Abstract
This study investigated the mechanisms underlying the propagation of cytoplasmic calcium waves and the genesis of systolic Ca2+ alternans in cardiac myocytes lacking transverse tubules (t-tubules). These correspond to atrial cells of either small mammals or large mammals that have lost their t-tubules due to disease-induced structural remodeling (e.g., atrial fibrillation). A mathematical model was developed for a cluster of ryanodine receptors distributed on the cross section of a cell that was divided into 13 elements with a spatial resolution of 2 μm. Due to the absence of t-tubules, L-type Ca2+ channels were only located in the peripheral elements close to the cell-membrane surface and produced Ca2+ signals that propagated toward central elements by triggering successive Ca2+-induced Ca2+ release (CICR) via Ca2+ diffusion between adjacent elements. Under control conditions, the Ca2+ signals did not fully propagate to the central region of the cell. However, with modulation of several factors responsible for Ca2+ handling, such as the L-type Ca2+ channels (Ca2+ influx), SERCA pumps (sarcoplasmic reticulum (SR) Ca2+ uptake), and ryanodine receptors (SR Ca2+ release), Ca2+ wave propagation to the center of the cell could occur. These simulation results are consistent with previous experimental data from atrial cells of small mammals. The model further reveals that spatially functional heterogeneity in Ca2+ diffusion within the cell produced a steep relationship between the SR Ca2+ content and the cytoplasmic Ca2+ concentration. This played an important role in the genesis of Ca2+ alternans that were more obvious in central than in peripheral elements. Possible association between the occurrence of Ca2+ alternans and the model parameters of Ca2+ handling was comprehensively explored in a wide range of one- and two-parameter spaces. In addition, the model revealed a spontaneous second Ca2+ release in response to a single voltage stimulus pulse with SR Ca2+ overloading and augmented Ca2+ influx. This study provides what to our knowledge are new insights into the genesis of Ca2+ alternans and spontaneous second Ca2+ release in cardiac myocytes that lack t-tubules.
Introduction
Ca2+ plays a crucial role in cardiac electrical activity, which triggers mechanical contraction of the cell. Systolic [Ca2+]i alternans (1,2) can produce alternans in action-potential duration, which may predispose to atrial or ventricular fibrillations (3–6). Previous experimental and simulation studies have revealed that a steep relationship between systolic [Ca2+]i transient and sarcoplasmic reticulum (SR) Ca2+ content can produce fluctuation in the SR Ca2+ content. This in turn is responsible for generating [Ca2+]i alternans in ventricular myocytes with transverse (t)-tubules (2,7,8).
However, the mechanisms underlying Ca2+ wave propagation and [Ca2+]i alternans in cardiac myocytes lacking t-tubules are still unclear. In small mammals, a key structural difference between atrial and ventricular myocytes is that atrial cells lack t-tubules (9). In ventricular cells, or those from the atria of large mammals, t-tubules may be lost during remodeling processes associated with some diseases, such as atrial fibrillation (10) or heart failure (11–13). In myocytes devoid of t-tubules, voltage-operated calcium channels (VOCCs) are only located on the peripheral cell membrane (9), and therefore, Ca2+ signals produced by depolarization of cell-membrane potential first appear at the subsarcolemmal region and then move to the central region of the cell by triggering successive calcium-induced calcium release (CICR) (14,15). It is unclear whether the dramatic difference in Ca2+ handling between cells with and those without t-tubules has a different impact on Ca2+ wave propagation and therefore on the genesis of [Ca2+]i alternans.
In the work presented here, we attempted to develop a biophysically detailed computer model for Ca2+ release and Ca2+ wave propagation in cardiac myocytes lacking t-tubules. The model was used to investigate possible mechanisms underlying the emergence of Ca2+ alternans in cardiac cells.
Methods
Mathematical model of Ca2+ wave propagation in atrial myocytes
The model was based on equations describing the dynamics of intracellular-Ca2+ handling for a generic cardiac myocyte that were implemented by Kurata et al. (16) for sinoatrial node cells. The Kurata et al. model was later modified by Tao et al. (8) to simulate intracellular Ca2+-wave propagation in ventricular cells. In the study presented here, we further modified the Tao et al. equations (8) to simulate intracellular Ca2+-wave propagation in atrial myocytes that lack t-tubules. In this atrial model, the cross section of a cell (with a diameter of 26 μm) is divided into 13 elements with a spatial resolution of 2 μm (Fig. 1 A i), which is close to the distance between neighboring Ca2+-release sites seen in the central and peripheral regions of an atrial cell (9). VOCCs are localized at the two peripheral units close to the cell membrane surface. The Ca2+ cycling scheme in the peripheral elements is shown in Fig. 1 A ii. In the central elements, Ca2+ cycling is similar to that of the peripheral units but without those elements present in the surface membrane, e.g., VOCCs. These elements are coupled by Ca2+ diffusion between neighboring cytoplasmic spaces adjacent to junctional and nonjunctional ryanodine receptors (RyRs) and between neighboring network SR spaces (Fig. 1 A i). The diffusion parameter, D, used in the model produces a Ca2+-wave conduction velocity of ∼230 μm s−1, which is consistent with experimental data (10) and previous models (17,18). Details of Ca2+ handling, diffusion equations, and parameters are given in the Supporting Material.
Figure 1.
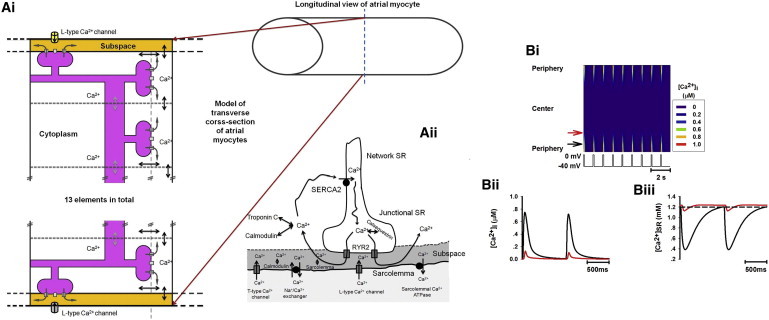
Schematic model of Ca2+ handling in atrial myocytes lacking t-tubules, and simulated Ca2+-wave propagation under control condition. (A i) A cluster of coupled RyRs on a transverse cross section of an atrial myocyte. The cross section is divided into 13 coupled elements, each of which represents a cluster of RyRs. (A ii) Schematic model of calcium cycling for the peripheral elements of the cluster shown in A i. (B i) Line-scan image of cytoplasmic [Ca2+]i transients throughout the atrial myocyte. The voltage trace below marks each of the stimulus pulses (similar in subsequent figures). (B ii and B iii) Time traces of cytoplasmic [Ca2+]i transient (B ii) and SR Ca2+ content (B iii) corresponding to different regions of the cell (black, peripheral region; red, central region; see online color figure).
