Abstract
In this study, we have investigated the potential role of placental growth factor (PlGF) in hypoxia-induced brain angiogenesis. To this end, PlGF wild-type (PlGF+/+) and PlGF knockout (PlGF−/−) mice were exposed to whole body hypoxia (10% oxygen) for 7, 14, and 21 days. PlGF+/+ animals exhibited a significant ∼40% increase in angiogenesis after 7 days of hypoxia compared with controls, while in PlGF−/− this effect only occurred after 14 days of hypoxia. No differences in pericyte/smooth muscle cell (SMC) coverage between the two genotypes were observed. After 14 days of hypoxia, PlGF−/− microvessels had a significant increase in fibrinogen accumulation and extravasation compared with those of PlGF+/+, which correlated with endothelial cell disruption of the tight junction protein claudin-5. These vessels displayed large lumens, were surrounded by reactive astrocytes, lacked both pericyte/SMC coverage and endothelial vascular endothelial growth factor expression, and regressed after 21 days of hypoxia. Vascular endothelial growth factor expression levels were found to be significantly lower in the frontal cortex of PlGF−/− compared with those in PlGF+/+ animals during the first 5 days of hypoxia, which in combination with the lack of PlGF may have contributed to the delayed angiogenic response and the prothrombotic phenotype observed in the PlGF−/−animals.
Keywords: angiogenesis, brain endothelial cells, hypoxia, pericytes, placental growth factor
Introduction
Neurons are highly sensitive to oxygen levels and a constant supply is necessary for their proper function and survival. However, high oxygen levels can also have a detrimental effect on neurons due to the formation of reactive oxygen species. Therefore, the oxygen balance in the brain is tightly controlled, via the cerebral blood flow, by the neurovascular unit, a functional group of cells that includes neurons, astrocytes, brain endothelial cells, vascular smooth muscle cells (VSMCs), and pericytes (Hamel, 2006). Under pathological conditions, such as stroke, interruption of cerebral blood flow leads to a reduction in oxygen delivery that results in neuronal death. In this situation, the brain has developed several adaptive mechanisms to protect and/or promote brain recovery including angiogenesis, the growth of new capillaries from preexisting vessels (LaManna et al, 1992).
Angiogenesis is a complex process that occurs over several days after a hypoxic event (Krupinski et al, 1994; LaManna et al, 1992) and that involves all the cellular constituents of the neurovascular unit (del Zoppo, 2010). Several angiogenic factors have been identified in the ischemic/hypoxic brain; however, the interplay between these factors and how they interact within the neurovascular unit, have not been completely characterized.
Vascular endothelial growth factor (VEGF), a potent pro-angiogenic factor, is upregulated in the hypoxic/ischemic brain (Beck et al, 2002; Kuo et al, 1999). Vascular endothelial growth factor binds to the tyrosine kinase receptors VEGFR-1 and VEGFR-2 and to neuropilin-1 but VEGFR-2 is the primary transducer of VEGF signals, activating several intracellular signaling pathways including the Raf-Mek-Erk, involved in cell proliferation (Takahashi et al, 1999), and the PI-3 kinase/Akt, involved in cell survival (Kilic et al, 2006). In addition to having beneficial angiogenic and neuroprotective effects, VEGF is also a potent permeability factor and has been associated with brain edema during angiogenesis (Kilic et al, 2006).
In contrast to the permeability effects of VEGF, placental growth factor (PlGF), a homolog of VEGF (Maglione et al, 1991), has been shown to have a role in vessel stabilization under pathological conditions (Autiero et al, 2003a; Du et al, 2010; Liu et al, 2006; Luttun et al, 2002). Moreover, systemic delivery of transfected mesenchymal stem cells expressing PlGF in rats subjected to middle cerebral artery occlusion showed that PlGF significantly increased angiogenesis without increasing cerebral edema (Liu et al, 2006).
Under pathological conditions, PlGF has been shown to synergistically enhance VEGF angiogenic activity in the systemic vascular system (Autiero et al, 2003b; Carmeliet et al, 2001) through various mechanisms: (1) displacing VEGF from VEGFR-1, thus increasing the availability of VEGF to bind and activate VEGFR-2 (Carmeliet et al, 2001); (2) heterodimerizing with VEGF (VEGF/PlGF), which leads to the activation and transmission of angiogenic signals through the VEGFR-2/VEGFR-1 heterodimer receptor complex (Autiero et al, 2003b); and (3) directly activating VEGFR-1 which, through transphosphorylation of VEGFR-2, enhances VEGFR-2 activity (Autiero et al, 2003b). Placental growth factor binding to VEGFR-1 has also been shown to induce its own signaling pathways, which result in the increased expression of c-Fos, FosB, and Survivin (Adini et al, 2002; Holmes and Zachary, 2004).
While several lines of evidence indicate that hypoxia is a potent inducer of VEGF in vitro and in vivo (Ferrara et al, 2003) studies investigating the effect of hypoxia on PlGF expression have rendered contradictory results, showing either no effect (Cao et al, 1996), PlGF upregulation (Cramer et al, 2005), or PlGF downregulation (Ahmed et al, 2000) depending on the cell type. However, the presence of endogenous PlGF mRNA and protein has been detected in the ischemic brain, suggesting an important role for PlGF during pathological conditions in the CNS (central nervous system) (Beck et al, 2002; Du et al, 2010; Hayashi et al, 2003). Recent studies have demonstrated the neuroprotective properties of PlGF in ischemic conditions both in vitro and in vivo (Du et al, 2010; Liu et al, 2006). Collectively, these findings indicate that PlGF has a role in hypoxic/ischemic brain, but the exact nature of its modulatory effect in hypoxia-induced brain angiogenesis remains unclear.
The purpose of this study was to investigate the role of PlGF in brain angiogenesis and blood–brain barrier (BBB) permeability using PlGF−/− animals. The current study shows that lack of PlGF results in a delayed angiogenic response to hypoxia, accumulation of fibrinogen in cerebral microvessels, and vessel regression after 21 days of hypoxia.
