Abstract
Combination therapy has been identified as a promising strategy to improve stroke management. We conducted a systematic review and meta-analysis of evidence from animal models of ischemic stroke to determine whether combining treatments improved efficacy. Multiple databases were searched and data were extracted from focal ischemia experiments comparing control groups, single treatments, and combination treatments. Of 11,430 papers identified, 142 met the inclusion criteria; these tested 126 treatments in 373 experiments using 8,037 animals (I2=85 to 96%). Taken together, single treatments reduced infarct size by 20% and improved neurological score by 12% compared with control; a second therapy improved efficacy by an additional 18% and 25%, respectively. Publication bias may affect combination efficacy for infarct size but not neurological score. Combining thrombolysis with other therapies may extend the time window from 4.4 to 8 hours in animal models, although testing beyond 6 hours is required to confirm this. Benefits of additional therapy decreased as the efficacy of the primary treatment increased, with combination efficacy reaching a ceiling at 60% to 80% protection. Combining treatments may bring benefits and extend the time window for treatment. More evidence is needed due to potential publication bias and heterogeneity.
Keywords: animal models, cerebral ischemia, neuroprotection, stroke, therapeutics
Introduction
With no single ‘magic bullet' for stroke, combination therapy is an increasingly appealing strategy (Cheng et al, 2004; Danton and Dietrich, 2004; Adams et al, 2003; Rogalewski et al, 2006; Fisher, 2003; Fisher et al, 2005, 2007). Combination therapies might raise the efficacy above levels achievable with single therapies; or extend the time window for thrombolysis with tissue plasminogen activator (tPA). Indeed, the multifaceted manifestations of stroke and its complications mean that most patients already receive a number of different interventions, notwithstanding that they may be administered for purposes ranging from management of hypertension, blood sugar levels, infection, and fluid balance through to those interventions aimed at improving mobility and speech. Testing combination therapies with the intention of reducing brain injury requires increased trial complexity and may present significant intellectual property and regulatory issues (European Ad Hoc Consensus Group, 1998; Saver and Kalafut, 2001). Combination therapies also raise the specter of a Pyrrhic victory where the adverse effects of multiple treatments outweigh real but small benefits; where patients take more than four medications, the risk of adverse events increases substantially (Byrne, 2003).
Combination therapy has been used in the treatment of blood pressure (Brown et al, 2003), cancer (Corn, 2004), and coronary artery disease (Ramanath and Eagle, 2007). Currently, there is little evidence to support the routine use of specific combinations to reduce brain injury in acute stroke (Adams et al, 2007), but this is an active area of research—driven by scientific and commercial imperatives—and is the subject of many patent applications (Brimble and Levi, 2006; Novartis, 2007; Moleac, 2007; NeurokeyA/S, 2007).
Meta-analysis allows the aggregation of data from a number of experiments to give a summary estimate of efficacy. We aimed to use meta-analysis of data from experiments testing combination therapies in animal models of ischemic stroke—identified using systematic review—to establish first, whether the use of therapies in combination resulted in greater efficacy than might be expected from their use as monotherapy; and second, whether the use of therapies in combination with thrombolysis extended the delays to treatment at which efficacy was seen.
To protect from the risks of post hoc data exploration, we limited our analyses to a small number of predefined hypotheses, and our statistical methods were chosen to test and reflect diversity in treatment effects. Because we expected substantial heterogeneity between studies, we also performed further analyses of a more uniform subset (tPA thrombolysis data); and we provide tables of source data for all included therapies to allow the reader to examine the data in detail.
The specific aims of the study were to examine (1) overall effect: whether a combination of two therapies is more effective than a single treatment or no treatment; (2) individual treatments: efficacies of individual therapies alone and in combination; (3) ceiling effect: whether there was a ceiling to the benefit from additional treatments and whether the overall level of efficacy depends on how effective the first therapy is alone; (4) tPA thrombolysis: whether combination therapy can extend the time window for tPA thrombolysis; and (5) classes of treatments: which particular classes of therapies are effective in combination.
Materials and methods
Scope
We included nonhuman animal studies in focal cerebral ischemia experiments that reported infarct size in a control group (no treatment), a single treatment group, and a combination group receiving the single treatment plus an additional treatment. Experiments without both single and dual groups were excluded since both were necessary to establish the additional effect of the second treatment. Experiments investigating treatment mechanisms using a second agent intended to counteract the effect of the first were also excluded. Where studies measured behavior, inclusion of data was restricted to neurological scores (results aggregating data from two or more behavioral test items into a single score).
Search Strategy
The following databases were searched: Web of Science, Current Contents, Biosis Previews, Pubmed, CAB abstracts, and Society for Neuroscience (search 21 September 2010). Search criteria were (1) combination neuroprotection; (2) neuroprotection AND (thrombolysis OR hypothermia); (3) (drug interaction OR combination therapy OR synergistic effect OR drug combination OR multiple drugs OR additive effect OR combinatorial treatment OR combinatorial therapy OR multimodal therapy) AND (stroke OR focal ischemia OR focal ischaemia OR cerebral ischaemia OR cerebral ischemia OR neuroprotection). Where possible, searches were restricted to animals.
Study Selection and Data Extraction
Studies were included if delivery and dose regimes for the single treatment were replicated in the combination condition. Information extracted included infarct size and neurological score summary statistics (mean, standard deviation/error, sample size); Intervention (treatment time, delivery method, mechanism of action); Study characteristics (species, animal age, and stroke model) and study quality. Animals of 6 months or older were defined as ‘old.' Study quality was rated as a composite of 10 criteria (Supplementary Table 1) (Macleod et al, 2004). Where volumetric data were not available, estimates were based on areas. Where data were presented only graphically, values were estimated using an electronic ruler.
Sensitivity Analysis and Meta-Analysis of Therapeutic Effect
Behavioral data were transformed to the same direction of effect and normalized to the size of the scale. Within each experiment, means and standard deviations (s.d.) in the single and combination groups were expressed as a percentage of control group outcomes (Macleod et al, 2004). Sample sizes in the control groups were adjusted for multiple comparisons (Macleod et al, 2004). We did not use a standardized mean difference approach because where sample size is small the observed s.d. is an imprecise measure of the population s.d., thereby introducing measurement error and reducing the power of the analysis. Mean outcomes were extracted to obtain: (1) single treatment effect (single treatment versus control); (2) combination treatment effect (combined treatment versus control); (3) additive effect (difference between the combination and single treatment effect for the other therapy); and (4) synergistic effect (the extent to which the combination efficacy exceeds the sum of the individual therapeutic efficacies of the constituents when tested individually). For instance, if treatment A and B tested individually reduced infarct size by 10% and 40%, respectively, and by 45% when used in combination, then the single treatment effect for A is 10%, the additive effect for A is 5% (45% to 40%), and the synergistic effect for A is –5% (45%–10%–40%). Because this calculation of synergy requires reporting of the single treatment effect of both treatments, it was not possible to calculate this for all included combinations.
