Abstract
Parkinson's disease (PD) is associated with a characteristic regional metabolic covariance pattern that is modulated by treatment. To determine whether a homologous metabolic pattern is also present in nonhuman primate models of parkinsonism, 11 adult macaque monkeys with parkinsonism secondary to chronic systemic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 12 age-matched healthy animals were scanned with [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET). A subgroup comprising five parkinsonian and six control animals was used to identify a parkinsonism-related pattern (PRP). For validation, analogous topographies were derived from other subsets of parkinsonian and control animals. The PRP topography was characterized by metabolic increases in putamen/pallidum, thalamus, pons, and sensorimotor cortex, as well as reductions in the posterior parietal-occipital region. Pattern expression was significantly elevated in parkinsonian relative to healthy animals (P<0.00001). Parkinsonism-related topographies identified in the other derivation sets were very similar, with significant pairwise correlations of region weights (r>0.88; P<0.0001) and subject scores (r>0.74; P<0.01). Moreover, pattern expression in parkinsonian animals correlated with motor ratings (r>0.71; P<0.05). Thus, homologous parkinsonism-related metabolic networks are demonstrable in PD patients and in monkeys with experimental parkinsonism. Network quantification may provide a useful biomarker for the evaluation of new therapeutic agents in preclinical models of PD.
Keywords: animal models, brain imaging, glucose, Parkinson's disease, positron emission tomography
Introduction
The major clinical manifestations of Parkinson's disease (PD) have been attributed to progressive loss of nigrostriatal dopaminergic projections and to concomitant changes in the activity of cortico-striato-thalamo-cortical circuits and related neural pathways. Resting-state metabolic brain imaging with [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) has been used in conjunction with spatial covariance analysis to identify the abnormal functional networks that underlie this disorder (Eidelberg, 2009). Specifically, parkinsonian akinesia and rigidity have been associated with a PD motor-related spatial covariance pattern (PDRP) (Eidelberg, 2009; Ma et al, 2007; Spetsieris and Eidelberg, 2011) characterized by increased metabolic activity in pallidothalamic, pontocerebellar, and motor cortical regions, and reduced metabolic activity in premotor, prefrontal, and parietal association regions. Expression of PDRP has been found to be abnormally elevated in individual PD patients (Ma and Eidelberg, 2007; Ma et al, 2010; Moeller et al, 1999), correlating with increasing motor disability and declining presynaptic nigrostriatal dopaminergic function in these subjects (Huang et al, 2007; Tang et al, 2010). Moreover, significant reductions in PDRP expression have been noted during effective dopamine replacement therapy (Asanuma et al, 2006; Hirano et al, 2008; Mattis et al, 2011) with the degree of treatment-mediated network modulation correlating significantly with concurrent improvement in clinical ratings (Asanuma et al, 2006; Feigin et al, 2001).
An experimental disease model in which nonhuman primates are treated with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) recapitulates the key clinical features of classical PD, with many of the pharmacologic and neurochemical features of the human disorder. In nonhuman primates, MPTP administration is associated with markedly reduced striatal uptake of radiotracers targeting presynaptic monoaminergic terminals (Brownell et al, 2003; Doudet et al, 1998), as is also seen in humans exposed to this neurotoxin (Snow et al, 2000). Moreover, quantitative autoradiography with [11C]-2-deoxyglucose (Guigoni et al, 2005; Mitchell et al, 1989), as well as in-vivo metabolic imaging with FDG PET (Brownell et al, 2003; Emborg et al, 2007), have been used to quantify changes in regional glucose utilization in experimental primate models of parkinsonism. While these studies have described significant regional metabolic abnormalities in MPTP-lesioned primates, no data exist concerning changes occurring at the network level in these animals. In particular, it is not known whether this experimental model of parkinsonism is associated with the expression of a distinct regional metabolic pattern akin to that observed in actual PD patients.
To address this issue, we used resting-state metabolic imaging in conjunction with spatial covariance mapping to identify a parkinsonism-related pattern (PRP) in a nonhuman primate model of PD. In addition to demonstrating the replicability of this pattern in prospective samples of MPTP-lesioned and control monkeys, we examined the direct effects of nigrostriatal dopaminergic lesioning by quantifying PRP expression in animals scanned before and after the induction of parkinsonian motor signs by systemic MPTP exposure.
Materials and methods
Eighteen adult macaque monkeys (12 males and 6 females, age 8 to 22 years, weight 5 to 8 kg) were scanned with FDG PET as described below. The PET scans from these monkeys were divided into two separate cohorts of parkinsonian and control animals as illustrated in Figure 1. The parkinsonian and control animals were of similar age (12.4±4.6 versus 10.0±3.9 years; P=0.20) and weight (9.8±2.2 versus 8.8±2.6 kg; P=0.31). All nonhuman primate experiments were conducted in accordance with the relevant guidelines and regulations of Canadian federal government on animal welfare and were approved by the Committee on Animal Care at the University of British Columbia.
Figure 1.
Schematic illustrating the relationships between each of the five parkinsonism-related covariance patterns (PRPs) and the scans from the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned and control animals (Cohorts A and B) used for pattern characterization (see text). The number of scans or hemispheres used for the derivation of each PRP (parkinsonism-related pattern) topography is marked on the flow chart from Cohorts A and B. PRP5 resulted from a whole-brain analysis of data from the animals in Cohort A. (Of note, the seven untreated hemispheres from the MPTP animals with contralateral transplants were included in Cohort B. Five of the parkinsonian monkeys in Cohort A were scanned before MPTP lesioning. These baseline images were used as control scans in Cohort B.)
Cohort A was comprised of five monkeys (age=9.60±0.89 years) who developed bilateral parkinsonism after chronic intravenous administration of MPTP (Doudet et al, 1998; Doudet et al, 2004). (These postMPTP scans were acquired from five of six monkeys whose baseline (i.e., preMPTP) scans served as controls for the parkinsonian monkey scans in Cohort B.) These MPTP-lesioned animals were scanned 2.2 to 6.3 months (mean 3.74 months) after baseline imaging. The 10 postMPTP hemispheres of the parkinsonian monkeys in Cohort A were combined with 12 control hemispheres from 6 healthy monkeys (age=8.3±0.5 years) for spatial covariance analysis (see below).