Materials and methods
Whole Body Hypoxia and Tissue Collection
Placental growth factor wild-type and knockout mice (generated by Carmeliet et al (2001); Vesalius Research Center, Leuven, Belgium) were bred at the NRCC (National Research Council of Canada) Institute for Biological Sciences Animal Facility (Ottawa, ON, Canada). Experiments were approved by the NRCC animal care committee in accordance with the Canadian Council on Animal Care guidelines. In all, 19-day-old mice were housed in cages inside a normobaric hypoxic chamber (Forma Anaerobic System model 1024; ThermoForma, Marietta, OH, USA) at 10% O2 and N2 balance fed into the chamber. Oxygen levels were measured with MI-730 Micro-Oxygen Electrode (Microelectrodes, Bedford, MA, USA) and maintained at 10% O2 inside the chamber. Normoxic control mice were housed in cages immediately adjacent to the hypoxic chamber, and were fed and changed on the same schedule as the hypoxic mice. At the beginning of the experiment and after each time point (7, 14, and 21 days), hypoxic and control mice were weighed and venous blood samples were collected for hematocrit. At the end of the experimental period, mice were deeply anesthetized with 4% halothane B.P. (MTC Pharmaceuticals, Cambridge, ON, Canada) under an oxygen flow rate of 2 L/min, and perfused transcardially with an 18-gauge needle connected to a syringe with cold heparinized saline. The brains from PlGF+/+ and PlGF−/− hypoxic and control mice from each time point were dissected and immediately frozen in dry ice and stored at −80°C for cryosectioning.
Immunofluorescence
The brains were sectioned at 10-μm thickness using a cryostat (Jung CM3000; Leica, Richmond Hill, ON, Canada). Sections were placed on Superfrost Plus microscope slides (Fisher Scientific, Nepean, ON, Canada), fixed in ice-cold methanol for 10 minutes and washed twice in ice-cold phosphate-buffered saline (PBS). Sections were permeabilized by incubation in 0.25% Triton X-100 for 10 minutes, followed by three 5 minutes washes in PBS. Nonspecific staining was blocked by preincubation of sections in PBS containing 0.25% Triton X-100 and 10% normal goat serum or 1% bovine serum albumin for 1 hour at room temperature. Incubation of primary antibodies were performed overnight at 4°C, in PBS+5% goat serum. Primary antibodies and their dilutions used were as follows: monoclonal rat anti-mouse CD31 (1:100; BD Bioscience, Mississauga, ON, Canada) for endothelial cells, polyclonal goat anti-rat VEGF (1:20; R&D Systems, Minneapolis, MN, USA) for mouse VEGF120 and VEGF164, polyclonal rabbit anti-nerve/glial antigen 2 (NG2) chondroitin sulfate proteoglycan (1:300; Millipore, Billerica, MA, USA), monoclonal mouse anti-human desmin (1:100; DakoCytomation, Burlington, ON, Canada) for pericytes, monoclonal mouse anti-α-smooth muscle actin (α-SMA) clone 1A4 (1:400; Sigma-Aldrich, Oakville, ON, Canada) for SMCs, polyclonal rabbit anti-human fibrinogen/FITC (1:60; DakoCytomation) for native fibrinogen as well as fibrinogen fragments D and E, and polyclonal rabbit anti-glial fibrillary acidic protein (1:500; DakoCytomation) for astrocytes, rabbit polyclonal to human claudin-5 (1:100; Abcam, Cambridge, MA, USA) for tight junction protein. Appropriate secondary Alexafluor antibodies (Invitrogen, Burlington, ON, Canada) were used at 1:500 dilution in PBS for 1 hour at room temperature. Samples were washed three times in PBS for 5 minutes and stained with Hoechst in PBS (Sigma-Aldrich) for 15 minutes. Sections were rinsed with PBS and coverslip mounted with a drop of Dako fluorescence mounting media. Omission of primary antibodies served as negative controls.
Quantitative Analysis
Four mice per genotype (PlGF+/+ and PlGF−/−), treatment condition (normoxia and hypoxia) and time point (7, 14, and 21 days) were analyzed (total of 24 mice per genotype). Three brain sections from the frontal cortex (Bregma 2.80 mm, Bregma 1.70 mm, and Bregma 1.18 mm) were used per animal for immunofluorescent analysis. Double or triple immunostaining was performed in each section (see Figure legends of Figures 3, 4 and 5). Four images per section, two from the right and two from the left side of the cerebral cortex (12 images/animal) were taken using Olympus fluorescent microscope with a × 10 objective, attached to Q-imaging Retiga EXi digital camera (Olympus Canada, Markham, ON, Canada). Total area, length, number, and frequency distribution analysis of immunopositive cells for a particular marker were calculated from the three sections per animal using an image analysis program, Image-Pro Plus v.6.2 software (MediaCybernetics). The average of each parameter for the four mice per group was then calculated.
Gene Expression Analysis
Placental growth factor and β-actin gene expression was analyzed in the brain samples from PlGF+/+ and PlGF−/− mice submitted to hypoxia for 1–5 days by reverse transcriptase polymerase chain reaction as described previously (Freitas-Andrade et al, 2008). The sequences of the primers used were as follows: PlGF (accession no. NM_008827) 5′-CAGCCAACATCACTATGCAG-3′ (forward) and 5′-GGGTGACGGTAATAAATACG-3′ (reverse), yielding a 268-bp product; β-actin (accession no. NM_007393) 5′-GGAGATTACTGCTCTGGCTC-3′ (forward) and 5′-GGACTCATCGTACTCCTGCT-3′ (reverse), yielding a 131-bp product.
Protein Expression Analysis by Western Blot
Western blot analysis was used to determine the levels of VEGF and PlGF in the brain lysates. Briefly, the brains were removed from PlGF+/+ and PlGF−/− hypoxic mice, and immediately frozen in dry ice and stored in −80°C until further use. A section of the frontal cortex from each animal was isolated and placed into a tube with ice-cold RIPA lysis buffer (1%NP40 (IGEPAL), 0.5% deoxycholate, 0.1% SDS, and protease inhibitor 100 μL/10 mL RIPA in PBS) (Sigma-Aldrich), and homogenized with an electric homogenizer at 4°C. The lysates were centrifuged at 14,000 r.p.m. for 10 minutes at 4°C, and the supernatants were collected. Protein concentration was measured in each of the brain lysates by BCA Protein Assay Reagent Kit (Pierce Biotechnology, Rockford, IL, USA), and 50 to 100 μg of protein were denatured in protein loading buffer for 5 minutes at 100°C, and then separated on a 15% sodium dodecyl sulfate-polyacrylamide gel. Separated proteins were then transferred to polyvinylidene difluoride membranes. Blocking was performed by incubating membranes in Tris-buffered saline Tween-20 (TBST; 20 mmol/L Tris-buffer (pH 8.0) and 150 mmol/L NaCl with 0.1% Tween-20) containing 5% BSA (Sigma-Aldrich) for 1 hour at room temperature. Membranes were then incubated overnight at 4°C with either primary rabbit polyclonal anti-human VEGF(A20) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1/50 in TBST containing 1% skim milk, or rabbit polyclonal human PlGF (Abcam) diluted 1/100 in TBST containing 1% skim milk. The membranes were extensively washed with TBST and incubated for 1 hour with anti-rabbit IgG (whole molecule)-peroxidase antibody (Sigma-Aldrich) diluted 1/5,000 in TBST containing 5% skim milk. Immunoreactive proteins were visualized by Amersham ECL Plus Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK). Membranes were also probed for β-actin protein expression using monoclonal anti-β-actin-peroxidase antibody (Sigma-Aldrich) at a concentration of 1/50,000, according to the manufacturers' instruction.