Sensitivity analysis was undertaken by sequentially removing experiments—individually and in blocks—to determine their contribution to heterogeneity as measured by their effect on Cochran's Q before and after removal of studies. Heterogeneity was also tested using I2, a statistic derived from Cochran's Q but adjusting for degrees of freedom (df) (Higgins et al, 2003). Studies were removed in blocks reflecting treatment class, as defined below. A second block based sensitivity analysis was based on how stroke was induced (see Table 1 stroke model column). Inclusion and removal of data outliers was also tested.
Table 1. Results of meta-analysis of efficacy of therapies tested alone and in combination with a second therapy in animal models of ischemic stroke.
| Therapy | Tested with (NP/NA) | NP/NA | Q | Time M (Med) | Stroke models | Species |
Therapeutic efficacy infarct (neurological score) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Alone | Additive | Combined | Synergy | |||||||
| M±s.e. | M±s.e. | M±s.e. | M±s.e. | |||||||
| Pharmacological neuroprotection | 231/242 | 4 | −738 (45) | 22±1 (14±1) | 18±0 (25±1) | 41±1 (41±1) | −10±1 (−4±1) | |||
| 1,5-Isoquinolinediol (ISO) | FeTMPyP (1/1) | 1/1 | 6 | 60 | Filament | Rat | 61±5 (60±12) | 28±2 (20±10) | 82±5 (59±12) | −33±2 (−41±10) |
| 3-Aminobenzamide | Melatonin (1/1), z-VAD-fmk (1/1) | 2/2 | 4 | 45 | Filament | Rat | 45±7 (41±11) | 16±2 (21±2) | 82±6(62±11) | −37±2 (−20±2) |
| AG490 | DMTU (1/1) | 1/1 | 5 | −20 | Filament | Rat | 32±9 | 39±5 | 65±8 | 7±5 |
| Albumin | tPA (1/1) | 1/1 | 5 | 120 | Filament | Rat | 43±17(42±19) | 61±16 (39±14) | 18±18 (14±17) | 18±16 |
| Alcohol | Caffeine (2/2) | 2/2 | 5 | −75 | Clip/wire | Rat | −69±10 | 99±9 | 74±5 | 170±12 |
| Alpha –PBN | tPA (0/1) | 0/1 | 4 | 360 | Embolic | Rat | — | 30±9 | 6±9 | — |
| Ang-1 | Stem cells (0/1) | 0/1 | 3 | 360 | Filament | Rat | — | 1±5 | 38±5 | — |
| Anti-CD 18 | tPA (2/2) | 2/2 | 4 | 120 | Embolic | Rat | 26±12 (0±71) | 23±10 (0±24) | 30±12 (50±71) | −3±10 (0±24) |
| Atorvastatin | Hypothermia (3/3), tPA (1/1) | 4/4 | 5 | −10,740 | Filament, embolic | Rat | 36±3 (19±6) | 38±3 (30±6) | 63±3 (40±6) | 2±3 |
| Basic fibroblast growth factor (BFGF) | Citicholine (1/1), Heparin (1/1), z-DEVD.FMK (2/1), z-VAD.FMK (1/1) | 5/4 | 6 | 30 | Filament | Mice, rat | 3±6 (10±2) | 37±6 (47±2) | 41±6 (52±3) | 38±6 (35±3) |
| Batroxobin | Urokinase (2/2) | 2/2 | 2 | 120 | Filament | Rat | 50±15 (−3±50) | 61±8 (−17±39) | 27±15 (−55±52) | −69±9 (−8±27) |
| BB-823 | Sipatrigine (1/1) | 1/1 | 4 | −15 | Filament | Rat | 29±20 (24±24) | 6±18 (53±20) | 47±16 (25±23) | −23±18 (29±32) |
| BDNF | Hypothermia (1/1), Ox26 (2/2), stem cells (1/1) | 4/4 | 5 | 398 | Filament | Rat | 14±5 | 18±3 | 43±5 | 0±3 |
| Butoxamine | Clenbuterol (1/2) | 1/2 | 3 | −300 | Coagulation | Mice | −2±6 | −8±4 | −1±6 | −11±6 |
| Cadesartan | Pioglitazone (1/1), Rosuvastatin (2/2) | 3/3 | 6 | −1,960 | Filament | Rat | 61±4 (44±5) | 19±4 (9±6) | 54±5 (37±6) | −42±4 (−36±6) |
| Caffeine | Alcohol (2/2), CNS-1102 (0/1), MK801 (0/1), MgSO4 (0/1) | 2/5 | 5 | −180 | Clip/wire | Rat | −21±18 | 88±6 | 78±16 | 170±12 |
| Citicholine | BFGF (1/1), Lamotrigine (2/2), MK801 (2/2), Nimodipine (1/1), tPA (7/7), Urokinase (1/1) | 14/14 | 4 | 60 | Filament, embolic, clip/ligation | Rat | 22±3 (6±8) | 17±2 (1±6) | 45±3 (24±8) | −12±2 (−4±6) |
| Clenbuterol | Butoxamine (2/1), Memantine (9/9) Metoprolol (2/1), Propranolol (1/1) | 14/12 | 2 | −6 | Electrocoagulation | Mice | 8±1 | 7±1 | 17±1 | 1±1 |
| CNS-1102 | Caffeine (1/0) | 1/0 | 4 | 15 | Wire/clip | Rat | 42±22 | — | 92±17 | — |
| CP101,606-27 | tPA (2/1) | 2/1 | 4 | 120 | Embolic | Rat | 63±11 (17±8) | 15±10 (−11±12) | 57±11 (11±9) | −57±10 (−26±13) |
| Cycloheximide | Dextrorphan (1/1) | 1/1 | 2 | −18 | Clip/ligation | Rat | 52±13 | 34±16 | 87±11 | −18±16 |
| Cyclosporine-A | Methylprednisolone (2/2) | 2/2 | 5 | 180 | Filament | Rat | 2±9 (−2±3) | −4±7 (−1.3±5) | −1±8 (−3±5) | −7±7 (7±5) |
| DETA NONOate | Stem cells (2/2) | 2/2 | 6 | 1,440 | Filament | Rat, mice | 11±29 (13±31) | 6±16 (5±13) | 23±26 (42±30) | −4±16 (−7±13) |
| Dexamethasone | Mannitol (0/1) | 0/1 | 1 | 0 | Implanted occluder | Cat | — | −4±9 | −5±9 | — |
| Dexanabinol | Tempol (2/2) | 2/2 | 5 | 60 | Electrocoagulation | Rat | 54±12 (72±18) | −2±8 (29±15) | 47±11 (77±16) | −56±8 (−44±15) |
| Dextromethorphan | Tirilazad (1/1) | 1/1 | 7 | −15 | Filament | Rat | 45±14 | −36±12 | 12±16 | −80±12 |
| Dextrorphan | Cycloheximide (1/1) | 1/1 | 2 | −15 | Clip/ligation | Rat | 53±17 | 35±11 | 87±11 | −18±11 |
| Diaspirin crosslinked hemoglobin (DCLHb) | Lubeluzole (1/1) | 1/1 | 4 | 15 | Clip | Rat | 11±3 | 19±3 | 72±3 | 8±3 |