Cohort B was comprised of six monkeys (age=14.7±5.3 years) treated systemically with MPTP. Five of these bilaterally parkinsonian monkeys received unilateral striatal implantation of cultured dopaminergic tissue (Ma et al, 2008). Of the 12 resulting MPTP-lesioned hemispheres, the 5 hemispheres with implants were excluded from further analysis. The nonimplanted hemispheres were selected for network analysis based on the assumption that unilateral implantation did not alter glucose utilization in the contralateral hemispheres. In this cohort, the remaining 7 MPTP-lesioned hemispheres were combined with 12 control hemispheres from 6 other healthy monkeys (age=11.7±5.1 years) for spatial covariance analysis.
In both cohorts, the lesioned animals were injected repeatedly, at varying intervals, with intravenous doses of MPTP (0.5 mg/kg or less) until the appearance and maintenance of robust parkinsonian symptoms with varying degrees of severity. The animals were used in the imaging studies after at least 3 months of stable motor deficits. Spontaneous motor rating scores were obtained weekly from each animal using a clinical rating scale (CRS) that included measures for daily activity, hypokinesia, bradykinesia, rigidity, posture, tremor, and amimia (Doudet et al, 2004). This scale has a maximal score of 26 and was shown to have an interrater reliability >90%. The scores used in the current report are an average of the scores obtained in the 2 weeks preceding and following the imaging studies.
All MPTP animals presented classic, bilateral parkinsonian symptoms with generally reduced activity, and varying degrees of hypokinesia, bradykinesia, deficits in balance and coordination, and hypomimia. Tremor was manifested generally only during retrieval of treats but was not a genuine rest tremor. At the time of imaging, the parkinsonian animals in Cohort A were rated as mild-moderate (n=1) or severe (n=4) according to the CRS (mean score 21.6±5.3; range 13 to 25). Parkinsonian animals in Cohort B were rated as mild-moderate (n=5) or severe (n=1) by the same scale (mean score 12.9±5.8; range 8 to 23). Four parkinsonian animals in Cohort A were euthanized shortly after PET imaging due to the severity of their motor disability. Although maintaining some degree of disability, the remaining parkinsonian animals were able to care for themselves independently after recovery from the acute effects of MPTP.
Positron Emission Tomography Imaging
Positron emission tomography imaging studies were performed on a high-resolution research tomograph (ECAT HRRT, CPS Innovations, Knoxville, TN, USA) at the University of British Columbia. This dedicated brain PET camera is made of lutetium-oxy-orthosilicate crystals yielding a 3D (three-dimensional) image volume with fields of view of 24 cm axially and 31.2 cm in-plane and an intrinsic resolution of 2.5 mm (de Jong et al, 2007). The night before imaging, each monkey was transferred from its large housing cage/group to a smaller squeeze cage in a procedure room to allow easier access for radiotracer administration. At least one of the animal's housing partners was also placed in a transfer cage and remained with the subject in the procedure room during the study, to reduce the effect of isolation and separation anxiety and maintain visual, vocal and auditory contact keeping the animal calm and quiet during the procedure.
The dose of FDG (4 to 6 mCi in 2 to 4 mL sterile saline) was brought to the procedure room in a shielded syringe holder and was injected intramuscularly with a 25-gauge needle in a thigh muscle of the animal—a practice to which the animals were accustomed for experimental and veterinary procedures performed while conscious. (Although preferable, intravenous administration of FDG is not easily feasible in large numbers of awake monkeys. This is because of concerns regarding the safety of both the animal and the handler, as well as the time needed to train each animal to comply with the procedure. By contrast, intramuscular injection provides an easily implemented alternative to intravenous administration and is more reliable and reproducible than oral dosing in the conscious primate.) The animal and its partner stayed awake and alone in the quiet, dim-lighted room during an uptake period of ∼40 minutes. In each animal, tracer uptake occurred during a period of rest in the transfer cage. The animals were videotaped during the entire length of the uptake period, and none displayed abnormal behavior or significant motor activity during this time interval. The timing of radiotracer administration also served to minimize the potential effects of anesthesia on cerebral metabolism (cf. Brownell et al, 2003; Emborg et al, 2007). The injected dose of radiotracer was similar (P=0.60) for parkinsonian (5.63±0.98 mCi) and control (5.44±0.67 mCi) monkeys.
At the end of the uptake period, the monkeys were rapidly sedated (ketamine 10 mg/kg intramuscularly) and brought to the PET suite where they were rapidly intubated and placed under isoflurane anesthesia for the remainder of the study. A single blood sample was taken on arrival in the PET suite (i.e., ∼60 minutes after FDG injection) to measure the plasma glucose concentration. This measure was found not to differ significantly across groups (MPTP: 3.50±0.84 mmol/L; control: 3.63±0.66 mmol/L; P=0.67). A 30-minute scan was acquired starting 80 minutes after radiotracer injection. A transmission scan was also acquired at the end of the emission session (i.e., a simultaneous transmission+emission protocol) with a Cs-137 source for precise photo attenuation correction. Images were then reconstructed using the OSEM (ordered subsets expectation maximization) algorithm with six iterations, after performing corrections for physical effects of photo attenuation, scatter, and random coincidences. The image has a matrix dimension of 256 × 256 × 207 and a voxel size of 1.2 × 1.2 × 1.2 mm3.
Image Processing
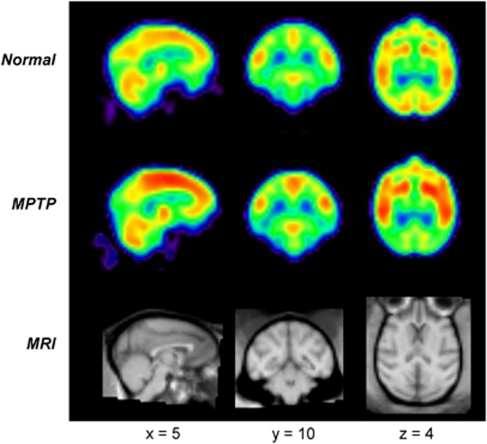
Image processing was performed using customized in-house software (ScanVP: freely available at http://www.feinsteinneuroscience.org/software) and statistical parametric mapping routines (SPM: Wellcome Department of Cognitive Neurology, London, UK). The PET images from all animals were exported into DICOM files, converted into Analyze format and reoriented into Neurological Convention (i.e., the left side of the image is the left side of the brain). The images were then cropped onto a small matrix and thresholded to remove voxels outside the brain. To facilitate voxel-based brain mapping analysis, metabolic images were spatially normalized with a nonlinear warping algorithm into a macaque brain template (Black et al, 2001). They were then smoothed with a 4-mm Gaussian filter to enhance the signal-to-noise ratio and reduce between-animal variability in brain morphology. Figure 2 (top and middle panels) depicts summed 3D maps of relative regional cerebral glucose metabolism in normal and parkinsonian monkeys. The individual scans were further analyzed on a hemisphere-by-hemisphere basis (Tang et al, 2010). Right hemisphere scans were flipped to the left so that all hemispheres had the same orientation in the subsequent network computations.
