Abstract
Activation of the NADPH oxidase subunit, NOX2, and increased oxidative stress are associated with neuronal death after cerebral ischemia and reperfusion. Inhibition of NOX2 by casein kinase 2 (CK2) leads to neuronal survival, but the mechanism is unknown. In this study, we show that in copper/zinc-superoxide dismutase transgenic (SOD1 Tg) mice, degradation of CK2α and CK2α′ and dephosphorylation of CK2β against oxidative stress were markedly reduced compared with wild-type (WT) mice that underwent middle cerebral artery occlusion. Inhibition of CK2 pharmacologically or by ischemic reperfusion facilitated accumulation of poly(ADP-ribose) polymers, the translocation of apoptosis-inducing factor (AIF), and cytochrome c release from mitochondria after ischemic injury. The eventual enhancement of CK2 inhibition under ischemic injury strongly increased 8-hydroxy-2′-deoxyguanosine and phosphorylation of H2A.X. Furthermore, CK2 inhibition by tetrabromocinnamic acid (TBCA) in SOD1 Tg and gp91 knockout (KO) mice after ischemia reperfusion induced less release of AIF and cytochrome c than in TBCA-treated WT mice. Inhibition of CK2 in gp91 KO mice subjected to ischemia reperfusion did not increase brain infarction compared with TBCA-treated WT mice. These results strongly suggest that NOX2 activation releases reactive oxygen species after CK2 inhibition, triggering release of apoptogenic factors from mitochondria and inducing DNA damage after ischemic brain injury.
Keywords: casein kinase 2, middle cerebral artery occlusion, NADPH oxidase, NOX2, reactive oxygen species
Introduction
Oxidative stress induced by reperfusion after occlusion of brain vessels is a major contributor to secondary brain injury (Jung et al, 2010; Moskowitz et al, 2010). Thus, many approaches have been used (antioxidants or inhibitors of cell-death signaling molecules) to reduce production of reactive oxygen species (ROS) and to block ROS-mediated neuronal cell death induced by ischemic insults (Chan et al, 1996; Murakami et al, 1998; Suzuki, 2009). Despite intensive research on the neuroprotective mechanisms of therapeutic antioxidants and inhibitors using rodent stroke models, there is no FDA-approved effective drug other than tissue plasminogen activator for reducing brain damage after stroke (Hacke et al, 2008).
Apoptosis-inducing factor (AIF) participates in caspase-independent apoptosis in various cells and neurodegenerative disease models including stroke, amyotrophic lateral sclerosis, spinal cord injury, and Alzheimer's disease, which are caused by various stressors (Cregan et al, 2002; Culmsee et al, 2005; Wang et al, 2009). Under normal physiological conditions, AIF is embedded in mitochondrial intermembrane space. With stress, AIF can be released from mitochondria and translocated to the nucleus where it can fragment DNA. Recent studies indicate that AIF resides in two different compartments in mitochondria (Yu et al, 2009). One pool of AIF is located in the intermembrane space of mitochondria, and the other pool is in the outer mitochondrial membrane. That pool of AIF, which is 62 kDa, appears to participate in the rapid release of AIF from mitochondria to the cytoplasm and nucleus (Wang et al, 2009). Recent studies show that AIF translocation can be caused by poly(ADP-ribose) (PAR) polymerase-1 (PARP-1) activation after cell-death stimulus. One recent study showed that AIF can be released by a PARP-1-independent mechanism (Kondo et al, 2010), but in general, excessive activation of PARP-1 by oxidative stress is believed to be responsible for the release of AIF to the cytoplasm and nucleus (Yu et al, 2002).
Poly(ADP-ribose) polymerase-1 was originally identified as a repair enzyme and acts when DNA is damaged by oxidative stress such as superoxide anions, hydroxyl radicals, nitrogen dioxide radicals, and peroxynitrite, which can cleave or modify DNA strands. As a result of PARP-1 activation, the PAR polymer is generated as a by-product and is regarded as a death signal to neurons after various types of oxidative stress (Andrabi et al, 2006). Poly(ADP-ribose) can bind AIF, and this physical interaction can facilitate the release of AIF from the mitochondrial outer membrane (Wang et al, 2011). Blockage of this binding between AIF and PAR could be a promising molecular target for inhibition of neuronal cell death. The PAR polymer accumulates less in transgenic (Tg) rats that overexpress copper/zinc-superoxide dismutase (SOD1) than in wild-type (WT) rats after hypoglycemia/glucose reperfusion (Suh et al, 2008). Upregulation of PARP-1 expression after focal ischemia is also reduced in SOD1 Tg mice (Narasimhan et al, 2003). These reports support our belief that ROS lead to activation of PARP-1, which eventually causes energy failure, characterized by the depletion of NAD+ and ATP, and cell death. Mitochondrial membrane permeability caused by an unbalanced oxidative status after cell-death insults accounts for the release from the membrane of pro-apoptotic molecules after ischemic reperfusion injury. These molecules include cytochrome c, AIF, and second mitochondria-derived activator of caspase/direct inhibitor-of-apoptosis protein binding protein with low pI (Smac/Diablo). This release leads to executive caspase-3 activation and cell death.
Recently, we showed that casein kinase 2 (CK2) has a pivotal role in the modulation of ROS at the cellular level via the NADPH oxidase subunit, NOX2, after ischemic reperfusion injury (Kim et al, 2009). We showed that NOX2 produced ROS after CK2 inhibition, and that ROS facilitated an increase in brain lesions. However, the downstream events, which lead to neuronal cell death by the mitochondrial-dependent signaling pathway, have not been fully elucidated. We show here that NOX2-induced ROS, produced by CK2 inhibition, strongly participates in mitochondrial dysfunction and nuclear damage, characterized by the release of pro-apoptotic molecules from mitochondria to the cytoplasm and by DNA oxidative damage after cerebral ischemia and reperfusion. In this study, we used SOD1 Tg mice and gp91phox (NOX2) knockout (KO) mice to show the role of ROS produced by NOX2 in mitochondrial and nuclear damage after ischemic brain injury. We also show that reciprocal feedback exists between CK2 and ROS in mouse cerebral ischemic injury.