| Dizocilpine | Caffeine (1/1), Citicholine (2/2), EG761 (1/1), Hypothermia (1/1), Muscimol (2/2), NBQX (1/1), Nimodipine (1/0), RSR13 (2/1), tPA (1/1), zVAD-fmk (7/7) | 18/16 | 5 | 32 | Filament, clip/wire/ligation, embolic, electrocoagulation | Rat, mice, cat, gerbil | 21±4 (22±8) | 9±2 | 39±3 (56±8) | −15±2 |
| Dimethylthiourea | AG490 (1/1) | 1/1 | 5 | −60 | Filament | Rat | 26±8 | 33±5 | 65±8 | 7±5 |
| DtBHB | -NAME (1/1) | 1/1 | 2 | 240 | Filament | Rat | −4±24 | 49±24 | 45±26 | 53±24 |
| Edavarone | Normobaric hyperoxia (1/1), tPA (0/1) | 1/2 | 4 | 2 | Filament | Rat, mice | 13±16 (16±20) | 29±7 (8±17) | 45±17 (58±21) | 8±14 |
| EGb 761 | Dipyrone (2/2), FK506 (1/0), MgSO4 (1/0), MK801 (1/0) | 5/2 | 4 | −10,092 | Filament, ligation | Rat, gerbil | 18±5 | −3±5 | 17±4 | −14±5 |
| Eliprodil | tPA (1/1) | 1/1 | 4 | 10 | Embolic | Rat | 49±40 (48±18) | 31±22 (16±12) | 88±36 (70±18) | −18±22 (−32±12) |
| Enlimolab | tPA (0/1) | 0/1 | 4 | 240 | Embolic | Rat | — | 35±13 | 25±13 | — |
| EPC-K1 | Heparin (1/1) | 1/1 | 5 | 180 | Photochemical | Rabbit | 28±10 (16±13) | 72±9 (129±7) | 56±8 (69±14) | 44±10 (112±13) |
| Erythropoietin (EPO) | GCSF (1/1), IGF-1 (1/1), stem cells (1/1), tPA (3/3) | 6/6 | 5 | 585 | Filament, embolic, ligation/clip | Rat, mice | 5±7 (22±38) | 4±5 (2+29) | 14±7 (17±35) | −8±5 |
| Estrogen | Progesterone (1/1), tPA (0/1) | 1/2 | 5 | −10,080 | Filament | Rat | 63±26 | 64±12 | 67±26 | −17±21 |
| Ethyl pyruvate | Aspirin (4/4) | 4/4 | 5 | 413 | Filament | Rat | 71±11 (67±29) | 20±4 | 74±11 (78±29) | −55±4 |
| FeTMPyP | 1,5-Isoquinolinediol (1/1) | 1/1 | 6 | 115 | Filament | Rat | 54±5 (39±13) | 21±1 (−1±8) | 82±5 (59±12) | −33±1 (−41±8) |
| Fluosol | Risperidone (1/0) | 1/0 | 1 | 0 | Balloon occlusion | Cat | −171±64 | — | 6±16 | — |
| Fluoxetine | Risperidone (1/1) | 1/1 | 4 | −10,080 | Photochemical | Rat | −34±36 | −30±19 | −16±32 | 4±19 |
| Glucose | Insulin (0/1) | 0/1 | 3 | −50 | Electrocoagulation | Rat | − | −20±4 | −7±6 | — |
| Glyercol | NS-7 (1/1) | 1/1 | 5 | 5 | Electrocoagulation | Rat | 20±16 | −1±11 | 64±16 | −21±11 |
| Granulocyte colony stimulating factor | EPO (1/1), stem cells (0/1), tPA (1/1) | 2/3 | 5 | 750 | Embolic, clip/ligation, cauterization | Rat, mice | 55±5 | −4±4 | 63±5 | −33±22 |
| IGF-1 | EPO (1/1) | 1/1 | 7 | 60 | Filament | Mice | 22±20 | 23±11 | 55±17 | 1±11 |
| Insulin | Glucose (1/0), Hypothermia (1/1), MgCl2 (0/1), tPA (1/1) | 3/3 | 3 | −30 | Embolic, electrocoagulation | Rat | 15±4 | 11±3 | 15±4 | −12±5 |
| Isoflurane | z-IETD-fmk (1/1), zVAD-fmk (1/1) | 2/2 | 7 | 8 | Filament | Rat | 9±11 | 31±6 | 70±9 | 23±6 |
| Ketamine | Nicotinamide (1/1) | 1/1 | 3 | −10 | Filament | Rat | −4±8 | 68±12 | 63±5 | 72±12 |
| Lamotrimine | Citicholine (2/2) | 2/2 | 7 | 90 | Filament | Rat | 14±12 (25±10) | 5±6 (22±5) | 45±12 (24±9) | −10±6 (−1±5) |
| -Name | DtBHB (1/1) | 1/1 | 2 | 240 | Filament | Rat | −4±27 | 49±21 | 45±26 | 53±21 |
| Lubeluzole | DCLHb (1/1) | 1/1 | 4 | 15 | Clip | Rat | 53±3 | 61±3 | 72±3 | 8±3 |
| Magnesium chloride (MgCl2) | Insulin (1/0), Tirilazad (1/1) | 2/1 | 6 | −10 | Electrocoagulation, filament | Rat | 26±6 | 11±10 | 46±5 | −14±10 |
| Magnesium sulphate (MgSO4) | Caffeine (1/0), EGB761 (0/1), FK506 (1/1), Hypothermia (1/1), Mexiletine (1/1), Nicardipine (0/1) | 4/5 | 4 | 28 | Filament, ligation, wire/clip | Rat, gerbil, | 23±9 | 18±3 | 44±9 | −11±7 |
| Mannitol | Dexamethasone (1/1), Fluosol (1/1), Hypothermia (1/1), Tirilazad (1/1) | 4/4 | 4 | −1 | Filament, electrocoagulation, balloon occlusion | Rat, cat, rabbit | 11±4 (49±13) | 11±3 (21±9) | 40±4 (83±10) | −5±3 (−27±9) |
| MC-1 | tPA (1/1) | 1/1 | 3 | 60 | Embolic | Rat | 37±16 (17±58) | 3±11 (−17±48) | 41±14 (17±59) | −34±11 (−33±48) |
| Melatonin | 3-AB (1/1), Meloxicam (1/1), Nicotinamide (0/1), tPA (1/1) | 3/4 | 4 | 68 | Filament | Rat, mice | 45±8 (41±11) | 23±2 (21±2) | 60±8 (62±11) | −33±2 (−20±2) |
| Meloxicam | Melatonin (1/1) | 1/1 | 3 | 240 | Filament | Rat | 23±16 | 18±8 | 64±15 | −5±8 |
| Memantine | Clenbuterol (9/9) | 9/9 | 2 | −3 | Electrocoagulation | Mice | 11±2 | 16±2 | 19±2 | 5±2 |
| Methohexital | Hypothermia (1/1) | 1/1 | 5 | −30 | Filament | Rat | 32±25 (38±23) | −4±10 (−8±23) | 66±24 (69±22) | −36±10 (−50±23) |
| Methylprednisolone | Cyclosporine (2/2) | 2/2 | 5 | 180 | Filament | Rat | 3±9 (−2±3) | −4±6 (−1±5) | −1±8 (−3±5) | −7±6 (1±5) |
| Metoprolol | Clenbuterol (1/2) | 1/2 | 3 | −300 | Coagulation | Mice | 5±4 | 4±3 | 24±3 | 3±4 |
| Mexiletine | MgSO4 (1/1) | 1/1 | 4 | −60 | Filament | Rat | 36±17 | −17±19 | 16±16 | −53±19 |