Stimulation protocol and simulated intracellular Ca2+-wave propagation
In simulations, [Ca2+]i transients were produced by a series of voltage-clamp pulses (1 Hz), as used in previous experimental studies (7). In each of the voltage-clamp pulses, cell membrane potential was clamped from a holding potential of −40 mV to a test potential of 0 mV for 100 ms.
In response to each voltage-clamp pulse, the model produced intracellular Ca2+ diffusion and subsequent Ca2+-wave propagation (Fig. 1 B i). The simulated time traces of [Ca2+]i and [Ca2+]SR in both the peripheral (black arrow) and central (red arrow) regions (Fig. 1 B i) are shown in Fig. 1, B ii and B iii, respectively. Under control conditions, on each voltage-clamp pulse, Ca2+ influx via VOCCs triggered CICR at the peripheral units, resulting in large [Ca2+]i transients in these regions. These localized [Ca2+]i transients diffused inward toward the central region, provoking successive CICR that led to Ca2+-wave propagation. However, the inward Ca2+ wave did not fully propagate to the center (Fig. 1 B i), because the gradually decreasing [Ca2+]i transient amplitude (Fig. 1 B ii) provided an insufficient trigger to generate further CICR, causing the wave to terminate. Consequently, the [Ca2+]i transients (Fig. 1 B ii) and the SR Ca2+ release (Fig. 1 B iii) were large in the periphery of the cell but small in the central region, leading to a spatially heterogeneous distribution of [Ca2+]i transients and Ca2+ wave propagation (Fig. 1 B i). These simulation results matched experimental observations in rat (15,19), guinea-pig (20), and cat (4) atrial myocytes that lack t-tubules.
Model validation
For the purpose of validation, a series of simulations was performed and compared to previous experimental observations from small mammal atrial myocytes that lack t-tubules. These simulations included the effects of sustaining the intracellular Ca2+ waves through increasing Ca2+ influx by elevating the extracellular Ca2+ concentration ([Ca2+]o, (Fig. S1)), increasing the RyR sensitivity by decreasing the threshold of RyR for CICR (Fig. S2), and elevating the SR content by pausing pacing for 10 s while increasing SERCA Ca2+ uptake (Pup increased by 75%) to allow SR Ca2+ content accumulation (Fig. S3). In the model, it was shown that increases in the Ca2+ influx, the RyR sensitivity, or the SR content helped to sustain full Ca2+-wave propagation from the periphery to the center of the cell, producing a more homogeneous [Ca2+]i transient across the cell. These simulation results were similar to experimental observations (Fig. S1, Fig. S2, and Fig. S3).
Results
Effect of increasing Ca2+ influx
An increase in Ca2+ influx via elevating [Ca2+]o has been shown to help facilitate full Ca2+-wave propagation toward the center region and to reduce the [Ca2+]i spatial heterogeneity in atrial cells (15). This experimental observation was reproduced by the model (Fig. S1). In this study, we investigated the effect of an augmented Ca2+ influx from a different approach, i.e., by increasing the maximum channel conductance of the VOCCs (gCaL). Results are shown in Fig. 2. Fig. 2 A shows a line scan of the [Ca2+]i transient across the cell, with an expanded plot of the line-scan image for the time period marked by the horizontal bracket and asterisk (inset A). In the simulation, the cell was paced under control conditions for several beats before gCaL was increased by a factor of 5. Upon the increase of gCaL, full propagation of the Ca2+ wave to the center was seen (Fig. 2 A). Fig. 2, B and C, shows the time courses of the [Ca2+]i transient and the SR Ca2+ content ([Ca2+]SR) recorded from sites near the periphery (black; see online color figure) and in the central region (red; see online color figure) for control (Fig. 2 B) and increased-gCaL (Fig. 2 C) conditions. The [Ca2+]i transient was heterogeneous across the cell under the control condition (Fig. 2 B) but more homogeneous when full inward Ca2+-wave propagation was evoked by increasing gCaL (Fig. 2 C). Insets B and C (Fig. 2, B and C, respectively) clearly illustrate that there is a time delay in the [Ca2+]i and [Ca2+]SR transients between the peripheral and central regions of the cell, indicating Ca2+-wave propagation from the peripheral toward the central region of the cell that triggered successive Ca2+ release, rather than a synchronized Ca2+ release across the cell.
Figure 2.
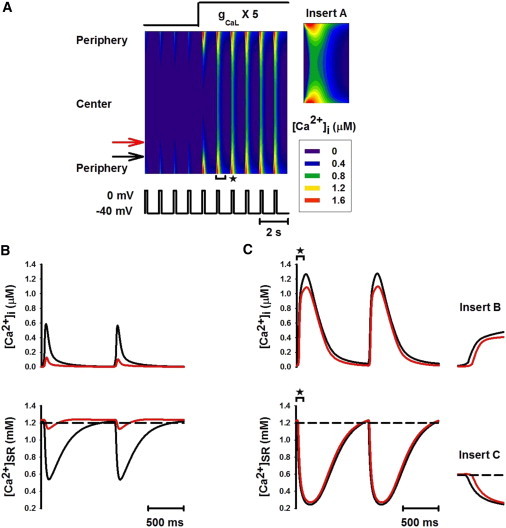
Fully propagated Ca2+ wave and homogeneous [Ca2+]i transients induced by increasing gCaL. (A) Line-scan image of cytoplasmic [Ca2+]i transients. gCaL was increased to five times its control value after the first four pulses. (Inset A) Expanded plot from the line-scan image for the time period marked by the bracket with asterisk in A (similar in subsequent figures). (B and C) Traces of [Ca2+]i transients (upper) and Ca2+ concentration in the SR space (lower) recorded from peripheral (black; see online color figure) and central (red; see online color figure) regions of the cell before (B) and after (C) increasing the L-type calcium current. (Insets B and C) Expanded plots of [Ca2+]i and [Ca2+]SR traces for the time periods marked by the horizontal brackets with asterisks in C.
The generation of a full Ca2+ wave upon increasing gCaL is attributable to a large [Ca2+]i transient in the peripheral region (Fig. 2, B and C, upper), rather than to altered SR Ca2+ content (Fig. 2, B and C, lower). An increased Ca2+ influx produced a large [Ca2+]i transient in the peripheral region, thus promoting enhanced centerward Ca2+ diffusion, which activated successive CICR in elements toward the central region.