Materials and methods
Transient Focal Cerebral Ischemia
All experiments with mice were performed in accordance with National Institutes of Health guidelines, and the animal protocols were approved by Stanford University's Administrative Panel on Laboratory Animal Care. CD1 mice (2-month-old male, 30 to 35 g) were purchased from Charles River Laboratories (Wilmington, MA, USA). C57BL/6J WT and gp91phox KO mice with a C57BL/6J background were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The SOD1 Tg mice with a CD1 background were derived from the founder stock described earlier (Epstein et al, 1987).
Male mice (30 to 35 g) were subjected to 45 minutes of focal cerebral ischemia as previously described (Jung et al, 2009). Rectal temperature was controlled with a homeothermic blanket and kept at 37°C. Physiological parameters were monitored throughout the surgeries. Sham controls underwent the same procedure without insertion of the suture and occlusion of the vessels.
Primary Cortical Neuron Culture
Pregnant CD1 mouse (E16) primary cortical neurons were prepared and cultured as previously described (Narasimhan et al, 2003). Cultures were maintained in a humidified 5% CO2 incubator at 37°C. On day 2, medium was changed from minimum essential medium with 5% horse serum, GlutaMAX, and 25 mmol/L glucose to neurobasal medium containing B27 supplement and glutamate. Experiments were performed on neurons grown in culture for 7 days.
Oxygen-Glucose Deprivation
The medium of cortical neurons containing B27 and glucose was replaced with minimum essential medium without glucose, and cortical neurons were placed in a gas-tight humidified anoxic chamber (PlasLabs, Lansing, MI, USA) at 37°C for 4 hours. Oxygen tension was kept under 0.02%. After oxygen-glucose deprivation (OGD), the medium was changed to a neuronal medium containing B27 with glucose and the plates were returned to a 5% CO2/95% air incubator for reoxygenation for 3 hours.
Transfection with Small-Interfering RNA
Primary neuronal cells, grown on 6-well plates, were transfected for 48 hours with 25 nmol/L of CK2 small-interfering RNA (siRNA) using HiPerFect Transfection Reagent (Qiagen, Valencia, CA, USA), and then subjected to OGD. Allstars Negative Control siRNA (1027280; Qiagen) that does not target any mRNA sequence was used to control siRNA. The target sequence of CK2α siRNA is 5′-CTGGGTGGGTGTCTCATTCAA-3′ (Mm_Csnk2a1_3; S100961037; Qiagen).
Drug Injection
The mice were anesthetized and tetrabromocinnamic acid (TBCA; 20 nmol in 2 μl of 50% dimethyl sulfoxide in PBS) was injected intracerebroventricularly 1 hour before ischemia (bregma: 1.0 mm lateral, 0.2 mm posterior, 3.1 mm deep) as described previously (Kim et al, 2009). Fifty percent dimethyl sulfoxide in PBS was used as a vehicle. The drug was injected intracerebroventricularly 1 hour before onset of ischemia.
Measurement of Infarction Volume
Twenty-four hours after reperfusion, the mice were anesthetized and decapitated. Coronal brain slices were cut at 1 mm intervals using a mouse brain matrix and were incubated in 2% 2,3,5-triphenyltetrazolium chloride in 0.1 mol/L PBS (pH adjusted to 7.4) for 30 minutes. The total infarct volume was calculated and quantified using Adobe Photoshop (Adobe Systems, San Jose, CA, USA).
Immunofluorescent Staining
The brain sections were prepared for immunofluorescent staining as described previously (Kim et al, 2009). The primary antibodies we used were rabbit anti-PAR (1:200; Trevigen, Gaithersburg, MD, USA) and goat anti-AIF (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Secondary antibodies were used and the sections were mounted on glass slides and covered with mounting medium.
Immunohistochemistry
The animals were cardially perfused with heparinized (10 U/ml) saline and subsequently with 4% formaldehyde in PBS. The brains were sectioned at 50 μm using a vibratome and the slices were stored at −20°C. The sections were probed with an antibody against 8-hydroxy-2′-deoxyguanosine (8-OHdG) (1:200; JalCA, Japan Institute for the Control of Aging, Fukuroi, Japan) followed by M.O.M. biotinylated anti-mouse IgG reagent (Vector Laboratories, Burlingame, CA, USA). Staining was visualized by VECTASTAIN ABC reagent (Vector Laboratories) and diaminobenzidine. Images were processed using a Zeiss optical microscope (Axioplan2; Thornwood, NY, USA).
Subcellular Fractionation
Brain tissue was gently homogenized by douncing 20 times in ice-cold resuspension buffer containing 20 mmol/L HEPES-KOH (pH 7.5), 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L EGTA, protease inhibitor cocktail, phosphatase inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA), and 8.59% sucrose. Homogenates were centrifuged for 10 minutes at 750 × g at 4°C. The supernatant was further centrifuged for 20 minutes at 10,000 × g at 4°C. The pellet was used as a mitochondrial fraction, which was suspended with suspension buffer without sucrose and homogenized with an ultrasonic homogenizer. This pellet (10,000 g) also contains synaptosomes, thus, there is some possibility of contamination with cytosolic proteins in the mitochondrial fraction. The supernatant of 10,000 g was further centrifuged at 100,000 × g for 60 minutes at 4°C and the resultant supernatant was used as cytosolic fractions. Pellets of 750 × g were suspended using a nuclear lysis buffer from a ProteoJET cytoplasmic and nuclear protein extraction kit (Fermentas International, Glen Burnie, MD, USA) to isolate and purify the nuclear fractions.