| Minocycline | Hypothermia (2/2), tPA (1/2) | 3/4 | 4 | 120 | Filament, embolic | Rat | 29±6 (50±13) | 29±4 (34±7) | 49±6 (54±13) | −15±4 (−13±8) |
| Muscimol | Dizocilpine (2/2) | 2/2 | 6 | 123 | Embolic, filament | Rat | 7±32 | −16±14 | 30±30 | −12±14 |
| NBQX | Dizocilpine (1/1), tPA (2/2) | 3/3 | 3 | 0 | Embolic, electrocoagulation | Rat, mice | 23±4 | 7±3 | 29±5 | −17±3 |
| NEP 1-40 | Motor training (1/1) | 1/1 | 4 | 10,080 | ET-1 | Rat | −5±20 | 5±20 | −9±21 | 10±20 |
| Nerve growth factor (NGF) | Electroacupuncture (1/1) | 1/1 | 6 | 120 | Filament | Rat | 21±15 (23±11) | 34±9 (21±5) | 59±15 (44±11) | 13±9 |
| Nicardipine | Magnesium sulphate (1/0) | 1/0 | 3 | −120 | Electrocoagulation, filament | Rat | 58±10 | — | 79±9 | — |
| Nicotinamide | Ketamine (1/1), Melatonin (1/0) | 2/1 | 4 | 75 | Filament | Rat | 33±8 (44±13) | 67±9 | 67±4 (59±12) | 72±9 |
| Nimodipine | Citicholine (1/1), MK801 (0/1) | 1/2 | 4 | 45 | Clip/ligation | Rat, cat, rabbit | 34±19 | 18±5 | 60±19 | −17±9 |
| NS-7 | Glycerol (1/1) | 1/1 | 5 | 5 | Electrocoagulation | Rat | 65±16 | 44±11 | 64±16 | −21±11 |
| Ox26 | BDNF (2/2) | 2/2 | 5 | 60 | Filament | Rat | −1±16 | 46±7 | 54±8 | 47±7 |
| Pentasaccharide | tPA (1/1) | 1/1 | 7 | 10 | Embolic | Rat | 6±17 | −40±7 | 40±17 | −46±7 |
| Pioglitazone | Candesartan (1/1) | 1/1 | 5 | −7,200 | Filament | Rat | 43±9 (30±16) | −4±12 (5±18) | 39±11 (32±18) | −47±12 (−25±18) |
| Pravastatin | tPA (1/1) | 1/1 | 0 | −40,320 | Filament | Rat | 65±5 | 61±4 | 81±5 | −4±4 |
| Progesterone | Estrogen (1/1) | 1/1 | 5 | −30 | Filament | Rat | 21±31 | 4±12 | 67±26 | −17±12 |
| Propranolol | Clenbuterol (1/1) | 1/1 | 3 | −300 | Electrocoagulation | Mice | −2±6 | −18±5 | −12±6 | −16±5 |
| PS519 | tPA (3/2) | 3/2 | 5 | 240 | Embolic | Rat | 25±16 (44±17) | 37±9 (29±13) | 44±17 (46±16) | −2±9 (−27±13) |
| Qingpi | Hypothermia (1/1) | 1/1 | 3 | 120 | Filament | Rat | 41±28 (32±17) | 22±15 (12±13) | 65±22 (40±15) | −19±15 (−20±13) |
| Rapamycin | Tacrolimus (1/1) | 1/1 | 4 | −20 | Filament | Rat | 5±23 | −62±15 | −18±24 | −67±15 |
| Risperidone | Fluoxetine (1/1) | 1/1 | 4 | −10,080 | Photochemical | Rat | 14±31 | 18±27 | −16±32 | 4±27 |
| Rosiglitazone | tPA (0/2) | 0/2 | 3 | −20,160 | Embolic | Rat | — | 9±2 | 38±3 | — |
| Rosuvastatin | Candesartan (2/2), tPA (1/1) | 3/3 | 5 | −6,690 | Filament | Rat, mice | 36±5 (29±7) | −6±4 (−7±4) | 55±5 (38±7) | −43±4 (−36±4) |
| RSR13 | Dizocipline (1/2) | 1/2 | 6 | −30 | Filament | Rat | 15±16 | 19±6 | 72±10 | 6±9 |
| S-0139 | tPA (1/1) | 1/1 | 7 | 120 | Embolic | Rat | 19±2 (45±4) | 30±2 (48±3) | 28±2 (57±4) | 12±2 |
| Simvastatin | Stem cells (1/1) | 1/1 | 7 | 1,440 | Not stated | Rat | 16±26 (15±17) | 13±22 (36±17) | 38±26 (38±17) | −3±22 |
| Sipatrimine (BW619C89) | BB-823 (1/1) | 1/1 | 4 | −15 | Filament | Rat | 41±18 (−29±21) | 18±19 (0.4±23) | 47±16 (25±23) | −23±19 (29±23) |
| Tacrolimus (FK506) | EGB761 (0/1), Hypothermia (1/1), MgSO4 (1/1), Rapamycine (1/1), tPA (5/5) | 8/9 | 4 | 83 | Filament, ligation, photochemical | Rat, gerbil | 20±4 | 13±3 | 30±4 | −7±3 |
| Tempol | Dexanabinol (2/2) | 2/2 | 5 | 60 | Electrocoagulation | Rat | 51±10 (51±19) | −9±8 (6±11) | 47±11 (77±16) | −54±8 (−42±11) |
| Tirilazad | Dextromethorphan (1/1), Mannitol (1/1), MgCl2 (1/1), tPA (2/2) | 5/5 | 6 | 22 | Electrocoagulation, embolic, filament | Rat, rabbit | 44±6 | 38±4 | 48±6 | −6±4 |
| Topiramate | Urokinase (2/2) | 2/2 | 3 | 120 | Embolic | Rat | 63±10 (40±10) | 34±7 (7±14) | 82±10 (45+14) | −29±7 (−39±14) |
| TS-011 | tPA (1/1) | 1/1 | 3 | 1 | Embolic | Primate | 26±19 (10±7) | 54±16 (12±6) | 38±22 (16±8) | 28±16 (2±6) |
| U-101033E | U-74389G (1/1) | 1/1 | 6 | −15 | Filament | Rat | 53±17 | 29±13 | 54±18 | −24±13 |
| U-74389G | U-101033E (1/1) | 1/1 | 6 | −15 | Filament | Rat | 25±18 | 1±11 | 54±18 | −24±11 |
| UK-279, 276 | tPA (2/2) | 2/2 | 7 | 180 | Embolic | Rat | 3±11 (7±9) | 44±6 (9±7) | 57±9 (22±8) | 41±6 (1±7) |
| Vapiprost | SUN9216 (0/1) | 0/1 | 3 | 60 | Photochemical | Rat | — | 14±18 | 11±17 | — |
| VEGF | Stem cells (0/1) | 0/1 | 2 | 360 | Filament | Rat | — | −42±5 | −5±6 | — |
| Velcade | tPA (1/1) | 1/1 | 6 | 120 | Embolic | Rat | 33±20 (19±12) | 40±15 (34±12) | 55±19 (36±13) | 7±15 (15±12) |
| Xg-102 | Hyperbaric oxygen (1/1), tPA (1/1) | 2/2 | 7 | 105 | Filament | Rat, mice | 46±6 (38±9) | 49±5 | 76±6 (42±14) | −6±5 (−33±15) |
| YM872 | tPA (1/1) | 1/1 | 3 | 120 | Embolic | Rat | 46±18 | 38±9 | 82±13 | −8±9 |
| z-DEVD.FMK | BFGF (2/2), Dizocipline (4/4) | 6/6 | 5 | 33 | Filament | Mice | 14±7 (3±3) | 43±5 | 45±6 (54±4) | 25±4 (44±2) |
| z-IETD-fmk | z-IETD-fmk (1/1) | 1/1 | 6 | −30 | Filament | Rat | 35±14 | 54±11 | 67±13 | 20±11 |
