Abstract
Intake of excess amounts of fluoride during tooth development cause enamel fluorosis, a developmental disturbance that makes enamel more porous. In mild fluorosis, there are white opaque striations across the enamel surface, whereas in more severe cases, the porous regions increase in size, with enamel pitting, and secondary discoloration of the enamel surface. The effects of fluoride on enamel formation suggest that fluoride affects the enamel-forming cells, the ameloblasts. Studies investigating the effects of fluoride on ameloblasts and the mechanisms of fluorosis are based on in vitro cultures as well as animal models. The use of these model systems requires a biologically relevant fluoride dose, and must be carefully interpreted in relation to human tooth formation. Based on these studies, we propose that fluoride can directly affect the ameloblasts, particularly at high fluoride levels, while at lower fluoride levels, the ameloblasts may respond to local effects of fluoride on the mineralizing matrix. A new working model is presented, focused on the assumption that fluoride increases the rate of mineral formation, resulting in a greater release of protons into the forming enamel matrix.
Keywords: fluoride, enamel fluorosis, amelogenin, ameloblasts, review
Introduction
An excess ingestion of fluoride induces multiple changes in the developing enamel, and is referred to as enamel fluorosis. Changes vary from chalky white opaque areas, resulting from subsurface hypomineralization, to pits and grooves, and with increased severity, post-eruption staining. Fluorotic enamel is softer and chips easily (for reviews, see Fejerskov et al., 1977, 1994; Giambro et al., 1995). Enamel fluorosis is observed in young children at fluoride intakes as low as 0.03 mg F/kg body weight, and there is a clear linear relationship between fluoride dose and the development of dental fluorosis, regardless of whether fluoride is ingested from drinking water, from supplements, or from other sources (Fejerskov et al., 1994; Warren et al., 2009). Although fluoride may have different effects in the various stages of enamel formation, its effect is greatest when exposure occurs during all stages of formation (Ishii and Suckling, 1986; DenBesten, 1999; Hong et al., 2006).
During the last four decades, many experimental animal and organ culture studies, as well as more recent cell culture studies, have investigated mechanisms by which fluoride affects ameloblasts and enamel formation. In these and future studies, important issues to consider include: how fluoride causes these changes in forming enamel, how fluoride affects different stages of development, and how fluoride affects ameloblast function. The effects of fluoride are highly dose-dependent, and proper interpretation of the cellular effects of fluoride, including effects on the ameloblasts, requires a careful analysis of the in vivo or in vitro model systems and the dose of fluoride used. The present review is focused on studies aimed at elucidating the effects of fluoride on the ameloblasts, and the objectives of the review are:
to evaluate the efficacy of animal model systems for the study of enamel fluorosis in humans;
to define the relevance of various fluoride doses in studies related to enamel fluorosis and how fluoride doses relate to fluoride plasma levels;
to describe which stages of enamel development are sensitive to fluoride and what types of mineralization disturbances occur at each stage; and
to present a working model that explains the observed effects of fluoride on the developing enamel organ.
Model Systems Used to Study The Effects of Fluoride On Human Tooth Development
The effects of fluoride on enamel development have been studied in a wide range of animal species and tooth types. In most studies, rat incisors (Angmar-Månsson et al., 1976; Angmar-Månsson and Whitford, 1982; DenBesten, 1986) and molars (Kruger, 1966, 1967; Mörnstad and Hammarström, 1978; Kardos et al., 1989) were used. Other studies used incisors (Suckling et al., 1988; Nelson et al., 1989) and molars of sheep (Milhaud et al., 1992), pig molars (Andersen et al., 1986; Richards et al., 1986; Kierdorf et al., 2004), rabbit incisors and molars (Susheela and Bhatnagar, 1993), hamster molars (Bronckers et al., 1984a,b; Lyaruu et al., 1986, 1987), mouse incisors (Everett et al., 2002; Vieira et al., 2005), and zebrafish teeth (Bartlett et al., 2005). With the exception of zebrafish, which do not form true enamel, most species develop enamel in similar ways, although there are some differences between species and the type of tooth studied.
Rodent incisors have been used as a model system for several reasons, including their rapid and continuous eruption, allowing for the study of the effects of fluoride on amelogenesis even in adult animals. Amelogenesis in rat and mouse incisors has been well-mapped (Smith and Nanci, 1996; Smith et al., 2005), and rat incisor enamel tissue is also sufficient to be sampled by micro-dissection. Molar tooth development in rodents is complete after the first 2 to 3 post-natal wks, and therefore the use of this tooth model has generally used fluoride administration as an acute dose by injections or gavage, instead of by drinking water, since rodents are weaned after this time period.
Essentially, small rodents, the rat in particular, have proved to be good models for the study of human dental fluorosis, since they show the same fluorotic disturbances at similar plasma levels as humans (Angmar-Månsson et al., 1976; Angmar-Månsson and Whitford, 1984, 1985; DenBesten, 1986; Everett et al., 2002). However, for reasons not fully understood, but possibly related to increased urinary secretion or more rapid bone growth by rodents, the doses of fluoride in the drinking water of rats and mice used for the study of fluorosis in the continuously erupting incisor are approximately 10 times higher than those required for humans to achieve similar fluoride plasma levels.
The mouse model has gained in importance as differences in fluoride susceptibility have been found between mouse strains (Everett et al., 2002). Mice will continue to be useful models, since transgenic and knockout mouse strains are becoming increasingly available, and will likely yield further insights into mechanisms by which fluoride affects enamel formation. Other useful models are developing molar teeth of hamsters (with faster tooth development than rats, and with a cusp morphology more similar to that of human than of rat and mouse molars), as well as teeth of larger animal species, such as sheep and pigs. Larger animal species are particularly useful for the study of proteins and proteinases isolated from the developing enamel.
Ameloblast cell culture models have been developed, and some studies have used isolated primary and SV40 transformed cells grown in vitro to address questions of specific effects of fluoride on ameloblasts (Kubota et al., 2005; Zhang et al., 2006; Yan et al., 2007). Cultured cells are useful tools, but must be carefully interpreted with physiologically relevant levels of fluoride and an understanding of how the cells, isolated and grown in vitro, reflect stages of ameloblast differentiation in vivo.
The Rat as a Model for Enamel Fluorosis
Fluoride at high doses is known to have acute toxic effects, including those that result in cell death. Therefore, in studying the effects of fluoride on ameloblasts, it is critical that one determine the appropriateness of the fluoride dose used in a particular study, as related to the questions addressed in that study. The rat has been the most frequently used model for studies of enamel fluorosis. However, differences in fluoride dose, administration, and age of the animals have made comparisons among published studies difficult.
In this section, we review the literature related to fluoride toxicity in the rat model, to better define biologically relevant fluoride levels in both in vivo and in vitro model systems. Fluoride can be administered either parenterally or in food or drinking water. Parenteral exposure by intraperitoneal, intravenous, intramuscular, or subcutaneous injection generally leads rapidly to a high, but transient, fluoride plasma peak level. These high peak fluoride plasma levels can induce dose-dependent systemic changes. Fluoride given in drinking water or food causes fluorosis at lower, but more sustained, plasma fluoride levels, without obvious systemic side-effects.
For appropriate fluoride levels to be evaluated, and the effects of fluoride on enamel development, it is relevant that one know the toxic doses of fluoride, including doses that induce systemic changes. LD50 values for fluoride in the rat (a measure for acute toxicity indicated by the dose that is lethal to 50% of the animals after a single intraperitoneal injection) depend on the age of the animals used (Mörnstad, 1975). The LD50 values for fluoride in rats are in the range of 40-45 mg F/kg body weight for 4-day-old pups, 28-30 mg/kg for 60-day-old young adult rats, and 20-21 mg/kg for 90-day-old rats (see Table 1). The younger the rats, the more resistant they are to fluoride, most probably due to their ability to clear fluoride rapidly from the circulation because of a high bone formation rate (Whitford, 1994).
Table 1.
Plasma Fluoride and Plasma Calcium after a Single Fluoride Injection into Rats at Different Ages
| Dose of F | Plasma Fluoride | Plasma Calcium | ||
|---|---|---|---|---|
| Rats’ Age | (mg/kg bw) | Peak, µmol/L | (% change) | References |
| Adult | 0.065 | 5 | ND | Angmar-Månsson and Whitford, 1982 |
| Adult | 0.10 | 10 | ND | Angmar-Månsson et al., 1976 |
| Adult | 0.13 | 9 | ND | Angmar-Månsson and Whitford, 1982 |
| Adult | 0.75 | 67 | normal | Angmar-Månsson and Whitford, 1985 |
| Adult | 4.0 | 527 | −8% | Angmar-Månsson and Whitford, 1985 |
| Adult | 4.5 | 373 | normal | Larsen et al., 1981 |
| Neonatal | 4.5 | 387 | normal | Larsen et al., 1981 |
| Adult | 7-9 | 1600** | −15%* | Monsour et al., 1985; Appleton, 1995 |
| Neonatal | 9.0 | 958 | −14% | Larsen et al., 1981 |
| Adult | 9.0 | 888 | −17% | Larsen et al., 1981 |
| Adult | 14 | 1981 | −48% | Angmar-Månsson and Whitford, 1985 |
| Neonatal | 18 | 1700 | −38% | Larsen et al., 1981 |
| Adult | 18 | 1750 | −58% | Larsen et al., 1981 |
| Adult | 20-21 | LD50 | ND | Mörnstad, 1975 |
| Young adult | 28-30 | LD50 | ND | Mörnstad, 1975 |
| Young | 47-51 | LD50 | ND | Mörnstad, 1975 |
| Neonatal | 40-45 | LD50 | ND | Mörnstad, 1975 |
1 part per million fluoride (1 ppm F, 1 mg/L) is equivalent to 52 µmol/L, or, vice versa, 1 µmol/L is 0.019 ppm fluoride.
bw, body weight; ND, not determined; LD50 lethal dose for 50% of the rats after one intraperitoneal injection.
Also rise in plasma values for creatinine, urea, and glucose.
Injection given intravenously and peak attained within 1 min. In most other reports, injection was given intraperitoneally and peak level attained after 30 min.
On the basis of these data, some of the older reports of the effects of parenterally applied fluoride on enamel formation in rats show levels that appear to be within the toxic range, and need to be interpreted with caution (Neiman and Eisenmann, 1975; Chen and Eisenmann, 1984; Ashrafi et al., 1988). Indeed, some studies have reported toxic effects after fluoride injection, including a delay in growth (less gain in body weight), immobility, and change of skin color (Mörnstad and Hammarström, 1978), or even death a few hours after injection of fluoride (Larsen et al., 1981). Rats fed a diet containing up to 11 mg F/day for 2 yrs showed a 30% decrease in body weight, with toxic changes in teeth, bones, and stomach (Maurer et al., 1990).
No adverse changes or effects on growth have been found in rat pups daily receiving 0.3-4 mg F/kg, delivered by either intraperitoneal injection, subcutaneous release devices, or gastric intubation (Drinkard et al., 1987). In rat pups and adult rats, an injection of 4.5 mg F/kg body weight does not change plasma calcium levels or induce other undesired systemic changes (Larsen et al., 1981; Angmar-Månsson and Whitford, 1985). A dose of 9 mg F/kg weight, however, starts to depress plasma calcium transiently by 15% (Larsen et al., 1981). Plasma calcium levels further decrease to 48% of control values in adult rats at a dose 14 mg F/kg; plasma calcium levels, however, normalize within 1 day (Angmar-Månsson and Whitford, 1985) (see Table 1).
Importantly, a parenteral dose in the range of 7-9 mg F/kg body weight also affects renal functions in adult rats, as indicated by a fast rise in plasma values for creatinine and urea (Monsour et al., 1985; Appleton, 1995). In this respect, it is important to know that amelogenesis of rat incisors is seriously disrupted in uremic rats (Lyaruu et al., 2008b). Furthermore, fluoride injection increases plasma phosphate and induces hyperglycemia, suggesting effects on kidneys and general stress (Monsour et al., 1985).
Systemic changes have not been found in rats exposed for 3-6 wks to fluoride in drinking water, at levels up to 100 ppm (mg/L)—a dose widely used in studies of enamel fluorosis in rodents. This dose does not show clear toxic effects (Smith et al., 1993; Zhou et al., 1996). Concentrations of 125 ppm F in drinking water, starting at weaning, do not change body weight when given during the first 6 wks, but reduce gain in body weight after 20 wks of exposure. A level of 175 ppm F in drinking water is lethal for about one-third of the rats within 10 days when started at weaning; the remaining rats have stunted growth (Mullenix et al., 1995). No changes in plasma calcium were found in adult rats fed food pellets containing 450 mg F/kg for 8 wks (Appleton, 1994) or pigs receiving a daily oral dose of 2 mg F/kg body weight for 6 mos (Andersen et al., 1986). In the pigs, no changes in immunoreactive PTH and 1,25 vitamin D or 24,25 vitamin D metabolites could be found (Andersen et al., 1986). This indicates that, at those doses, fluoride does not interfere with calcium homeostasis.
Fluoride Intake in Relation to Plasma Fluoride Levels
In an effort to determine biologically relevant doses in animal, organ, and cell culture models, in this section we review fluoride intake and plasma fluoride levels. These plasma fluoride levels will then be related to enamel mineralization defects in the next section.
Fluoride chronically applied in drinking water induces slightly elevated but sustained plasma fluoride levels, while parenteral application induces short-lasting but high plasma peak levels that can induce systemic and toxic changes. These differences present different fluorotic effects on forming enamel and on the ameloblasts, as discussed below.