Figure 2.
Mean images of relative cerebral glucose metabolism in healthy and parkinsonian macaques acquired using a high-resolution positron emission tomography (PET) instrument (see text). The regional distribution of radiotracer uptake was highly symmetrical in scans from normal (top) and parkinsonian (middle) macaques. (Each image was obtained by averaging the [18F]fluorodeoxyglucose (FDG) PET scans from each group following spatial registration to a standard brain template (Black et al, 2001). The PET images were compared with magnetic resonance imaging scans (bottom) registered to the same anatomical space.)
Network Identification: Parkinsonism-Related Pattern
To identify distinct metabolic patterns associated with parkinsonism in MPTP-lesioned monkeys, FDG PET scans from combined groups of MPTP-treated and control animals were analyzed using a spatial covariance mapping algorithm as described elsewhere (Eidelberg, 2009; Ma et al, 2007; Spetsieris and Eidelberg, 2011). In this study, we used an automated software package (SSMPCA Toolbox) available online (http://www.fil.ion.ucl.ac.uk/spm/ext) to perform Principal Component Analysis rapidly on groups of brain images transformed into a common anatomical space.
To delineate covariance topographies (i.e., metabolic networks) associated with experimental parkinsonism, we performed separate voxel-level analysis of MPTP-lesioned and control scans from Cohorts A and B (Figure 1). The search for a significant parkinsonism-related pattern (PRP) in each cohort was limited to the space spanned by the first and second principal components (i.e., PC1 and PC2) or a linear combination of the two (Moeller et al, 1999). The resulting spatial covariance patterns were considered to be parkinsonism-related if the associated pattern expression values (i.e., the PC scalars or ‘subject scores') discriminated between MPTP-lesioned and control animals at a prespecified threshold of P<0.001 (Student's t-test). The PRP voxel weights (i.e., the regional loadings on candidate patterns) underwent cross-validation using a bootstrap resampling algorithm (Habeck and Stern 2010). This procedure yields a reliability map of voxel-weight point estimates expressed as the inverse coefficient of variation (ICV) at each voxel. For anatomical visualization and localization, maps of the PRP voxel weights and the corresponding ICV measures were superimposed on a macaque magnetic resonance imaging brain template (Black et al, 2001). Significant network regions were localized post hoc by reference to a primate brain atlas (Martin and Browden, 2000).
Prospective Validation
For each combined group of MPTP and control animals used to identify a candidate PRP topography, the other group was used for prospective validation of the derived pattern. Thus, PRP expression was quantified in each member of the testing cohort using an automated single scan routine on an individual hemisphere basis (Ma et al, 2007; Tang et al, 2010). These computations were performed blind to group (Cohorts A and B), MPTP-lesion status (parkinsonian and normal), and degree of clinical disability (CRS ratings). Network values were z-transformed with respect to the relevant derivation sample (MPTP-lesioned and control hemispheres) and adjusted so that the mean for the control animals was zero.
Cross-Cohort Validation of Parkinsonism-Related Pattern Topographies
To assess the reproducibility of the PRP pattern derived from scans in Cohort A (PRP1), we generated a second PRP topography (PRP2) from the 10 MPTP-lesioned hemispheres of Cohort A (see Figure 1) and the 10 normal hemispheres of Cohort B (corresponding to the preMPTP scans of the same five animals). For comparison, we generated two additional topographic patterns. PRP3 was generated by spatial covariance analysis of the 7 MPTP-lesioned hemispheres and the 12 control hemispheres of Cohort B. Similarly, PRP4 was generated from the MPTP-lesioned hemispheres of Cohort B and the 12 control hemispheres of Cohort A. In summary, PRP1 and PRP2 were identified in the analysis of MPTP-lesioned animals with moderate to severe motor symptoms (mean CRS 21.6) with different sets of control scans. By contrast, PRP3 and PRP4 were derived from scans acquired in animals with less severe motor symptoms (mean CRS 12.9) and different sets of control hemispheres. Similarities and differences in these spatial topographies were assessed by voxel-level correlation of the region weights (for values that are reliable at P<0.001 based on the bootstrapping tests; see above) on each pair of PRPs (Spetsieris and Eidelberg, 2011). Similarly, subject scores for each PRP were correlated pairwise in each group of MPTP-lesioned and control animals. Network values in the MPTP-lesioned monkeys were correlated with corresponding clinical motor ratings from the same animals. To compare with the whole-brain pattern (PRP5) described below, subject scores from PRPs 1 to 4 were computed on a whole-brain basis in individual monkeys by averaging values from the left and right hemispheres.
Finally, to ensure that the hemispheric topographies were applicable to the whole brain, we performed an exploratory PRP derivation using the entire brain volume of the images acquired in Cohort A. The resulting whole-brain PRP candidate pattern (PRP5) was then projected onto the scan data from Cohort B. Pattern expression in this data set was computed prospectively in the untreated hemispheres of the MPTP-lesioned monkeys that had undergone unilateral transplantation surgery (n=6), and in the whole-brain images of the corresponding control animals (n=6) in this cohort. The resulting network values were z-transformed as described above. Network scores in the parkinsonian animals were then compared with those from the control animals and correlated with the corresponding clinical motor ratings. These scores were also compared with the corresponding whole-brain values computed from PRPs 1 to 4. For direct comparison with the PRP topographies derived from half-brain images, we constructed a mean hemi-PRP5. This was accomplished by dividing PRP5 into left and right hemisphere patterns. The latter was flipped and averaged with left hemisphere voxel weights to produce a mean left-oriented pattern, which was correlated on a voxel-by-voxel basis with corresponding hemispheric loadings on PRPs 1 to 4 as described above.