| zVAD-fmk | 3-AB (1/1), BFGF (1/1), Dizocilpine (3/3), Isoflurane (1/1) | 6/6 | 5 | −3 | Filament | Rat, mice | 31±7 (10±4) | 42±3 | 70±5 (48±7) | 3±4 (19±7) |
| Reperfusion therapy | 100/92 | 4 | 136 (120) | 15±1 (6±2) | 13±1 (15±1) | 35±1 (37±2) | −6±1 (10±2) | |||
| Abciximab | tPA (4/4) | 4/4 | 6 | 210 | Embolic | Rat | 36±7 (15±5) | 18±4 (23±3) | 47±7 (28±5) | −10±4 (18±3) |
| Annexin (rA2) | tPA (1/1) | 1/1 | 8 | 120 | Embolic | Rat | 2±14 | 45±13 | 49±13 | 43±13 |
| Argatroban | Hypothermia, tPA | 3/3 | 5 | 162 | Clip, embolic, filament | Rat | 5±14 | 31±8 | 35±15 | 20±8 |
| Aspirin | Ethyl pyruvate (4/4), Ticlopidine (1/1) | 5/5 | 5 | 318 | ADP ephinephrine, filament | Rat, guinea pig | 53±12 (2±13) | 3±2 (9±10) | 72±11 (37±14) | −48±2 (−10±10) |
| Cilostazol | Normobaric hyperoxia (2/2) | 2/2 | 6 | 1 | Filament | Mice | 18±13 | 33±10 | 50±12 | 16±10 |
| Clopidogrel | tPA (1/1) | 1/1 | 4 | 5 | Embolic | Rat | −2±5 (43±25) | 2±4 (−17±18) | −1±5 (30±22) | 4±4 (−60±18) |
| Dipyrone | EGB 761 (2/2) | 2/2 | 3 | −30 | Filament | Rat | 14±5 | 1±6 | 6±5 | −14±6 |
| Heparin | BFGF (0/1), EPC-K1 (1//1), Ozagrel (1/1), tPA (2/3) | 4/5 | 5 | 91 | Embolic, filament, photochemical | Rat, rabbit | −12±10 (−60±12) | 20±5 (52±14) | 55±7 (70±9) | 19±5 (112±9) |
| Melagatran | tPA (2/2) | 2/2 | 2 | 180 | Embolic | Rat | 22±10 | 17±8 | 38±11 | −8±8 |
| Ozagrel | Heparin (1/1) | 1/1 | 3 | 5 | Photochemical | Rat | 30±13 | 48±21 | 40±20 | 17±21 |
| SUN9216 | Vapiprost (1/0) | 1/0 | 3 | 60 | Photochemical | Rat | −3±7 | — | 11±17 | — |
| Ticlopidine | Aspirin (1/1) | 1/1 | 3 | −180 | ADP ephinephrine | Guinea pig | 47±37 (20±17) | 26±30 (33±15) | 47±39 (27±16) | −21±30 (13±15) |
| Tissue plasminogen activiator (tPA) | Abciximab (4/4), Albumin (1/1), Alpha-PBN (1/0), Annexin (1/1), Anti-CD18 (2/2), Aortic occlusion (1/1), Argatroban (2/2), Atorvastatin (1/1), Citicholine (7/7), Clopidogrel (1/1) , CP101,606-27 (1/2), Dizocilpine (4/1), Edaravone (1/0), Eliprodil (1/1), Enlimomab (1/0), EPO (3/3), Estrogen (1/0), GCSF (1/1), Heparin (2/2), Hypothermia (3/3), Insulin (1/1) , MC-1 (1/1), Melagatran (2/2), Melatonin (1/1), Minocycline (2/1), NBQX (1/2), Normobaric oxygen (2/2), Pentasaccharide (1/1), Pravastatin (1/1), PS519 (2/3), Rosiglitazone (2/0), Rosuvastatin (1/1), S-0139 (1/1), Tacrolimus (5/5), Tirilizad (2/2), TS-011 (1/1), UK-279,276 (2/2), Velcade (1/1), XG-102 (1/1), YM872 (1/1) | 68/63 | 4 | 138 | Filament, embolic, photochemical | Rat, mice, primate, rabbit | 15±1 (6±2) | 13±1 (12±2) | 36±1 (39±2) | −3±1 (5±2) |
| Urokinase | Batroxobin (2/2), Citicholine (1/1), Topiramate (2/2) | 5/5 | 3 | 120 | Filament, embolic | Rat | 26±7 (34±13) | 7±5 (−5±12) | 65±7 (38±14) | 19±5 (−32±13) |
| Nonpharmacological neuroprotection | 30/29 | 6 | 733 (60) | 24±2 (28±3) | 21±1 (19±2) | 50±2 (53±3) | −6±1 (−6±4) | |||
| Electroacupunture | NGF (1/1) | 1/1 | 6 | 120 | Filament | Rat | 25±15 (18±5) | 38±9 (21±5) | 59±15 (44±6) | 13±9 (3±5) |
| Exercise/environmental enrichment | NEP 1-40 (1/1), stem cells (1/1) | 2/2 | 5 | 10,080 | ET-1, cauterization | Rat | −16±6 | −3±3 | −14±6 | 13±3 |
| Hyperbaric oxygen | XG-102 (1/1) | 1/1 | 8 | 190 | Filament | Rat | 63±7 (37±9) | 34±5 | 76±6 (42±14) | −29±5 |
| Hypothermia | Argatroban (1/1), Atorvastatin (3/3), BDNF (1/1), Craniectomy (3/2), Insulin (1/1), MgSO4 (1/1), Mannitol (2/2), Methohexital (1/1), Minocycline (2/2), MK801 (1/1), Qingpi (1/1), Tacrolimus (1/1), tPA (3/3) | 21/20 | 4 | 68 | Filament, electrocoagulation, embolic | Rat | 28±2 (32±5) | 24±1 | 55±2 (59±5) | −10±1 (−10±4) |
| Normobaric oxygen | Cilostazol, Edaravone, tPA | 5/5 | 7 | 19 | Embolic, filament | Rat, mice | 15±4 (50±21) | 26±4 | 43±4 (58±21) | 10±4 |
| Surgical therapy | 3/4 | 6 | 230 (60) | 43±5 (24±12) | 40±3 (33±2) | 63±6 (36±12) | 1±5 (9±6) | |||
| Aortic occlusion | tPA (1/1) | 1/1 | 6 | 90 | Embolic | Rat | 43±20 | 21±13 | 73±17 | −21±13 |
| Craniectomy | Hypothermia (2/3) | 2/3 | 6 | 300 | Filament | Rat | 43±7 (24±12) | 42±4 (33±10) | 61±6 (36±12) | 4±5 (9±9) |
| Cellular therapy | 9/6 | 5 | 3,120 (1,440) | 22±4 (12±15) | 4±3 (22±2) | 10±3 (36±14) | 8±3 (1±23) | |||
| Stem cells | Ang-1 (1/0), BDNF (1/1), DETA-NONOate (2/2), EPO (1/1), environmental enrichment (1/1), GCSF (1/0), Simvastatin (1/1), VEGF (1/0) | 9/6 | 5 | 3,120 | Filament, cauterization | Rat, mice | 22±3 (15±14) | 4±3 (22±9) | 10±3 (36±12) | 8±3 (1±9) |