Statistical Analysis
Effects of hypoxia on PlGF+/+ and PlGF−/− mice over the three time points were evaluated; two × three (genotype × time) analysis of variances were applied to detect differences between PlGF+/+ and PlGF−/− hypoxic groups followed by Bonferroni post-test method. To detect differences among hypoxic and normoxic PlGF+/+ and PlGF−/−, two × two (genotype × treatment) analysis of variances were applied followed by Bonferroni post-test method with the statistical package GraphPad Prism 5 (La Jolla, CA, USA). P values of <0.05 were considered significant. All data are presented as mean±s.e.m.
Results
Effect of Hypoxia on Body Weight and Hematocrit in PlGF+/+ and PlGF−/− Mice
To investigate the role of PlGF in hypoxia-induced brain angiogenesis, PlGF+/+ and PlGF−/− mice were subjected to chronic hypoxia (10% oxygen), which mimics the effect of exposure to high altitude (∼18,000 ft).
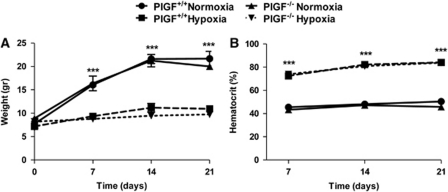
Both PlGF+/+ and PlGF−/− normoxic animals gained about 60% of the initial weight over the 21 days of the experiment, with no significant differences between the two genotypes (Figure 1A). In contrast, PlGF+/+ and PlGF−/− mice failed to gain weight when exposed to long-term hypoxia (7, 14, and 21 days); however, no differences were observed between the two genotypes (Figure 1A).
Figure 1.
Effects of 7-, 14-, and 21-day exposure to 10% hypoxia on body weight (A) and hematocrit (B) of PlGF+/+ and PlGF−/− mice. Data presented are mean values±s.e.m. of four mice per genotype and time point. ***indicates significant difference (P<0.001) between hypoxic groups and the corresponding normoxic controls (analysis of variance (ANOVA) followed by Bonferroni post-test). PlGF, placental growth factor.
PlGF+/+ and PlGF−/− mice exposed to normoxia exhibited similar levels of hematocrit (∼ 46%) over the 21 days of experiment (Figure 1B). Under hypoxic conditions, both PlGF+/+ and PlGF−/− animals developed polycythemia as indicated by the twofold increase in hematocrit levels in both genotypes (Figure 1B).
Effect of Placental Growth Factor Knockout on Brain Angiogenesis
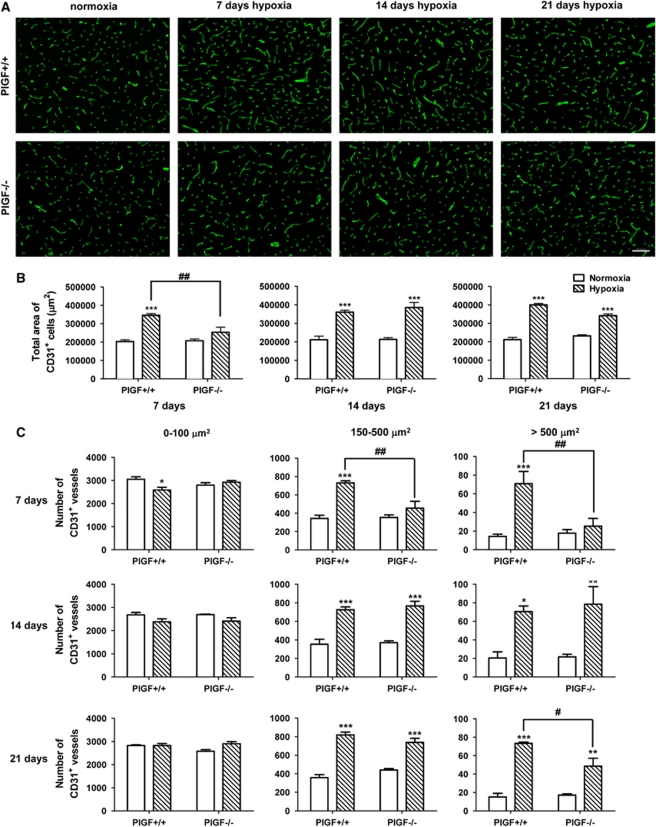
Brain angiogenesis was evaluated in PlGF+/+ and PlGF−/− normoxic and hypoxic mice by quantifying the number, total length, and total area of CD31+ cells in frontal brain sections of the cerebral cortex. Data obtained from the analysis of both total area and total length of CD31+ cells showed similar trends of changes in PlGF+/+ and PlGF−/− animals in all conditions. The total area of CD31+ cells is therefore presented as a representative measure of angiogenesis (Supplementary Figure 1). This parameter measures the changes in all three types of angiogenesis including sprouting, intercalated, and intussusceptive angiogenesis (Ward et al, 2007).
Total area of CD31+ cells was not significantly different in normoxic PlGF+/+ and PlGF−/− mice at any time point (Figures 2A and 2B). After 7 days exposure to hypoxia, PlGF+/+ mice showed a significant ∼40% increase in total area of CD31+ cells compared with normoxic controls (Figures 2A and 2B); however, no such increase was observed in PlGF−/− animals under the same conditions (Figures 2A and 2B). After 14 and 21 days, both hypoxic PlGF+/+ and PlGF−/− mice showed ∼40% higher vessel area compared with their normoxic controls, with no significant differences between the two genotypes (Figures 2A and 2B).