In drinking water, fluoride levels of at least 10-30 ppm are necessary to induce lasting enamel disturbances in rodents, and levels used in most experimental studies are in the range of 25-100 ppm (Shinoda, 1975; Angmar-Månsson et al., 1976; Fejerskov et al., 1979; Ekstrand et al., 1981; Angmar-Månsson and Whitford, 1984; DenBesten, 1986; Kubota et al., 2005). The high fluoride levels in drinking water needed to cause enamel fluorosis in rats (about 10 times the amount of humans) have raised the question as to whether studies in rats have relevance for the development of enamel fluorosis in humans. However, measurements of plasma F levels in rats exposed to fluoride in drinking water (from 10-100 ppm) revealed that these levels are in the same range as in humans exposed to much lower fluoride doses (1-8 ppm) in drinking water (Singer and Ophaug, 1982; Martínez-Mier et al., 2003).
The plasma levels in rats also show a linear dose-dependent increase in plasma fluoride levels, from 1 µmol/L (no fluoride) to 9-10 µmol/L, with increases in fluoride in drinking water from 0 to 100 ppm in rats given a low-fluoride diet (DenBesten, 1986). Adult rats receiving drinking water containing 25-100 ppm fluoride reach a steady plasma fluoride level in about 2 wks (Ekstrand et al., 1981). Similar data were obtained for pigs when fluoride was given orally during feeding (Richards et al., 1985; Andersen et al., 1986).
There is a daily variation in the plasma fluoride levels when food is denied to rats; the plasma fluoride peak levels were as high as 25 µmol/L in non-fasting conditions at 150 ppm fluoride in drinking water, while after 6-12 hrs of fasting, this plasma level was down to a steady level of 10 µmol/L (Ekstrand et al., 1981). At 100 ppm fluoride in drinking water, these levels were 6.5 µmol/L (non-fasting) and 5.4 µmol/L (fasting). In pigs fed a diet containing 2 mg F/kg, fluoride reached a short-lasting plasma peak of approximately 60 µmol/L, which rapidly decreased to a steady level of 12.7 µmol/L for 48 hrs after discontinuation of the fluoride application (Richards et al., 1985; Andersen et al., 1986). Analysis of these data suggests that plasma fluoride levels can vary considerably within a day, depending on feeding and drinking behavior.
Plasma fluoride levels rapidly return to baseline levels after discontinuation of a fluoride regime. Removing fluoride from the drinking water of rats given 25 ppm fluoride shows a rapid decrease of plasma fluoride levels to pre-exposure levels in about 2-3 days. Plasma levels remain slightly elevated at recovery from moderate to high doses of fluoride (50-100 ppm). However, plasma fluoride levels remain elevated significantly and over extended periods at recovery from very high (150 ppm) levels of fluoride in drinking water (Ekstrand et al., 1981; Larsen et al., 1981).
The plasma fluoride levels obtained after a single parenteral application of fluoride are proportional to the fluoride dose (Larsen et al., 1981), and are much higher than those obtained following ingestion of fluoride-containing drinking water (Table 1). After intraperitoneal injection of fluoride into rats, a plasma fluoride peak level is attained within 30 min, followed by a rapid decline due to clearance from the circulatory system by kidney and skeletal tissues (plasma fluoride half-life in rats is approximately 45 min to 1 hr; Larsen et al., 1981).
Injections of 7-9 mg F/kg body weight start to reduce plasma calcium levels, while plasma calcium levels drop very significantly at twice that fluoride dose (Table 1). Rats on a low-calcium diet also show a more pronounced decrease in plasma calcium and a delay in fluoride clearance, as compared with rats on a normal calcium-rich diet (Larsen et al., 1981).
Plasma fluoride levels also depend on the rate of fluoride clearance by the kidneys (Whitford, 1994), and renal insufficiency delays fluoride clearance. Partial nephrectomy decreases fluoride clearance 7-fold, and plasma fluoride levels increase as high as 70 µmol/L, 7-fold the values in sham-operated control rats under a fluoride regime in drinking water for 3-6 mos (Dunipace et al., 1998). Changes in amelogenesis of partially nephrectomized rats are more severe when these rats are also exposed to low doses of fluoride in drinking water (Lyaruu et al., 2008b). Increased plasma fluoride levels are also obtained during metabolic acidosis, which makes the kidneys re-absorb more fluoride, when rats are exposed to fluoride in drinking water. Despite a lower total fluoride intake by acidotic rats, as compared with normal or alkalotic rats, plasma fluoride levels in acidotic rats are substantially higher than in normal or alkalotic rats (Whitford and Reynolds, 1979). This indicates that plasma fluoride levels can be independent of, or even inversely related to, fluoride intake and strongly points out the necessity of using plasma fluoride levels, rather than total fluoride intake, to assess dose-effect relations (Whitford and Reynolds, 1979).
Different plasma fluoride levels have been reported for male and female rats given the same amount of fluoride in drinking water for 6 wks, suggesting gender-related differences (Mullenix et al., 1995). Lower plasma fluoride levels were measured in female rats than in age-matched males when fluoride exposure started at weaning, but higher levels were found in females when exposure started at the age of 3 mos. The latter finding may be related to continuous growth of male rats; female rats cease growth when they reach adulthood, as indicated by a two-fold-higher body weight of male rats at the end of the experiment (Mullenix et al., 1995). Similar gender-related differences in plasma fluoride levels have not been reported in humans.
When plasma fluoride levels associated with enamel fluorosis in different species are compared, the values appear in the same range in rodents, humans, sheep, and pigs (Angmar-Månsson et al., 1976; Fejerskov et al., 1979; Angmar-Månsson and Whitford, 1982, 1984, 1985; Andersen et al., 1986; Bawden et al., 1992; Milhaud et al., 1992). Hence, plasma fluoride values are currently the best way to compare effects of fluoride in relation to the development of enamel fluorosis between and among different species.
Doses of Fluoride that Cause Mineralization Defects in Developing Rat Enamel
Parenteral Administration of Fluoride
A single injection with a low (2 mg F/kg body weight) to high dose of fluoride (14 mg F/kg body weight) induces a characteristic double-response event in secretory enamel, consisting of an inner hyper- followed by an outer hypo-mineralized line (Kruger, 1967; Fejerskov et al., 1974; Neiman and Eisenmann, 1975; Shinoda, 1975; Tros et al., 1990; Lyaruu et al., 2006). The intensity of the double-response line is dose-dependent. The formation of the response lines continues until fluoride is cleared from the plasma, after which normal mineralization resumes (Kruger, 1970b; Walton and Eisenmann, 1974, 1975). Injections given some time apart result in corresponding multiple alternating double-response lines, running in the enamel almost parallel to the enamel surface (e.g., Kruger, 1967; Walton and Eisenmann, 1974). The lines reflect the mineralization front of secretory enamel at time of injection and can be seen at any depth of enamel. Weak double-response lines gradually fade with time during progressive pre-eruptive mineralization, but strong response lines may persist in erupted enamel, seen as dark (hypomineralized) lines or bands on microradiographs (Angmar-Månsson and Whitford, 1985). To address the relevance of these hypo- and hypermineralized responses to fluoride injections to the mechanisms of enamel fluorosis, one must know the lowest dose of fluoride that can induce such mineralization disturbances.
The height of the plasma fluoride peak and the exposure time determine whether or not lasting mineralization disturbances will develop. A single injected dose of fluoride once a day that transiently increases plasma level to 10 µmol F/L (0.13 mg F/kg body weight per day) for 1 wk induces alternating layers of hypo- and hypermineralization and a slight subsurface hypomineralization in rat incisor enamel (Angmar-Månsson and Whitford, 1982). However, the same amount of fluoride, divided into 2 injections per day for 1 wk, results in 2 short-lasting peak levels of approximately 5 µmol F/L. which do not cause such defects. This observation demonstrates that the magnitude of fluoride plasma peak levels, and not the total daily dose, is the factor that determines whether or not this type of enamel mineralization defect will form.
However, if the same 5 µmol/L plasma fluoride levels are kept at a continuously sustained level for 1 wk, by means of subcutaneously implanted mini-pumps, enamel defects become apparent, consisting of double-response lines and a reduction in mineral content in subsurface enamel (Angmar-Månsson and Whitford, 1982). This indicates that even low but sustained levels of fluoride can induce such mineralization defects. Such low sustained plasma levels may be related to prolonged release of fluoride from bone after an initial high fluoride uptake into a bone reservoir (Angmar-Månsson and Whitford, 1982, 1985), or a build-up of a fluoride reservoir within the enamel matrix.
Chronic Fluoride Intake from Drinking Water
The lowest dose of fluoride in drinking water that induces visible and lasting defects in fully mature rat incisor enamel is 25-30 ppm (1.3-1.6 mM) fluoride (Angmar-Månsson and Whitford, 1984; DenBesten et al., 1985). The minimal fluorotic dose also depends on the time of exposure: Exposure to 9 ppm (0.5 mM) fluoride for 70 days significantly decreases the hardness of the outer enamel of erupted rat incisors, indicating that a prolonged exposure to a low fluoride dose already induces functional defects in the enamel (Shinoda, 1975).
The various mineralization defects (seen by microradiography) that develop in rat incisor enamel when fluoride is delivered for 1 or 8 wks, either by a mini-pump or in drinking water, are shown in Table 2. The defects consist of 3 types of disturbances that may persist in mature enamel: (1) a thin but highly mineralized outer enamel surface that develops pre-eruptively (Angmar-Månsson and Whitford, 1984; Richards et al., 1992); (2) a less-mineralized subsurface that expands inward at a higher fluoride dose; and (3) multiple alternating but weak lines of higher and lower mineral content throughout the enamel width, almost parallel to the enamel surface. These are similar to, but much weaker than, the lines seen formed in the secretory stage after multiple injections of low to high doses of fluoride. These lines are probably associated with the daily fluctuations in plasma fluoride when rats are on a fluoridated drinking water regime, depending on periods of feeding and drinking. Defects are dose-dependent and become more serious at higher fluoride levels or longer exposure times.
Table 2.
Plasma Fluoride Levels and Enamel Mineralization Defects in Rat Incisors after Chronic Exposure to Fluoride
| F Delivery, Exposure Time | Dose of F per Day per kg bw or Total Intake per Day | Plasma F µmol/L | Mineralization Defects | References |
|---|---|---|---|---|
| Minipump control(7 days) | 0 mg/kg* | 1.8 | Sound enamel | Angmar-ånsson and Whitford, 1982 |
| Minipump (7 days) | 0.68 mg/kg* | 3.3 | Mature enamel subsurface hypomineralized multiple double-response lines in 25% of the cases | Angmar-Månsson and Whitford, 1982 |
| Minipump (7 days) | 1.37 mg/kg* | 4.7 | Mature enamel subsurface hypomineralized multiple double-response lines | Angmar-Månsson and Whitford, 1982 |
| Control | 0 mg** | <1.0 | Sound enamel | Angmar-Månsson and Whitford, 1984 |
| Minipump (56 days) | 0.14 mg** | 1.5 | Early maturation (very weak) subsurface hypomineralizedMature enamel normal | Angmar-Månsson and Whitford, 1984 |
| Minipump (56 days) | 0.32 mg** | 3.1 | Early maturation (weak) surface hypermineralized subsurface hypomineralized multiple double-response lines Mature enamel normal | Angmar-Månsson and Whitford, 1984 |
| 10-ppm drinking water (56 days) | 0.41 mg** | 3.4 | Early maturation (weak) subsurface hypomineralized Mature enamel normal | Angmar-Månsson and Whitford, 1984 |
| 25-ppm drinking water (56 days) | 0.93 mg** | 6.8 | Early maturation surface hypermineralized subsurface hypomineralized multiple double-response lines Mature enamel moderate defects | Angmar-Månsson and Whitford, 1984 |
| 60-ppm drinking water (56 days) | 2.25 mg** | 12 | Early maturation: surface hypermineralized subsurface hypomineralized multiple double-response lines Mature enamel severe defects | Angmar-Månsson and Whitford, 1984 |
Throughout the manuscript, the expression ‘double response lines’ is used to describe the typical reaction of secretory enamel to fluoride peak levels. ‘Double response lines’ represents an inner hypermineralized line and an outer hypomineralized line.
Fluoride levels in food: 28 ppm.
Fluoride levels in food: 0.5 ppm (0.009 mg F/day).
Sustained plasma fluoride levels (as attained by mini-pumps) are more potent in inducing enamel defects than transient levels reached by the intake of fluoridated water. At low plasma fluoride levels (1.5-3.1 µmol F/L), the defects are temporary, seen only in transitional-stage to early-maturation-stage, but not in fully mature, enamel (Table 2). This suggests that small defects can be healed at successive stages of pre-eruptive enamel maturation, to the point at which they are not visible by microradiography. Enamel defects are worse and become permanent at higher fluoride doses and at prolonged exposure (Table 2).
Basal plasma fluoride levels in non-fluoridated control animals are in the range of 0.5-2.0 µmol/L, but may show some variation, likely depending on fluoride in the diet. The finding in some reports that baseline plasma fluoride levels of unexposed animals are already within the fluorotic range—e.g., in the mouse (9.4 µmol/L) (Kubota et al., 2005), sheep (5 µmol/L) (Milhaud et al., 1992), and pigs (5 µmol/L) (Richards et al., 1985)—is somewhat difficult to explain. These baseline differences may be related to fluoride content in the diet, or the method used for fluoride analysis. Measurement of fluoride levels in plasma is a complex assay, and requires that fluoride be diffused from the plasma into a buffered solution to allow for accurate measurement of fluoride by an ion-specific electrode (Taves, 1968; Fry and Taves, 1970).