Statistical Analysis
Between-group differences in PRP expression were assessed using Student's t-tests. Differences in network values before and after MPTP lesioning were assessed using paired Student's t-tests. Correlations of PRP scores with one another and with corresponding CRS ratings within each group were assessed by computing Pearson's correlation coefficients. These calculations were performed using JMP software (SAS Institute, Cary, NC, USA). All analyses were considered significant for P<0.05.
Results
Parkinsonism-Related Pattern: Identification and Prospective Validation
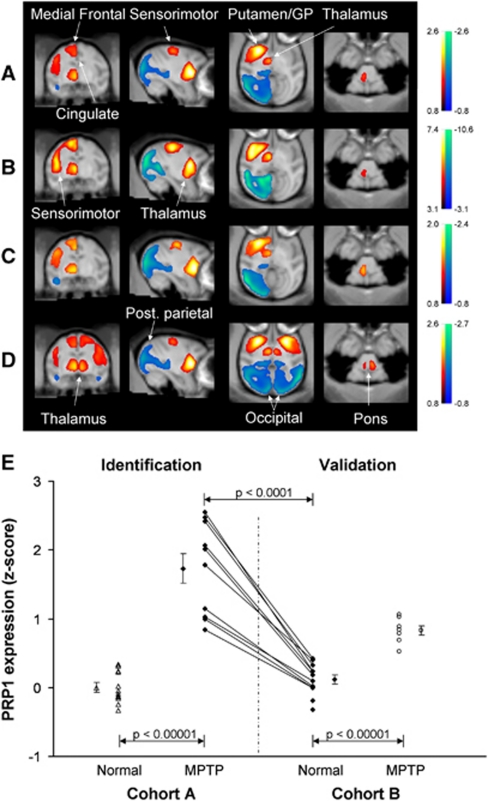
Spatial covariance analysis of the hemispheric data from Cohort A (10 hemispheres from MPTP-lesioned animals with moderate-severe motor signs and 12 hemispheres from control animals) disclosed a significant regional metabolic pattern (PC1, accounting for 42.9% of the subject × voxel variance) that was characterized by relatively increased activity in the putamen, globus pallidus (GP), ventral thalamus, pons, and in the medial frontal/cingulate and sensorimotor cortical regions, associated with relatively reduced activity in the parietal-occipital cortex (Figure 3A). Region weights on this PRP were found to be highly reliable (ICV⩾±3.09, range −13.0 to 10.3, P=0.001) on bootstrap resampling (Figure 3B). Subject scores, reflecting the expression of this pattern in individual hemispheres, were consistently increased (Table 1; Figure 3E) in MPTP relative to control hemispheres of both the derivation and validation samples (P<0.0001; Student's t-tests). The PRP scores were also elevated (P<0.0001; paired Student's t-test) relative to the preMPTP baseline in the postMPTP scans of the five animals who were imaged before and after lesioning. There were no differences in PRP expression in the scans from the two cohorts of healthy animals.
Figure 3.
Abnormal metabolic covariance patterns associated with experimental parkinsonism. (A) Voxel-based spatial covariance analysis of high-resolution [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) images from five parkinsonian and six healthy macaques (Cohort A). Hemispheric analysis revealed a spatial covariance pattern (PRP1) characterized by increased metabolic activity (red–yellow) in the putamen and globus pallidus (GP), thalamus, pons, medial frontal/cingulate areas, and sensorimotor cortex, as well as relative reductions (blue–green) in the posterior parietal-occipital cortex. (B) Reliability of PRP1 at each voxel according to a bootstrapping estimation procedure (Habeck and Stern, 2010). This map of inverse coefficient of variation (ICV) was thresholded at ICV=3.09 (P=0.001). (C) Abnormal metabolic covariance pattern (PRP2) from the hemispheres of the five monkeys scanned before and after chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration. (D) Candidate whole-brain PRP topography (PRP5) identified over the entire image volume of FDG PET scans from the MPTP-lesioned and normal monkeys included in Cohort A. There was a high degree of topographic similarity between patterns identified using either the hemispheric or the whole-brain spatial covariance approach. (E) Network activity of the hemispheric PRP (PRP1) in individual hemispheres discriminated MPTP animals and normal controls in the derivation sample (P<0.00001). In the validation sample, network activity computed prospectively also separated (P<0.00001) MPTP and control animals. Pattern expression in the MPTP-lesioned animals in the validation sample was lower than those used for pattern derivation (P=0.002), consistent with the difference in motor severity ratings for the two groups (see text). Compared with preMPTP baseline, network activity increased significantly (P<0.0001) in the five parkinsonian animals in the derivation sample who subsequently underwent MPTP lesioning. (Maps of PRP voxel weights and ICV values were displayed on a standard magnetic resonance imaging brain template. Error bars in the graph refer to the standard error of the mean.) PRP, parkinsonism-related pattern.
Table 1. Subject scores for the parkinsonism-related metabolic patterns in normal and MPTP-lesioned monkeys.
|
Cohort A |
Cohort B |
|||
|---|---|---|---|---|
| Control | MPTP | Control | MPTP | |
| Hemispheric analysisa | ||||
| PRP1 (42.9) | 0.00±0.07 | 1.73±0.21 | 0.12±0.07 | 0.83±0.07 |
| PRP2 (43.3) | 0.09±0.07 | 1.67±0.23 | −0.04±0.06 | 0.81±0.07 |
| PRP3 (27.8) | 0.42±0.12 | 2.05±0.29 | 0.00±0.16 | 1.77±0.12 |
| PRP4 (27.2) | 0.00±0.19 | 2.10±0.29 | 0.38± 0.16 | 1.68±0.15 |
| Whole-brain analysisb | ||||
| PRP1 | 0.00±0.09 | 1.73±0.31 | 0.12±0.09 | 0.83±0.08 |
| PRP2 | 0.09±0.09 | 1.67±0.33 | −0.04±0.09 | 0.81±0.08 |
| PRP3 | 0.42±0.16 | 2.05±0.42 | 0.00±0.20 | 1.77±0.14 |
| PRP4 | 0.00±0.22 | 2.10±0.41 | 0.38± 0.15 | 1.71±0.16 |
| PRP5c (48.2) | 0.00±0.09 | 1.70±0.31 | 0.13±0.09 | 0.78±0.04 |
MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PRP, parkinsonism-related pattern.