Figure 2.
(A) Representative images of sections of the cerebral cortex from PlGF+/+ and PlGF−/− mice exposed to normoxia or 7-, 14-, or 21-day hypoxia. Sections were immunostained for the endothelial marker, CD31 (green). Scale bar=100 μm. (B) Quantification of total area of CD31+ cells after 7-, 14-, or 21-day normoxia or hypoxia as described in Materials and methods. (C) Number of CD31+ vessels separated in size intervals of 0 to 100 μm2, 150 to 500 μm2, and >500 μm2 for each time point. White solid bars represent normoxic controls and hatched bars represent hypoxic groups. Results expressed are mean values±s.e.m. ‘*' indicates significant difference (*P<0.05, **P<0.01, ***P<0.001) between hypoxic groups and the corresponding normoxic control groups and ‘#' indicates significant difference (#P<0.05; ##P<0.01) between hypoxic PlGF+/+ and PlGF−/− groups (analysis of variance (ANOVA) followed by Bonferroni post-test). PlGF, placental growth factor.
The total number of CD31+ cells did not significantly change between the normoxic and hypoxic groups for either PlGF+/+ or PlGF−/− mice at 7- or 14-day time points (data not shown). However, at 21 days of exposure to hypoxia, a significant ∼15% increase in total number of CD31+ cells was observed in both PlGF+/+ and PlGF−/− mice (data not shown). In PlGF+/+ mice subjected to 7 days of hypoxia, a greater number of vessels exhibited an increase in size and branching compared with either normoxic controls or the PlGF−/− counterparts (Figure 2A).
To further evaluate the shift in vessels size, a frequency distribution analysis of CD31+ cells with area ranging between 0 to 100 μm2, 150 to 500 μm2, and >500 μm2 was performed (Figure 2C). In both PlGF+/+ and PlGF−/− normoxic mice, the number of CD31+ vessels was similar and inversely proportional to the vessel size with ∼3,000 CD31+ vessels of 0 to 100 μm2, ∼350 CD31+ vessels of 150 to 500 μm2, and ∼20 CD31+ vessels of >500 μm2 (Figure 2C).
After 7 days of hypoxia, PlGF+/+ mice showed a significant decrease (∼400 vessels) in the number of 0 to 100 μm2 CD31+ vessels compared with normoxic controls (Figure 2C). This was correlated with a significant increase (∼400 vessels) in the number of CD31+ vessels measuring 150 to 500 μm2 and >500 μm2 in hypoxic PlGF+/+ mice compared with normoxic animals (Figure 2C). Interestingly, no changes in the number of vessels were observed in any of the vessel-size distribution groups between hypoxic PlGF−/− mice and its corresponding normoxic controls (Figure 2C).
After a 14-day hypoxia, both PlGF+/+ and PlGF−/− mice showed a small, nonsignificant, decrease in the number of 0 to 100 μm2 CD31+ vessels but a significant increase in the number of 150 to 500 μm2 and >500 μm2 CD31+ vessels compared with their respective normoxic groups (Figure 2C). No differences between hypoxic PlGF+/+ and PlGF−/− was observed in either of the three vessel-size distribution groups (0 to 100, 150 to 500, and >500 μm2; Figure 2C).
At day 21, a similar number of 0 to 100 μm2 CD31+ vessels was measured in both PlGF+/+ and PlGF−/− normoxic and hypoxic animals (Figure 2C); a significant increase in the number of 150 to 500 μm2 CD31+ vessels was observed in hypoxic PlGF+/+ and PlGF−/− animals compared with their normoxic counterparts with no significant differences between the two genotypes; a significant increase in the number of >500 μm2 CD31+ vessels was also measured in hypoxic PlGF+/+ and PlGF−/− animals compared with their normoxic counterparts; however, PlGF−/− mice demonstrated a significantly reduced number of >500 μm2 CD31+ vessels compared with hypoxic PlGF+/+ animals (Figure 2C).
Effect of Placental Growth Factor Knockout on Pericyte and Smooth Muscle Cell Vessel Coverage
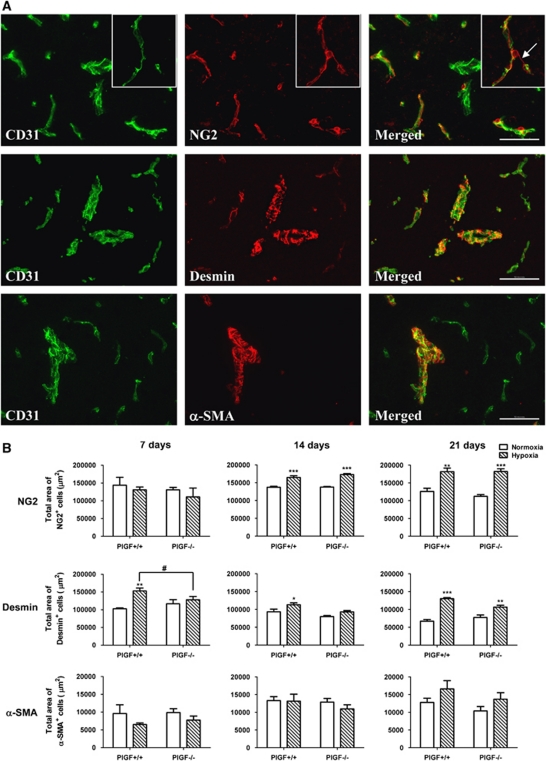
Placental growth factor has been shown to stimulate pericyte/VSMC recruitment under pathological ischemic conditions (Luttun et al, 2002; Takeda et al, 2009). To investigate whether PlGF knockout affects pericyte/VSMC vessel coverage, total area of NG2+ and Desmin+ cells (pericyte markers) and α-SMA+ cells (pericyte/VSMC marker) were evaluated in both PlGF+/+ and PlGF−/− normoxic and hypoxic brain sections by immunofluorescence.
Immunofluorescent staining of PlGF+/+ and PlGF−/− brain sections showed no morphological differences in NG2+, desmin+, and α-SMA+ cells in either normoxic or hypoxic conditions. NG2 and desmin markers stained the pericyte layer that was intimately apposed to CD31-labeled endothelial cells (Figure 3A). NG2-stained vessels in a continuous pattern, while desmin staining was interrupted, showing frequent gaps and finger-like projections around the vessels (Figure 3A). α-Smooth muscle actin antibody selectively stained large microvessels (>500 μm2) and did not stain the relatively smaller microvessels (Figure 3A). α-Smooth muscle actin labeling showed a circumferentially arranged striated appearance that ran perpendicular to the longitudinal axis of the CD31-labeled vessels. The morphological features that were observed using the above pericyte and VSMC markers in this study are in agreement with those reported by other groups (Kurz et al, 2008; Virgintino et al, 2007).