An interesting recent development concerning the relationship between plasma fluoride levels and fluorosis is a possible genetic influence on the susceptibility to fluoride (Everett et al., 2002, 2009; Vieira et al., 2005). Screening of 12 different mouse strains for loss of yellow pigmentation of erupted incisors showed that some strains are very susceptible to fluoride, while others are relatively resistant (Everett et al., 2002), in spite of the fact that plasma fluoride levels in all strains were not significantly different (Everett et al., 2009). A dental fluorosis-associated trait for susceptibility to fluoride locates on mouse chromosomes 2 and 11 (Everett et al., 2009). However, later functional analysis indicated that the enamel of the most susceptible strain was, from the beginning, already significantly softer and retained more proteins than the most resistant strain (Vieira et al., 2005). This suggests that ‘normal’ enamel mineralization in the susceptible strain is already different from non-susceptible strains (Vieira et al., 2005).
In summary, fluoride induces mineralization disturbances in a dose- and time-dependent manner. Plasma fluoride levels correlate with enamel mineralization disturbances. Chronic plasma fluoride levels in the range of 2-12 µmol/L, attained by fluoride in drinking water for extended periods of time, induce disturbances in forming enamel in most species, including humans.
Stages of Enamel Development that are Affected By Fluoride
Experimental studies show that fluoride influences ameloblasts and enamel formation differently at different stages of the life cycle, resulting in different types of enamel defects. This is illustrated, for instance, by the formation of cysts at particular stages of ameloblast development after a single injection with a high dose of fluoride (Fig. 1). Generally, the fluoride doses required to induce cellular changes in ameloblasts are at least two orders of magnitude higher than the very low doses of fluoride that induce mineralization disturbances. The effect of fluoride on each stage of enamel formation will therefore be discussed separately. We will also address the type of permanent enamel lesion resulting from each disturbance. First, a brief description of the stages of amelogenesis is presented.
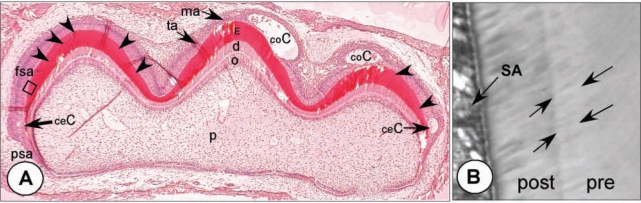
Figure 1.
Cyst formation after single injection of a high dose of fluoride. (A) Hamster first maxillary molar tooth germ, at post-natal day 4, from an animal injected with 9 mg F/kg body weight and killed 24 hrs later. Undecalcified, hematoxylin-eosin staining (25x). Cysts have formed near the cervical area (ceC) at early-secretory stages and coronally (coC) at late-secretory/transitional stages. Enamel surface under the cysts is intensely mineralized. Fully secretory ameloblasts (fsa) are not overtly affected, but a thin weak response line (position indicated by arrowheads) runs almost parallel to the surface through secretory-stage enamel (E). At the right cusp, this line connects the bases of both coronal and cervical cysts. Late-secretory-stage cells in the left cusp are not affected (Lyaruu et al., 2006). (B) Higher magnification of boxed area with double-response in adjacent section stained with toluidine blue (400x). Between arrows is the pre-exposure (‘pre’) secretory enamel that hypermineralized during exposure and stained less intensely with toluidine blue. The enamel layer secreted after the F peak insult (‘post’) by secretory ameloblasts (SA) is less mineralized and stains darker with toluidine blue. Fig. 4d illustrates a similar area with a sharp transition of hyper- to hypomineralized enamel at the ultrastructural level. Psa, pre-secretory ameloblasts; fsa, fully secretory ameloblasts; ta, transitional ameloblasts; ma, maturation ameloblasts; d, dentin; o, odontoblasts, p, pulp.
Amelogenesis (a brief summary)
During their life cycle, ameloblasts go through different stages of differentiation (Fig. 2a). Pre-secretory ameloblasts differentiate into secretory ameloblasts that deposit a protein matrix, which acts as a temporary protein scaffold on which enamel crystals can form (Smith and Nanci, 1996). The first thin layer of enamel deposited against mantle dentin is aprismatic, formed by early-secretory ameloblasts that have not yet developed a Tomes’ process. The inner enamel layer, which constitutes the bulk of enamel, consists of prismatic enamel with rod (or prisms) and interrod structures (interprismatic enamel) formed by the Tomes’ processes of fully differentiated secretory ameloblasts. These cells secrete large quantities of protein matrix (predominantly amelogenins) into the enamel space. Thin but long enamel crystals grow preferentially in length in the wake of the retreating cells. At the end of secretion, the ameloblasts lose their Tomes’ process and deposit a final layer of aprismatic enamel with small crystals. The cells transform via a short transitional stage, where enamel matrix proteins undergo proteolysis and gradual removal from the matrix, into maturation ameloblasts. In the maturation stage, the ameloblasts modulate cyclically from cells with a smooth-ended into ruffle-ended distal membrane, the latter with characteristics of resorbing cells. During this modulation, matrix proteins continue to be removed from the extracellular space, and mineralization increases progressively until the tooth erupts. After eruption, the enamel is exposed to exchange with mineral ions of the oral fluids that can influence the composition of the outer layers of enamel.
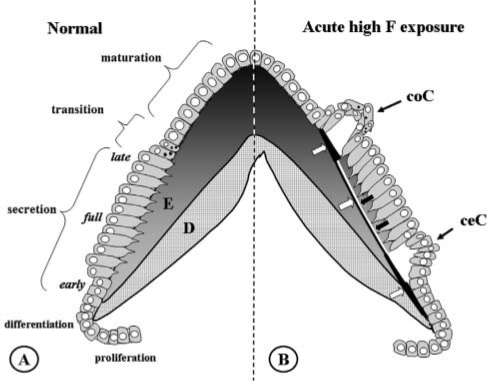
Figure 2.
Normal amelogenesis (A) and amelogenesis 24 hrs after an acute high exposure to fluoride (B). (A) Schematic drawing of normal amelogenesis in an imaginary cusp of a molar. D, dentin; E, enamel. Increasing greyness in enamel represents increasing mineral content. Successive stages of development from bottom to top. Aprismatic enamel with small crystallites is produced by early- and late-secretory ameloblasts. The bulk of (inner) enamel consists of prisms containing large crystals and is deposited by fully differentiated ameloblasts. (B) Amelogenesis 24 hrs after injection of 9 mg F/kg. Fluoride induces an (inner) hypermineralized layer (black line, white arrows) in fully secretory-stage enamel, running almost parallel to the surface of the enamel. It represents the mineralization front 24 hrs earlier at the time of fluoride injection. A later-formed (outer) layer is a hypomineralized layer (white line, black arrows); together, these lines form the double-response typical of fluoride. Two areas of intense hypermineralization are formed at both ends of these lines: one where the lines intersect the enamel-dentin junction in the inner aprismatic enamel, the other where the lines intersect the enamel surface with outer aprismatic enamel below late-secretory and transitional ameloblasts. Cyst formation occurs only under some groups of transitional ameloblasts (coronal cyst, coC) and early-secretory ameloblasts (cervical cyst, ceC) that detach from enamel surface. Maturation ameloblasts seem structurally unaltered. Fully secretory-stage ameloblasts recover completely after 24 hrs; only the double-response lines are reminiscent of the insult.
Effects of Fluoride on Amelogenesis
Proliferating and Differentiating Ameloblasts
There is no evidence that exposure of developing teeth to physiological levels of fluoride, in vivo (Smith et al., 1993) and in organ culture (Kerley and Kollar, 1977; Levenson, 1980; Bronckers et al., 1984a; Bawden et al., 1992), affects tooth morphogenesis, cell proliferation, or differentiation of ameloblasts. Even in highly fluorotic teeth, the size and form of the teeth are not changed (e.g., Kierdorf and Kierdorf, 1997). In organ culture, proliferating and differentiating pre-ameloblasts are very resistant to fluoride, and fluoride’s effects on cell morphology and matrix synthesis have been reported only in the range of 1.3-5.2 mmol F/L (Kerley and Kollar, 1977; Levenson, 1980; Bronckers et al., 1984a; Bronckers and Wöltgens, 1985; Li et al., 2005), which is far higher than the plasma levels that can induce enamel fluorosis in vivo.
There are data indicating that in vitro ameloblast-like cells can be sensitive to low levels of fluoride. Studies of human primary enamel organ epithelial cells, grown in culture, have shown that 16 µmol F/L significantly increases cell proliferation, while 1 mmol F/L inhibits cell proliferation (Yan et al., 2007). In addition, the apoptotic index for human primary enamel organ cells, identified by cell sorting, is increased at both 10 and 20 µmol F/L in culture, though these fluoride levels are higher than the plasma fluoride levels to which early-differentiating ameloblasts would likely be exposed. Also, exposure of human primary enamel organ epithelial cells to fluoride levels as low as 5 µmol/L fluoride results in reduced expression of MMP-20, normally synthesized in secretory ameloblasts (Zhang et al., 2006) and mediated by JNK/c-Jun signaling (Zhang et al., 2007). The significance of these in vitro data is not yet understood. Although these fluoride levels are still relatively high compared with plasma fluoride levels, they are in the physiologic range, and may have an effect on enamel formation in vivo in the long run. More detailed studies are needed to confirm the effects of such low levels of fluoride on enamel formation in vivo.
Studies in an SV40-transformed mouse enamel organ epithelial cell line showed no effect of fluoride on cell proliferation, but high fluoride levels (125 µmol/L to 1 mmol/L) initiated an endoplasmic reticulum (ER) stress-response in these cells (Sharma et al., 2008). These fluoride levels are much higher than serum fluoride levels and may be in the toxic range, suggesting that the effects of fluoride on ER stress require further study at lower doses.
These cell-culture studies underline the importance of understanding the relationship between fluoride given in vivo, either in the drinking water or through parenteral injection (millimolar levels resulting in micromolar plasma levels), and fluoride exposure in either cell or organ culture at micromolar levels similar to what would likely be found in vivo.
Early-secretory Ameloblasts
Injections of moderate doses (3-7 mg F/kg body weight) affect cell structure of early-secretory ameloblasts and reduce protein synthesis dose-dependently and transiently (Kruger, 1970a,b). A single higher dose of fluoride (9 mg F/kg body weight) induces cyst formation by early-secretory ameloblasts in the cervical loop area of developing hamster molars, and affects the structure of these ameloblasts (Figs. 1, 2b). The aprismatic enamel under these cyst-forming layers of early-secretory cells is extremely hypermineralized (Lyaruu et al., 1989a,b, 1990). In contrast, enamel matrix secreted during fluoride exposure fails to mineralize (referred to as ‘fluorotic matrix’). The same observations are seen in tooth organ cultures exposed to fluoride (Bronckers et al., 1984b). In vitro, these effects are more severe when calcium levels in the culture media, during exposure to fluoride, are low. At high media calcium levels, the fluoride effects on matrix and on cell structure are much less severe, or absent (Bronckers et al., 1989). These results suggest that, in vivo, the drop of plasma calcium associated with injection of a high dose of fluoride may enhance the severity of fluorotic lesions.
How do such lesions relate to the defects seen in mature enamel? Various fluorotic lesions that can be observed in erupted teeth after chronic exposure to low levels of fluoride are depicted schematically in Fig. 3a, or acute but high levels of fluoride (Fig. 3b) during pre-eruptive stages of enamel development. Most severe lesions are pits and grooves in the enamel seen occlusally or cervically. Cervically located deep, narrow, hypoplastic pits have been found in highly fluorotic molars and premolars in two species of deer (Kierdorf and Kierdorf, 1997). Cervical defects (chalky white and diffuse opacities) have also been found in erupted fluorotic teeth of sheep (Suckling and Thurley, 1984), adding further evidence that ameloblasts at this early secretory stage are highly susceptible to fluoride-related effects. It has been suggested that cervical pit formation is associated with a specific effect of fluoride at the transition of pre-secretory into secretory ameloblasts (Kierdorf and Kierdorf, 1997), cell stages that also form cysts in hamster molars (Lyaruu et al., 2006). Thus, the formation of cervical cysts seen in pre-eruptive stages conceivably leads to the cervical pits found post-eruptively.
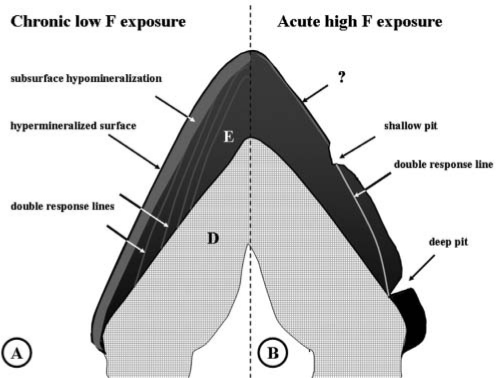
Figure 3.