Subject scores were presented as mean±standard error. Bold indicated the scans used to identify each of the significant PRP networks. In each cohort, subject scores for these networks were elevated in the parkinsonian animals compared with the controls. The eigenvalue associated with each PRP derivation is given in parentheses as the percent of the subject × voxel variance accounted for (Spetsieris and Eidelberg, 2011).
Subject scores for PRPs generated by hemispheric analysis (see text).
Subject scores for the whole brain computed as the left/right average of subject scores for PRPs generated on a hemispheric basis.
Subject scores for PRP5 were computed as part of a whole-brain spatial covariance analysis.
Cross-Validation of Parkinsonism-Related Pattern Topographies from Different Derivation Samples
The PRP covariance patterns generated from different samples of MPTP-lesioned and control hemispheres (i.e., PRPs 1 to 4) were compared with one another through pairwise correlation of the voxel weights on each of the topographies, as well as their corresponding pattern expression values (i.e., subject scores). These findings are summarized in Tables 2 and 3. There was evidence of a close correlation between voxel weights on PRP1 with those on PRPs 2 to 4 (r⩾0.90, P<0.0001; see Figures 3A and 3C). Similarly, hemispheric expression of the PRP1 pattern was highly correlated with corresponding values computed for PRPs 2 to 4 in the two groups of MPTP-lesioned monkeys (r⩾0.77, P<0.001; Supplementary Table 1). In each animal group, these hemispheric values were of comparable magnitude to corresponding measures computed over the whole brain (cf. Table 1). Between-pattern subject score correlations were generally similar for whole-brain and hemispheric values (Table 3).
Table 2. Voxel-based pairwise correlations of parkinsonism-related metabolic pattern topographies.
| PRP1 | PRP2 | PRP3 | PRP4 | PRP5L | PRP5R | PRP5A | |
|---|---|---|---|---|---|---|---|
| PRP1a | 1.000b | — | — | — | — | — | — |
| PRP2a | 0.974 | 1.000 | — | — | — | — | — |
| PRP3a | 0.898 | 0.942 | 1.000 | — | — | — | — |
| PRP4a | 0.919 | 0.882 | 0.913 | 1.000 | — | — | — |
| PRP5Lc | 0.991 | 0.970 | 0.893 | 0.913 | 1.000 | — | — |
| PRP5Rc | 0.993 | 0.962 | 0.889 | 0.916 | 0.969 | 1.000 | — |
| PRP5Ac | 1.000 | 0.974 | 0.899 | 0.921 | 0.992 | 0.993 | 1.000 |
PRP, parkinsonism-related pattern.
PRP topographies derived on a hemispheric basis.
Pearson's correlation coefficient computed on a hemispheric basis for voxels with loadings ⩾0.8.
PRP5L, PRP5R, and PRP5A represented the left and right hemispheres and the left/right average of voxel weights on PRP5, which was derived on a whole-brain basis (see text).
Table 3. Pairwise correlations of whole-brain parkinsonism-related metabolic pattern subject scores for parkinsonian macaques.
| 11 MPTP | PRP1 | PRP2 | PRP3 | PRP4 | PRP5 |
|---|---|---|---|---|---|
| PRP1a | 1.000b | — | — | — | — |
| PRP2a | 0.994++ | 1.000 | — | — | — |
| PRP3a | 0.756+ | 0.794** | 1.000 | — | — |
| PRP4a | 0.823** | 0.812** | 0.886++ | 1.000 | — |
| PRP5c | 0.992++ | 0.991++ | 0.744+ | 0.792** | 1.000 |
| Motor | 0.760+ | 0.711* | 0.311 | 0.550 | 0.758+ |
MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PRP, parkinsonism-related pattern.
Whole-brain subject scores reflect the left/right average of pattern expression values for PRPs 1 to 4, which were generated on a hemispheric basis.
Pearson's correlation coefficient.
Subject score of PRP5, which was generated on a whole-brain basis.
*P<0.05.
+P<0.01.
**P<0.005.
++P=0.0001.
A significant positive correlation was evident (r>0.71, P<0.05) between PRP1 and PRP2 subject scores computed for the whole brain across the combined sample of parkinsonian animals (n=11) with concurrent motor severity ratings (correlations between CRS ratings and subject scores for the PRP3 and PRP4 patterns did not reach significance; P>0.08). Thus, the PRP topographies generated from different combinations of parkinsonian and control animals were spatially similar to one another but differed in the degree to which their expression in individual MPTP-lesioned animals correlated with motor disability ratings.
Whole-Brain Metabolic Network Pattern
Whole-brain network analysis yielded similar results to those identified on a hemisphere-by-hemisphere basis. The topography (PRP5) generated from the whole-brain PET images of the five bilaterally MPTP-lesioned animals and the six normal animals in Cohort A (PC1, accounting for 48.2% subject × voxel variance) was symmetrical and topographically similar to patterns obtained by hemispheric analysis (Table 2; cf. Figure 3D versus Figures 3A and 3C). Voxel weights on the left and right hemispheres of PRP5 were highly intercorrelated (r>0.96, P=0.0001). These values also correlated closely with voxel weights on PRP1 and PRP2, and to a lesser extent with those on PRP3 and PRP4 (Table 2). Subject scores for this pattern (Supplementary Figure 1A) were elevated in the parkinsonian relative to the healthy animals (P<0.001). In addition, consistent increases in PRP5 expression were present (P<0.005) in the five monkeys who were scanned before and after lesioning. Moreover, PRP5 scores correlated strongly with values computed from the left/right average of hemispheric scores for PRPs 1 to 4, particularly those for PRP1 and PRP2 (Table 3). A positive correlation was evident (r=0.76, P<0.01) between PRP5 scores and individual motor ratings obtained in the combined group of MPTP-lesioned animals assessed at the time of imaging (see Supplementary Figure 1B).
Discussion
In this study, we report the presence of a parkinsonism-related metabolic covariance pattern (i.e., PRP) in a nonhuman primate model of nigrostriatal dopamine loss. Of note, the animals remained awake and at rest in a dimly lit room during the 40-minute FDG uptake period; they underwent anesthesia and PET imaging only after the completion of radiotracer uptake. This ensured that the functional state of the animals was not altered in any way by anesthesia and that imaging accurately measured the physiological activity of the animals. This approach also minimized the potentially large variability in local and global metabolic activity associated with differences between animals in their responses to anesthesia. Hence, the physiological condition of the monkeys scanned in this study was similar to that typically employed in metabolic PET imaging of human subjects.