Effect of increasing RyR sensitivity
Fig. 3 shows the impact of enhancing the RyR sensitivity by reducing the threshold of RyR for CICR (Krel). In this simulation, the cell was initially stimulated under control conditions for 2 s before Krel was reduced by 10% (Fig. 3, A i and B i) and 60% (Fig. 3, A ii and B ii). The corresponding line-scan images of cytoplasmic Ca2+ are shown in Fig. 3, A i and A ii, respectively. [Ca2+]i transients and SR Ca2+ contents, recorded from both peripheral and central regions, are shown in Fig. 3, B i and B ii, respectively, which are compared to those obtained under control conditions (Fig. 3 B). When Krel was reduced by 10%, a complete Ca2+ wave spreading toward the interior region of the cell was established temporarily (Fig. 3 A i). However, the amplitude of the [Ca2+]i transient recorded from either the peripheral or the central region was smaller (Fig. 3 B i, upper) than that seen in Fig. S2, where RyR sensitivity was increased while SR content was maintained. This was attributed to a reduced level of [Ca2+]SR (Fig. 3 B i, lower) compared to control and Fig. S2 conditions, as more Ca2+ in the SR was released to the cytoplasmic space. Due to the reduced SR content, propagation of the Ca2+ wave toward the center of the cell was unstable, leading to alternans between a complete Ca2+ wave and an incomplete Ca2+ wave after a few pulses (Fig. 3, A i and B i). This produced [Ca2+]i alternans in both peripheral and central regions (Fig. 3 B i; upper), which were associated with alternating SR Ca2+ content (Fig. 3 B i, lower). However, a stable complete Ca2+ wave was observed when Krel was reduced by >60% (Fig. 3 A ii), with even smaller amplitude of [Ca2+]i across the cell due to even greater reduction of SR Ca2+ content (Fig. 3 B ii).
Figure 3.
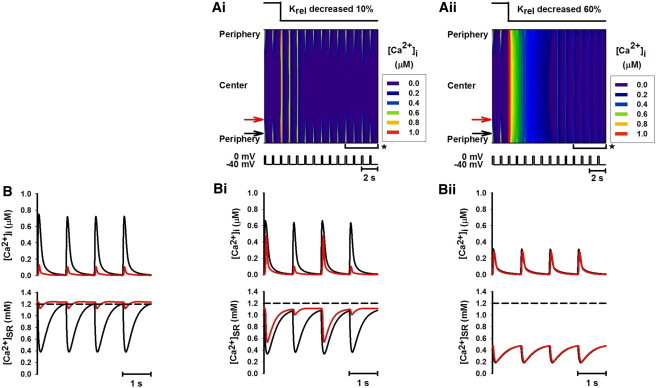
Effect of increasing the sensitivity of RyRs by decreasing Krel by 10% and 60%. (A i and A ii) Line-scan images of spatial patterns of [Ca2+]i transients produced by decreasing Krel by 10% and 60%, respectively. (B, B i, and B ii) Traces of [Ca2+]i transients (upper) and Ca2+ concentration in the SR space (lower) recorded from peripheral (black; see online color figure) and central (red; see online color figure) regions of the cell before (B) and after (B i and B ii) increasing the sensitivity of RyRs. B i and B ii are the time traces recorded during the time period marked by the horizontal brackets with asterisks shown in A i and A ii.
Effect of increased SR content
The effects of increased SR Ca2+ content on Ca2+-wave propagation were investigated in two ways. In one approach, the SR Ca2+ content was increased by pausing voltage-clamp pulse for 10 s after the initial 3 s of stimulations. In the other, the SR content was increased by increasing the SR Ca2+ uptake rate (Pup) by 70%. The results are shown in Fig. 4.
Figure 4.
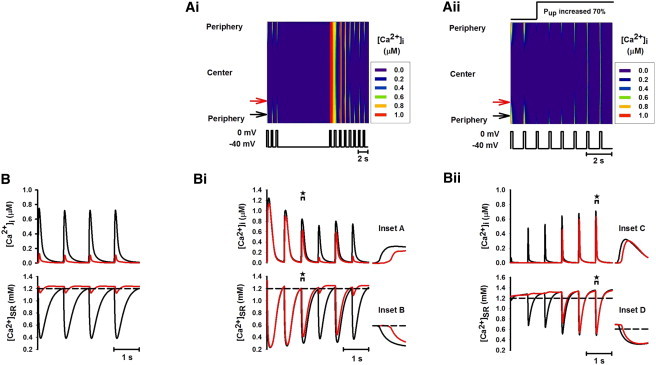
Effects of elevated SR Ca2+ content on Ca2+-wave propagation by pausing pacing for 10 s when [Ca2+]o was increased from 1 mM to 10 mM and by increasing SR uptake rate Pup by 70%. (A i and A ii) Line-scan images of spatial patterns of [Ca2+]i transients produced by pausing voltage-clamp pulse and increasing Pup, respectively. (B, B i, and B ii) Traces of [Ca2+]i (upper) and [Ca2+]SR (lower) recorded from peripheral (black; see online color figure) and central (red; see online color figure) regions of the cell before (B) and after (B i and B ii) elevating the SR Ca2+ content. (Insets A–D) Expanded plots of the time traces for the time periods marked by the brackets with asterisks shown in B i and B ii.
Fig. 4 A i shows a line-scan image of the effects of pausing stimulation. The corresponding time traces for the [Ca2+]i transient and the SR Ca2+ contents recorded from the peripheral and central regions before (Fig. 4 B) and after (Fig. 4 B i) pausing stimulation are shown. Stopping the voltage-clamp pulse for 10 s increased SR Ca2+ content (Fig. 4 B i, lower), leading to a period of complete Ca2+ wave propagation into the center of the cell (Fig. 4 A i), which was followed by alternating complete and incomplete Ca2+ waves (Fig. 4, A i and B i). This produced [Ca2+]i alternans in both peripheral and central regions (Fig. 4 B i, upper). Insets A and B (Fig. 4, B i, upper and lower, respectively) demonstrate the time delays in the [Ca2+]i and [Ca2+]SR transients between the peripheral and central regions of the cell, illustrating that Ca2+ transients were not invoked homogeneously across the cell.
Fig. 4 A ii is a line-scan image of cytoplasmic Ca2+ after increasing Pup, and Fig. 4 B ii shows the corresponding time series for [Ca2+]i and [Ca2+]SR recorded from the peripheral and central regions. It is clear that the SR Ca2+ content was gradually elevated in both the peripheral and central regions by enhancing the SR Ca2+ uptake (Fig. 4 B ii). During the first 3-s voltage-clamp pulse after increasing Pup, the elevation of the SR Ca2+ content in the central region was small. Thus, increasing SR Ca2+ uptake expedited the decline of local [Ca2+]i and hindered the inward Ca2+ wave propagation (Fig. 4 A ii). However, 5 s after elevating Pup, a stable complete Ca2+ wave was observed (Fig. 4 A ii) when the SR Ca2+ content was significantly elevated in both central and peripheral regions (Fig. 4 B ii, lower). Insets C and D (Fig. 4 B ii, upper and lower, respectively) represent the time delay in the [Ca2+]i and [Ca2+]SR transients between the peripheral and central regions of the cell, indicating that the Ca2+ wave was first initiated in the periphery and then conducted toward the center of the cell.