Permanent lesions induced by chronic or acute exposure to fluoride found or expected in erupted fluorotic enamel. During amelogenesis, the tooth was chronically exposed to low-dose fluoride in drinking water (A) or to a single high parenteral dose of fluoride (B). Black represents the fully mineralized enamel, grey, hypomineralized. E, enamel; D, dentin. (A) Characteristic of low chronic doses of fluoride acting on the maturation stage is development of the hypomineralized subsurface area (grey), and a thin hypermineralized outer surface layer (black). Multiple weak hypo- and hypermineralized (grey) lines run through the enamel, the double-response lines, formed at the secretory stage. The enamel-dentin junction also contains a double-response (not shown). (B) Post-eruptive defects after a single high plasma peak level of fluoride (9 mg F/kg injection) are deep (hypothetical) and shallow pits that result from sub-ameloblastic cysts formed by damaged early- and late-secretory ameloblasts. The altered mineralization pattern seen in the double-response line partially recovers during post-exposure maturation, but remains hypomineralized. Maturation stages may also be affected (indicated by question mark) by chronic low but sustained levels of fluoride over time released by bone remodeling after clearance of F plasma peak levels.
It is relevant to note that the damage to early-secretory ameloblasts is correlated with the amount of mineral deposited in the aprismatic enamel and inversely with the thickness of this enamel present before fluoride was given. The thinner the layer of enamel, the more intensely it hypermineralized (Figs. 4a, 4b), and the more severely the adjacent ameloblasts are affected (Bronckers et al., 1984b; Lyaruu et al., 2006). Analysis of these data points to the possible molecular mechanism of fluorosis discussed below.
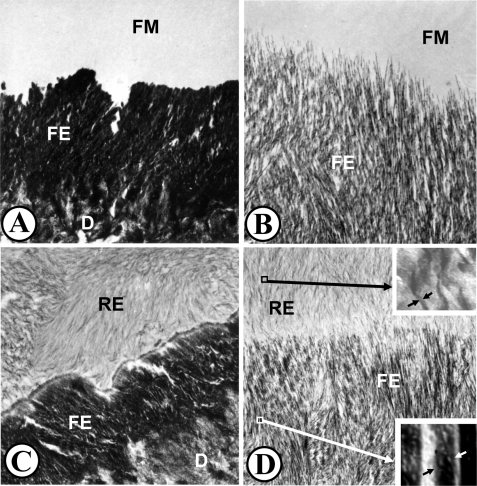
Figure 4.
Ultrastructural micrographs of hamster molar tooth germs cultured for 24 hrs in the presence of 5 mg F/L (A,B) followed by a 24-hour recovery in fluoride-free media (C,D). (A) Fluorotic matrix (FM) fails to mineralize, whereas the initial aprismatic enamel (FE) deposited prior to fluoride exposure hypermineralizes intensely (about 15-fold of the pre-exposure control values). All the enamel crystals are laterally fused [see Lyaruu et al. (1989a) for details]. D, dentin. Original magnification: 26,400x. (B) Prismatic secretory enamel (FE) situated more occlusally also hypermineralizes, but to a lesser extent (about five-fold of control levels). Although hypermineralized, there are no lateral enamel crystal fusions. The new fluorotic matrix (FM) fails to mineralize. Original magnification: 34,000x. (C) Same enamel region as that shown in Fig. 4A after recovery. The fluorotic matrix has recovered (RE) after 24-hour culture in fluoride-free media. The new crystals are thin compared with the fluorotic crystals. The crystals show preferred orientation within the matrix, but are not continuous with crystals in the fluorotic enamel (FE). This is a potential fracture plane after tooth eruption. D, Dentin. Original magnification: 20,500x. (D) Enamel region comparable with that shown in 4B, but after a 24-hour “recovery” culture in fluoride-free medium. The fluorotic matrix has recovered (RE), i.e., mineralized. The new crystals are thin and ribbon-like (upper inset, side view of one crystal between arrowheads) and initiate and extend from the fluorotic enamel (FE) crystals. The degree of hypermineralization of the fluorotic enamel crystals did not change during the recovery period (lower inset, side view of one crystal between arrowheads; same magnification as upper inset; note difference in thickness with upper inset). D = dentin. Original magnification: 20,500x. Insets: 100,000x.
Fully Secretory Ameloblasts
Secretory ameloblasts are relatively resistant to the effects of acute fluoride exposure in comparison with early- and late-secretory cells, and cellular changes in secretory ameloblasts in vivo are reported only after the injection of high levels of fluoride. One or multiple injections of moderate (4-9 mg F/kg body weight) doses of fluoride into adult rats can transiently disrupt the structure of secretory ameloblasts in incisors, causing accumulation of matrix proteins, generating clear vacuoles, decreasing deposition of matrix, and inducing the typical double-response of hypermineralized followed by hypomineralized lines or bands in secretory enamel (Walton and Eisenmann, 1974; Neiman and Eisenmann, 1975; Mörnstad and Hammarström, 1978; Chen and Eisenmann, 1984; Ashrafi et al., 1988; Monsour et al., 1989; Matsuo et al., 1996). Double-response lines and disturbances in mineralization patterns are apparent in secretory enamel after a single injection of 2.2-9 mg F/kg body weight, but without significant structural changes in fully secretory ameloblasts 24 hrs after injection (Lyaruu et al., 2006). It should be noted that where these double-response lines intersect the dentin-enamel junction cervically and the enamel surface occlusally, the response lines are highly mineralized and represent the locations where cyst formation sometimes occurs. The highly mineralized areas under the cysts thus are part of the double-response reaction (Fig. 2b).
Chronic exposure to fluoride in drinking water or repeated injections of moderate fluoride doses reduces the thickness of enamel by about 10% (Smith et al., 1993; Zhou et al., 1996). Although this suggests that chronic exposure to fluoride reduces biosynthesis of matrix by secretory ameloblasts, there is no evidence for that (Bronckers and Wöltgens, 1985; DenBesten, 1986; Aoba et al., 1990). The small reduction in enamel thickness is possibly attributed to a limited disruption of vesicular transport in fluorotic secretory ameloblasts and subsequent intracellular degradation of a minor portion of the matrix by the lysosomal system (Monsour et al., 1989; Matsuo et al., 1996; Bronckers et al., 2002). Chronic fluoride exposure also affects mineralization patterns during the secretory stage, but much less obviously than with acute high levels of fluoride. In rats, chronic exposure in vivo to 25-100 ppm fluoride in drinking water induces multiple weak hypo- and hypermineralized lines (double response) (Angmar-Månsson and Whitford, 1984), without causing gross morphological changes in secretory ameloblasts (Smith et al., 1993). Such lines remain as the accentuated hypomineralized (‘incremental’) lines seen by microradiography in erupted enamel (Fig. 3a). In organ culture, where fluoride levels are sustained, similar defects (an inner hypermineralized band and an outer band of completely unmineralized fluorotic matrix) are found at a minimum effective dose of 1 ppm or 52 µmol F/L (Levenson, 1980; Bronckers et al., 1984a; Bronckers and Wöltgens, 1985; Lyaruu et al., 1989a). However, lower levels (10 µmol F/L) can generate the same fluorotic effects if culture time is prolonged to 8 days (Bronckers et al., 1984a). Thus, the severity of the mineralization defects depends on fluoride dose and exposure time.
Late-secretory, Transitional Ameloblasts
This cell stage appears more sensitive to peak fluoride levels than early- and fully secretory ameloblasts. In hamster molar tooth germs, a moderate dose of fluoride (4.5 mg F/kg body weight) induces the late-secretory to transitional cells, but not early-secretory ameloblasts, to detach occasionally from the surface and form subameloblastic cysts. The aprismatic layer near these sites is extremely hypermineralized (Lyaruu et al., 1989a,b). Not all transitional ameloblasts, however, are equally sensitive to fluoride, and it appears that only some isolated groups are affected.
Cyst formation after fluoride insult has also been reported in rat molar tooth germs (Nordlund et al., 1986; Nordlund and Lindskog, 1986). Remarkably, cysts are not seen in incisors of the same rat pups that form cysts in molar tooth germs (Mörnstad and Hammarström, 1978), or incisors of adult rats after multiple injections with fluoride (Walton and Eisenmann, 1974), or after prolonged exposed to fluoride in drinking water (Smith et al., 1993). It may be that late-secretory ameloblasts of rat incisors are less susceptible to fluoride than those in molar tooth germs.
In rat molars, these cysts develop into serious hypomineralization defects that are associated with shallow pitting in erupted enamel, as seen by scanning microscopy (Nordlund and Lindskog, 1986). Pits at the enamel surface are underlain by a hypermineralized layer similar to those seen below subameloblastic cysts in severely fluorosed teeth of sheep, deer, and wild boars (Suckling and Thurley, 1984; Kierdorf and Kierdorf, 1997; Kierdorf et al., 2000). It is thus conceivable that the enamel below the cysts under late-secretory ameloblasts will give rise to the shallow occlusal pits (Fig. 3b) often seen in severely fluorosed teeth in various species (Richards et al., 1986; Suckling et al., 1988; Kardos et al., 1989; Nelson et al., 1989; Milhaud et al., 1992; Susheela and Bhatnagar, 1993; Fejerskov et al., 1994; Kierdorf and Kierdorf, 1997).
Whether such lesions erupt as pits (Kierdorf et al., 2004), or develop into pits post-eruptively by mechanical damage of the surface layer that covers the porous subsurface defect (Fejerskov et al., 1994), remains to be investigated. Studies in sheep suggest that both options are possible (Suckling and Thurley, 1984). Cyst formation and pitting also happen after exposure to other agents, but the fluoride-induced cysts (Lyaruu et al., 2006) and pits (Kierdorf et al., 2004) are underlain by a particularly accentuated highly mineralized response line containing incorporated fluoride that distinguishes them as induced by fluoride (Lyaruu et al., 1989b).
This stage of development is likely also associated with formation of accentuated perikymata during chronic exposure to fluoride, clinically the first signs of enamel fluorosis. Perikymata are the openings of the incremental lines at the outer enamel surface, seen in erupted human enamel as small horizontal lines. At very low doses of fluoride, these lines become more pronounced. At slightly higher doses, these defects will expand along the enamel surface and fuse with such defects in neighboring perikymata to form the characteristic white opaque areas.
Maturation Ameloblasts
Although maturation-stage enamel seems to be most sensitive to fluoride, there are remarkably few structural changes in the maturation ameloblasts exposed to fluoride. No major structural changes have been found in maturation ameloblasts of molar tooth germs of rodent pups shortly after injection of a single high dose of fluoride that severely damages early- and late-secretory ameloblasts seen in the same teeth (Richards et al., 1986; Suckling et al., 1988; Lyaruu et al., 2006). Only repeated injections of fluoride over several days seriously change the structure of maturation ameloblasts of rat incisors (Walton and Eisenmann, 1974).
Chronic exposure to fluoridated drinking water slightly shortens the maturation ameloblasts of adult rat incisors (Smith et al., 1993), reduces or abolishes the typical orange pigmentation, slightly diminishes the numbers of lysosomes and phagosomes (Ribeiro et al., 2006), reduces lysosomal activity (Smid et al., 1990), and induces expression of ER stress proteins in mouse incisors (Sharma et al., 2008). This suggests that cellular changes in the maturation stage are gradual, and cell activity in general is decreased in the presence of fluoride, reminiscent of a more general toxic effect of fluoride.
Fluoride also disrupts the modulation of maturation ameloblasts (DenBesten et al., 1985; Nishikawa and Josephsen, 1987; Smith et al., 1993). There are fewer modulation bands representing groups of smooth-ended ameloblasts—visualized by calcein or GBHA staining—than in unexposed controls, and stained bands consisting of groups of ruffle-ended ameloblasts gradually become longer (DenBesten et al., 1985; Smith et al., 1993). The first modulation bands that disappear during fluoride exposure are the most incisal smooth-ended ameloblasts. At prolonged exposure, other smooth-ended bands disappear one by one in an incisal-to-apical direction (DenBesten et al., 1985). In addition to changes in modulation, fluoride also reduces the cyclic uptake of 45Ca labeling in a similar pattern (DenBesten et al., 1985). This change correlates with retention of protein, suggesting a delay in proteolytic breakdown, and a lower mineral content than in unexposed controls (DenBesten et al., 1985). When fluoride exposure is discontinued, smooth-ended bands reappear, starting from the youngest, most apical, part toward older, more incisal, bands. This suggests that the fluoride effect on modulation is reversible, and that the young modulating cells recover more rapidly than older ameloblasts.
The maturation stage of enamel development can be affected by fluoride, even if there is no fluoride exposure in earlier stages, and is considered the stage most susceptible to fluoride exposure (Table 2). The outer surface of the enamel progressively hypermineralizes during the maturation stage under chronic fluoride exposure, seen on microradiographs as white lines not observed in non-fluorosed enamel (Angmar-Månsson et al., 1976; Suga et al., 1987; Richards et al., 1992). Line-scan analysis of calcium and fluoride measured by electron microprobe confirmed the increased calcium and F content at the outermost layer of fluorotic enamel (Suga et al., 1987). Mineralization defects in rat incisor maturation-stage enamel can develop during prolonged exposure to fluoridated drinking water at levels as low as 9-10 ppm fluoride (Shinoda, 1975; Angmar-Månsson et al., 1976), and are characterized by the development of a generalized hypomineralized porous subsurface area along the entire crown enamel (Fig. 3a) (Shinoda, 1975; Angmar-Månsson et al., 1976; Angmar-Månsson and Whitford, 1982, 1984; Richards et al., 1992; Kierdorf et al., 2004). This type of defect correlates to the porous white opacities seen clinically. With increased fluoride exposure, the extent and degree of this hypomineralization area increase inward toward the enamel-dentin junction (Shinoda, 1975; Angmar-Månsson and Whitford, 1985; Milhaud et al., 1992; Fejerskov et al., 1994), particularly in the cervical portion of the teeth (Richards et al., 1985; Suckling et al., 1988; Kierdorf and Kierdorf, 1997).
