Abstract
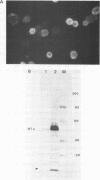
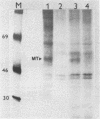
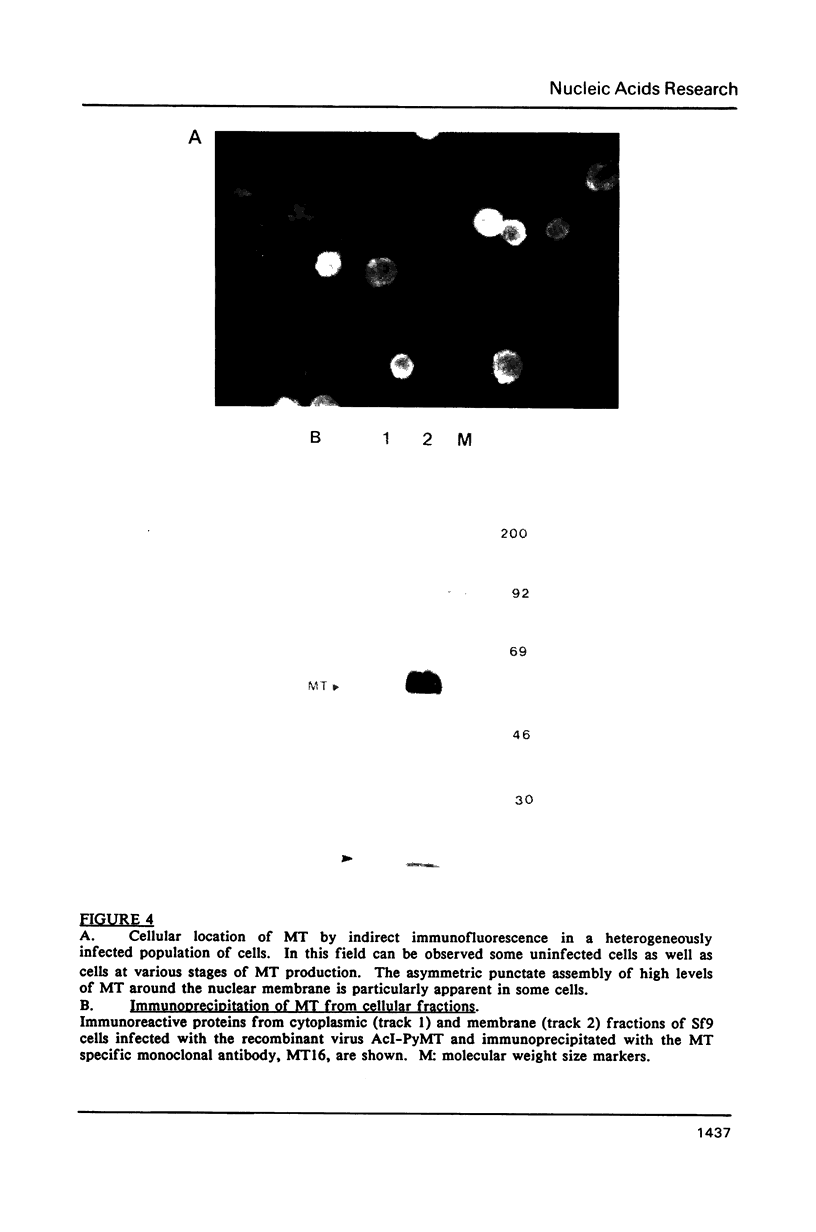
Two different recombinant baculoviruses have been generated for expressing the middle T antigen (MT) of polyoma virus in insect (Sf9) cells. One (pAcI-PyMT) produces moderate levels of MT and the other (pVL-PyMT) high levels. Indirect immunofluorescence and cellular fractionation studies with pAcI-PyMT infected Sf9 cells give results similar to those observed with wild type polyoma virus infected mouse cells, and show MT to be mainly associated with cytoplasmic membranes in the insect cell. In the latter, a sub-population of MT is phosphorylated in in vitro protein kinase assays. The yields of MT from pVL-PyMT infected cells are high enough to suggest that this protein can now be produced by this method in sufficient amounts for definitive biochemical and crystallographic analyses.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L., Schaffhausen B. S., Roberts T. M., Sharp P. A. Abundant expression of polyomavirus middle T antigen and dihydrofolate reductase in an adenovirus recombinant. J Virol. 1987 Apr;61(4):1213–1220. doi: 10.1128/jvi.61.4.1213-1220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Transformation by polyoma virus middle T antigen. Cancer Surv. 1986;5(2):173–182. [PubMed] [Google Scholar]
- Courtneidge S., Ralston R., Alitalo K., Bishop J. M. Subcellular location of an abundant substrate (p36) for tyrosine-specific protein kinases. Mol Cell Biol. 1983 Mar;3(3):340–350. doi: 10.1128/mcb.3.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzin F. The polyoma virus oncogenes. Coordinated functions of three distinct proteins in the transformation of rodent cells in culture. Biochim Biophys Acta. 1984 Apr 5;781(3):193–204. doi: 10.1016/0167-4781(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Davidson D., Hassell J. A. Overproduction of polyomavirus middle T antigen in mammalian cells through the use of an adenovirus vector. J Virol. 1987 Apr;61(4):1226–1239. doi: 10.1128/jvi.61.4.1226-1239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M., Griffin B. E. Monoclonal antibodies against polyoma virus tumor antigens. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1059–1063. doi: 10.1073/pnas.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M., Griffin B. E. The transforming gene of polyoma virus. Arch Geschwulstforsch. 1983;53(3):187–195. [PubMed] [Google Scholar]
- Dilworth S. M., Hansson H. A., Darnfors C., Bjursell G., Streuli C. H., Griffin B. E. Subcellular localisation of the middle and large T-antigens of polyoma virus. EMBO J. 1986 Mar;5(3):491–499. doi: 10.1002/j.1460-2075.1986.tb04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M. Protein kinase activities associated with distinct antigenic forms of polyoma virus middle T-antigen. EMBO J. 1982;1(11):1319–1328. doi: 10.1002/j.1460-2075.1982.tb01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield C., Patel G., Clark S., Jones N., Waterfield M. D. Expression of the human EGF receptor with ligand-stimulatable kinase activity in insect cells using a baculovirus vector. EMBO J. 1988 Jan;7(1):139–146. doi: 10.1002/j.1460-2075.1988.tb02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grussenmeyer T., Carbone-Wiley A., Scheidtmann K. H., Walter G. Interactions between polyomavirus medium T antigen and three cellular proteins of 88, 61, and 37 kilodaltons. J Virol. 