Effect of partial inhibition of SERCA pump
In simulations, two different approaches were implemented to reduce the SERCA pump activity. In one approach, SERCA pump rate (Pup) was decreased by 10%. The results are shown in Fig. 5. After Pup was decreased, the reduced SR Ca2+ uptake enhanced Ca2+-wave propagation (Fig. 5 A i) when the SR Ca2+ content was comparable to that under control conditions (Fig. 5, B and B i, lower). However, in a subsequent voltage-clamp pulse, the SR Ca2+ contents in both the peripheral and central regions declined due to defective SERCA pump activity. This led to a smaller Ca2+ release in the peripheral region and a diminished Ca2+ wave in the central region (Fig. 5, A i and B i), and produced [Ca2+]i transient alternans (Fig. 5 B i, upper).
Figure 5.
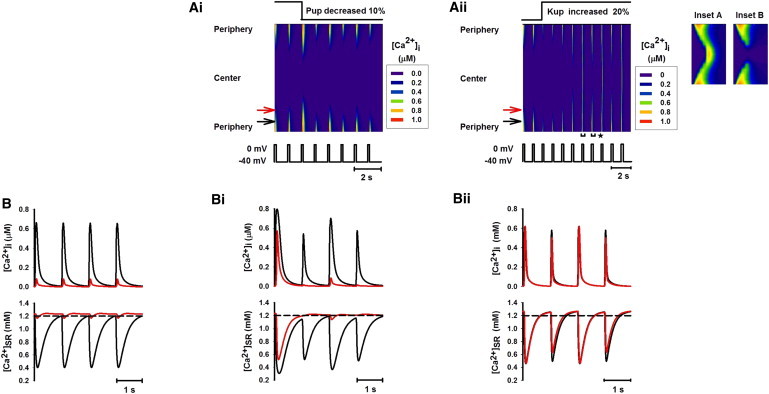
Ca2+ propagation with partial inhibition of SR Ca2+ uptake by decreasing the SR uptake rate (Pup) by 10% and increasing the SERCA uptake threshold (Kup) by 20%. In the latter case, Pup was increased by 50% to keep SR Ca2+ content comparable to that of the control condition. (A i and A ii) Line-scan images of spatial patterns of [Ca2+]i transients produced by decreasing Pup and increasing Krel, respectively. (Insets A and B) Expanded plots from the line-scan image in A ii for the time periods marked by the horizontal brackets with asterisks (left, Inset A; right, Inset B). (B, B i, and B ii) Time traces of [Ca2+]i (upper) and [Ca2+]SR (lower) recorded from peripheral (black; see online color figure) and central (red; see online color figure) regions of the cell before (B) and after (B i and B ii) inhibiting SERCA activity.
In the other approach, the SERCA pump activity was inhibited by increasing the threshold of SERCA Ca2+ uptake (Kup) by 20% (Fig. 5 A ii). Meanwhile the SERCA pump rate (Pup) was increased by 50% such that the SR Ca2+ content was maintained close to that in control condition. An enhanced Ca2+-wave propagation was established, as shown in Fig. 5 A ii. In this case, [Ca2+]i transients at the peripheral sites remained almost unchanged (Fig. 5, B and B ii). However, the amplitude of [Ca2+]i transients was greatly enhanced in the interior region of the cell (Fig. 5 B ii, upper), leading to full Ca2+-wave propagation, though the SR Ca2+ content was comparable to that in control condition (Fig. 5, B and B ii, lower). This was attributed to the fact that a partial inhibition of SERCA activity while the SR Ca2+ content was maintained resulted in more diffusion of Ca2+ throughout the cell. In addition to the increased [Ca2+]i transients in the central region, the Ca2+ signal displayed alternans (Fig. 5 B ii, upper) that was more obvious in the central region than in the periphery (Fig. 5 A ii, insets A and B). These Ca2+ alternans arose because the Ca2+ release propagated toward the interior. The resulting large Ca2+ release depleted the SR content in the central region, which did not fully recover by the next voltage-clamp pulse, resulting in a reduced Ca2+ release. In this way, an alternating large-small pattern of [Ca2+]i transients was produced.
Exploring the mechanisms for Ca2+ alternans
Further simulations were performed to explore possible mechanisms by which Ca2+ alternans in cardiac myocytes without t-tubules could be generated in response to a linear change in the parameters associated with various aspects of Ca2+ handling.
Role of Ca2+ diffusion
Fig. 6 A shows the effect of a linear increase of the time constant of the Ca2+ diffusion (τ) on generation of [Ca2+]i alternans. When τ was varied from 100% to 130% of its control value, various patterns of Ca2+ alternans were observed (Fig. 6 A, upper). Transition to such variant [Ca2+]i alternans was via a bifurcation process (Fig. 6 A, lower), which occurred when τ was 115% of its control value, with each large Ca2+ transient being followed first by four (Fig. 6 A i), then two (Fig. 6 A ii), then one (Fig. 6 A iii) small one. This suggested that a more complicated pattern of [Ca2+]i alternans than that typically observed (1:1 [Ca2+]i alternans) was possible. Note that such a complicated pattern of alternans has been observed experimentally in the electrical activity of the heart with period doubling and tripling and even more complicated 1:n patterns generated by a cascade effect of the bifurcation process (21).
Figure 6.
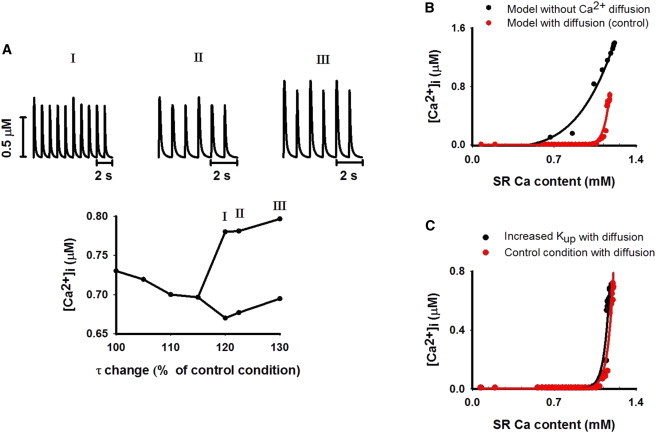
Role of Ca2+ diffusion in generating [Ca2+]i alternans. (A, upper) Various patterns of [Ca2+]i alternans generated by varying the Ca2+ diffusion constant (τ). (A, lower) Systolic [Ca2+]i amplitudes as a function of τ increased over the range 100% to 130% of its control value. A bifurcation process occurred, leading to [Ca2+]i alternans with complicated patterns of 1:4 (I), 1:2 (II), and 1:1 (III) alternans. (B) Relationship between systolic [Ca2+]i amplitude and SR Ca2+ content with (red; see online color figure) and without (black; see online color figure) Ca2+ diffusion. The solid lines were generated by using the formula for curve fitting (amplitude of systolic [Ca2+]i = a + b × [SR Ca2+ content]n). n = 4.0 for the model without Ca2+ diffusion, and n = 25.3 for the model with Ca2+ diffusion. (C) Relationship between systolic [Ca2+]i amplitude and SR calcium content in a model with Ca2+ diffusion, but with (black dots; see online color figure) (n = 25.3) or without (red dots; see online color figure) (n = 25.3) partial inhibition of SERCA pump activity.