Figure 3.
(A) Double-immunofluorescence staining performed on sections of cerebral cortex from normoxic or hypoxic PlGF+/+ and PlGF−/− mice, using the endothelial marker CD31 (green) with either of the following pericyte markers: NG2 (red), desmin (red), or vascular smooth muscle cell (VSMC) marker α-smooth muscle actin (α-SMA) (red). Scale bar=100 μm. Insert in the right upper corner represents a magnified image of an NG2+ reactive pericyte. (B) Quantitative analysis of total area of NG2+, desmin+, and α-SMA+ cells as described in Materials and methods. White solid bars represent normoxic controls and hatched bars represent hypoxic groups. Results expressed are mean values±s.e.m. ‘*' indicates significant difference (*P<0.05, **P<0.01, ***P<0.001) between hypoxic groups and the corresponding normoxic control groups and # indicates significant difference (P<0.05) between hypoxic PlGF+/+ and PlGF−/− groups (analysis of variance (ANOVA) followed by Bonferroni post-test). PlGF, placental growth factor.
Quantitative analysis of total area covered by NG2+, desmin+, and α-SMA+ cells showed that NG2+ and desmin+ cells are 10-times more abundant than α-SMA+ cells in the brain vasculature (Figure 3B), which correlates with the observed selective staining of α-SMA+ cells in large (>500 μm2) microvessels (Figure 3A). No significant differences in total area covered by any of the three markers was observed between PlGF+/+ and PlGF−/− mice under normoxic conditions. Hypoxia did not affect the total area of α-SMA+ cells at any time point in either PlGF+/+ and PlGF−/− mice.
Total area covered by NG2+ cells was not affected by 7 days hypoxia in either PlGF+/+ or PlGF−/− animals; however, it was increased in both genotypes by ∼20% and 30% after 14 and 21 days of hypoxia, respectively (Figure 3B).
Total area covered by desmin+ cells was significantly higher in PlGF+/+ mice after 7, 14, and 21 days of hypoxia compared with the corresponding normoxic controls, while in PlGF−/− mice, the area was significantly higher than in the control group only after 21 days of hypoxia (Figure 3B).
Effect of Placental Growth Factor Knockout on Blood–Brain Barrier Permeability
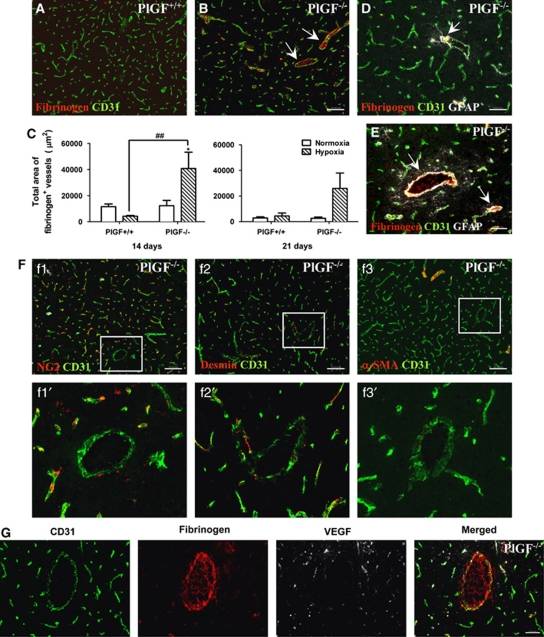
Several lines of evidence indicated that PlGF has a role in vessel stabilization under pathological conditions (Autiero et al, 2003a; Du et al, 2010; Liu et al, 2006; Luttun et al, 2002). To evaluate whether PlGF knockout can affect vessel permeability, vessel extravasation of fibrinogen was investigated in frontal cortical brain sections of PlGF+/+ and PlGF−/− normoxic and hypoxic mice by immunofluorescence. In all cases, fibrinogen colocalized with CD31+ vessels, which consistently exhibited intraluminal fibrinogen accumulation (Figures 4A, 4B, 4D, 4E, and 4G) and a certain degree of fibrinogen extravasation (Figure 4G).
Figure 4.
(A, B) Double-immunofluorescence staining was performed on sections of cerebral cortex from normoxic and hypoxic PlGF+/+ and PlGF−/− mice, using the endothelial marker CD31 (green) and fibrinogen (red). Arrows indicate the presence of fibrinogen in the lumen of PlGF−/− vessels after 14 days hypoxia (B). Scale bar=100 μm. (C) Quantitative analysis of total area of fibrinogen+ microvessels from PlGF+/+ and PlGF−/− mice exposed to 14- and 21-day normoxia or hypoxia. White solid bars represent normoxic controls and hatched bars represent hypoxic groups. Results expressed are mean values±s.e.m. * indicates significant difference (P<0.05) between hypoxic groups and the corresponding normoxic control groups, and ## indicates significant difference (P<0.01) between hypoxic PlGF+/+ and PlGF−/− groups (analysis of variance (ANOVA) followed by Bonferroni post-test). (D, E) Triple-immunofluorescence for fibrinogen (red), endothelial cells (green), and astrocytes (white) on frozen sections of cerebral cortex from PlGF−/− mice exposed to 14 days of hypoxia. Scale bar=50 μm. Arrows indicate reactive astrocytes surrounding fibrinogen+ vessels. (F) Double-immunofluorescence of sections of cerebral cortex from PlGF−/− mice exposed to 14-day hypoxia, using the endothelial marker CD31 (green) and pericyte/vascular smooth muscle cell (VSMC) markers NG2, desmin, and α-smooth muscle actin (α-SMA) (red). Boxes in f1, f2, f3 outline areas magnified in f1′, f2′, f3′, showing the lack of pericyte/VSMC markers in enlarged microvessels (>500 μm2 CD31+ vessel group). Scale bar=100 μm. (G) Triple-immunofluorescence staining of fibrinogen (red), endothelial cells (green), and vascular endothelial growth factor (VEGF) (white) in sections of cerebral cortex from PlGF−/− mice after 14 days of hypoxia. Scale bar=50 μm. PlGF, placental growth factor.