Micro-indentation studies have indicated that these subsurface areas (containing the opaque zone) are softer with increasing fluoride doses in drinking water (Shinoda, 1975; Suckling and Thurley, 1984; Suckling et al., 1988; Milhaud et al., 1992). Matrix proteins disappear from non-fluorosed enamel in the maturation phase, but are retained in subsurface enamel of fluorosed teeth at fluoride levels, beginning at 25 ppm F in drinking water (DenBesten, 1986; Zhou et al., 1996).
Some of these fluorotic defects likely result from carry-over effects by ameloblasts affected in previous stages. There is convincing evidence, however, that fluoride-induced subsurface hypomineralization can occur independently in the maturation state only (DenBesten et al., 1985; Richards et al., 1986; Suckling et al., 1988).
Effects of Fluoride on Enamel Crystal Growth
Crystals in sound enamel are extremely long, and the dynamics of enamel crystal growth, sizes of the crystals, and their shape are well-controlled by matrix proteins during enamel formation (Simmer and Fincham, 1995; Smith and Nanci, 1996; Moradian-Oldak, 2001). It has long been known that fluoride causes mineralization defects and decreases enamel hardness. This suggests that fluoride may change crystal size, number, shape, or quality by interfering with their formation, as reported for bone crystals (Eanes and Hailer, 1998). Is there evidence that enamel crystals in fluorotic human teeth are different from those in sound enamel?
Unfortunately, there is not much detailed information available on enamel crystals in mature human fluorotic enamel. The few published studies disagree with each other with respect to the dimensions and morphology of fluorotic crystals. Four studies reported that fluorotic crystals have a significantly greater diameter than crystals in sound enamel, as determined by high-resolution electron microscopy (60-70 nm for fluorotic crystals vs. 40-50 nm for normal crystals; Kérébel and Daculsi, 1976), by x-ray diffraction of powdered enamel samples (average values 21 nm wide for fluorotic crystals and 16 nm for normal crystals; Vieira et al., 2005), or by scanning microscopy of fractured inner-enamel specimens (average values 10 nm for fluorotic crystals and about 4 nm wide for normal crystals; Sundstrom et al., 1978). In a detailed study of severely fluorosed human enamel, the strongly hypermineralized surface was found to contain large flattened hexagonal crystals (30-50 nm thick and 60-100 nm wide) and many extremely small irregularly shaped crystals, often even less than 10 nm wide and 10 nm thick (Yanagisawa et al., 1989). High-resolution images also showed extremely small crystals growing on or attaching to the surfaces of the large crystals. In the hypomineralized subsurface layer, crystals were far less abundant, most of them large, measuring 22 nm thick and 70 nm wide, and only a few small crystals. Some of the large crystals in this area had large central perforations along the c-axis, while several of the small crystals seemed partly dissolved (along their a- and b-axes), reminiscent of caries defects. Whether these caries-like defects had been formed pre- or post-eruptively is unknown. Unfortunately, no controls of sound enamel were included in several of these studies.
No differences between fluorotic and normal human crystals were found in two other studies (Fejerskov et al., 1974; Robinson et al., 2006). These six studies are difficult to compare with each other, and vary in many aspects, including state of eruption, severity of fluorosis, magnitude of crystal dimensions, analytical procedures, inclusion of non-fluorotic controls, or information on the exact position in the enamel where the fluorotic crystals were sampled. Though some of these studies suggest that crystals in human fluorotic teeth are different in morphology and dimension, the data are yet inconclusive. The issue whether crystals in human fluorotic enamel have characteristics different from those in sound enamel still needs to be resolved in well-defined samples of fluorotic enamel, preferably unerupted, to avoid post-eruptive changes.
With respect to the effect of fluoride on crystal morphology, more is known from experimental studies with rodents. In rat incisors, the dimensions of fluorotic crystals, isolated from secretory- and maturation-stage rat incisor enamel exposed to 75 ppm fluoride in drinking water, were not grossly different from those of control crystals. However, at the nanoscale level, viewed by atomic force microscopy, fluorotic crystals had a significantly rougher surface than non-fluorotic crystals (Kirkham et al., 2001). The crystal surface roughness—likely the extremely small crystals seen at the surfaces of large crystals (Yanagisawa et al., 1989)—increased with higher fluoride levels in the drinking water (Chen et al., 2006). The first small (though not statistically significant) rise in roughness was noted at a drinking water level of 25 ppm fluoride (Chen et al., 2006), the minimal level that induces obvious fluorotic changes in the rat incisor. Therefore, low sustained levels of fluoride in the micromolar range that induce the first clinical signs of fluorosis do not appear to change crystal size at microscale levels, but do affect the crystal surface structure at the nanoscale level.
At higher levels of exposure, fluoride may also change the shapes and sizes of the crystals at the microscale level. In organ culture explants, the crystals in early-secretory prismatic enamel formed under the influence of fluoride are much thicker (at least 5 times at 5 ppm fluoride; Fig. 4d, insets), but shorter in comparison with non-fluoride-containing control cultures (Lyaruu et al., 1986, 1987). The crystals in the prismatic enamel are still separated from each other by an intercrystalline space. However, fluorotic crystallites in aprismatic enamel are small, short, and thick and completely fused laterally, without leaving any intercrystalline space (Fig. 4a). The mineral content in these layers of aprismatic enamel can be up to 15 times that of control value areas, with increased calcium-to-phosphate ratios, and a high fluoride content (Lyaruu et al., 1989b, 1990, 2006; Tros et al., 1990).
Similar changes in crystal structure probably also occur in the presence of fluoride in late-secretory transitional enamel exposed to fluoride, which is extremely brittle and difficult to section (Fejerskov et al., 1974; Lyaruu et al., 1986, 1987, 2006). Fluorotic crystals in the prismatic secretory enamel in the same explants are thicker, but far less affected, remaining as individual crystals with greater intercrystalline distance than at the early-secretory stage (Fig. 4b) (Lyaruu et al., 1986, 1987).
The recovery of the fluorotic hypomineralized areas in organ cultures, after the removal of fluoride, shows that crystal growth, orientation, and organization of rod-interrod structures depend on the nature of the crystals in the hypermineralized areas, the presence of the (fluorotic) matrix, and the organizing influence of the Tomes’ processes. If the fluorotic crystals are small and completely fused into a mass, new crystals start to form in the fluorotic matrix at some distance from the interface matrix-crystal mass (Fig. 4c); they then extend in a random direction until new rod and interrod structures are formed by the Tomes’ processes of the recovered ameloblasts. If the hypermineralized crystals are still present as separate entities, crystal growth starts at these crystals, and the new crystals then grow in length in a well-ordered and controlled fashion (Fig. 4d).
Possible Mechanisms of Fluoride Action on Enamel Formation
Does Fluoride Change the Composition of the Matrix?
It has long been assumed that fluoride alters the composition of the enamel matrix, resulting in altered crystal growth. Ultrastructural studies show that the matrix secreted after fluoride injections appears more amorphous and stippled, with only sparsely distributed crystallites and increased intercrystalline spaces as compared with the normal enamel matrix (Neiman and Eisenmann, 1975; Chen and Eisenmann, 1984; Monsour et al., 1989). In severe cases, prismless enamel is found within prismatic inner enamel. At these locations, formation of prismatic enamel had been abruptly interrupted during fluoride insult, during which prismless enamel had formed; after recovery, the prismatic enamel continued to form (e.g., Neiman and Eisenmann, 1975; Chen and Eisenmann, 1984; Suckling et al., 1988; Monsour et al., 1989; Kierdorf and Kierdorf, 1997; Kierdorf et al., 2000, 2004).
Biochemical analyses of fluorotic secretory matrix have not identified changes in composition or quality of the matrix proteins (Bronckers and Wöltgens, 1985; DenBesten, 1986; Aoba et al., 1990). Furthermore, functional studies in organ culture have indicated that the fluorotic matrix retains the capacity to form mineral, as seen in forming enamel in vivo that recovers from a fluoride insult (Lyaruu et al., 1987). Removal of fluoride from the culture medium—mimicking fluoride clearance from the plasma—restores ameloblast structure, and allows for the initiation of crystal formation in the fluorotic matrix.
So, how can fluoride alter crystal formation in the enamel matrix, if protein synthesis is not altered? Studies have shown that fluoride does not directly bind to amelogenin proteins, but that fluoride is likely bound to calcium contained within the protein matrix (Tanimoto et al., 2008). Therefore, at high fluoride levels, fluoride may bind to amelogenins and other matrix proteins through protein-bound calcium. Though reversible, this interaction could alter matrix-mediated crystal growth. When fluoride levels decrease, fluoride would no longer bind calcium in the matrix, and normal crystal growth would proceed (Lyaruu et al., 1987).
Does Fluoride Impair Degradation of the Matrix?
Major effects of chronic fluoride intake are induced during the maturation stage, resulting in a hypomineralized subsurface enamel that contains less mineral and retains matrix. Many studies have proposed that fluoride impairs degradation of matrix proteins, with a resultant inhibition of crystal growth. The following mechanisms have been proposed:
Fluoride reduces degradation of matrix proteins by lowering the output of proteases by the ameloblasts (Tanabe et al., 1988; DenBesten and Heffernan, 1989; DenBesten et al., 2002; Robinson et al., 2006; Tanimoto et al., 2008).
Fluoride acts directly on protease activity in the extracellular matrix and inhibits matrix degradation (Suga, 1970; Robinson and Kirkham, 1984; DenBesten and Heffernan, 1989; DenBesten et al., 2002). Some studies show no such effect (Gerlach et al., 2000).
Fluoride changes the adsorption characteristics, surface area, or surface properties of enamel crystals to which matrix proteins adhere. This might influence their proteolytic degradation and cause matrix retention (Tanabe et al., 1988; Kirkham et al., 2001; Robinson et al., 2006; Tanimoto et al., 2008).
Fluoride reduces calcium in the enamel fluid required for protease activity (Crenshaw and Bawden, 1984).
Fluoride impairs endocytosis and intracellular degradation of matrix by modulating ameloblasts (Smid et al., 1990).
Fluoride increases apoptosis (Bartlett et al., 2005; Kubota et al., 2005; Yan et al., 2007) or stimulates some of the maturation ameloblasts to migrate from the ameloblastic layer (Nishikawa and Josephsen, 1987).
Such mechanisms would reduce the number of maturation ameloblasts and thus the capacity to degrade and remove the matrix and complete mineralization.
Arguments for involvement in fluorosis have been found for each of these factors, but it remains unclear which is the primary molecular mechanism that underlies the disturbance of enamel development.
Is the Matrix Physically Trapped within Fluorotic Enamel?
A possibility that has not received much attention is that the hypermineralized layers could act as a physical barrier, impairing the supply of mineral ions to deeper layers of enamel (Suga et al., 1987). The outer surface layer of the aprismatic fluorotic enamel progressively accumulates fluoride and hypermineralizes during the maturation stage, while the subsurface becomes porous (Suga et al., 1987; Nelson et al., 1989; Richards et al., 1992). Perhaps the hypermineralized surface layer that forms in the presence of fluoride, especially at locations where cysts are formed, impedes transfer of mineral ions and proteases into the enamel subsurface and prevents enamel matrix proteins in deeper layers from leaving the enamel compartment.
Does Calcium Modulate the Effect of Fluoride on Amelogenesis?
This review of the various ways in which fluoride can affect ameloblast function and alter enamel mineralization has discussed the effects that fluoride can have on ameloblasts. Many studies also point to the dynamic relationship between fluoride and calcium, and suggest a relationship between calcium bioavailability and fluorosis.
A clue to how fluoride may indirectly affect ameloblasts at low concentrations of fluoride can be found in studies of the effects of calcium on enamel formation, in the presence of fluoride. In hamster tooth organ-culture studies, the calcium concentration in the medium affects the amount of enamel formed in both control and fluoride-exposed tooth organs. At low calcium (0.9 mmol/L) concentrations in the culture media, no enamel is deposited, while at standard (2.6 mmol/L) and high (4.5 mmol/L) levels of calcium, normal amounts of enamel are formed (Wöltgens et al., 1987). Likewise, in the presence of fluoride, supplementation of extra calcium to the media enables the newly secreted ‘fluorotic’ matrix to mineralize and decreases or abolishes the fluoride-induced changes in ameloblast morphology (Bronckers et al., 1989, 2006). The effect of a significant reduction of ionic calcium in the medium (from 2.6 mmol/L to 0.9 mmol/L) on ameloblast structure, however, is far less severe than the supplementation of only 1-2 ppm F (52-104 µmol/L) to the medium.
Enamel crystal formation in this (fluorotic) matrix in a high-calcium environment is associated with increased amelogenin secretion (Bronckers et al., 2006). Recent studies of the effects of calcium on human primary ameloblast lineage cells grown in vitro showed up-regulation of amelogenin in the presence of calcium (Chen et al., 2009). The mechanism by which calcium regulates amelogenin remains to be determined. However, these studies support the relationship between calcium and amelogenin, in promoting enamel matrix secretion and protein-mediated biomineralization.