Western Blot Analysis
Brain tissue from the ipsilateral hemisphere was homogenized in ice-cold lysis buffer and centrifuged, and western analysis was performed as described (Kim et al, 2009). Primary antibodies and titers used in this study are as follows: anti-CK2α antibody, anti-CK2α′ antibody, anti-CK2β, anti-AIF, and anti-cyclooxygenase 4 antibody (all five from Santa Cruz Biotechnology); anti-Lamin A/C antibody and anti-phospho-H2A.X antibody (both from Cell Signaling Technology, Beverly, MA, USA); anti-cytochrome c antibody (BD Biosciences Pharmingen, San Diego, CA, USA); and anti-β-tubulin antibody (Sigma-Aldrich). Horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology.
Statistical Analysis
All data represent mean±s.e.m. Statistical analysis was performed using Student's t-test or analysis of variance (ANOVA) (SigmaPlot; Systat Software, Chicago, IL, USA) with post hoc analyses (Tukey's test or Bonferroni correction). A P value <0.05 was accepted as statistically significant.
Results
Reactive Oxygen Species Are Important Mediators of Casein Kinase 2 Dysfunction
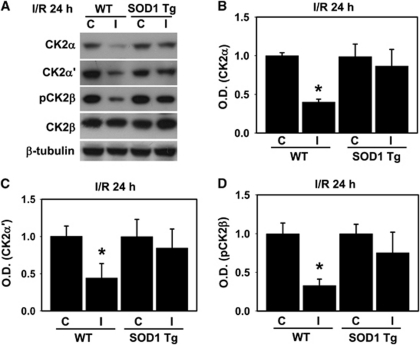
We have shown in our earlier study that each subunit of CK2 is affected differentially by oxidative stress caused by ischemia reperfusion, resulting in the degradation of catalytic subunits CK2α and α′ only, and not the regulatory subunit CK2β (Kim et al, 2009). To further elucidate the direct interplay between ROS and CK2 under oxidative stress after ischemic injury, and the role of ROS in the degradation of CK2, we first examined the degree of degradation of the CK2 subunits after ischemic injury using WT and SOD1 Tg mice. As shown in Figure 1A, the protein levels of CK2α and α′ and the phosphorylation of CK2β were significantly reduced by 45 minutes of occlusion and 24 hours of reperfusion. However, in the mouse brains overexpressing the SOD1 gene, degradation of the catalytic subunits CK2α and α′ was potentially blocked compared with the WT mice subjected to middle cerebral artery occlusion (MCAO; Figures 1A–C). Phosphorylation of CK2β was also not reduced by ischemic injury in the SOD1 Tg mouse brains compared with the WT brains (Figures 1A and 1D). These data suggest that an acute influx of ROS via reperfusion after MCAO is involved in the degradation and dysfunction of CK2. Interestingly, the results shown in Figure 1, and the results of our previous study (Kim et al, 2009), imply that there is a reciprocal feedback between ROS and CK2 activity during ischemic brain injury. Activity of CK2 is immediately downregulated by ROS, and this ROS overproduction induced by inactivated CK2 is likely associated with NADPH oxidase.
Figure 1.
Reactive oxygen species (ROS) mediate casein kinase 2 (CK2) downregulation in brain injury. (A) Protein samples from the ipsilateral and contralateral hemispheres of WT mice or mice that overexpress SOD1 that were harvested 24 hours after 45 minutes of transient focal cerebral ischemia. The samples were separated by SDS-PAGE and immunoblotted with anti-CK2α, CK2α′, phosphorylated CK2β (Ser209), and CK2β antibodies. β-Tubulin was used as a loading control. Quantitative data show that expression of CK2α (B), CK2α′ (C), and phosphorylated CK2β (D) was markedly reduced at 24 hours in the ipsilateral hemispheres compared with the contralateral hemispheres in the WT mice, but not in the ipsilateral hemispheres of the superoxide dismutase transgenic (SOD1 Tg) mice after transient focal cerebral ischemia (n=5). One-way analysis of variance (ANOVA) followed by Bonferroni correction. *P<0.05 compared with the ipsilateral hemispheres of the WT mice. I/R, ischemic reperfusion; C, contralateral; I, ipsilateral; OD, optical density; WT, wild type.
Casein Kinase 2 Inhibition Facilitates Poly(ADP-ribose) Polymer Accumulation and Apoptosis-Inducing Factor Translocation to the Nucleus
To define the specific signaling pathway that is involved in CK2 inhibition-induced neuronal cell death in ischemic brains, we examined the relationship between CK2 and PARP-1 activation via ROS production. Inhibition of CK2 by ischemic reperfusion induces NADPH oxidase activation and ROS overproduction in ischemic mouse brains (Kim et al, 2009). Many reports have showed that ROS induce activation of PARP-1 under oxidative stress and that production of ROS is an upstream event in PARP-1 activation in oxidative stress-induced cell death (Esposito and Cuzzocrea, 2009; Suh et al, 2008). Once PARP-1 is activated, PAR polymers accumulate in cells. This is recognized as an indicator of cell-death signaling (Andrabi et al, 2006). As shown in Figure 2, 3 hours after ischemic reperfusion, PARP-1 activation, assessed by immunofluorescence staining with the PAR polymer antibody, was increased compared with sham controls. Moreover, CK2 inhibition after administration of TBCA markedly enhanced PAR polymer immunoreactivity in the ischemic cortex more than in brains injected with the vehicle 3 hours after ischemic reperfusion injury (Figure 2).