Tested with=names of the other therapies with which the therapy is tested in combination, together with the number of times it has been tested with the other therapy. NP/NA=number of experiments the therapy is tested individually as the primary therapy/number of experiments in which it is tested as the additional therapy. Q=average quality across experiments. Time=mean (median) time of delivery of the therapy postocclusion, measured in minutes. Stroke models=experimental models in which the therapy was tested. Species=species in which the therapy was tested. Therapeutic efficacy=effect size and standard error of the therapeutic efficacy derived from random effects meta-analyses. Alone=therapeutic efficacy of the treatment tested individually versus control. Additive=therapeutic efficacy of the treatment derived from the therapy in combination with another therapy versus the individual effect of the other therapy. Combined=therapeutic efficacy of the treatment in combination versus control. Synergy=therapeutic ‘Gestalt,' that is, the extent to which efficacy in combination exceeds efficacy of the constituents. Please refer to the Supplementary Materials for the references for each therapy.
Both fixed effect and random effect meta-analysis were undertaken, and due to heterogeneity, results from the conservative random effects model are presented (DerSimonian and Laird, 1986; Macleod et al, 2004, 2005a, 2005b). Overall estimates of effect were calculated for (1) single treatment therapy; (2) combination therapy; and (3) synergistic efficacy. The effect size for the combination does not necessarily equal the sum of the single and additive effects, because combination treatment effect was calculated for each experiment and then a summary estimate derived using random effects meta-analysis, an approach that is more sophisticated than a simple averaging.
Data were tested for publication bias using Egger's test, a statistical analog of the funnel plot (Metabias, Stata v.10, Statacorp, TX, USA) (Egger et al, 1997); significant results (P<0.05) suggest bias. Bias-corrected estimates were calculated with the trim and fill technique (STATA, v.10), which accounts for unpublished data by imputing the most likely values for missing data (Duval and Tweedie, 2000).
Ceiling Effect
To investigate overall limits to acute stroke therapy (ceiling effects), meta-analysis results were partitioned into 10% bands on the basis of the level of efficacy of a single treatment when tested individually (designated here as the ‘primary treatment'). Where both treatments were tested individually, both could alternatively be considered as the primary treatment for the purpose of this analysis, with additional therapy building on this base. Meta-regression (STATA v.10) (Thompson and Sharp, 1999; STB-42, 1998) was used to test whether combination or synergistic efficacy were influenced by the effectiveness of the primary treatment. Note, the designation of primary and additional therapies was created for the purpose of this analysis and was not a classification described within individual papers themselves.
Tissue Plasminogen Activator Thrombolysis
To determine whether combination therapies extended the time window for efficacy, we examined tPA thrombolysis in greater detail. We carried out an unweighted meta-regression; treatment effects for thrombolysis alone and in combination were regressed against time of drug delivery (measured relative to stroke onset) with treatment as the within-subject factor and time of delivery as a covariate (SPSS v18, PASW Statistics, Chicago, IL, USA). We also conducted a stratified analysis of the relationship between tPA efficacy and the model of stroke (embolic, filament, photochemical) and the timing of the other treatment (pre, post, or simultaneously with tPA).
Efficacy by Treatment and Class
Data were partitioned to obtain therapeutic efficacy for individual treatments. For each therapy, the following information was tabulated: treatments with which it had been combined; extent of testing individually as the primary therapy (number of experimental contrasts (NP)), or as the additional therapy (NA); stroke models and species in which it was tested; mean time of delivery postocclusion (minutes) and experimental quality (Q).
Treatments were categorized by treatment class: (1) pharmacological neuroprotection (e.g. citicholine); (2) reperfusion therapy (e.g. tPA); (3) nonpharmacological neuroprotection (e.g. hypothermia); (4) surgical therapy (e.g. craniectomy); or (5) cell-based therapies (e.g. stem cells) (see Table 1 for the categorization of individual therapies). We used partitioned analyses to investigate the association between outcome and the treatment class of the primary treatment or the combination. Significance was tested using meta-regression (STATA v.10) (Thompson and Sharp, 1999) (STB-42, 1998).