Analysis of these data suggests that calcium acts on secretory ameloblasts in two ways: (1) It modulates synthesis and secretion of amelogenins by the ameloblasts, with increased amelogenin synthesis correlated to higher calcium concentrations; and (2) calcium also improves transcellular calcium influx to provide mineral ions for crystal growth in the layer being deposited. On the basis of these data, it can be speculated that amelogenins protect against the formation of non-mineralizing fluorotic matrix and allow the matrix to mineralize, even in the presence of fluoride (Bronckers et al., 2006). These observations suggest that calcium and its effect on ameloblasts, including matrix secretion, may be a primary underlying mechanism for the effects of fluoride, and furthermore, the relationship among calcium, fluoride, and amelogenins seems to be key in the formation of fluorosed enamel.
How to explain the effects of calcium and amelogenins on fluoride? One hypothesis for the failure of new crystals to form in the fluorotic matrix is that there is a local shortage of calcium secondary to rapid calcium consumption at the mineralization front. However, the observation that the structure of secretory ameloblasts is far less affected by substantial lowering of medium calcium than by a relatively lower supplement of fluoride argues against a significant role of local hypocalcemia in fluoride-induced effects during the secretory phase.
Another hypothesis that accounts for the protective action of amelogenins is that amelogenins can bind fluoride and thus buffer against the effects of fluoride on crystallites. Though amelogenins do not directly bind fluoride (DenBesten et al., 1992), such effects may be possible through amelogenin-mediated calcium binding (Tanimoto et al., 2008).
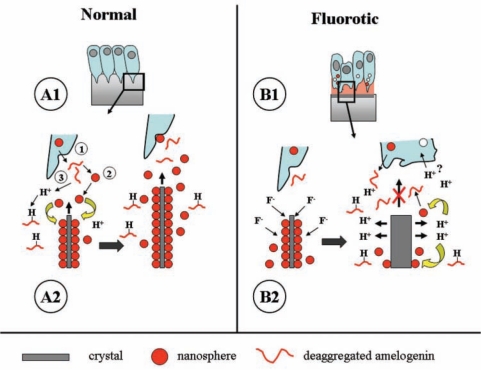
A third hypothesis that accounts for the ‘protective’ effects of amelogenins in preventing fluorotic changes is that amelogenins regulate pH at the site where fluoride enhances mineral formation (Bronckers et al., 2006). Central to this hypothesis is that fluoride accelerates the growth rate of existing crystals by directly increasing mineral deposition and enhancing the hydrolysis of octacalcium phosphate into hydroxyapatite crystals with a higher calcium phosphate ratio (Brown, 1964; Iijima et al., 1992). This process (hypermineralization and the formation of the first component of the typical double-response to fluoride) generates an excess of protons that exceeds the buffering capacity of the enamel matrix. This results in an acidification of the matrix (Fig. 5a), which may change the dynamics of cell/matrix interactions in enamel formation.
Figure 5.
Proposed molecular mechanism for enamel fluorosis. (A) Early-secretory amelogenesis in non-fluorotic conditions (adapted from Fincham et al., 1995). (A1) Light-microscope level. Boxed area is enlarged in A2 and represents crystal formation at the molecular level. (A2) Tomes’ process (1) secretes a secretory vesicle containing amelogenins. At neutral pH, amelogenins (for simplicity, drawn as a thread) form nanospheres (2) that adhere to the surfaces of growing crystals and foster preferential crystal growth in length, but reduce growth in width. Crystal growth generates protons that need to be neutralized to drive further crystal growth. Amelogenins bind and neutralize protons (3). The result of this action is an increase in crystal length, seen at the right side of this picture. (B) Disruption of secretory amelogenesis by fluoride as seen in vitro. (B1) Light-microscopic level. The boxed area in B1 is magnified in B2. (B2) Fluoride primarily accelerates crystal growth in thickness (left drawing). This forms the hypermineralized line, the initial event of the double-response. Accelerated mineral deposition strongly enhances proton production (right side drawing) that cannot be buffered by the available amelogenins. Amelogenin nanospheres at acid pH deaggregate and detach from the crystal surface. Also, newly secreted matrix will not form nanospheres at low pH and remains monomeric (fluid). Control of preferential crystal growth in length is then lost at acid pH, and newly secreted matrix will not foster crystal growth until neutral pH is restored. This layer represents the hypomineralized line, the outer component of the double-response event. These fluoride-related changes in the matrix could further alter ameloblast cell function.
The Role of Amelogenin Buffering in Modulating the Effects of Fluoride: a Hypothesis
During apatite crystal formation, substantial numbers of protons are formed (10 Ca2+ + 6 HPO42- + 2 H2O → Ca10(PO4)6(OH)2 + 8H+) that need to be neutralized. Amelogenins bind as many as 12 protons per molecule (Ryu et al., 1998). However, if this amelogenin-buffering system is either not available or is saturated, it is conceivable that a fluoride-induced pH drop could alter the amelogenin’s tertiary structure and affects its function. At neutral pH, amelogenins form nanospheres that coat crystal surfaces selectively in the secretory phase and act as spacer molecules between crystals (Fincham et al., 1995; Moradian-Oldak, 2001). Selective coating by amelogenins allows crystals to grow in length (c-axis) and prevents lateral fusion of crystals (Moradian-Oldak, 2001). These nanospheres are extremely sensitive to pH changes between pH 6 and 8 (Moradian-Oldak et al., 1998). At neutral pH, they form large aggregates that de-aggregate into small ones at pH 6; this makes the matrix ‘fluid’ (Fig. 5b). Therefore, after hypermineralization and subsequent drop in pH, an amelogenin’s tertiary structure would be affected, with a resultant hypomineralization, the second component of the fluoride-induced double-response in secretory enamel.
At low sustained plasma levels of fluoride, these effects on the matrix structure of secretory-stage enamel would be small, due to circadian peak levels that generate weak hyper- and hypomineralized lines at the mineralization front. The model could explain why early- and late-secretory ameloblasts are more susceptible to fluoride than ameloblasts at other stages. In early-secretory enamel, amelogenin synthesis is minimal, and aprismatic enamel forms, with many smaller-sized immature crystals. In the presence of fluoride, this relatively large mineralized surface area would rapidly further mineralize, resulting in a rapid pH drop. Likewise, in the late-secretory/transition stage, when amelogenins are hydrolyzed, calcium and fluoride rapidly diffuse into the porous enamel (see radioautography of 45Ca and 18F in transitional stage; Hammarström, 1971). This infusion of fluoride and calcium would promote a similar hypermineralized crystal formation at the late-secretory/transition stage. In both of these stages, the low amelogenin content is unfavorable for buffering an excess of protons.
This hypothesized role of amelogenin-mediated buffering would explain why cysts form preferentially at the two transition stages of ameloblasts exposed to high fluoride concentration. The fully differentiated secretory ameloblasts are relatively well-protected by the presence of excess amelogenins. The maturation-stage ameloblasts presumably buffer protons with bicarbonate generated by cytosolic carbonic anhydrase (Lin et al., 1994; Toyosawa et al., 1996; Smith, 1998). In the transition stage, the buffering systems in the enamel compartment likely change from amelogenin into bicarbonate, and this change may make some cells vulnerable to a drop in pH.
Fluoride-induced acidification as a result of stimulation of mineral deposition may also be a contributing factor in the formation of fluorosed enamel at the maturation stage. At the maturation stage, massive amounts of protons are produced (Smith et al., 2005), and the amount of protons would be even greater than in the secretory phase. Initial fluoride-induced hypermineralization happens at the very outer surface layer, just below the apical membrane of the maturation ameloblasts (Nelson et al., 1989; Richards et al., 1992; Suckling et al., 1995), and this process seems associated with the development of subsurface hypomineralization lesions. Whether surface hypermineralization at physiological plasma fluoride levels will generate enough protons to gradually influence the functional activity of the maturation ameloblasts remains to be investigated. The presence of stress-related proteins in maturation ameloblasts under fluorotic conditions suggests that these cells are undergoing stress (Sharma et al., 2008). Any acidification in the enamel compartment at this stage would also influence protease kinetics, as, for instance, MMP-20; e.g., low pH potentiates the inhibitory effect of fluoride on amelogenin degradation by MMP-20 (DenBesten et al., 2002).
The importance of pH regulation in enamel formation has been emphasized in recent studies of alterations to functional exchangers, co-transporters, and chloride channels found to be present in ameloblasts (Wright et al., 1996a,b; Lyaruu et al., 2008a; Paine et al., 2008; Bronckers et al., 2009). Mutations in or deletions of these molecules present enamel phenotypes quite similar to those seen in enamel fluorosis (without hypermineralization lines), and suggest that these genes are potential factors that may be related to susceptibility to fluoride.
This working model of the effects of fluoride on enamel mineralization is speculative, but accounts for numerous observations, the most important of which is how to explain how such low levels of fluoride affect enamel mineralization, while higher levels of fluoride lead to many diverse cellular and biosynthetic changes. The fluoride-induced change in pH could provide a general molecular mechanism that operates basically in the same way, whether in the secretory or the maturation phase. It also suggests that some of the mechanisms by which fluoride affects developing cells and matrix are unique to enamel formation. It does not exclude other working mechanisms in which fluoride at micromolar levels inhibits various pathways and changes molecular processes. The model forms a framework for future studies on the molecular mechanism of enamel fluorosis.
Summary and Conclusions
The following conclusions were drawn from this review of the literature:
Enamel formation in rodents, particularly in rats, is an appropriate model for studying the development of human enamel fluorosis.
Plasma levels of fluoride are better associated with fluorotic changes than total fluoride intake alone.
Chronic low plasma fluoride levels (2 µmol/L and higher) induce enamel fluorosis in a dose- and time-dependent manner. Clinically relevant doses that experimentally induce fluorosis in rat incisors are sustained plasma levels of 2-12 µmol/L or peak levels exceeding 10 µmol/L.
Maturation-stage enamel is most sensitive to chronic exposure to low levels of fluoride. The characteristic defect for this stage is the development of highly porous hypomineralized subsurface enamel, which extends deeper into the enamel with increased fluoride intake. This is clinically seen as the white opaque areas. These changes occur independently from fluoride effects in preceding stages and can be found when fluoride is present only during the maturation stage.
Fluorotic changes in enamel are more severe if both secretory and maturation stages are exposed to fluoride.
Shallow coronal and deep cervical pits at the surface of highly fluorotic enamel likely result from cystic lesions due to local disruption of late-secretory/transitional and early-secretory ameloblasts, respectively.
Multiple mechanisms including direct fluoride related effects on the ameloblasts and indirect fluoride related effects on the forming matrix can result in dental fluorosis, depending on the dose and duration of fluoride exposure.
We propose that a primary effect of fluoride is the stimulation of crystal formation, resulting in the generation of excess protons. The effective buffering of this fluoride-induced acidification of the enamel matrix modulates fluorotic changes in the forming enamel.
Acknowledgments
We thank Mr. J. Alberga and Mrs. N. Kwee for technical assistance in preparing Fig. 1.
Footnotes
This work was supported by NIH grant R01 DE013508 (AB, DL, and PDB).
