Tissue-Based Biology Provides Insight into Pregnancy-Associated Breast Cancer
For the last several decades, the field of experimental carcinogenesis has been focused on identifying the intricacies of single-cell transformation. A complement to this epithelial cell-centric theory proposes that cellular transformation and progression are driven by deregulation at the tissue level.1,2 Interest in the tissue-based theory of carcinogenesis has been increased by abundant evidence that stromal and immune cells dictate epithelial cancer outcomes.3 We have utilized a tissue-based framework to investigate the increased incidence and poor prognosis of pregnancy-associated breast cancers (PABC) that arise in the postpartum setting. Large epidemiologic studies show that a woman is at increased risk for breast cancer for five to ten years after a completed pregnancy.4 Importantly, patients diagnosed with postpartum breast cancer also have poorer prognosis than nulliparous women even after matching for patient age and tumor stage. If PABC is defined using these epidemiologic data, up to 12,500 recent mothers per year in the United States may have breast cancer confounded by pregnancy.4 In spite of these numbers, PABC remains largely under-recognized and understudied.
Recently, we determined that normal physiologic mechanisms of postpartum mammary gland involution likely account for the poor prognosis of PABC. We found that programs similar to those involved in wound healing are activated to remodel the lactationally competent mammary gland back to a non-secretory state. An undesired consequence of this tissue remodeling is the generation of a pro-tumorigenic microenvironment.5,6 One identified mechanism links the fibrillar collagen deposition seen during normal involution to high cyclooxygenase-2 (COX-2) expression in tumor cells and subsequent tumor cell metastasis.6 In a xenograft model, non-steroidal antiinflammatory drug (NSAID) treatments, limited to the window of postpartum mammary gland involution, were sufficient to eliminate the tumor-promotional attributes of involution. Remarkably, in addition to targeting tumor cells directly, NSAID treatment prevented fibrillar collagen deposition characteristic of the postpartum involuting mammary gland, highlighting the role of a normal stromal change, i.e., collagen, in driving carcinogenesis. While an NSAID-based strategy for PABC prevention and treatment is one possibility, in the background of human breast tumor heterogeneity and host differences (women's age, genetics, reproductive history, immune status, etc.,), mining the biology of postpartum breast involution for new targets is essential.
Involution-Associated Macrophages as a Potential Pharmacologic Target for PABC
Further characterization of weaning-induced mammary gland involution in rodents has identified additional factors that likely contribute to mammary tumor promotion, and as such represent unexplored avenues for prevention and targeted treatment of PABC. During involution, macrophages that express arginase-1 and mannose receptor, both markers of alternative activation, are recruited into the mammary gland.5 Importantly, alternatively activated macrophages share characteristics with tumor promotional macrophages (tumor-associated macrophages, TAMs), and thus may contribute to PABC promotion.5 For example, TAMs can be directly tumor promotional through secretion of growth factors.7 Additionally, immature macrophages are capable of suppressing cytotoxic T cell function, creating an immunosuppressive microenvironment further conducive to tumor cell promotion.8 We propose a similar immunomodulatory role for normal involutionassociated macrophages, thus permitting immune evasion by the tumor in the postpartum period. Recent published data provide support for this hypothesis, as high tumor macrophage infiltrate and low cytotoxic T cell numbers can predict decreased survival in breast cancer patients.9 By determining how involution-associated macrophages obtain their alternatively-activated phenotype, as well as identifying their potential role in T cell suppression, we hope to identify novel immune-based targets for PABC.
Mammary Epithelial Cells as Potential Drivers of the Involution Macrophage Phenotype
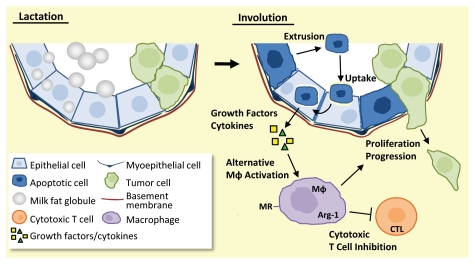
One possible mechanism accounting for the phenotype of involution macrophages is that they become alternatively activated as a consequence of apoptotic epithelial cell clearance. During involution, an estimated 50–80% of the milk-producing epithelial cells undergo programmed cell death,7 and surprisingly, mammary epithelial cells themselves have been identified as the primary phagocyte responsible for clearance of their apoptotic neighbors.10 Importantly, to minimize self-antigen exposure, apoptotic cell clearance by professional phagocytes (macrophages and dendritic cells) promotes local immune suppression, including the alternative activation of macrophages.11 Though studies regarding phagocytic mammary epithelial cells are limited, initial data suggest these amateur phagocytes (epithelial cells) utilize immune suppressive mechanisms comparable to their professional counterparts.10 Here, we expand on this idea to predict that phagocytic mammary epithelial cells promote alternative macrophage activation, and thus drive local immune suppression during involution (Fig. 1). Using this physiology-based, whole-tissue paradigm, we hope to expand upon our current knowledge of the dynamic interactions between phagocytic mammary epithelial cells and the local immune milieu of the involuting gland. Importantly, interventions that sway the postpartum mammary gland microenvironment from a tumor cell promotional environment to more neutral territory are anticipated to reduce PABC incidence and progression.
Figure 1.
Model of pro-tumorigenic immune modulation by phagocytic mammary epithelial cells. Upon the switch from lactation (left) to involution (right), milk-producing mammary epithelial cells undergo apoptosis (dark blue cells) and are extruded into the alveolar lumen. Other milk-producing mammary epithelial cells transition to become phagocytic (light blue cells) and engulf the shed apoptotic cells. Clearance of apoptotic cells is predicted to result in release of Th2-like cytokines and growth factors from the phagocytic mammary epithelial cells. This cytokine milieu is anticipated to promote alternative activation of macrophages (Mφ) and inhibition of cytotoxic T cells (CTL), resulting in an immunosuppressed, tumor-supportive microenvironment (green cells).
Abbreviations
- PABC
pregnancy-associated breast cancer
- COX-2
cyclooxygenase-2
- NSAID
non-steroidal anti-inflammatory drug
- TAMs
tumor-associated macrophages
Comment on: Lyons TR, et al. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416.
References
- 1.Bissell MJ, et al. Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soto AM, et al. Bioessays. 2011;33:332–340. doi: 10.1002/bies.201100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tlsty TD, et al. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 4.Schedin P. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien J, et al. Am J Pathol. 2010;176:1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyons TR, et al. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien J, et al. J Mammary Gland Biol Neoplasia. 2009;14:145–157. doi: 10.1007/s10911-009-9118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusmartsev S, et al. J Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denardo DG, et al. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monks J, et al. Cell Death Differ. 2005;12:107–114. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 11.Freire-de-Lima CG, et al. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]