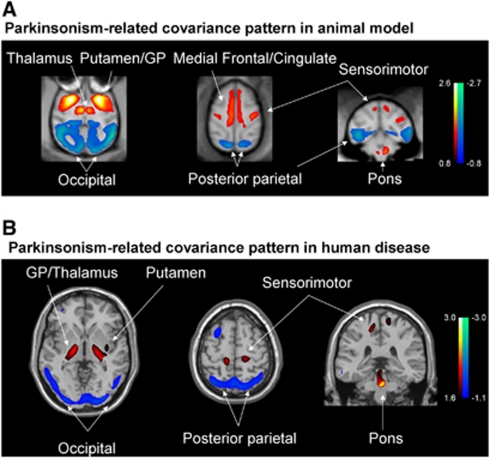
The PRP metabolic topography discerned by spatial covariance analysis in MPTP-lesioned parkinsonian macaques was similar to that of the homologous PDRP network described consistently in humans with PD (Eidelberg, 2009; Ma et al, 2007; Spetsieris and Eidelberg, 2011). The most salient features of the PD topography are recapitulated in the primate model, including network-related metabolic increases in the GP, ventrolateral thalamus, pons, and sensorimotor cortex, as well as relative decreases in parietal association regions (Figure 4). Both of these disease-specific metabolic topographies in monkeys and humans were found to be highly reliable voxel-wise as determined by bootstrap resampling. Likewise, PRP subject scores representing the expression of the pattern in individual hemispheres/animals consistently discriminated between MPTP-lesioned and control monkeys in both cohorts. Indeed, as in PD patients, higher network expression in parkinsonian monkeys was associated with greater motor disability (Eidelberg, 2009), a finding consistent with the higher network activity observed in the more severely affected MPTP-lesioned animals in Cohort A.
Figure 4.
Overlays illustrating regional homologies between the abnormal metabolic covariance patterns identified in monkeys with experimental parkinsonism and human patients with Parkinson's disease (PD). (A) Voxel-based whole-brain spatial covariance analysis of high-resolution [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) images from a combined group of parkinsonian and healthy macaques (Cohort A). This analysis revealed a significant metabolic pattern (PC1, 48% variance accounted for) that accurately discriminated the two groups of animals (P<0.00001; Supplementary Figure 1A). (B) The topography of this pattern (PRP5) resembled that of the human PD-related covariance pattern (PDRP) described consistently in multiple PD patient cohorts (Eidelberg, 2009). Both topographies were characterized by homologous regional components of the motor cortico-striato-pallido-thalamo-cortical loop and related pathways. (Both spatial covariance patterns were displayed on standard magnetic resonance imaging brain templates. Voxels with positive loadings (metabolic increases) are color coded from red to yellow; those with negative loadings (relative metabolic reductions) are color coded from blue to green). PC, principal component; PRP, parkinsonism-related pattern.
Interestingly, the monkey and human spatial covariance patterns diverged with respect to contributions to network activity (i.e., region weights) from the medial frontal and cingulate cortical areas, which exhibited relative metabolic increases in the former topography but not in the latter (cf. Figure 4). This apparent discrepancy cannot be simply attributed to differences in the physiological state of the subjects during imaging in that monkeys and humans were studied under comparable experimental conditions. Rather, it is more likely that the interspecies differences stem from the fact that MPTP lesioning of the nigrostriatal dopamine system does not recapitulate the entire histopathologic picture of human PD, particularly the involvement of mesocortical and mesolimbic dopamine systems, as well as the characteristic deposits of protein aggregates in specific cortical regions. Moreover, the awake human is apt to exhibit substantial between-subject variability in resting frontal metabolism, which may or may not be present in the macaque. The normal variability in these areas is likely to make it more difficult to detect homologous regional changes in human subjects.
The unique design of this study enabled us to perform a direct comparison of the networks identified by cross-sectional group comparison with that generated within group by analyzing data from a subset of normal macaques scanned before and after MPTP administration. Although only five animals were available for this paired analysis, we found that the network derived in this cohort (PRP2) was topographically similar (r=0.97 on voxel-wise correlational analysis) to that identified in the analysis of the same five MPTP-lesioned monkeys and a separate group of control animals (PRP1). The expression of both PRP networks was found to increase in the five monkeys scanned after MPTP administration relative to their baseline scans. Indeed, the mean values of the computed network scores and the magnitude of correlations between these values and concurrent clinical ratings were comparable for the two pattern derivations. It will be useful to study a larger number of animals before and after MPTP administration, in conjunction with recently developed within-subject network mapping approaches (Habeck et al, 2005; Moeller and Habeck, 2006), to delineate more accurately the specific network changes associated with experimental parkinsonism.
The reproducibility of these PRP networks was further assessed across other combined samples of parkinsonian monkeys and control animals. Two additional networks (PRP3 and PRP4) were identified in cohorts including MPTP animals with mild to moderate motor symptoms. These patterns proved to be very similar in terms of topographic correlation and group discrimination. Of note, correlations between the expression of these patterns in individual animals and the corresponding clinical motor ratings did not reach significance (in contrast to subject scores for PRP1 and PRP2 from the more severely affected cohorts). That said, correlation analysis of these network topographies, and the associated subject scores for these patterns, revealed that each of these PRPs closely resembled the two other metabolic brain networks (i.e., PRP1 and PRP2) described above. The similarity in the brain networks identified in independent parkinsonian animals across the two samples lends credence to the assumption that unilateral implants did not affect PRP measurements obtained in the contralateral hemispheres of the five operated parkinsonian monkeys. However, correlations between PRP1 and PRP2 subject scores (as well as between-subject scores for PRP3 and PRP4) were stronger than those observed in other pairwise comparisons. Given that the eigenvalues for PRP1 and PRP2 were overall greater than for PRP3 and PRP4 (43% versus 27% variance accounted for), it is likely that the former two networks represent greater and more robust parkinsonism-related effects than the latter.