References
- Andersen L, Richards A, Care AD, Andersen HM, Kragstrup J, Fejerskov O. (1986). Parathyroid glands, calcium, and vitamin D in experimental fluorosis in pigs. Calcif Tissue Int 38:222-226 [DOI] [PubMed] [Google Scholar]
- Angmar-Månsson B, Whitford GM. (1982). Plasma fluoride levels and enamel fluorosis in the rat. Caries Res 16:334-339 [DOI] [PubMed] [Google Scholar]
- Angmar-Månsson B, Whitford GM. (1984). Enamel fluorosis related to plasma F levels in the rat. Caries Res 18:25-32 [DOI] [PubMed] [Google Scholar]
- Angmar-Månsson B, Whitford GM. (1985). Single fluoride doses and enamel fluorosis in the rat. Caries Res 19:145-152 [DOI] [PubMed] [Google Scholar]
- Angmar-Månsson B, Ericsson Y, Ekberg O. (1976). Plasma fluoride and enamel fluorosis. Calcif Tissue Res 22:77-84 [DOI] [PubMed] [Google Scholar]
- Aoba T, Moreno EC, Tanabe T, Fukae M. (1990). Effects of fluoride on matrix proteins and their properties in rat secretory enamel. J Dent Res 69:1248-1250 [DOI] [PubMed] [Google Scholar]
- Appleton J. (1994) Formation and structure of dentine in the rat incisor after chronic exposure to sodium fluoride. Scanning Microsc 8:711-719 [PubMed] [Google Scholar]
- Appleton J. (1995). Changes in the plasma electrolytes and metabolites of the rat following acute exposure to sodium fluoride and strontium chloride. Arch Oral Biol 40:265-268 [DOI] [PubMed] [Google Scholar]
- Ashrafi SH, Eisenmann DR, Zaki AE, Liss R. (1988). Effect of fluoride and cobalt on forming enamel: scanning electron microscope and x-ray microanalysis study. Scanning Microsc 2:1527-1534 [PubMed] [Google Scholar]
- Bartlett JD, Dwyer SE, Beniash E, Skobe Z, Payne-Ferreira TL. (2005). Fluorosis: a new model and new insights. J Dent Res 84:832-836 [DOI] [PubMed] [Google Scholar]
- Bawden JW, Deaton TG, Crawford BP. (1992). Fluoride and calcium content of enamel organ, muscle, liver and plasma in rats. Caries Res 26:263-267 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Wöltgens JH. (1985). Short term effects of fluoride on biosynthesis of enamel-matrix proteins and dentin collagens and on mineralization during hamster tooth-germ development in organ culture. Arch Oral Biol 30:181-185 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Jansen LL, Wöltgens JH. (1984a). A histological study of the short-term effects of fluoride on enamel and dentine formation in hamster tooth-germs in organ culture in vitro. Arch Oral Biol 29:803-810 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Jansen LL, Wöltgens JH. (1984b). Long-term (8 days) effects of exposure to low concentrations of fluoride on enamel formation in hamster tooth-germs in organ culture in vitro. Arch Oral Biol 29:811-819 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Bervoets TJ, Lyaruu DM, Wöltgens JH. (1989). Antagonism of fluoride toxicity by high levels of calcium but not of inorganic phosphate during secretory amelogenesis in the hamster tooth germ in vitro. Arch Oral Biol 34:625-636 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Lyaruu DM, Bervoets TJ, Wöltgens JH. (2002). Fluoride enhances intracellular degradation of amelogenins during secretory phase of amelogenesis of hamster teeth in organ culture. Connect Tissue Res 43:456-465 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Bervoets TJ, Wöltgens JH, Lyaruu DM. (2006). Effect of calcium, given before or after a fluoride insult, on hamster secretory amelogenesis in vitro. Eur J Oral Sci 114(Suppl 1):116-122 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Lyaruu DM, Jansen ID, Medina JF, Kellokumpu S, Hoeben KA, et al. (2009). Localization and function of the anion exchanger Ae2 in developing teeth and orofacial bone in rodents. J Exp Zool Mol Dev Evol 312(B):375-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WE. (1964). A mechanism for growth of apatite crystals. In: Tooth enamel II. Stack MV, Fearnhead RW.editors. Bristol: John Wright & Sons, Ltd, pp. 11-14 [Google Scholar]
- Chen H, Czajka-Jakubowska A, Spencer NJ, Mansfield JF, Robinson C, Clarkson BH. (2006). Effects of systemic fluoride and in vitro fluoride treatment on enamel crystals. J Dent Res 85:1042-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang Y, Mendoza J, DenBesten P. (2009). Calcium mediated differentiation of ameloblast lineage cells in vitro. J Exp Zool Part B Mol Dev Evol 312(B):458-464 [DOI] [PubMed] [Google Scholar]
- Chen S, Eisenmann DR. (1984). Ultrastructural study of the effects of fixation and fluoride injection on stippled material during amelogenesis in the rat. Arch Oral Biol 29:681-686 [DOI] [PubMed] [Google Scholar]
- Crenshaw MA, Bawden JW. (1984). Proteolytic activity in embryonic bovine secretory enamel. In: Tooth enamel IV. Fearnhead RW, Suga S, editors. Amsterdam: Elsiever Science, pp. 109-113 [Google Scholar]
- DenBesten PK. (1986). Effects of fluoride on protein secretion and removal during enamel development in the rat. J Dent Res 65:1272-1277 [DOI] [PubMed] [Google Scholar]
- DenBesten PK. (1999). Biological mechanisms of dental fluorosis relevant to the use of fluoride supplements. Community Dent Oral Epidemiol 27:41-47 [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Heffernan LM. (1989). Enamel proteases in secretory and maturation incisor enamel of rats ingesting 0 and 100 ppm fluoride in drinking water. Adv Dent Res 3:199-202 [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Crenshaw MA, Wilson MH. (1985). Changes in the fluoride-induced modulation of maturation stage ameloblasts of rats. J Dent Res 64:1365-1370 [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Heffernan LM, Featherstone JD, Shields CP. (1992). Fluoride binding by matrix proteins in rat mineralizing tissue. Arch Oral Biol 37:459-462 [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Yan Y, Featherstone JD, Hilton JF, Smith CE, Li W. (2002). Effects of fluoride on rat dental enamel matrix proteinases. Arch Oral Biol 47:763-770 [DOI] [PubMed] [Google Scholar]
- Drinkard CR, Deaton TG, Bawden JW. (1987). A comparison of the effects of continuous and periodic fluoride delivery on fluoride levels in plasma, enamel, and bones of nursing rats. J Dent Res 66:19-22 [DOI] [PubMed] [Google Scholar]
- Dunipace A, Brizendine E, Wilson M, Zhang W, Wilson C, Katz B, et al. (1998). Effect of chronic fluoride exposure in uremic rats. Nephron 78:96-103 [DOI] [PubMed] [Google Scholar]
- Eanes ED, Hailer AW. (1998). The effect of fluoride on the size and morphology of apatite crystals grown from physiologic solutions. Calcif Tissue Int 63:250-257 [DOI] [PubMed] [Google Scholar]
- Ekstrand J, Lange A, Ekberg O, Hammarström L. (1981). Relationship between plasma, dentin and bone fluoride concentrations in rats following long-term fluoride administration. Acta Pharmacol Toxicol (Copenh) 48:433-437 [DOI] [PubMed] [Google Scholar]
- Everett ET, McHenry MA, Reynolds N, Eggertsson H, Sullivan J, Kantmann C, et al. (2002). Dental fluorosis: variability among different inbred mouse strains. J Dent Res 81:794-798 [DOI] [PubMed] [Google Scholar]
- Everett ET, Yan D, Weaver M, Liu L, Foroud T, Martinez-Mier EA. (2009). Detection of dental fluorosis-associated quantitative trait loci on mouse chromosomes 2 and 11. Cells Tissues Organs 189:212-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejerskov O, Johnson NW, Silverstone LM. (1974). The ultrastructure of fluorosed human dental enamel. Scand J Dent Res 82:357-372 [DOI] [PubMed] [Google Scholar]
- Fejerskov O, Thylstrup A, Larsen MJ. (1977). Clinical and structural features and possible pathogenic mechanisms of dental fluorosis. Scand J Dent Res 85:510-534 [DOI] [PubMed] [Google Scholar]
- Fejerskov O, Larsen MJ, Josephsen K, Thylstrup A. (1979). Effect of long-term administration of fluoride on plasma fluoride and calcium in relation to forming enamel and dentin in rats. Scand J Dent Res 87:98-104 [DOI] [PubMed] [Google Scholar]
- Fejerskov O, Larsen MJ, Richards A, Baelum V. (1994). Dental tissue effects of fluoride. Adv Dent Res 8:15-31 [DOI] [PubMed] [Google Scholar]
- Fincham AG, Moradian-Oldak J, Diekwisch TG, Lyaruu DM, Wright JT, Bringas P, Jr, et al. (1995). Evidence for amelogenin “nanospheres” as functional components of secretory-stage enamel matrix. J Struct Biol 115:50-59 [DOI] [PubMed] [Google Scholar]
- Fry BW, Taves DR. (1970). Serum fluoride analysis with the fluoride electrode. J Lab Clin Med 75:1020-1025 [PubMed] [Google Scholar]
- Gerlach RF, de Souza AP, Cury JA, Line SR. (2000). Fluoride effect on the activity of enamel matrix proteinases in vitro. Eur J Oral Sci 108:48-53; erratum in Eur J Oral Sci 108:464, 2000 [DOI] [PubMed] [Google Scholar]
- Giambro NJ, Prostak K, DenBesten PK. (1995). Characterization of fluorosed human enamel by color reflectance, ultrastructure, and elemental composition. Caries Res 29:251-257 [DOI] [PubMed] [Google Scholar]
- Hammarström L. (1971). Distribution in developing rat enamel of simultaneously injected fluoride and calcium. Scand J Dent Res 79:369-376 [DOI] [PubMed] [Google Scholar]
- Hong L, Levy SM, Warren JJ, Broffitt B, Cavanaugh J. (2006). Fluoride intake levels in relation to fluorosis development in permanent maxillary central incisors and first molars. Caries Res 40:494-500 [DOI] [PubMed] [Google Scholar]
- Iijima M, Tohda H, Suzuki H, Yanagisawa T, Moriwaki Y. (1992). Effects of F- on apatite-octacalcium phosphate intergrowth and crystal morphology in a model system of tooth enamel formation. Calcif Tissue Int 50:357-361 [DOI] [PubMed] [Google Scholar]
- Ishii T, Suckling G. (1986). The appearance of tooth enamel in children ingesting water with a high fluoride content for a limited period during early tooth development. J Dent Res 65:974-977 [DOI] [PubMed] [Google Scholar]
- Kardos TB, Hunter AR, Hubbard MJ. (1989). Scanning electron microscopy of trypsin-treated enamel from fluorosed rat molars. Adv Dent Res 3:183-187 [DOI] [PubMed] [Google Scholar]
- Kérébel B, Daculsi G. (1976). Ultrastructural and crystallographic study of human enamel in endemic fluorosis. J Biol Buccale 4:143-154 [PubMed] [Google Scholar]
- Kerley MA, Kollar EJ. (1977). Regeneration of tooth development in vitro following sodium fluoride treatment. Am J Anat 149:181-195 [DOI] [PubMed] [Google Scholar]
- Kierdorf H, Kierdorf U. (1997). Disturbances of the secretory stage of amelogenesis in fluorosed deer teeth: a scanning electron-microscopic study. Cell Tissue Res 289:125-135 [DOI] [PubMed] [Google Scholar]
- Kierdorf H, Kierdorf U, Richards A, Sedlacek F. (2000). Disturbed enamel formation in wild boars (Sus scrofa L.) from fluoride polluted areas in Central Europe. Anat Rec 259:12-24 [DOI] [PubMed] [Google Scholar]
- Kierdorf H, Kierdorf U, Richards A, Josephsen K. (2004). Fluoride-induced alterations of enamel structure: an experimental study in the miniature pig. Anat Embryol (Berl) 207:463-474 [DOI] [PubMed] [Google Scholar]
- Kirkham J, Brookes SJ, Zhang J, Wood SR, Shore RC, Smith DA, et al. (2001). Effect of experimental fluorosis on the surface topography of developing enamel crystals. Caries Res 35:50-56 [DOI] [PubMed] [Google Scholar]
- Kruger BJ. (1966). Interaction of fluoride and molybdenum on dental morphology in the rat. J Dent Res 45:714-725 [Google Scholar]
- Kruger BJ. (1967). Histological effect of fluoride and molybdenum on developing dental tissues. Aust Dent J 12:54-60 [Google Scholar]
- Kruger BJ. (1970a). An autoradiographic assessment of the effect of fluoride on the uptake of 3H-proline by ameloblasts in the rat. Arch Oral Biol 15:103-108 [DOI] [PubMed] [Google Scholar]
- Kruger BJ. (1970b). The effect of different levels of fluoride on the ultrastructure of ameloblasts in the rat. Arch Oral Biol 15:109-114 [DOI] [PubMed] [Google Scholar]
- Kubota K, Lee DH, Tsuchiya M, Young CS, Everett ET, Martinez-Mier EA, et al. (2005). Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. J Biol Chem 280:23194-23202 [DOI] [PubMed] [Google Scholar]
- Larsen MJ, Richards A, Fejerskov O. (1981). Effect of intraperitoneally injected fluoride on plasma calcium in suckling and adult rats. Calcif Tissue Int 33:541-544 [DOI] [PubMed] [Google Scholar]
- Levenson GE. (1980). The effect of fluoride on ameloblasts of mouse molar tooth germs in vitro. J Biol Buccale 8:255-263 [PubMed] [Google Scholar]
- Li Y, Decker S, Yuan ZA, DenBesten PK, Aragon MA, Jordan-Sciutto K, et al. (2005). Effects of sodium fluoride on the actin cytoskeleton of murine ameloblasts. Arch Oral Biol 50:681-688 [DOI] [PubMed] [Google Scholar]
- Lin HM, Nakamura H, Noda T, Ozawa H. (1994). Localization of H(+)-ATPase and carbonic anhydrase II in ameloblasts at maturation. Calcif Tissue Int 55:38-45 [DOI] [PubMed] [Google Scholar]
- Lyaruu DM, de Jong M, Bronckers AL, Wöltgens JH. (1986). Ultrastructural study of fluoride induced in vitro hypermineralzation of enamel in hamster tooth germs explanted during the secretory phase of amelogenesis. Arch Oral Biol 31:109-117 [DOI] [PubMed] [Google Scholar]
- Lyaruu DM, de Jong M, Bronckers AL, Wöltgens JH. (1987). Ultrastructure of in-vitro recovery of mineralization capacity of fluorotic enamel matrix in hamster tooth germs pre-exposed to fluoride in organ culture during the secretory phase of amelogenesis. Arch Oral Biol 32:107-115 [DOI] [PubMed] [Google Scholar]