1987 Dec;61(12):3902–3909. doi: 10.1128/jvi.61.12.3902-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink W. F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970 May 2;226(5244):466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- Ito Y., Brocklehurst J. R., Dulbecco R. Virus-specific proteins in the plasma membrane of cells lytically infected or transformed by pol-oma virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4666–4670. doi: 10.1073/pnas.74.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Bockus B., Roberts T. M., Bolen J., Israel M., Schaffhausen B. S. Large-scale production of polyoma middle T antigen by using genetically engineered tumors. Mol Cell Biol. 1985 Jul;5(7):1795–1799. doi: 10.1128/mcb.5.7.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Hauser C., Rott R., Klenk H. D., Doerfler W. Expression of the influenza virus haemagglutinin in insect cells by a baculovirus vector. EMBO J. 1986 Jun;5(6):1359–1365. doi: 10.1002/j.1460-2075.1986.tb04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E. Expression of simian virus 40 T antigen in insect cells using a baculovirus expression vector. Virology. 1988 Nov;167(1):72–81. doi: 10.1016/0042-6822(88)90055-4. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. Signals important for high-level expression of foreign genes in Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1988 Nov;167(1):56–71. doi: 10.1016/0042-6822(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Bishop D. H. Expression of the S-coded genes of lymphocytic choriomeningitis arenavirus using a baculovirus vector. J Gen Virol. 1986 Aug;67(Pt 8):1515–1529. doi: 10.1099/0022-1317-67-8-1515. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miyamoto C., Smith G. E., Farrell-Towt J., Chizzonite R., Summers M. D., Ju G. Production of human c-myc protein in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1985 Oct;5(10):2860–2865. doi: 10.1128/mcb.5.10.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak U., Griffin B. E. Requirement for the C-terminal region of middle T-antigen in cellular transformation by polyoma virus. Nucleic Acids Res. 1981 May 11;9(9):2055–2073. doi: 10.1093/nar/9.9.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Bockus B. J., Berkner K. L., Kaplan D., Roberts T. M. Characterization of middle T antigen expressed by using an adenovirus expression system. J Virol. 1987 Apr;61(4):1221–1225. doi: 10.1128/jvi.61.4.1221-1225.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L., Lodge J., Kaplan D., Roberts T. M. Expression of polyoma early gene products in E. coli. Nucleic Acids Res. 1985 Jan 25;13(2):501–519. doi: 10.1093/nar/13.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Ju G., Ericson B. L., Moschera J., Lahm H. W., Chizzonite R., Summers M. D. Modification and secretion of human interleukin 2 produced in insect cells by a baculovirus expression vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8404–8408. doi: 10.1073/pnas.82.24.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M., Streuli C. H., Griffin B. E. Efficient oligodeoxyribonucleotide-directed deletion mutagenesis using pEMBL vectors: removal of early region introns from polyoma virus mutants. Gene. 1986;49(3):331–340. doi: 10.1016/0378-1119(86)90369-0. [DOI] [PubMed] [Google Scholar]
- Street A. J., Griffin B. E. Immunological variation in the 'common region' of the T antigens of polyoma virus. J Gen Virol. 1987 Dec;68(Pt 12):3153–3164. doi: 10.1099/0022-1317-68-12-3153. [DOI] [PubMed] [Google Scholar]
- Templeton D., Eckhart W. N-terminal amino acid sequences of the polyoma middle-size T antigen are important for protein kinase activity and cell transformation. Mol Cell Biol. 1984 May;4(5):817–821. doi: 10.1128/mcb.4.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]