Results
Characteristics of Experiments
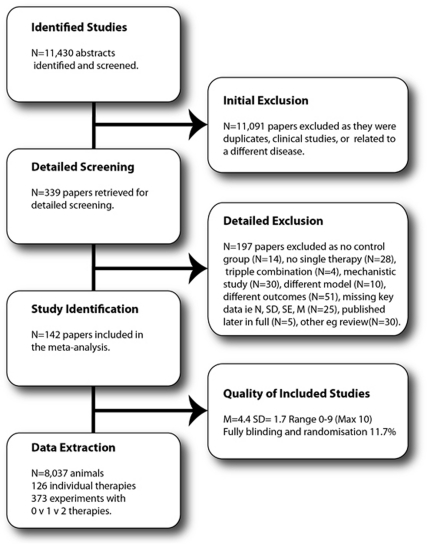
The search identified 11,430 publications of which 142 met the inclusion criteria (Figure 1). From these publications, we extracted 373 three-way comparisons of a control group (1,811 animals), a single treatment (3,888 animals) and a combination of that single treatment with an additional therapy (2,268 animals). In total, data from 8,037 animals were included, with a median number of animals per treatment arm of 10 (Control IQR (interquartile range): 8 to 14; Single treatment IQR: 7 to 13; Combination treatment IQR: 7 to 13).
Figure 1.
Flowchart of inclusions and exclusions in the systematic review.
Experiments were predominantly undertaken in young (98%), male animals (89% male, 2% female, or both, 9% unstated) without comorbidities (97% healthy, 3% hypertensive). The following papers used older animals (Toung et al, 2004; Zhao et al, 2005; Zhang et al, 2010; Fang et al, 2010), female animals (Toung et al, 2004; Carter et al, 1992; Liu et al, 2010), or hypertensive animals (Teichner et al, 2003; Bochelen et al, 1999; Asahi et al, 2000; Fujiwara et al, 2009; Maeda et al, 2009; Murata et al, 2008). Most experiments were conducted in rats (76% of experiments) or mice (18%), with some studies in rabbits (3%), gerbils (1.3%), guinea pigs (0.5%), cats (0.8%), or primates (0.5%). Of note, stroke was induced in gerbils using an uncommon (for gerbils) distal ischemia model rather than the standard model of global ischemia typically employed in this species. Quality ranged from 0 to 9 with a median of 4 (M=4.4, s.d.=1.7) with 52% of experiments reporting randomization, 43% the blinded assessment of outcome, 19% the blinded induction of ischemia, and 11% reporting all three measures.
Characteristics of Treatments
Overall, 126 single interventions were reported, with 118 therapies tested alone and in combination and 8 tested only in combination (Table 1). Most individual therapies were not tested extensively (median of one experiment as the first treatment, mean 2.9), with the exception of tPA (n=68), hypothermia (n=21), dizocilipine (n=18), citicholine (n=14), and clenbuterol (n=14). The estimates of effect size for individual treatments are given in Table 1, together with the circumstances under which they were tested. Treatments are listed alphabetically, under each therapeutic class, with the summary estimate for each class at the top of each section. References are provided in Supplementary Table 2.
Sensitivity Analysis
Substantial heterogeneity exists within the data, with I2=85% for single and combination treatment effects and I2=96% for synergistic effects. Sensitivity analysis demonstrated that Cochrane's Q could be reduced by 33% by removing six studies, but this had no effect on estimates of effect, and hence the studies were retained. For single and combination treatment data, treatment class and method of occlusion accounted for a significant proportion of the observed heterogeneity as determined by Cochrane's Q but had no effect on I2, which is less sensitive to sample size but does not reflect the original Q (Higgins et al, 2003).
Meta-Analysis of Therapeutic Effect
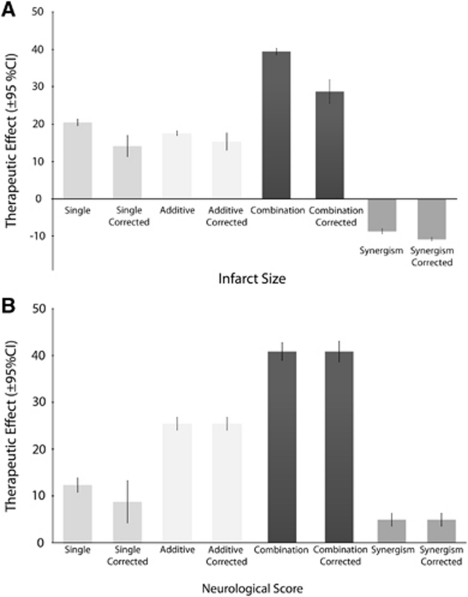
Estimates of therapeutic efficacy are shown in Figure 2A, infarct size and 2B, neurological score), together with estimates corrected for potential publication bias. Overall, administration of a single treatment improved infarct size by 20.5% (95% confidence interval (CI): 19.6 to 21.3, 373 experiments) and neurological score by 12.3% (95% CI: 10.86 to 13.8, n=108). Combination therapy improved infarct size by 39.4% (95% CI: 38.6 to 40.2, n=373) and neurological score by 40.9% (95% CI: 39.0% to 42.8%, n=108) compared with the untreated control. The addition of the second therapy improved outcome by a further 17.6% (95% CI: 16.9 to 18.1, n=373) for infarct size and by 25.5% (95% CI: 24.1 to 26.8, n=108) for neurological score. For infarct size, two therapies in combination were 8.7% (95% CI: −9.3 to −8.0, n=336) less effective than the sum of efficacies when given alone but for neurological outcome there was significant synergism, with efficacy 4.9% (95% CI: 3.6 to 6.3, n=100) higher than the sum of individual efficacies in monotherapy.
Figure 2.
Overall estimates of treatment efficacy: raw and adjusted for potential bias. Estimates for the single treatment effect, the additive effect, and the combination treatment effect derived from DerSimonian and Laird meta-analysis and after correcting for potential publication bias using the Trim and Fill technique (A) infarct efficacy: n=373 experiments using 8,037 animals; (B) neurological score efficacy: n=108 experiments using 2,208 animals). Higher values reflect greater therapeutic effect. Error bars represent 95% confidence intervals. Refer to text for values.
Egger's test found evidence for publication bias in the estimation of infarct effect size in single treatments (P=0.01) and combination treatments (P=0.01) and in the estimation of neurobehavioral effect size for single (P=0.002) treatments. After correcting for possible publication bias, the effects sizes for infarct volume were reduced to 14.1% (from 20.5%) for single therapies and to 28.7% from 39.4% for combination therapies. The effect size for neurological score was reduced from 12.3% to 8.7% for single therapies. There was a moderate correlation between infarct size and neurological score effect size, with changes in infarct size explaining 33% (r=0.58) of the change in neurological score in the single treatment groups and 25% (r=0.50) of neurological score in combination therapy groups.
Ceiling Effect
Combination efficacy increased as primary efficacy increased and plateaued when primary efficacy reached around 60% protection (Figure 3A, infarct size). When the primary efficacy exceeded 80%, there was little benefit from the addition of a second therapy, although CIs were wide for neurological score estimates (Figure 3B, neurological score). Synergistic effects were most pronounced when the efficacy of individual treatments was <30%.
Figure 3.