Figure 2.
PARP-1 activation is more intense in tetrabromocinnamic acid (TBCA)-treated mice (CD1) after ischemic injury. Brain sections from sham mice and mice (CD1) that underwent middle cerebral artery occlusion (MCAO) after vehicle or TBCA treatment were incubated with an anti-PAR polymer antibody (green), followed by incubation with a secondary antibody conjugated with Alexa-488. The sections were then mounted with medium containing DAPI (blue) (n=6). Scale bar=50 μmol/L. I/R, ischemic reperfusion; DAPI, 4′,6 diamidino-2-phenylindole; PAR, poly(ADP-ribose); PARP-1, PAR polymerase-1.
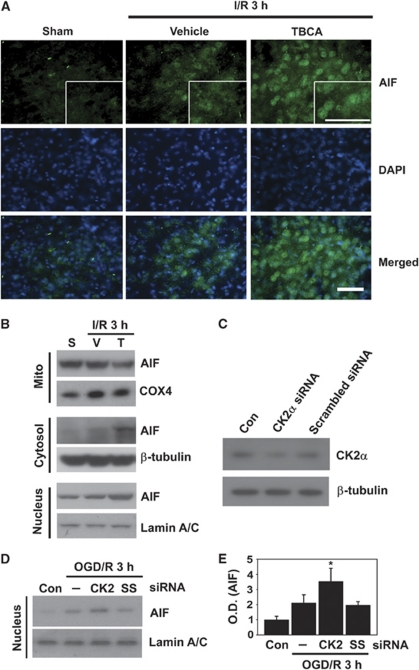
Much evidence suggests that PARP-1 activation is followed by AIF processes, which are mediated by ROS (Yu et al, 2006; Zhang et al, 2005). Thus, we examined whether the ROS/PARP-1 cascade caused by CK2 inhibition leads to translocation of AIF from mitochondria to the nucleus after ischemic brain injury. Immunofluorescent images showed that nuclear localization of AIF-positive cells was increased in the ischemic cortex 3 hours after ischemic reperfusion compared with sham controls (Figure 3A). Moreover, in brain cortices injected with TBCA, nuclear localization of AIF-positive cells increased more than in the brains injected with the vehicle under ischemic reperfusion conditions (Figure 3A). To further confirm whether AIF translocation is facilitated by CK2 inhibition after MCAO, we separated ischemic brain samples into three parts (mitochondrial, cytosolic, and nuclear fractions) and performed western blotting with the AIF antibody to trace the migration of AIF within the subcellular compartments. As shown in Figure 3B, in the mitochondrial fractions of the brains injected with TBCA under ischemic reperfusion, the mitochondrial level of AIF was reduced compared with the brains injected with the vehicle. In the cytosolic fractions, the level of AIF was increased in the TBCA-treated brains compared with the sham or vehicle-treated ischemic samples. Also, the level of AIF in the nuclei of the TBCA-treated ischemic brains was markedly increased compared with the vehicle-treated ischemic brains. Taken together, our data show that the death signals induced by CK2 inhibition cause the release of AIF from mitochondria to the nucleus via the CK2/ROS/PARP-1 axis in ischemic brain injury.
Figure 3.
Reactive oxygen species (ROS) generation through inhibition of casein kinase 2 (CK2) leads to facilitation of apoptosis-inducing factor (AIF) translocation to the nucleus. (A) AIF-positive cells were detected by an immunofluorescent technique with an anti-AIF antibody using slices from brains of vehicle- and tetrabromocinnamic acid (TBCA)-injected mice (CD1) 3 hours after ischemia and reperfusion. 4′,6 diamidino-2-phenylindole (DAPI) (blue) was used to counterstain the nucleus (n=6). Scale bar=50 μmol/L. The insets are magnification images showing AIF translocation to the nucleus. (B) Western blotting was performed with an anti-AIF antibody using fractionated samples (mitochondrial, cytosolic, and nuclear) from sham, vehicle- or TBCA-treated mice (CD1) 3 hours after ischemia and reperfusion injury. Cyclooxygenase 4 (COX4), Lamin A/C, and β-tubulin antibodies were used as mitochondrial, nuclear, and cytoplasmic markers, respectively (n=6). (C) Western blotting was performed using samples from control neuronal cells and CK2α small-interfering RNA (siRNA)-transfected and scrambled siRNA-transfected primary neuronal cells to confirm the effect of CK2α siRNA on downregulation of the CK2α protein. (D, E) Primary cortical neurons were transfected with CK2α siRNA or scrambled siRNA for 48 hours. These cells were then subjected to 4 hours of OGD and 3 hours of reoxygenation. AIF translocation to the nucleus was tested by western blotting using the nuclear fraction samples (n=4). One-way analysis of variance (ANOVA) followed by Tukey's test. *P<0.05 compared with OGD/3 hours of reoxygenation only or scrambled siRNA plus OGD and reoxygenation. I/R, ischemic reperfusion; S, sham; V, vehicle; T, TBCA; OGD/R, oxygen-glucose deprivation/reoxygenation; Con, control; —, OGD only (no siRNA transfection); SS, scrambled siRNA; OD, optical density.