One of the limitations in this study was that most of the network analyses were conducted on a hemisphere-by-hemisphere basis. While the purpose was to increase sample size and statistical power for covariance mapping, the approach was justified given the systemic nature of MPTP administration and the symmetrical distribution of the regional changes in glucose utilization seen in this bilateral model of nigrostriatal dopamine loss (Figure 2). This is borne out of the striking similarity of PRP subject scores computed in the individual hemispheres and those associated with the left/right average of these values. Indeed, in the bilaterally MPTP-lesioned animals comprising Cohort A, an exploratory full-brain PRP generated from a small sample was found to be highly symmetrical and very similar topographically to that derived in half-brain analyses in terms of both voxel weights and subject score values. Nevertheless, the results from the present study need to be replicated in a whole-brain analysis of FDG PET data from large, independent samples. It is also desirable to assess the test–retest reliability of PRP expression in individual animals, as reported previously in human subjects (Ma et al, 2007). This type of validation will be needed before PRP scores are employed to assess treatment-mediated network modulation in preclinical studies.
Comparison with Other Animal Studies and Discussion on Methodological Issues
The pattern of abnormal regional metabolic activity in MPTP-lesioned monkeys described in this study has also been observed previously in other experimental models of parkinsonism. For instance, relative subcortical hypermetabolism was reported in the GP and cerebellum in a previous FDG PET study that compared parkinsonian with healthy hemispheres in monkeys using voxel-based brain mapping analysis (cf. Emborg et al, 2007). Quantitative [11C]-2-deoxyglucose autoradiography has demonstrated abnormally increased glucose metabolism in the lateral striatum, ventral thalamus, and pedunculopontine nucleus (Mitchell et al, 1989), and in the internal/external GP, and ventral thalamus (Guigoni et al, 2005) of macaques with bilateral MPTP-induced parkinsonism. This was in line with another [11C]-2-deoxyglucose autoradiography study that specifically identified absolute metabolic increases in striatum/GP as well as in the pedunculopontine nucleus and sensorimotor cortex in the unilateral 6-OHDA rodent model (Carlson et al, 1999). The results from these early experiments support the findings reported in the present study.
Because of interspecies variation as well as methodological differences, published data from experimental animal models were generally inconsistent in subcortical and cortical glucose metabolism following nigrostriatal lesions. These discrepancies may relate to the use of different MPTP models and varying degrees of nigrostriatal dopamine depletion, as well as differences in imaging techniques and analytical methodologies. Note that we used bilateral parkinsonian macaques produced by chronic MPTP administration, although unilateral models have also been frequently used with acute MPTP injection (cf. Emborg et al, 2007; Mitchell et al, 1989). It is known that barbiturate administration influences cerebral glucose metabolism and newer anesthetics such as isoflurane or propofol also affect cerebral blood flow and, in itself, the depth of anesthesia could differentially affect regional and global cerebral blood flow and metabolism. In this study, animals were kept awake during radiotracer uptake, while anesthetized animals were used in most other studies.
Intravenous administration of FDG has been performed in monkeys studied under light sedation and following training in a primate chair (Blaizot et al, 2000; Rauchs et al, 2006). Very few animals tolerate intravenous administration while awake without sedation and extensive prior training. The intravenous procedure may therefore not be practical in terms of time and cost when population data are needed from a relatively large number of animals. Of note, FDG can also be administered orally to primates (Martinez et al, 1997). However, this method is complicated by the possibility that not all the animals swallow the dose and keep it in their jowls as they often do with food, making radiotracer uptake slow and unacceptably variable. By contrast, injection of the dose into a well-defined highly vascularized tissue, such as muscle, insures rapid transit into the circulation and avoids storage in fat with the attendant variability in tracer uptake. It is expected that more consistent results are likely to emerge from animal studies with the implementation of comparable methodology.
Conclusion
We report the first demonstration of an abnormal metabolic brain network in a nonhuman primate model of parkinsonism. The spatial topography of this network and its correlation with independent clinical ratings of motor symptom severity proved to be reproducible and consistent with homologous findings in human PD patients. The quantification of network expression may therefore provide an objective descriptor of parkinsonism in experimental disease models. Moreover, network values are likely to provide critical preclinical information concerning the effects of novel therapeutic interventions on brain function.
Acknowledgments
This study would not have been possible without the assistance of Ms S Jivan and M Pronk (chemists), C English and C Williams (technologists), S Blinder (reconstruction), and J Grant (AHT). Special thanks are due to the personnel of the UBC Animal Care Facilities for their exceptional care of the animals. We are grateful to Ms Toni Fitzpatrick at The Feinstein Institute for Medical Research for assistance with copyediting. The authors thank the UBC/TRIUMF PET program for their assistance in PET studies.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research was supported by Team Grant (CTP-79851) at the University of British Columbia from the Canadian Institute of Health Research. TRIUMF is funded by a contribution from the National Research Council of Canada. Drs Ma, Peng, Spetsieris, and Eidelberg were supported by the Morris K Udall Center of Excellence for Parkinson's Disease Research (P50 NS071675) at The Feinstein Institute for Medical Research.