After 7 days of either normoxia or hypoxia, no significant differences in the total area of fibrinogen+ vessels were observed in PlGF+/+ and PlGF−/− animals (data not shown). After 14 days of hypoxia, the total area of fibrinogen+ vessels in PlGF−/− mice was ∼10-fold higher than in PlGF+/+ hypoxic mice (Figure 4C). After 21 days, no significant differences in the total area of fibrinogen+ cells was observed between the normoxic and hypoxic PlGF+/+ animals but a significant increase was still quantified in two out of four PlGF−/− hypoxic mice compared with the normoxic group.
PlGF−/− hypoxic mice exhibited a greater number of vessels (>500 μm2 CD31+) with dilated lumen compared with those in PlGF+/+ hypoxic mice. These vessels frequently contained intraluminal fibrinogen accumulation (Figures 4A, 4B, 4D, 4E, and 4G) were surrounded by GFAP+ astrocytes (Figures 4D and 4E) and completely lacked or had sparse NG2+, desmin+, and/or α-SMA+ cell coverage (Figure 4F). In addition, these vessels presented very low-VEGF expression compared with that of smaller vessels in the same sections (Figure 4G).
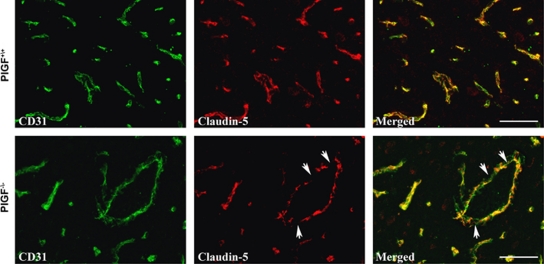
The status of tight junction proteins in the brain vessels was analyzed in PlGF+/+ and PlGF−/− mice at 14 days of hypoxia, when fibrinogen accumulation was highly abundant in PlGF−/− animals. Claudin-5, the major interendothelial junctional protein limiting paracellular permeability at the BBB, exhibited a highly regular and organized immunoreactive arrangement in PlGF+/+ vessels compared with the more disorganized and fragmented immunoreactivity pattern displayed in the PlGF−/− vessels (Figure 5). No significant changes were observed in zona occludens protein-1 (ZO-1) expression between both genotypes (data not shown).
Figure 5.
Double-immunofluorescence of sections of cerebral cortex from PlGF+/+ and PlGF−/− mice exposed to 14-day hypoxia, using the endothelial marker CD31 (green) and tight junction marker claudin-5 (red). Arrows indicate areas of disruption of claudin-5. Scale bar=50 μm. PlGF, placental growth factor.
Effect of Hypoxia on Placental Growth Factor and Vascular Endothelial Growth Factor Expression in PlGF+/+ and PlGF−/− Mice
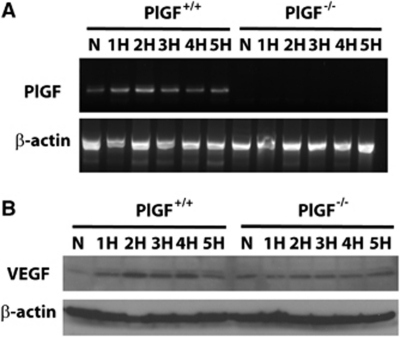
Since the delay in angiogenic response in PlGF−/− mice occurred during the first week of hypoxia, PlGF gene expression was analyzed in the brain homogenate of both genotypes during the course of the first 5 days of hypoxia (n=2 animals per time point). In PlGF+/+ mice, PlGF mRNA was expressed in the brain during normoxia as well as during the first 5 days of hypoxia. No PlGF expression was found in PlGF−/− mice in either normoxia or hypoxia, as expected (Figure 6A). Although a slight increase in PlGF expression was observed in the wild-type mice at specific time points during the course of hypoxia, the variability obtained between the two experiments do not allow us to firmly state that there is a significant change in PlGF expression during hypoxia in the brain of wild-type mice.
Figure 6.
(A) Agarose gel showing placental growth factor (PlGF) and β-actin RT-PCR (reverse transcriptase polymerase chain reaction) amplification products from the frontal brain region of PlGF+/+ and PlGF−/− mice exposed to 5 days of normoxia (N) or 1 to 5 days of hypoxia (H). (B) Vascular endothelial growth factor (VEGF) and β-actin protein expression analyzed by Western blot from PlGF+/+ and PlGF−/− mice exposed to 5 days of normoxia (N) or 1 to 5 days of hypoxia (H).
Vascular endothelial growth factor protein was upregulated during the first 5 days of hypoxia in PlGF+/+ animals; however, the peak of expression during those 5 days varied between 1 and 4 days in the two experiments performed. The expression of VEGF was consistently higher during hypoxia in PlGF+/+ animals compared with the PlGF−/− mice.
Discussion
In this study, the effect of PlGF in hypoxia-induced brain angiogenesis and BBB permeability was examined using PlGF+/+ and PlGF−/− mice subjected to chronic hypoxia (10% oxygen) for 7, 14, and 21 days. Placental growth factor knockout mice exhibited a delayed angiogenic response to mild hypoxia as well as fibrinogen accumulation and a certain degree of extravasation in a number of microvessels, some of which exhibited enlarged lumens. These fibrinogen+ microvessels presented claudin-5 disruption, were surrounded by reactive astrocytes, showed lack of both pericyte and VSMC coverage and VEGF expression, and regressed after 21 days of hypoxia.
Previous reports have shown that several adaptive mechanisms occur to protect the brain from the decrease in oxygen supply, including hyperventilation, an increase in packed red blood cell volume (hematocrit) and decrease in metabolism. Consistent with these reports, PlGF+/+ and PlGF−/− mice exhibited an increase in hematocrit and failed to gain weight during the period of hypoxia (Harik et al, 1996; LaManna et al, 1992). However, no significant differences were found between the two genotypes.
Chronic hypoxia also promotes angiogenesis in the brain (Harik et al, 1996; LaManna et al, 1992). Among the factors contributing to the vascularization of the hypoxic brain, VEGF, angiopoietin-1, and integrins have important roles (Milner et al, 2008). Placental growth factor expression has been shown to increase in the brain after middle cerebral artery occlusion (Beck et al, 2002; Du et al, 2010), but its contribution to chronic hypoxia-induced brain angiogenesis has not been fully elucidated.