To define the precise roles of CK2 dysfunction in the ischemic brain, we used specific siRNA targeting of the CK2α subunit in primary cortical neurons. Forty-eight hours after siRNA transfection, the neurons were harvested and the knockdown effect of CK2α by siRNA was evaluated by western blotting with the CK2α antibody. The protein level of CK2α was decreased by CK2α siRNA transfection compared with control and scrambled siRNA transfection (Figure 3C). Similarly to the results from the in vivo experiments using the pharmacological inhibitor TBCA, which is specific against CK2, CK2 siRNA transfection in cortical neurons followed by OGD and 3 hours of reoxygenation facilitated the translocation of AIF to the nucleus compared with control or scrambled siRNA transfection plus OGD and reoxygenation (Figures 3D and 3E). Using TBCA in vivo and CK2α siRNA transfection in vitro, we have strengthened our hypothesis that CK2 inhibition promotes AIF translocation from mitochondria.
Casein Kinase 2 Inhibition Enhances Mitochondrial Dysfunction via Release of Cytochrome c
Leakage of cytochrome c from mitochondria to the cytoplasm is caused by ischemic injuries such as focal or global cerebral ischemia (Fujimura et al, 1998; Sugawara et al, 1999). Therefore, we asked whether CK2 inhibition could also affect neuronal cell-death signaling associated with mitochondrial dysfunction such as the release of cytochrome c. To examine the degree of cytochrome c release from mitochondria to the cytoplasm, we isolated the cytosolic fractions from ischemic brains injected with the vehicle or TBCA. As shown in Figures 4A and 4B, 1 hour after ischemic reperfusion, the degree of cytochrome c release was not significantly different between the vehicle- and TBCA-treated ischemic brains. However, 3 hours after ischemic reperfusion injury, TBCA treatment showed a striking enhancement of cytochrome c release compared with vehicle treatment of the mouse brains (1.73±0.86 versus 3.42±1.71, P<0.05). These data show that CK2 inhibition by TBCA induces mitochondrial dysfunction through release of cytochrome c to the cytoplasm after ischemic reperfusion injury.
Figure 4.
Tetrabromocinnamic acid (TBCA) treatment promotes the release of cytochrome c to the cytoplasm after ischemic-reperfusion injury. (A) Mouse (CD1) brains were isolated 1 and 3 hours after ischemic reperfusion. Cytochrome c release was detected by western blotting using cytoplasmic protein samples from the sham and damaged brains, which were injected with the vehicle or TBCA. (B) Summary graph shows that 3 hours after ischemic reperfusion, cytochrome c release was increased in the TBCA-injected brains (n=4). One-way analysis of variance (ANOVA) followed by Bonferroni correction. *P<0.05 compared with vehicle-treated brains 3 hours after middle cerebral artery occlusion (MCAO). I/R, ischemic reperfusion; S, sham; V, vehicle; T, TBCA; OD, optical density.
Casein Kinase 2 Inhibition after Treatment with Tetrabromocinnamic Acid Enhances Oxidative DNA Damage
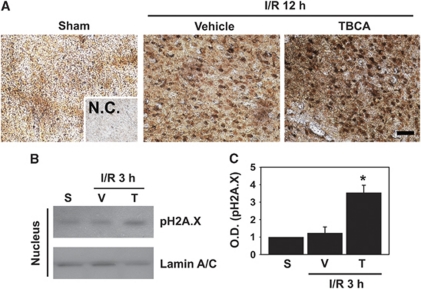
It is well known that ROS have a major role in DNA modification by an oxidative reaction, resulting in production of 8-OHdG, which is widely used as a marker of DNA damage after cell-death stimuli such as UV radiation and ischemic brain injury (Ames, 1989; Won et al, 1999). Thus, our next question was whether CK2 inhibition could invoke cell-death signals that could damage nuclear DNA by oxidation or nicking. To test this hypothesis, we first performed immunohistochemistry staining with the 8-OHdG antibody on slices from sham, vehicle-treated, or TBCA-treated mouse brains subjected to reperfusion for 12 hours after MCAO. As shown in Figure 5A, ischemic-reperfusion injury induced an increase in 8-OHdG-positive cells in the cortex of damaged mouse brains compared with sham controls. Moreover, in the slices from TBCA-injected brains subjected to 45 minutes of ischemia and reperfusion for 12 hours after MCAO, 8-OHdG-positive cells were markedly increased compared with vehicle-treated damaged brains (Figure 5A).
Figure 5.
Inhibition of casein kinase 2 (CK2) by tetrabromocinnamic acid (TBCA) increases DNA damage after brain injury. (A) Twelve hours after ischemic reperfusion, mouse (CD1) brains were isolated and sectioned. DNA damage was revealed by immunohistochemistry using an anti-8-OHdG antibody on undamaged or damaged brain sections from mice injected intracerebroventricularly with TBCA or the vehicle (n=4). Scale bar=50 μm. (B) Phosphorylation of H2A.X was assessed 3 hours after ischemic reperfusion by western blotting with an anti-phospho-H2A.X antibody using nuclear extracts from sham or damaged brains, which were injected with the vehicle or TBCA. Lamin A/C was used as an internal control and nuclear marker. (C) The graph shows that TBCA increased phosphorylation of H2A.X 3 hours after ischemic injury (n=4). One-way analysis of variance (ANOVA) followed by Tukey's test. *P<0.05 compared with 3 hours of ischemic reperfusion with vehicle treatment. I/R, ischemic reperfusion; NC, negative control; S, sham; V, vehicle; T, TBCA; OD, optical density; 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
To further confirm that CK2 inhibition affects enhancement of DNA damage after ischemic injury, we performed western blotting using the phospho-H2A.X antibody in nuclear fractions from mouse brains injected with the vehicle or TBCA and subjected to reperfusion for 3 hours after MCAO. It is widely accepted that phosphorylation by the ataxia telangiectasia mutant on the serine residue of the histone protein H2A.X is a relevant marker of DNA damage induced by various cell-death stimuli. Administration of TBCA for 1 hour before MCAO enhanced phosphorylation of H2A.X almost three times compared with vehicle-treated brains subjected to reperfusion for 3 hours after MCAO (1.23±0.35 versus 3.54±0.43, P<0.05; Figures 5B and 5C). These data strongly suggest that cell-death signals invoked by CK2 inhibition could induce DNA damage after ischemic injury.