We further examined the effect of Ca2+ diffusion on genesis of [Ca2+]i alternans by removing the Ca2+ diffusion in the cell. In the absence of Ca2+ diffusion (i.e., the model was considered as a single unit), partial inhibition of SERCA activity by increasing Kup by 20% or even by 40% did not produce Ca2+ alternans. This indicated an important role of Ca2+ diffusion in generating [Ca2+]i alternans.
Such an important role of Ca2+ diffusion in generating alternans can be understood by analyzing the dependence of the systolic [Ca2+]i amplitude on the SR Ca2+ content, which was derived by correlating the SR Ca2+ content to the systolic [Ca2+]i amplitude during refilling of the SR from the empty state. Results shown in Fig. 6 B were obtained at the control condition. In this case, diffusion dramatically increased the steepness of this dependence (from n = 4.0 in the absence of diffusion to 25.3 for diffusion).
It has been suggested previously that increased dependence of Ca2+ release on SR Ca2+ content increases the probability of alternans occurring (2,7). In ventricular myocytes, it was shown (7) that reducing the activation of ICa,L channels—thus producing a small Ca2+ signal for triggering CICR of RyR channels—increased the steepness of dependence of Ca2+ release on SR Ca2+ content, leading to genesis of [Ca2+]i alternans. In atrial myocytes devoid of t-tubules, the ICa,L channels are only located in the peripheral region. In the interior region of the cell, it is Ca2+ diffusion that provides a Ca2+ signal that triggers CICR of RyR channels. As the Ca2+ signal for triggering CICR via such Ca2+ diffusion is small, one would also expect a steep dependence of Ca2+ release on SR Ca2+ content.
In the model with Ca2+ diffusion, the effect of partial inhibition of SERCA activity (increasing Kup by 20%) on the steepness of the [Ca2+]i dependence on the SR Ca2+ content is shown in Fig. 6 C. Increasing Kup shifted the dependence curve leftward (i.e., toward smaller SR Ca2+ content region) compared with that under the control condition, indicating a more sensitive dependence of the systolic [Ca2+]i amplitude on the SR content, which enabled the genesis of [Ca2+]i alternans (Fig. 5 A ii).
Role of Ca2+ influx
The role of increased Ca2+ influx in generating [Ca2+]i alternans was investigated by systematically increasing gCaL. When gCaL was increased over the range 100%–500% of its control value, a cascade of bifurcations occurred, leading to [Ca2+]i alternans with various patterns (Fig. 7 A i). In all cases, [Ca2+]i alternans was more dramatic in the central region than in the peripheral region (Fig. 7, A ii and A iii). In the range 120%–150% of gCaL, a very small [Ca2+]i transient amplitude in the central region associated with 1:2 alternans (Fig. 7, A i I and A iv) indicated a partial propagation of a Ca2+ wave into the central region for every two out of three Ca2+ waves, each of which resulted in a marked spatial gradient in [Ca2+]i amplitude across the cell. In the range 150–170% of gCaL, [Ca2+]i alternans disappeared (Fig. 7, A i II and A iv), but it reappeared when gCaL was further increased to 200%–400% of its control value (Fig. 7, A i III, A i IV, and A iv). In the latter case, although the amplitude of the [Ca2+]i transient alternated from beat to beat, its spatial gradient across the cell was reduced, as the amplitude of the [Ca2+]i transient in the central region was close to that in the peripheral region (Fig. 7 A iv). [Ca2+]i alternans disappeared when gCaL was increased to >450% (Fig. 7, A ii and A iii).
Figure 7.
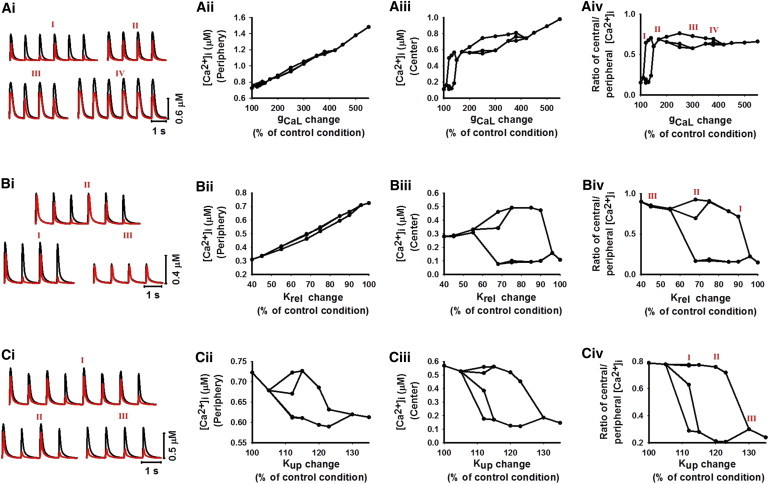
Roles of altered Ca2+ influx (A, i–iv), sensitivity of RyR (B, i–iv), and SERCA pump activity (C, i–iv) in generating [Ca2+]i alternans. The bifurcation diagram was drawn by plotting multiple peak values of the Ca2+ transient for a given gCa,L, Krel, or Kup. (A i, B i, and C i) Various profiles of Ca2+ transients recorded in peripheral (black; see online color figure) and central (red; see online color figure) regions of the cell. Profiles were generated by a cascade of bifurcation processes with a linear increase of gCa,L (A i) and Kup (C i) and a decrease of Krel (B i). (A ii, B ii, and C ii) Amplitude of systolic [Ca2+]i transient against gCa,L (A ii), Krel (B ii), and Kup (C ii) in the peripheral region of the cell. (A iii, B iii, and C iii) Amplitude of systolic [Ca2+]i transient versus gCa,L (A iii), Krel (B iii), and Kup (C iii) in the central region of the cell. (A iv, B iv, and C iv) Ca2+ gradient measured as the ratio between the central and peripheral Ca2+ amplitudes with a linear increase of gCa,L (A iv) and Krel (B iv) and a decrease of Kup (C iv). Small ratios represent a large gradient; ratios approaching 1 imply more homogeneous Ca2+ distribution in the cell.