In this study, 10% hypoxia promoted a rapid (from day 7) and sustained (until day 21) increase in vascularization of the cerebral cortex in wild-type mice, while in PlGF knockout animals angiogenesis was only measurable 14 days after hypoxia, reaching similar levels than those obtained in the wild-type. This delay in angiogenic response to hypoxia in PlGF knockout mice is most likely due to both the lack of PlGF and the significantly reduced levels of VEGF expression detected during the first 5 days of hypoxia compared with those in the PlGF+/+ animals. Although the PlGF expression was only detected at the RNA but not at the protein level, this does not exclude the possibility that PlGF protein is produced in low amounts or expressed only in specific cells in the brain at quantities in the brain cortex homogenate beyond the detection limit by Western blot. Experimental in vitro evidence obtained in our laboratory indicated that mouse brain endothelial cells from wild-type mice express PlGF when subjected to 6 hours hypoxia followed by 14 hours reoxygenation (data not shown). As previously shown (Autiero et al, 2003b; Park et al, 1994), very low levels of PlGF can synergistically enhance the angiogenic response induced by suboptimal concentrations of VEGF. As the angiogenic process is orchestrated by the activity of multiple growth factors, the lack of one of them may impact the timing of the response without affecting the final outcome.
Pericytes and VSMC have a role in vessel stabilization (Luttun et al, 2002), inhibit endothelial cell proliferation and migration (Orlidge and D'Amore, 1987; Sato and Rifkin, 1989), and promote BBB integrity (Armulik et al, 2010). Under angiogenic conditions, pericytes/VSMC interacts with endothelial cells and supports tube formation (Ozerdem and Stallcup, 2003). The PlGF receptor, VEGFR-1, has been shown to be expressed in both pericytes and VSMC (Luttun et al, 2002). Placental growth factor promotes vessel normalization, and reduces vascular leakage by inducing recruitment of VSMC and pericytes under ischemic conditions (Luttun et al, 2002). However, there are no cellular markers that are uniquely specific for pericytes, and none of them recognizes all pericytes; their expression is dynamic, varies between organs and developmental stages and have been shown to overlap as they are not mutually exclusive in most pericytes (Armulik et al, 2005). Desmin, NG2, and α-SMA are markers commonly used to identify pericytes and VSMC.
In both PlGF wild-type and knockout mice, the cerebrovascular coverage by NG2+ pericyte significantly increased after 14 days of hypoxia. This marker has been shown to specifically identify activated pericytes in angiogenic microvessels (Ozerdem and Stallcup, 2003; Virgintino et al, 2007). Since NG2 expression was still observed after 21 days of hypoxia, vascular remodeling at the pericyte level seems to be ongoing at least within this time frame.
Similarly, cerebrovascular coverage by desmin+ pericyte was significantly stimulated by hypoxia in wild-type mice, although occurring at an earlier time point (7 days) when compared with NG2+ cells. In PlGF−/− animals, this process was delayed until day 21 of hypoxia, which correlates with the late angiogenic response observed in PlGF−/− mice.
α-Smooth muscle actin cell staining was not affected by hypoxia in either PlGF+/+ or PlGF−/− mice. This may indicate that the neovasculature has not reached full maturity within the 21-day time frame of this study. This is supported by the presence of activated pericytes (NG2+) 21 days after hypoxia and the selective association of α-SMA+ cells with larger microvessels (⩾500 μm2 CD31+ vessels).
Fibrinogen extravasation has been used as a marker of cerebrovascular permeability (del Zoppo, 2008), and hypoxia has been shown to promote rapid microvascular thrombosis and fibrin deposition within the brain (Adhami et al, 2006; del Zoppo, 2008). Fibrinogen immunofluorescence was used in this study to investigate intravascular and extravascular fibrinogen accumulation in PlGF+/+ and PlGF−/− normoxic and hypoxic mice.
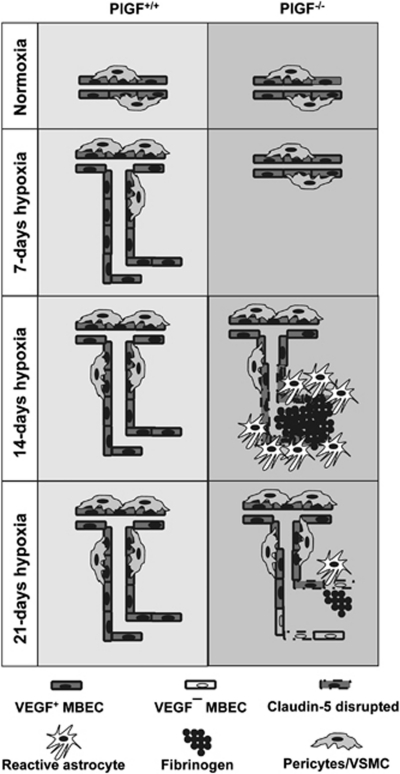
Despite no obvious and significant changes observed in pericyte/VSMC coverage between PlGF+/+ and PlGF−/− hypoxic mice, PlGF−/− mice showed intravascular fibrinogen accumulation and extravasation after 14 days of exposure to hypoxia. This correlated with vascular disruption of the tight junction protein claudin-5 in PlGF−/− animals. Vessels with fibrinogen+ lumens were surrounded by reactive astrocytes (GFAP+), and lacked pericyte/VSMC coverage. The size of these vessels were within the >500 μm2 CD31+ vessel group. Fibrinogen has been shown to inhibit neurite outgrowth, increase BBB permeability and endothelial cell disorganization, and promote neuroinflammation (del Zoppo, 2008; Schachtrup et al, 2007), and to induce astrocyte reactivity and scar formation (Schachtrup et al, 2010). In PlGF−/− animals, a high proportion of the fibrinogen+ vessels exhibited relatively large lumen. This may be due to the lack of pericytes/VSMC coverage in these vessels as these cells have an important role in inhibiting endothelial cell proliferation (Orlidge and D'Amore, 1987) and controlling the vascular tone (Diaz-Flores et al, 2009). Moreover, supporting trophic factors such as VEGF were not expressed in the fibrinogen+ vessels, while it was present in the fibrinogen-free microvessels. We hypothesize that the delayed angiogenic process observed in PlGF−/− animals leads to an increase in hypoxic stress that promotes fibrinogen accumulation within pericyte-denuded cerebral microvessels (Adhami et al, 2006) and gliosis (Schachtrup et al, 2010), which ultimately may lead to vessel regression (Figure 7). Interestingly, after 21 days of mild hypoxia, the overall extent of the brain vascularization in these animals appears to be similar to that of PlGF+/+ mice, indicating that, under these conditions, the lack of PlGF can be compensated against by other alternative factors/mechanisms in the CNS.