Reactive Oxygen Species Are the Pivotal Mediators of Mitochondrial Dysfunction
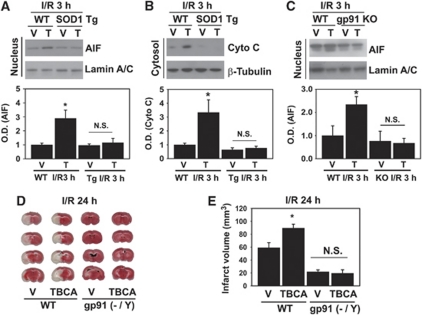
In our present study, we found that CK2 inhibition by ischemic reperfusion triggers the cell-death pathway via PAR polymer accumulation/AIF translocation to the nucleus and release of cytochrome c from mitochondria, and damages DNA in mouse cerebral ischemic injury. We hypothesized that all these phenomena were caused by ROS via CK2 inhibition-mediated NADPH oxidase activation after ischemic brain damage. Thus, to investigate whether ROS induced by CK2 inhibition indeed increases mitochondrial-mediated cell death, we used SOD1 Tg mice and gp91 KO mice. First, we investigated whether the translocation of AIF and cytochrome c by CK2 inhibition could be blocked in the SOD1 Tg mice that underwent MCAO. As shown in Figure 6A, inhibition of CK2 by TBCA significantly increased the translocation of AIF to the nucleus in WT mice (CD1) subjected to reperfusion for 3 hours after MCAO. However, TBCA did not increase the translocation of AIF to the nucleus in SOD1 Tg mice subjected to reperfusion for 3 hours after MCAO (Figure 6A). As with the data on AIF, CK2 inhibition by TBCA in the SOD1 Tg mouse brains did not enhance the release of cytochrome c from mitochondria to the cytoplasm compared with a significant increase in cytochrome c release by TBCA in the brains of WT mice subjected to reperfusion for 3 hours after MCAO (Figure 6B). These results indicate that ROS induced by CK2 inhibition increase mitochondrial-mediated cell death in cerebral ischemic injury. To test the direct involvement of NADPH oxidase (NOX2) in ROS production by CK2 inhibition and AIF translocation, we used gp91 (NOX2) KO mice. As shown in Figure 6C, TBCA did not promote AIF nuclear translocation in the gp91 KO mouse brains after ischemic reperfusion compared with WT mice (C57BL/6) injected with TBCA. These data show that NADPH oxidase is responsible for ROS production caused by CK2 inhibition and for AIF translocation.
Figure 6.
Reactive oxygen species (ROS) generated by NADPH oxidase induced by casein kinase 2 (CK2) inhibition are responsible for the increase in mitochondria-mediated brain damage. (A) Apoptosis-inducing factor (AIF) translocation to the nucleus by CK2 inhibition after ischemic injury was measured by western blotting with an anti-AIF antibody using the nuclear fraction from WT (CD1) and superoxide dismutase transgenic (SOD1 Tg) (CD1) mouse brains injected with the vehicle or tetrabromocinnamic acid (TBCA) 3 hours after ischemic reperfusion. Lamin A/C was used as an internal control and nuclear marker (n=6). One-way analysis of variance (ANOVA) followed by Bonferroni correction. *P<0.05 compared with the WT vehicle-injected group. (B) Cytochrome c release from mitochondria to the cytoplasm induced by CK2 inhibition after ischemic injury was assessed by western blotting with an anti-cytochrome c antibody using cytoplasmic samples from WT mouse brains and SOD1 Tg mouse brains injected with the vehicle or TBCA 3 hours after ischemic reperfusion. β-Tubulin was used as an internal control (n=6). One-way ANOVA followed by Bonferroni correction. *P<0.05 compared with the WT vehicle-injected group. (C) AIF translocation was measured by western blotting using samples from WT (C57BL/6J) or gp91phox knockout (KO) (C57BL/6J) mouse brains injected with the vehicle or TBCA (n=4). *P<0.05 compared with the WT vehicle-injected group. (D) The vehicle and TBCA were injected intracerebroventricularly in WT (C57BL/6J) or gp91 KO (C57BL/6J) mice 1 hour before onset of middle cerebral artery occlusion (MCAO). Twenty-four hours after ischemic reperfusion, brains were isolated and brain slices were analyzed to measure infarction volume using 2,3,5-triphenyltetrazolium chloride staining. (E) A summary graph shows comparative data on infarction volumes after vehicle or TBCA treatment in WT and gp91 KO mouse brains 24 hours after ischemic injury (n=5 to 6 in each group). One-way ANOVA followed by Bonferroni correction. *P<0.05 compared with the WT/vehicle group. I/R, ischemic reperfusion; V, vehicle; T, TBCA; NS, not significant; WT, wild type; OD, optical density.
Next, to demonstrate if brain damage after ischemic reperfusion is indeed caused by CK2 inhibition-mediated NADPH oxidase activation, we again used gp91 KO mice for the measurement of infarction volumes by 2,3,5-triphenyltetrazolium chloride staining. As shown in Figures 6D and 6E, greater inhibition of CK2 by TBCA injection significantly increased infarct volume in the WT mice (C57BL/6) subjected to reperfusion for 24 hours after MCAO (59.05±7.8 versus 89.49±6.0, WT vehicle versus WT TBCA, P<0.05). However, TBCA did not increase infarct volume in the gp91 KO mice subjected to reperfusion for 24 hours after MCAO (Figure 6D, second and fourth lanes). Moreover, there was no significant difference in infarct volume between vehicle-injected and TBCA-injected brains of the gp91 KO mice subjected to reperfusion for 24 hours after MCAO (Figure 6D, third and fourth lanes) (21.70±3.0 versus 19.49±5.38, gp91 KO vehicle versus gp91 KO TBCA). These results strongly suggest that brain damage by CK2 inhibition after ischemic injury is caused by NADPH oxidase-produced ROS.