Role of RyR sensitivity
The role of increased sensitivity of RyR to CICR in generating [Ca2+]i alternans was investigated by reducing Krel from 100% to 60% of its control value. With decreasing Krel, a cascade of bifurcations occurred, leading to [Ca2+]i alternans with various patterns (Fig. 7 B i). Similar to the case of increasing gCa,L, [Ca2+]i alternans was more significant in the central region (Fig. 7, B ii and B iii). When Krel was reduced to 90% of its control value, [Ca2+]i alternans began with a 1:1 pattern (Fig. 7, B i I and B iv). It became more complicated when Krel was reduced to 85%–55% (Fig. 7, B i II and B iv). Further reducing Krel to <40% caused [Ca2+]i alternans to disappear (Fig. 7, B i III and B iv). In the latter case, small [Ca2+]i transients were seen in both the peripheral and central regions, leading to a decreased spatial gradient of the [Ca2+]i transient in the cell. It was due to the fact that the increased sensitivity of RyRs in nonjunctional regions enabled them to be more easily triggered for CICR at a lower level of the SR content, producing small but homogeneous [Ca2+]i transients across the cell (Fig. 7 B iv).
Role of the SERCA pump
The role of a defective SERCA pump in generating [Ca2+]i alternans was investigated by modulating the half-maximal cytoplasmic [Ca2+]i for SERCA Ca2+ uptake (Kup). When Kup was increased from 100% to 140% of its control value, a cascade of bifurcations was triggered, leading to Ca2+ alternans with different patterns (Fig. 7 C i). When Kup was increased by 10%, [Ca2+]i alternans had a complicated pattern (Fig. 7, C i I and C iv), but it transited into 1:1 alternans when Kup was increased by 20% (Fig. 7, C i II and C iv). When Kup was increased by >30%, [Ca2+]i alternans disappeared (Fig. 7, C i III and C iv). In all cases, decreased SR uptake due to increased Kup resulted in a low level of SR Ca2+ content and, consequently, reduced [Ca2+]i transient amplitude (Fig. 7, C ii and C iii). However, the spatial gradient in [Ca2+]i transient across the cell was augmented by the defective SR uptake, which gradually depleted the SR content (Fig. 7 C iv).
Further simulations were performed to explore theoretically the genesis of [Ca2+]i alternans in a 2D parameter space, mimicking combined modulations of two different properties of Ca2+ handling, which may occur in some disease conditions, such as heart failure, where the sensitivity of RyRs is enhanced, whereas the SERCA activity is significantly reduced (22). Detailed results are shown in in the Supporting Material.
Spontaneous Ca2+ release
Overloading the SR Ca2+ content may produce spontaneous Ca2+ release from the SR. Fig. 8 shows recorded time traces of [Ca2+]i (Fig. 8 A i), [Ca2+]SR (Fig. 8 A ii), Ca2+-Na+ exchange current (INCX; Fig. 8 A iii), and ICaL (Fig. 8 A iv) recorded from the cell under the condition of increased Ca2+ influx (gCaL increased to 370%) and SERCA pump activity (Pup increased to 150%). With dramatic increases in both the Ca2+ influx and the SR uptake efficiency, an irregular pattern of [Ca2+]i transient was observed in response to a series of stimuli. A most interesting observation was the spontaneous second SR Ca2+ releases in response to one stimulus (Fig. 8 A i, asterisks).
Figure 8.
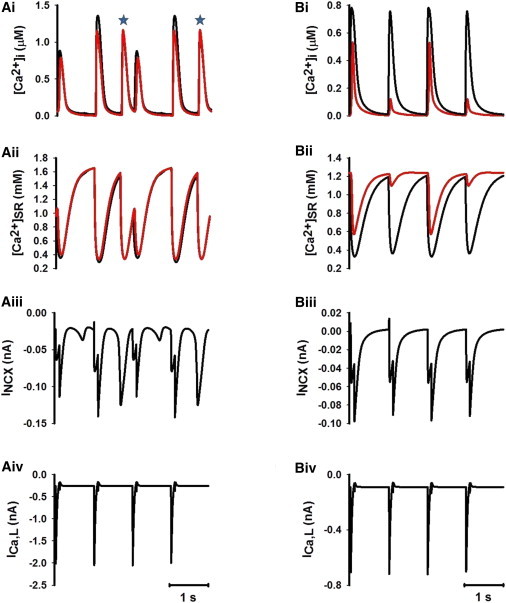
(A i–A iv) Time traces of [Ca2+]i (A i), SR Ca2+ content (A ii), Na+-Ca2+ exchange current (INCX) (A iii), and ICa,L (A iv) under the condition of SR Ca2+ overload (Pup increased to 150%) and increased Ca2+ influx (gCa,L increased to 370%) for peripheral (black; see online color figure) and central (red; see online color figure) regions of the cell. Irregular [Ca2+]i transients were produced, as were spontaneous secondary SR Ca2+ releases in response to a single voltage-clamp pulse (asterisks). (B i–B iv) Time traces of [Ca2+]i (B i), SR Ca2+ content (B ii), Na+-Ca2+ exchange current (INCX) (B iii), and ICa,L (B iv) under the condition of increasing gCa,L by 30% of its control value. Highly sensitive dependence of [Ca2+]i transient on SR Ca2+ content led to genesis of [Ca2+]i alternans.
Discussion
Summary of major findings
We have developed a mathematical model for Ca2+-wave propagation in cardiac atrial myocytes that lack t-tubules, thereby representing atrial myocytes of small mammals, or atrial cells of larger mammals, that have lost t-tubules due to disease-induced structural remodeling. The developed model was validated by its ability to reproduce typical Ca2+-wave propagation patterns of atrial myocytes without t-tubules. It was also validated by its ability to reproduce experimentally observed effects of modulations of various aspects of Ca2+ cycling, such as Ca2+ influx, SERCA pumps (SR Ca2+ uptake), and RyRs (SR Ca2+ release), on spatial distribution of Ca2+ transients (15). Using the model, we explored possible factors responsible for generating Ca2+ alternans in cardiac cells devoid of t-tubules. The major findings of this study are as follows. 1), The functional spatial heterogeneity in Ca2+ diffusion due to gradually decreased amplitudes of Ca2+ transients across the cell produced a steep relationship between the SR Ca2+ content and the cytoplasmic Ca2+ concentration. Together with Ca2+ wave propagation, this contributed to the genesis of Ca2+ alternans, which was more obvious in central than in peripheral elements. 2), Analyses on one-parameter space and two-parameter space (see Supporting Material) were performed to provide a theoretical exploration of possible associations between the occurrence of Ca2+ alternans and parameters related to calcium handling. In these analyses, either one or two parameters associated with Ca2+ handling were altered alone or together. 3), Under the condition of SR Ca2+ overload and augmented Ca2+ influx, the model predicted a spontaneous second Ca2+ release in response to a single voltage-stimulus pulse. Spontaneous Ca2+ release may be responsible for ectopic activities, leading to atrial fibrillations (23). This study provides, for the first time to our knowledge, a biophysically detailed mathematical model of intracellular Ca2+ handling that underpins the mechanisms of Ca2+-wave propagation in cardiac myocytes that lack t-tubules. It also offers what to our knowledge are new insights into the genesis of Ca2+ alternans and spontaneous Ca2+ release, both of which are proarrhythmic.