Supplementary Material
References
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson's disease. Brain. 2006;129:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KJ, Koller JM, Snyder AZ, Perlmutter JS. Template images for nonhuman primate neuroimaging: 2. Macaque. Neuroimage. 2001;14:744–748. doi: 10.1006/nimg.2001.0871. [DOI] [PubMed] [Google Scholar]
- Blaizot X, Landeau B, Baron JC, Chavoix C. Mapping the visual recognition memory network with PET in the behaving baboon. J Cereb Blood Flow Metab. 2000;20:213–219. doi: 10.1097/00004647-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Brownell AL, Canales K, Chen YI, Jenkins BG, Owen C, Livni E, Yu M, Cicchetti F, Sanchez-Pernaute R, Isacson O. Mapping of brain function after MPTP-induced neurotoxicity in a primate Parkinson′s disease model. Neuroimage. 2003;20:1064–1075. doi: 10.1016/S1053-8119(03)00348-3. [DOI] [PubMed] [Google Scholar]
- Carlson JD, Pearlstein RD, Buchholz J, Iacono RP, Maeda G. Regional metabolic changes in the pedunculopontine nucleus of unilateral 6-hydroxydopamine Parkinson′s model rats. Brain Res. 1999;828:12–19. doi: 10.1016/s0006-8993(99)01268-8. [DOI] [PubMed] [Google Scholar]
- de Jong HW, van Velden FH, Kloet RW, Buijs FL, Boellaard R, Lammertsma AA. Performance evaluation of the ECAT HRRT: an LSO-LYSO double layer high resolution, high sensitivity scanner. Phys Med Biol. 2007;52:1505–1526. doi: 10.1088/0031-9155/52/5/019. [DOI] [PubMed] [Google Scholar]
- Doudet DJ, Chan GL, Holden JE, McGeer EG, Aigner TA, Wyatt RJ, Ruth TJ. 6-[18F]Fluoro-L-DOPA PET studies of the turnover of dopamine in MPTP-induced parkinsonism in monkeys. Synapse. 1998;29:225–232. doi: 10.1002/(SICI)1098-2396(199807)29:3<225::AID-SYN4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Doudet DJ, Cornfeldt ML, Honey CR, Schweikert AW, Allen RC. PET imaging of implanted human retinal pigment epithelial cells in the MPTP-induced primate model of Parkinson′s disease. Exp Neurol. 2004;189:361–368. doi: 10.1016/j.expneurol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg ME, Carbon M, Holden JE, During MJ, Ma Y, Tang C, Moirano J, Fitzsimons H, Roitberg BZ, Tuccar E, Roberts A, Kaplitt MG, Eidelberg D. Subthalamic glutamic acid decarboxylase gene therapy: changes in motor function and cortical metabolism. J Cereb Blood Flow Metab. 2007;27:501–509. doi: 10.1038/sj.jcbfm.9600364. [DOI] [PubMed] [Google Scholar]
- Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, Moeller JR, Eidelberg D. Metabolic correlates of levodopa response in Parkinson's disease. Neurology. 2001;57:2083–2088. doi: 10.1212/wnl.57.11.2083. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Li Q, Aubert I, Dovero S, Bioulac BH, Bloch B, Crossman AR, Gross CE, Bezard E. Involvement of sensorimotor, limbic, and associative basal ganglia domains in L-3,4-dihydroxyphenylalanine-induced dyskinesia. J Neurosci. 2005;25:2102–2107. doi: 10.1523/JNEUROSCI.5059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, Moeller JR. A new approach to spatial covariance modeling of functional brain imaging data: ordinal trend analysis. Neural Comput. 2005;17:1602–1645. doi: 10.1162/0899766053723023. [DOI] [PubMed] [Google Scholar]
- Habeck C, Stern Y. Multivariate data analysis for neuroimaging data: overview and application to Alzheimer's disease. Cell Biochem Biophys. 2010;58:53–67. doi: 10.1007/s12013-010-9093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Asanuma K, Ma Y, Tang C, Feigin A, Dhawan V, Carbon M, Eidelberg D. Dissociation of metabolic and neurovascular responses to levodopa in the treatment of Parkinson′s disease. J Neurosci. 2008;28:4201–4209. doi: 10.1523/JNEUROSCI.0582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, Dhawan V, Eidelberg D. Changes in network activity with the progression of Parkinson's disease. Brain. 2007;130:1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Eidelberg D. Functional imaging of cerebral blood flow and glucose metabolism in Parkinson′s disease and Huntington's disease. Mol Imaging Biol. 2007;9:223–233. doi: 10.1007/s11307-007-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Huang C, Dyke JP, Pan H, Alsop D, Feigin A, Eidelberg D. Parkinson's disease spatial covariance pattern: noninvasive quantification with perfusion MRI. J Cereb Blood Flow Metab. 2010;30:505–509. doi: 10.1038/jcbfm.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Peng S, Flores J, Cornfeldt M, Mitrovic B, Eidelberg D, Doudet DJ. Abnormal metabolic brain network in parkinsonian macaques: Modulation by retinal pigment epithelial (RPE) cell implantation. Neurology. 2008;71:154–155. doi: 10.2967/jnumed.115.161513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J Cereb Blood Flow Metab. 2007;27:597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Browden D. Primate brain maps: structure of the macaque brain. Amsterdam, The Netherlands: Elsevier; 2000. [Google Scholar]
- Martinez ZA, Colgan M, Baxter LR, Jr, Quintana J, Siegel S, Chatziioannou A, Cherry SR, Mazziotta JC, Phelps ME. Oral 18F-fluoro-2-deoxyglucose for primate PET studies without behavioral restraint: demonstration of principle. Am J Primatol. 1997;42:215–224. doi: 10.1002/(SICI)1098-2345(1997)42:3<215::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mattis PJ, Tang CC, Ma Y, Dhawan V, Eidelberg D. Network correlates of the cognitive response to levodopa in Parkinson disease. Neurology. 2011;77:858–865. doi: 10.1212/WNL.0b013e31822c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell IJ, Clarke CE, Boyce S, Robertson RG, Peggs D, Sambrook MA, Crossman AR. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 1989;32:213–226. doi: 10.1016/0306-4522(89)90120-6. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Habeck C. Reciprocal benefits of mass-univariate and multivariate modeling in brain mapping: applications to event-related functional MRI, H215O-, and FDG-PET. Int J Biomed Imaging. 2006;2006:1–13. doi: 10.1155/IJBI/2006/79862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller JR, Nakamura T, Mentis MJ, Dhawan V, Spetsieres P, Antonini A, Missimer J, Leenders KL, Eidelberg D. Reproducibility of regional metabolic covariance patterns: comparison of four populations. J Nucl Med. 1999;40:1264–1269. [PubMed] [Google Scholar]
- Rauchs G, Blaizot X, Giffard C, Baron JC, Insausti R, Chavoix C. Imaging visual recognition memory network by PET in the baboon: perirhinal cortex heterogeneity and plasticity after perirhinal lesion. J Cereb Blood Flow Metab. 2006;26:301–309. doi: 10.1038/sj.jcbfm.9600203. [DOI] [PubMed] [Google Scholar]
- Snow BJ, Vingerhoets FJ, Langston JW, Tetrud JW, Sossi V, Calne DB. Pattern of dopaminergic loss in the striatum of humans with MPTP induced parkinsonism. J Neurol Neurosurg Psychiatry. 2000;68:313–316. doi: 10.1136/jnnp.68.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson's disease: methodological issues. Neuroimage. 2011;54:2899–2914. doi: 10.1016/j.neuroimage.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson's disease. J Neurosci. 2010;30:1049–1056. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.