Discussion
Evidence suggests that ROS produced by reperfusion have a very detrimental role in the progression of neuronal and glial cell death in the ischemic brain. It is widely accepted that one of the main sources of ROS in ischemia-injured brain cells is NADPH oxidase. In our previous study, we identified CK2 as a binding partner of NADPH oxidase, serving as a negative modulator of ROS production in NADPH oxidase in the ischemic brain. Thus, inhibition of CK2 by its specific pharmacological inhibitor promotes production of ROS by NADPH oxidase via Rac1 disassociation from CK2α, resulting in increased brain damage after ischemic reperfusion (Kim et al, 2009). However, the downstream events provoked by ROS induced by CK2 inhibition, and the reciprocal actions between ROS and CK2, remain to be elucidated.
To our knowledge, this is the first report showing that CK2 inhibition affects pro-apoptotic PARP-1, the AIF axis, and mitochondrial and DNA damage via ROS production by NADPH oxidase, and the mutual relationship between ROS and CK2 dysfunction after ischemic injury. We showed that pharmacological inhibition of CK2 by TBCA or inhibition by CK2α siRNA facilitates the accumulation of PAR polymers that represents the excess activation of PARP-1 and that induces more AIF translocation after ischemic reperfusion compared with vehicle treatment. Moreover, TBCA treatment before ischemic stress affected mitochondrial permeability transition, resulting in the release of cytochrome c from mitochondria to the cytoplasm. In contrast, CK2 inhibition seems to have an impact on DNA structure modification via ROS production. Using immunohistochemistry and western blots, we showed that CK2 inhibition increased 8-OHdG-immunopositive cells after ischemic reperfusion injury, which are a by-product generated when DNA is oxidatively modified by ROS. Also, CK2 inhibition by TBCA promoted phosphorylation of H2A.X. Interestingly, the release of cytochrome c and AIF by CK2 inhibition was suppressed in SOD1-overexpressing mice and gp91phox (NOX2) KO mice, and TBCA treatment did not increase infarction in these mice after ischemic reperfusion injury. These data strongly suggest that ROS generated by CK2 inhibition-mediated NADPH oxidase activation are responsible for facilitation of ischemic brain damage via the PARP-1/AIF axis and release of mitochondrial proteins, probably resulting in DNA damage after ischemic reperfusion injury.
Casein kinase 2 is a multifunctional kinase and in our previous report, we identified it as a neuroprotectant against ischemic brain damage. We showed that each of the CK2 subunits is degraded by ischemic injury. In that report, we did not provide direct evidence showing that ROS may be involved in CK2 subunit degradation. It is well known that oxidatively damaged proteins can be processed by the proteasome complex to avoid storage of useless, damaged proteins (Breusing and Grune, 2008; Jung et al, 2007). The oxidized parts of these proteins can serve as simple tags for recognizing the proteasome complex substrate (Giulivi et al, 1994). To define the direct role of ROS in CK2 subunit degradation, we used SOD1 Tg mice in which the SOD1 protein is overexpressed. Copper/Zinc-superoxide dismutase is a cytosolic antioxidant that can detoxify ROS. As shown in Figure 1, in SOD1 Tg mouse brains damaged by ischemic injury, CK2α and CK2α′ degradation and CK2β dephosphorylation were markedly blocked. These data indicate the strong involvement of ROS in CK2 protein dysfunction in ischemic injury. Taken together with the data that we previously reported showing that inhibition of CK2 increases ROS production by NADPH oxidase, we now suggest that there is an interplay between ROS and CK2 after ischemic injury.
It is noteworthy that CK2 inhibition can facilitate PARP-1 activation and AIF translocation after ischemic injury via ROS production. Emerging evidence suggests that AIF action is an indispensable process in the PARP-1-mediated cell-death pathway after various stimuli because PAR polymers (the product of PARP-1 activation) are known to facilitate the release of AIF by directly affecting mitochondrial function. In this study, we sought to discover whether CK2 inhibition could facilitate PARP-1 activation and AIF translocation after brain injury via ROS. We found that CK2 inhibition can promote PARP-1 activation and the release of AIF in ischemic brains. These events were inhibited in the brains of SOD1 Tg mice and gp91 KO mice. These data suggest that ROS produced by CK2 inhibition may be involved in the PARP-1/AIF cell-death pathway. We cannot rule out the possibility that CK2 directly affects PARP-1 and AIF activation via other mechanisms, such as modulation of the NAD+ level, by acting as an SIRT1 kinase in cells that then do not produce harmful ROS by NADPH oxidase (Kang et al, 2009; Rajamohan et al, 2009).
Reactive oxygen species generated by NADPH oxidase activation may affect outer membrane pore formation by loss of Bcl-2 or insertion of Bax, Bak, and tBid, resulting in an increase in release of AIF, cytochrome c, and Smac/Diablo, so-called apoptogenic proteins, that reside inside the mitochondrial intermembrane space. Even though a large body of evidence suggests that ROS can initiate the opening of these pores in many neurodegenerative disease models (Chan, 2005; Kirkland and Franklin, 2001; Kitazawa et al, 2002), little is known of the interplay between CK2 and outer membrane pore formation by Bcl-2 family interaction and requires further elucidation.