Mechanisms of incomplete Ca2+-wave propagation in cardiac cells without t-tubules
It has been shown that in atrial myocytes without t-tubules, the Ca2+ signal is restricted to the junctional subsarcolemmal compartment (9,15) due to incomplete Ca2+-wave propagation in the cell. It was hypothesized that this was due to the reduction in amplitude of Ca2+ transients from the peripheral to the central regions of the cell, leading to a gradual reduction in Ca2+ diffusion. Thus, moving toward the central region, the decreased Ca2+ signal produced smaller CICR and further reduced Ca2+ signals until CICR could not be induced in the central region of the cell. This led to an incomplete Ca2+ wave propagation (9). Our simulation results support this theory (Fig. 1). The model reproduced spatially inhomogeneous Ca2+ transients across the cell. With a gradual decrease in amplitude, Ca2+ diffusion becomes reduced from the peripheral region to the central region, leading to reduced CICR trigger at interior sites, which results in termination of the Ca2+ wave when the trigger is insufficient for further SR Ca2+ release at the interior region. However, modulations of Ca2+ handling that enhanced intracellular Ca2+ diffusion from the peripheral to the central region, such as increasing Ca2+ influx by increasing gCaL (Fig. 2), increasing the sensitivity of RyRs by lowering the threshold of RyRs (Fig. 3), increasing the SR Ca2+ content (Fig. 4), or reducing the SERCA pump activity (Fig. 5), helped to establish a complete Ca2+ wave across the cell.
Mechanisms of Ca2+ alternans in cardiac cells devoid of t-tubules
Roles of Ca2+ diffusion
The results of Fig. 6 clearly indicate an important contribution of Ca2+ diffusion in generating Ca2+ alternans. In the model, upon partial inhibition of SERCA pump activity, 1:1 Ca2+ alternans was produced. However, with the same set of model parameters, no Ca2+ alternans was observed if the cell was treated as a single release element, or with t-tubules across the cell with VOCC channels being coupled with all RyR elements, i.e., no Ca2+ diffusion in the model. Further analysis revealed that Ca2+ diffusion altered the relationship between the SR Ca2+ load and the systolic Ca2+ concentration in cytoplasm by sharply increasing the steepness of this relationship (Fig. 6 B), enabling the genesis of Ca2+ alternans. In the model, the Ca2+ diffusion was induced by a spatially inhomogeneous distribution of Ca2+ transients across the cell due to the lack of t-tubules in the central region. This was fundamentally different from ventricular myocytes, where the existence of t-tubules inside cells was expected to produce a more simultaneous and homogeneous distribution of Ca2+ transients in the cross section of the cell.
Roles of Ca2+-handling kinetics—insights from one-parameter analysis
Similar to previous modeling studies on ventricular cells with t-tubules (8), Ca2+ alternans can also be generated in cardiac myocytes absent of t-tubules by changing properties of Ca2+ cycling. Varying an individual parameter (Fig. 7) associated with Ca2+ influx (gCaL), SR Ca2+ release (Krel), and SR Ca2+ uptake (Kup) triggered a cascade bifurcation process, leading to complex patterns of Ca2+ alternans as a consequence of interactions between Ca2+ diffusion and altered Ca2+ cycling in individual elements. Such interactions produced a steep relationship between the SR Ca2+ content and cytoplasmic Ca2+ transients, resulting in a highly sensitive dependence of Ca2+ release on the SR content. This was illustrated in Fig. 8, B, i–iv, which plotted the time series of [Ca2+]i (Fig. 8 B i), [Ca2+]SR (Fig. 8 B ii), INCX (Fig. 8 B iii), and ICaL (Fig. 8 B iv) when [Ca2+]i alternans were produced by increasing gCaL to 130% of its control value (Fig. 7 A). It was obvious that the SR Ca2+ load was slightly lower before a small Ca2+ release than before a large release (Fig. 8 B ii). Corresponding to a larger [Ca2+]i transient, there was a greater removal of cytoplasmic Ca2+ by NCX current (Fig. 8 B iii), which led to incomplete refilling of the SR (Fig. 8 B ii), producing a smaller Ca2+ release (Fig. 8 B ii), minor Ca2+-wave propagation, and Ca2+ efflux by the next stimulus. All of these effects enabled SR Ca2+ content to recover to the normal level, producing a large Ca2+ release on the next stimulus (7,8,24). During all of the [Ca2+]i alternating processes, the amplitude of ICa,L remained unchanged (Fig. 8 B iv). In some conditions, several cycles might be needed for the SR Ca2+ content to recover to a normal level after a large Ca2+ release, producing more complicated patterns (1:n − 1, where n > 1) of Ca2+-transient alternans.
Significance of the study
It has been observed that t-tubule networks are dense and well organized in ventricular myocytes, are absent or less organized in other types of cardiac cells, including atrial cells (3,19). However, recent studies have identified t-tubules in atrial myocytes from a number of species, including sheep (10,11), dog (25), and human (26). Further studies have also shown that t-tubules of atrial and ventricular cells may be lost or disorganized by structural remodeling processes during chronic diseases (11,13). Loss of t-tubules of ventricular myocyctes has been observed in a number of animal models of heart failure, as well as in human heart failure (27). Dramatic loss of t-tubules was also observed in atrial myocytes in a sheep model of heart failure (12) and in patients with persistent atrial fibrillation (10).
Thus, this study provides insight into the mechanisms underlying Ca2+-wave propagation in cardiac myocytes, not only for atrial myocytes of small mammals that lack t-tubules but also for other cardiac myocytes (including ventricular myocytes) that lose t-tubules due to disease-induced structural remodeling. Experimental studies show that the loss of t-tubules in ventricular myocytes due to detubulation (9) is associated with desynchronized Ca2+ release across the cell, and Ca2+ wavelike propagation from the sarcolemma to the cell interior. In a similar way, loss of t-tubules in atrial myocytes during atrial fibrillation is also associated with spatially desynchronized Ca2+ release (10). All of these features observed in ventricular and atrial myocytes with loss of t-tubules were reproduced in the model.