Figure 7.
Schematic representation of the effect of hypoxia on the brain angiogenesis in PlGF+/+ and PlGF−/− mice. Under normoxic conditions, PlGF+/+ and PlGF−/− mice exhibit similar brain vascularity. After 7 days hypoxia, an angiogenic response is induced in PlGF+/+ mice, while in PlGF−/− mice, this effect is delayed until 14 days of hypoxia. The delayed angiogenic response likely promotes hypoxic stress in PlGF−/− mice, which leads to fibrinogen accumulation and a small amount of extravasation. This affects vessel integrity as indicated by the lack of endothelial vascular endothelial growth factor (VEGF) expression, pericyte coverage, and disruption of the tight junction protein claudin-5 in the fibrinogen+ vessels, which ultimately may result in vessel regression after 21 days of hypoxia. PlGF, placental growth factor.
In conclusion, PlGF knockout results in a delay in angiogenic response, vascular instability, and increase permeability in the brain under mild hypoxic conditions. This phenotype may not be exclusively due to the lack of PlGF but to additional molecular changes (reduced VEGF expression, alterations in receptor expression (Freitas-Andrade et al, 2008)) associated to the effect of the knockout. Since the chronic hypoxic conditions simulated in this study can be largely compensated by systemic changes such as hyperventilation, increased cerebral blood flow, reduced metabolism, and increased hematocrit, a full evaluation of the impact that PlGF knockout may have in the CNS warrants further analysis in acute ischemic conditions such as stroke in which the severity of the insult may lead to more prominent effects.
Acknowledgments
The authors thank Mr Tom Devecseri for image processing.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by a Grant from Heart and Stroke Foundation of Ontario (HSFO), a scholarship (MFA) from HSFO/Canadian Institute of Health Research (CIHR)/Canadian Stroke Network (CSN)/AstraZeneca, from long term Structural funding Methusalem - by the Flemish Government and from Interuniversity Attraction Poles Program - Federal Government - P06/30.
Supplementary Material
References
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–2752. [PubMed] [Google Scholar]
- Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen—a review. Placenta. 2000;21 (Suppl A:S16–S24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood--brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003a;1:1356–1370. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Communi D, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003b;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- Beck H, Acker T, Puschel AW, Fujisawa H, Carmeliet P, Plate KH. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. J Neuropathol Exp Neurol. 2002;61:339–350. doi: 10.1093/jnen/61.4.339. [DOI] [PubMed] [Google Scholar]
- Cao Y, Linden P, Shima D, Browne F, Folkman J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest. 1996;98:2507–2511. doi: 10.1172/JCI119069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- Cramer M, Nagy I, Murphy BJ, Gassmann M, Hottiger MO, Georgiev O, Schaffner W. NF-kappaB contributes to transcription of placenta growth factor and interacts with metal responsive transcription factor-1 in hypoxic human cells. Biol Chem. 2005;386:865–872. doi: 10.1515/BC.2005.101. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Virchow's triad: the vascular basis of cerebral injury. Rev Neurol Dis. 2008;5 (Suppl 1:S12–S21. [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- Du H, Li P, Pan Y, Li W, Hou J, Chen H, Wang J, Tang H. Vascular endothelial growth factor signaling implicated in neuroprotective effects of placental growth factor in an in vitro ischemic model. Brain Res. 2010;1357:1–8. doi: 10.1016/j.brainres.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Freitas-Andrade M, Carmeliet P, Stanimirovic DB, Moreno M. VEGFR-2-mediated increased proliferation and survival in response to oxygen and glucose deprivation in PlGF knockout astrocytes. J Neurochem. 2008;107:756–767. doi: 10.1111/j.1471-4159.2008.05660.x. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Harik N, Harik SI, Kuo NT, Sakai K, Przybylski RJ, LaManna JC. Time-course and reversibility of the hypoxia-induced alterations in cerebral vascularity and cerebral capillary glucose transporter density. Brain Res. 1996;737:335–338. doi: 10.1016/0006-8993(96)00965-1. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Holmes DI, Zachary I. Placental growth factor induces FosB and c-Fos gene expression via Flt-1 receptors. FEBS Lett. 2004;557:93–98. doi: 10.1016/s0014-5793(03)01452-2. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF′s neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20:1185–1187. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Kuo NT, Benhayon D, Przybylski RJ, Martin RJ, LaManna JC. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. J Appl Physiol. 1999;86:260–264. doi: 10.1152/jappl.1999.86.1.260. [DOI] [PubMed] [Google Scholar]
- Kurz H, Fehr J, Nitschke R, Burkhardt H. Pericytes in the mature chorioallantoic membrane capillary plexus contain desmin and alpha-smooth muscle actin: relevance for non-sprouting angiogenesis. Histochem Cell Biol. 2008;130:1027–1040. doi: 10.1007/s00418-008-0478-8. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J Appl Physiol. 1992;72:2238–2243. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, del Zoppo GJ. Increased expression of fibronectin and the alpha5beta1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci. 2008;38:43–52. doi: 10.1016/j.mcn.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlidge A, D′Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, Zheng B, Akassoglou K. Fibrinogen inhibits neurite outgrowth via beta 3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci USA. 2007;104:11814–11819. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Uemura S, Iwama H, Imagawa K, Nishida T, Onoue K, Takemoto Y, Soeda T, Okayama S, Somekawa S, Ishigami K, Takaoka M, Kawata H, Kubo A, Horii M, Nakajima T, Saito Y. Treatment with recombinant placental growth factor (PlGF) enhances both angiogenesis and arteriogenesis and improves survival after myocardial infarction. Circ J. 2009;73:1674–1682. doi: 10.1253/circj.cj-08-1067. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- Ward NL, Moore E, Noon K, Spassil N, Keenan E, Ivanco TL, LaManna JC. Cerebral angiogenic factors, angiogenesis, and physiological response to chronic hypoxia differ among four commonly used mouse strains. J Appl Physiol. 2007;102:1927–1935. doi: 10.1152/japplphysiol.00909.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.