- Lyaruu DM, Blijleven N, Hoeben-Schornagel K, Bronckers AL, Wöltgens JH. (1989a). X-ray micro-analysis of the mineralization patterns in developing enamel in hamster tooth germs exposed to fluoride in vitro during the secretory phase of amelogenesis. Adv Dent Res 3:211-218 [DOI] [PubMed] [Google Scholar]
- Lyaruu DM, Lenglet WJ, Wöltgens JH, Bronckers AL. (1989b). Micro-PIGE determination of fluorine distribution in developing hamster tooth germs. J Histochem Cytochem 37:581-587 [DOI] [PubMed] [Google Scholar]
- Lyaruu DM, Tros GH, Bronckers AL, Wöltgens JH. (1990). Micro-PIXE (proton-induced x-ray emission) study of the effects of fluoride on mineral distribution patterns in enamel and dentin in the developing hamster tooth germ. Scanning Microsc 4:315-322 [PubMed] [Google Scholar]
- Lyaruu DM, Bervoets TJ, Bronckers AL. (2006). Short exposure to high levels of fluoride induces stage-dependent structural changes in ameloblasts and enamel mineralization. Eur J Oral Sci 114(Suppl 1):111-115 [DOI] [PubMed] [Google Scholar]
- Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, et al. (2008a). The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol 27:119-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyaruu DM, Bronckers AL, Santos F, Mathias R, DenBesten P. (2008b). The effect of fluoride on enamel and dentin formation in the uremic rat incisor. Pediatr Nephrol 23:1973-1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Mier EA, Soto-Rojas AE, Ureña-Cirett JL, Stookey GK, Dunipace AJ. (2003). Fluoride intake from foods, beverages and dentifrice by children in Mexico. Community Dent Oral Epidemiol 31:221-230 [DOI] [PubMed] [Google Scholar]
- Matsuo S, Inai T, Kurisu K, Kiyomiya K, Kurebe M. (1996). Influence of fluoride on secretory pathway of the secretory ameloblast in rat incisor tooth germs exposed to sodium fluoride. Arch Toxicol 70:420-429 [DOI] [PubMed] [Google Scholar]
- Maurer JK, Cheng MC, Boysen BG, Andersen RL. (1990). Two year carcinogenic study of sodium fluoride in rats. J Natl Cancer Inst 82:1118-1126 [DOI] [PubMed] [Google Scholar]
- Milhaud GE, Charles E, Loubière ML, Kolf-Clauw M, Joubert C. (1992). Effects of fluoride on secretory and postsecretory phases of enamel formation in sheep molars. Am J Vet Res 53:1241-1247 [PubMed] [Google Scholar]
- Monsour P, Harbrow J, Warshawsky H. (1989). Effects of acute doses of sodium fluoride on the morphology and the detectable calcium associated with secretory ameloblasts in rat incisors. J Histochem Cytochem 37:463-471 [DOI] [PubMed] [Google Scholar]
- Monsour PA, Kruger BJ, Smid JR. (1985). Effects of a single intravenous dose of sodium fluoride on plasma electrolytes and metabolites in rats, rabbits, and cockerels. J Dent Res 64:1281-1285 [DOI] [PubMed] [Google Scholar]
- Moradian-Oldak J. (2001). Amelogenins: assembly, processing and control of crystal morphology. Matrix Biol 20:293-305 [DOI] [PubMed] [Google Scholar]
- Moradian-Oldak J, Leung W, Fincham AG. (1998). Temperature and pH-dependent supramolecular self-assembly of amelogenin molecules: a dynamic light-scattering analysis. J Struct Biol 122:320-327 [DOI] [PubMed] [Google Scholar]
- Mörnstad H. (1975). Acute sodium fluoride toxicity in rats in relation to age and sex. Acta Pharmacol Toxicol (Copenh) 37:425-428 [DOI] [PubMed] [Google Scholar]
- Mörnstad H, Hammarström L. (1978). Morphologic changes in the rat enamel organ following a single intraperitoneal injection of sodium fluoride. Scand J Dent Res 86:211-220 [DOI] [PubMed] [Google Scholar]
- Mullenix PJ, DenBesten PK, Schunior A, Kernan WJ. (1995). Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol 17:169-177 [DOI] [PubMed] [Google Scholar]
- Neiman A, Eisenmann DR. (1975). The effect of strontium, cobalt and fluoride on rat incisor enamel formation. Anat Rec 183:303-321 [DOI] [PubMed] [Google Scholar]
- Nelson DG, Coote GE, Vickridge IC, Suckling G. (1989). Proton microprobe determination of fluorine profiles in the enamel and dentine of erupting incisors from sheep given low and high daily doses of fluoride. Arch Oral Biol 34:419-429 [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Josephsen K. (1987). Cyclic localization of actin and its relationship to junctional complexes in maturation ameloblasts of the rat incisor. Anat Rec 219:21-31 [DOI] [PubMed] [Google Scholar]
- Nordlund AL, Lindskog S. (1986). Scanning electron microscopy of fluoride-induced disturbances on the enamel surface of rat molars. Scand J Dent Res 94:185-192 [DOI] [PubMed] [Google Scholar]
- Nordlund AL, Ekstrand JL, Hammarström L. (1986). Fluoride-induced cystic changes in the enamel organ of the rat molar. J Oral Pathol 15:87-92 [DOI] [PubMed] [Google Scholar]
- Paine ML, Snead ML, Wang HJ, Abuladze N, Pushkin A, Liu W, et al. (2008). Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res 87:391-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro DA, Hirota L, Cestari TM, Ceolin DS, Taga R, Assis GF. (2006). Ultrastructural morphometric analysis of ameloblasts exposed to fluoride during tooth development. J Mol Histol 37:361-367 [DOI] [PubMed] [Google Scholar]
- Richards A, Kragstrup J, Nielsen-Kudsk F. (1985). Pharmacokinetics of chronic fluoride ingestion in growing pigs. J Dent Res 64:425-430 [DOI] [PubMed] [Google Scholar]
- Richards A, Kragstrup J, Josephsen K, Fejerskov O. (1986). Dental fluorosis developed in post-secretory enamel. J Dent Res 65:1406-1409 [DOI] [PubMed] [Google Scholar]
- Richards A, Likimani S, Baelum V, Fejerskov O. (1992). Fluoride concentrations in unerupted fluorotic human enamel. Caries Res 26:328-332 [DOI] [PubMed] [Google Scholar]
- Robinson C, Kirkham J. (1984). Alterations during developent and possible interactions with the mineral phase. In: Tooth enamel IV. Fearnhead RW, Suga S, editors. Amsterdam: Elsevier Science, pp. 261-265 [Google Scholar]
- Robinson C, Yamamoto K, Connell SD, Kirkham J, Nakagaki H, Smith AD. (2006). The effects of fluoride on the nanostructure and surface pK of enamel crystals: an atomic force microscopy study of human and rat enamel. Eur J Oral Sci 114(Suppl 1):99-104 [DOI] [PubMed] [Google Scholar]
- Ryu OH, Hu CC, Simmer JP. (1998). Biochemical characterization of recombinant mouse amelogenins: protein quantitation, proton absorption, and relative affinity for enamel crystals. Connect Tissue Res 38:207-214 [DOI] [PubMed] [Google Scholar]
- Sharma R, Tsuchiya M, Bartlett JD. (2008). Fluoride induces endoplasmic reticulum stress and inhibits protein synthesis and secretion. Environ Health Perspect 116:1142-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda H. (1975). Effect of long-term administration of fluoride on physico-chemical properties of the rat incisor enamel. Calcif Tissue Res 18:91-100 [DOI] [PubMed] [Google Scholar]
- Simmer JP, Fincham AG. (1995). Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med 6:84-108 [DOI] [PubMed] [Google Scholar]
- Singer L, Ophaug R. (1982). Ionic and nonionic fluoride in plasma (or serum). Crit Rev Clin Lab Sci 18:111-140 [DOI] [PubMed] [Google Scholar]
- Smid JR, Monsour PA, Harbrow DJ, Young WG. (1990). A histochemical study of the effects of high doses of sodium fluoride on dipeptidyl peptidase II activity in the rat incisor ameloblast. Arch Oral Biol 35:671-675 [DOI] [PubMed] [Google Scholar]
- Smith CE. (1998). Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128-161 [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A. (1996). Protein dynamics of amelogenesis. Anat Rec 245:186-207 [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A, DenBesten PK. (1993). Effects of chronic fluoride exposure on morphometric parameters defining the stages of amelogenesis and ameloblast modulation in rat incisors. Anat Rec 237:243-258 [DOI] [PubMed] [Google Scholar]
- Smith CE, Chong DL, Bartlett JD, Margolis HC. (2005). Mineral acquisition rates in developing enamel on maxillary and mandibular incisors of rats and mice: implications to extracellular acid loading as apatite crystals mature. J Bone Miner Res 20:240-249 [DOI] [PubMed] [Google Scholar]
- Suckling G, Thurley DC. (1984). Histological, macroscopic and microhardness observations of fluoride-induced changes in the enamel organ and enamel of sheep incisor teeth. Arch Oral Biol 29:165-177 [DOI] [PubMed] [Google Scholar]
- Suckling G, Thurley DC, Nelson DG. (1988). The macroscopic and scanning electron-microscopic appearance and microhardness of the enamel, and the related histological changes in the enamel organ of erupting sheep incisors resulting from a prolonged low daily dose of fluoride. Arch Oral Biol 33:361-373 [DOI] [PubMed] [Google Scholar]
- Suckling G, Coote GE, Cutress TW, Gao J. (1995). Proton microprobe assessment of the distribution of fluoride in the enamel and dentine of developing central incisors of sheep and changes induced by daily fluoride supplements. Arch Oral Biol 40:439-446 [DOI] [PubMed] [Google Scholar]
- Suga S. (1970). Histochemical observation or proteolytic enzyme activity in the developing dental hard tissues of the rat. Arch Oral Biol 15:555-558 [DOI] [PubMed] [Google Scholar]
- Suga S, Aoki H, Yamashita Y, Tsuno M, Ogawa M. (1987) A comparative study of disturbed mineralization of rat incisor enamel induced by strontium and fluoride administration. Adv Dent Res 1:339-355 [DOI] [PubMed] [Google Scholar]
- Sundstrom B, Jongebloed WL, Arends J. (1978). Fluorosed human enamel. A SEM investigation of the anatomical surface and outer and inner regions of mildly fluorosed enamel. Caries Res 12:329-338 [DOI] [PubMed] [Google Scholar]
- Susheela AK, Bhatnagar M. (1993). Fluoride toxicity: a biochemical and scanning electron microscopic study of enamel surface of rabbit teeth. Arch Toxicol 67:573-579 [DOI] [PubMed] [Google Scholar]
- Tanabe T, Aoba T, Moreno EC, Fukae M. (1988). Effect of fluoride in the apatitic lattice on adsorption of enamel proteins onto calcium apatites. J Dent Res 67:536-542 [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Le T, Zhu L, Chen J, Featherstone JD, Li W, et al. (2008). Effects of fluoride on the interactions between amelogenin and apatite crystals. J Dent Res 87:39-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves DR. (1968). Determination of submicromolar concentrations of fluoride in biological samples. Talanta 15:1015-1023 [DOI] [PubMed] [Google Scholar]
- Toyosawa S, Ogawa Y, Inagaki T, Ijuhin N. (1996). Immunohistochemical localization of carbonic anhydrase isozyme II in rat incisor epithelial cells at various stages of amelogenesis. Cell Tissue Res 285:217-225 [DOI] [PubMed] [Google Scholar]
- Tros GHJ, Lyaruu DM, Vis RD. (1990). The effect of fluoride on mineralization during tooth germ development in neonatal hamsters. Nuclear Instrum Meth Phys Res 49(B):476-480 [Google Scholar]
- Vieira AP, Hanocock R, Eggertsson H, Everett ET, Grynpas MD. (2005). Tooth quality in dental fluorosis—genetic and environmental factors. Calcif Tissue Int 76:17-25 [DOI] [PubMed] [Google Scholar]
- Walton RE, Eisenmann DR. (1974). Ultrastructural examination of various stages of amelogenesis in the rat following parenteral fluoride administration. Arch Oral Biol 19:171-182 [DOI] [PubMed] [Google Scholar]
- Walton RE, Eisenmann DR. (1975). Ultrastructural examination of dentine formation in rat incisors following multiple fluoride injections. Arch Oral Biol 20:485-488 [DOI] [PubMed] [Google Scholar]
- Warren JJ, Levy SM, Broffitt B, Cavanaugh JE, Kanellis MJ, Weber-Gasparoni K. (2009). Considerations on optimal fluoride intake using dental fluorosis and dental caries outcomes—a longitudinal study. J Public Health Dent 69:111-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford GM. (1994). Intake and metabolism of fluoride. Adv Dent Res 8:5-14 [DOI] [PubMed] [Google Scholar]
- Whitford GM, Reynolds KE. (1979). Plasma and developing enamel fluoride concentrations during chronic acid-base disturbances. J Dent Res 58:2058-2065 [DOI] [PubMed] [Google Scholar]
- Wöltgens JH, Lyaruu DM, Bervoets TJ, Bronckers AL. (1987). Effects of calcium and phosphate on secretion of enamel matrix and its subsequent mineralization in vitro. Adv Dent Res 1:196-201 [DOI] [PubMed] [Google Scholar]
- Wright JT, Hall KI, Grubb BR. (1996a). Enamel mineral composition of normal and cystic fibrosis transgenic mice. Adv Dent Res 10:270-274 [DOI] [PubMed] [Google Scholar]
- Wright JT, Kiefer CL, Hall KI, Grubb BR. (1996b). Abnormal enamel development in a cystic fibrosis transgenic mouse model. J Dent Res 75:966-973 [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang Y, Li W, DenBesten PK. (2007). Micromolar fluoride alters ameloblast lineage cells in vitro. J Dent Res 86:336-340 [DOI] [PubMed] [Google Scholar]
- Yanagisawa T, Takuma S, Fejerskov O. (1989). Ultrastructure and composition of enamel in human dental fluorosis. Adv Dent Res 3:203-210 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan Q, Li W, DenBesten PK. (2006). Fluoride down-regulates the expression of matrix metalloproteinase-20 in human fetal tooth ameloblast-lineage cells in vitro. Eur J Oral Sci 114(Suppl 1):105-110 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li W, Chi HS, Chen J, DenBesten PK. (2007). JNK/c-Jun signaling pathway mediates the fluoride-induced down-regulation of MMP-20 in vitro. Matrix Biol 26:633-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Zaki AE, Eisenmann DR. (1996). Morphometry and autoradiography of altered rat enamel protein processing due to chronic exposure to fluoride. Arch Oral Biol 41:739-747 [DOI] [PubMed] [Google Scholar]