In summary, we have shown the detailed downstream events of ROS produced by CK2 inhibition that impact mitochondrial damage and subsequent nuclear DNA damage in ischemic brains. We found that ROS generated by CK2 dysfunction come from NADPH oxidase after ischemic brain injury. To our knowledge, this is the first report showing that CK2 is associated with neuronal mitochondrial and nuclear damage mediated by NADPH oxidase-generated ROS production after ischemia and reperfusion brain injury in mice. This study expands our knowledge of the interplay between CK2 and ROS mediated by NOX2 activation in ischemic brain injury, and provides clues for the development of therapeutic agents that target prevention of ROS generation via the modulation of CK2 activity.
Acknowledgments
The authors thank Liza Reola and Bernard Calagui for technical assistance.
The authors declare no conflict of interest.
Footnotes
This work was supported by Grants PO1 NS014543, RO1 NS036147, RO1 NS025372, and RO1 NS038653 from the National Institutes of Health, and by the James R Doty Endowment.
References
- Ames BN. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7:121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Andrabi SA, Kim NS, Yu S-W, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci USA. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem. 2008;389:203–209. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann NY Acad Sci. 2005;1042:203–209. doi: 10.1196/annals.1338.022. [DOI] [PubMed] [Google Scholar]
- Chan PH, Epstein CJ, Kinouchi H, Kamii H, Chen SF, Carlson E, Gafni J, Yang G, Reola L. Neuroprotective role of CuZn–superoxide dismutase in ischemic brain damage. Adv Neurol. 1996;71:271–280. [PubMed] [Google Scholar]
- Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu S-W, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol. 2002;158:507–517. doi: 10.1083/jcb.200202130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellechia M, Blomgren K, Plesnila N. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen–glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein CJ, Avraham KB, Lovett M, Smith S, Elroy-Stein O, Rotman G, Bry C, Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci USA. 1987;84:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E, Cuzzocrea S. Superoxide, NO, peroxynitrite and PARP in circulatory shock and inflammation. Front Biosci. 2009;14:263–296. doi: 10.2741/3244. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Morita-Fujimura Y, Murakami K, Kawase M, Chan PH. Cytosolic redistribution of cytochrome c after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1998;18:1239–1247. doi: 10.1097/00004647-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Giulivi C, Pacifici RE, Davies KJA. Exposure of hydrophobic moieties promotes the selective degradation of hydrogen peroxide-modified hemoglobin by the multicatalytic proteinase complex, proteasome. Arch Biochem Biophys. 1994;311:329–341. doi: 10.1006/abbi.1994.1245. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Bader N, Grune T. Oxidized proteins: intracellular distribution and recognition by the proteasome. Arch Biochem Biophys. 2007;462:231–237. doi: 10.1016/j.abb.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Kang H, Jung J-W, Kim MK, Chung JH. CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS One. 2009;4:e6611. doi: 10.1371/journal.pone.0006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GS, Jung JE, Niizuma K, Chan PH. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci. 2009;29:14779–14789. doi: 10.1523/JNEUROSCI.4161-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Franklin JL. Evidence for redox regulation of cytochrome c release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition. J Neurosci. 2001;21:1949–1963. doi: 10.1523/JNEUROSCI.21-06-01949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Wagner JR, Kirby ML, Anantharam V, Kanthasamy AG. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J Pharmacol Exp Ther. 2002;302:26–35. doi: 10.1124/jpet.302.1.26. [DOI] [PubMed] [Google Scholar]
- Kondo K, Obitsu S, Ohta S, Matsunami K, Otsuka H, Teshima R. Poly(ADP-ribose) polymerase (PARP)-1-independent apoptosis-inducing factor (AIF) release and cell death are induced by eleostearic acid and blocked by α-tocopherol and MEK inhibition. J Biol Chem. 2010;285:13079–13091. doi: 10.1074/jbc.M109.044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan P, Fujimura M, Noshita N, Chan PH. Role of superoxide in poly(ADP-ribose) polymerase upregulation after transient cerebral ischemia. Mol Brain Res. 2003;113:28–36. doi: 10.1016/s0169-328x(03)00062-7. [DOI] [PubMed] [Google Scholar]
- Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Birukov KG, Samant S, Hottiger MO, Gupta MP. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci. 1999;19:RC39. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, Chan PH, Swanson RA. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Anti-oxidants for therapeutic use: why are only a few drugs in clinical use. Adv Drug Deliv Rev. 2009;61:287–289. doi: 10.1016/j.addr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol. 2009;218:193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kim NS, Haince J-F, Kang HC, David KK, Andrabi SA, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos) Sci Signal. 2011;4:ra20. doi: 10.1126/scisignal.2000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won MH, Kang T-C, Jeon G-S, Lee J-C, Kim D-Y, Choi E-M, Lee KH, Choi CD, Chung M-H, Cho SS. Immunohistochemical detection of oxidative DNA damage induced by ischemia–reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999;836:70–78. doi: 10.1016/s0006-8993(99)01611-x. [DOI] [PubMed] [Google Scholar]
- Yu S-W, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci USA. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S-W, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1–dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Yu S-W, Wang Y, Frydenlund DS, Ottersen OP, Dawson VL, Dawson TM. Outer mitochondrial membrane localization of apoptosis-inducing factor: mechanistic implications for release. ASN Neuro. 2009;1:275–281. doi: 10.1042/AN20090046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Park TS, Gidday JM. Cerebral endothelial cell apoptosis after ischemia–reperfusion: role of PARP activation and AIF translocation. J Cereb Blood Flow Metab. 2005;25:868–877. doi: 10.1038/sj.jcbfm.9600081. [DOI] [PubMed] [Google Scholar]