In this study, we also investigated the mechanisms underlying the genesis of Ca2+ alternans and spontaneous RyR Ca2+ release, both of which are proarrhythmic, in cardiac cells devoid of t-tubules. It was shown that the absence of t-tubules promoted the genesis of Ca2+ alternans due to Ca2+ diffusion consequent to heterogeneous distribution of Ca2+ transients across the cell. Ca2+ alternans were generated for conditions under which no Ca2+ alternans would be observed if t-tubules were present. This may provide a partial explanation to the increased risk of arrhythmogenesis in cardiac tissues remodeled by chronic diseases such as heart failure and atrial fibrillation.
Limitations of the study
This model was based on the Kurate et al. (16) and Tao et al. (8) models and inherited the same limitations of that model, which have been discussed in detail in our previous study (8). The major limitation of this model was its use of one-dimensional RyR elements on the cross section of a cell, which is an idealized consideration of the cell. It therefore lacked the complex geometry of a whole cell, which imposes 3-dimensional structure and features complicated t-tubule networks and RyR arrangement. In simulations, the inositol-1,4,5-trisphosphate-receptor (IP3R) was not incorporated into the model, because the contribution of IP3R-mediated Ca2+ release to atrial [Ca2+]i remains controversial and is limited under the basal condition (28). On the one hand, the lack of consideration of IP3Rs in our model may be considered a potential limitation of this study. On the other hand, however, it makes clear the importance of Ca2+ diffusion and various factors of Ca2+ handling in the generation of Ca2+ waves and alternans in cardiac myocytes without t-tubules.
All of these limitations are now being addressed for future versions of the model. However, they do not alter our conclusions about the mechanisms that underlie the initiation and propagation of Ca2+ waves in cardiac myocytes lacking t-tubules or the genesis of Ca2+ alternans in this type of cell.
Acknowledgments
This work was supported by project grants from the Engineering and Physical Science Research Council UK (EP/I029826/1; EP/J00958X/1) and the National Science Foundation China (61179009). Q.L. was supported by an Overseas Research Scholarship from The University of Manchester.
Supporting Material
References
- 1.Chudin E., Goldhaber J., Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys. J. 1999;77:2930–2941. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisner D.A., Choi H.S., Trafford A.W. Integrative analysis of calcium cycling in cardiac muscle. Circ. Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- 3.Blatter L.A., Kockskämper J., Lipsius S.L. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J. Physiol. 2003;546:19–31. doi: 10.1113/jphysiol.2002.025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kockskämper J., Blatter L.A. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J. Physiol. 2002;545:65–79. doi: 10.1113/jphysiol.2002.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayan S.M., Bode F., Franz M.R. Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation. 2002;106:1968–1973. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 6.Pruvot E.J., Katra R.P., Laurita K.R. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ. Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 7.Díaz M.E., O'Neill S.C., Eisner D.A. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ. Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 8.Tao T., O'Neill S.C., Zhang H. Alternans of cardiac calcium cycling in a cluster of ryanodine receptors: a simulation study. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H595–H609. doi: 10.1152/ajpheart.01086.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brette F., Orchard C. T-tubule function in mammalian cardiac myocytes. Circ. Res. 2003;92:1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 10.Lenaerts I., Bito V., Willems R. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ. Res. 2009;105:876–885. doi: 10.1161/CIRCRESAHA.109.206276. [DOI] [PubMed] [Google Scholar]
- 11.Balijepalli R.C., Lokuta A.J., Kamp T.J. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc. Res. 2003;59:67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 12.Dibb K.M., Clarke J.D., Trafford A.W. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ. Heart Fail. 2009;2:482–489. doi: 10.1161/CIRCHEARTFAILURE.109.852228. [DOI] [PubMed] [Google Scholar]
- 13.Louch W.E., Mørk H.K., Sejersted O.M. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J. Physiol. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carl S.L., Felix K., Ferguson D.G. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J. Cell Biol. 1995;129:673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackenzie L., Roderick H.L., Bootman M.D. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J. Cell Sci. 2004;117:6327–6337. doi: 10.1242/jcs.01559. [DOI] [PubMed] [Google Scholar]
- 16.Kurata Y., Hisatome I., Shibamoto T. Dynamical description of sinoatrial node pacemaking: improved mathematical model for primary pacemaker cell. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2074–H2101. doi: 10.1152/ajpheart.00900.2001. [DOI] [PubMed] [Google Scholar]
- 17.Backx P.H., de Tombe P.P., ter Keurs H.E. A model of propagating calcium-induced calcium release mediated by calcium diffusion. J. Gen. Physiol. 1989;93:963–977. doi: 10.1085/jgp.93.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langer G.A., Peskoff A. Calcium concentration and movement in the diadic cleft space of the cardiac ventricular cell. Biophys. J. 1996;70:1169–1182. doi: 10.1016/S0006-3495(96)79677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo S.H., Cleemann L., Morad M. Ca2+ current-gated focal and local Ca2+ release in rat atrial myocytes: evidence from rapid 2-D confocal imaging. J. Physiol. 2002;543:439–453. doi: 10.1113/jphysiol.2002.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berlin J.R. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am. J. Physiol. 1995;269:H1165–H1170. doi: 10.1152/ajpheart.1995.269.3.H1165. [DOI] [PubMed] [Google Scholar]
- 21.Ritzenberg A.L., Adam D.R., Cohen R.J. Period multupling-evidence for nonlinear behaviour of the canine heart. Nature. 1984;307:159–161. doi: 10.1038/307159a0. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T., Yano M., Matsuzaki M. Abnormal Ca2+ release from cardiac sarcoplasmic reticulum in tachycardia-induced heart failure. Cardiovasc. Res. 1999;44:146–155. doi: 10.1016/s0008-6363(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 23.Hove-Madsen L., Llach A., Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 24.Shiferaw Y., Karma A. Turing instability mediated by voltage and calcium diffusion in paced cardiac cells. Proc. Natl. Acad. Sci. USA. 2006;103:5670–5675. doi: 10.1073/pnas.0511061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolber P.C., Bauman R.P., Greenfield J.C., Jr. Regional changes in myocyte structure in model of canine right atrial hypertrophy. Am. J. Physiol. 1994;267:H1279–H1287. doi: 10.1152/ajpheart.1994.267.4.H1279. [DOI] [PubMed] [Google Scholar]
- 26.Richards M.A., Clarke J.D., Dibb K.M. Transverse tubules are a common feature in large mammalian atrial myocytes including human. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1996–H2005. doi: 10.1152/ajpheart.00284.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyon A.R., MacLeod K.T., Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc. Natl. Acad. Sci. USA. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Zima A.V., Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ. Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


