Scholar]
- Yun PLW, Decarlo AA, Chapple CC, Hunter N. (2005). Functional implication of the hydrolysis of platelet endothelial cell adhesion molecule 1 (CD31) by gingipains of Porphyromonas gingivalis for the pathology of periodontal disease. Infect Immun 73:1386-1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba M, Górska R, Suwalski P, Kowalski J. (2007). Evaluation of the incidence of periodontitis-associated bacteria in the atherosclerotic plaque of coronary blood vessels. J Periodontol 78:322-327 [DOI] [PubMed] [Google Scholar]
- Zenovich AG, Taylor DA. (2008). Atherosclerosis as a disease of failed endogenous repair. Front Biosci 13:3621-3636 [DOI] [PMC free article] [PubMed] [Google Scholar]