Ceiling effect to level of protection evidenced by combination therapy. (A) The ceiling for infarct size efficacy: n=373 experiments using 8,037 animals. (B) The ceiling for neurological score efficacy: n=108 experiments using 2,208 animals). Data were partitioned on the basis of primary therapeutic efficacy (bands of 10%, depicted on the horizontal axis). At each level of efficacy of the first (primary) treatment, estimates of combination efficacy, additive efficacy, and synergism were calculated using random effects meta-analyses (effect size±standard error, depicted on the vertical axis). As the overall efficacy of the primary therapy increased, the efficacy of the combination therapy increased, plateauing as the primary therapy reached 60% to 70% protection. With increasing protection from the primary therapy, the marginal benefits from the second therapy tended to decrease. Overall, additional efficacy disappeared beyond 80% protection from the primary therapy. Synergistic benefits disappeared earlier, when primary efficacy rose above 20%.
For infarct size, there was a significant association between efficacy in primary therapy on the one hand, and efficacy in combination (β=0.50, P<0.001), synergistic efficacy (β=−0.92, P<0.001), and additive efficacy (β=−0.44, P<0.001) on the other hand. For neurological scores, there was a significant association between efficacy in primary therapy and efficacy in combination (β=0.47, P<0.001), synergistic efficacy (β=−0.91, P<0.001), and additive efficacy (β=−0.49, P<0.001).
Tissue Plasminogen Activator Thrombolysis in Combination
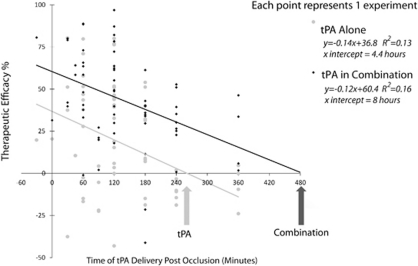
Tissue plasminogen activator was tested individually in 68 experiments and as the additional therapy in the combination in 63 experiments. When given alone, the ability of tPA to reduce infarct size was greatest in the embolic model, but when given in combination efficacy was greatest in the filament occlusion model (embolic model (n=54): alone 16.8 (95% CI: 14.8 to 19.0) versus combination 35.3 (95% CI: 33.3 to 37.2); filament model (n=9): alone 0.2 (95% CI: −6.4 to 6.9) versus combination 52.0 (95% CI: 45.4 to 58.7); photochemical model (n=5): alone 11.8 (95% CI: 1.7 to 21.8) versus combination 35.3 (95% CI: 14.1 to 33.7). The superior results for tPA in combination in the filament model may be due to tPA being combined with more effective therapies in the filament model, or because tPA started with such a low base in filament models that combination results reflect regression toward the mean, or alternatively, due to ceiling effects in the embolic model preventing recovery above a certain level.
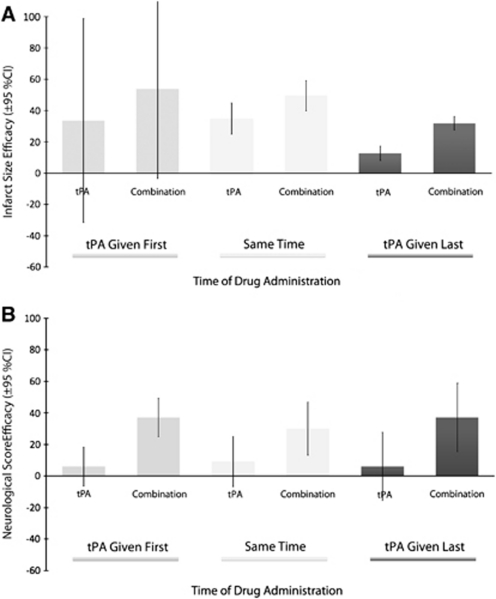
The effect of thrombolysis on infarct size was significantly related to treatment time (F(1,62)=13.1, P=0.001, partial η2=0.2), with lower estimates of efficacy seen at longer delays to treatment. Adding a second therapy led to a statistically significant improvement in outcome (F(1,62)=15.9, P<0.001, partial η2=0.2). Based on extrapolation of resulting regression curves, the point of no effect in animal models is reached for tPA alone at 4 hours 20 minutes postocclusion and for tPA in combination at 8 hours postocclusion (Figure 4). There was no interaction between therapy (single or combination) and treatment time (F(1,62)=0.2, P=0.6, partial η2=0.003), with efficacy declining with treatment delays for both single and combination therapy. Whether given before or at the time of thrombolysis, a second therapy increased efficacy (Figure 5), and this effect was largest with pretreatment.
Figure 4.
Time window for thrombolysis alone and in combination. Results from regression analysis of therapeutic efficacy of tissue plasminogen activator (tPA) thrombolysis alone and in combination with a second treatment. Therapeutic efficacy (vertical axis) is measured as the effect on infarct size with higher values indicating a better outcome in the treatment group compared with the control condition. The horizontal axis depicts the time of tPA treatment measured in minutes postocclusion (note, combination treatment time is graphed as timing of tPA in the combination, while the second treatment may have been given before or at the same time as tPA (see Figure 5 for mean differences for the second therapy). Each point represents the result from a single experiment and the lines represent the linear regression of tPA alone or in combination. With increasing delays between the time of treatment with tPA thrombolysis and the occlusion, the estimate of efficacy declines. Extrapolating from the regression curve, the predicted time of no effect for tPA is combination is extended from 4.4 to 8 hours.
Figure 5.
Thrombolysis in combination: relative timing of treatments. Effects sizes (with 95% confidence intervals) for tissue plasminogen activator (tPA) alone and in combination with a second treatment. Results are portioned on the basis of whether tPA was given first (n=23), concurrently (n=39), or after the additional treatment (n=2). (A) Infarct size efficacy (N=68 experiments using 1,515 animals). (B) Neurological score efficacy (N=20 experiments using 441 animals). Overall, efficacy is raised across all timing protocols when tPA is given in combination.
Efficacy by Class
The most common combination therapies reported were the use of two pharmacological neuroprotectants (n=152 experiments), followed by pharmacological neuroprotectants with reperfusion therapies (n=116), and two reperfusion therapies (n=29). The most effective combinations for reducing infarct size were surgery with reperfusion (effect size=73.1, 95% CI: 49.1 to 97.0, n=2), surgery with nonpharmacological (e.g. hypothermic) neuroprotection (56.8, 95% CI: 48.8 to 64.8, n=5), and nonpharmacological with pharmacological neuroprotection (56.5, 95% CI: 54.0 to 59.0, n=38). There were no significant differences between treatment classes for the estimated combination efficacy, but some variation in synergistic efficacy was attributable to the class of combination (β=−1.27, P=0.03).
The most effective combinations for improving neurological score were nonpharmacological with pharmacological neuroprotection (effect size=56.4, 95% CI: 51.2 to 61.1, n=18), followed by pharmacological neuroprotection with reperfusion (41.1, 95% CI: 37.7 to 44.5, n=38). There were no significant differences between the treatment classes for estimates of combination, additive or synergistic efficacy.
Discussion
The absence of a single magic bullet for stroke has encouraged exploration of combination therapy. We found 126 treatments that have been tested in combination in 373 experimental comparisons of control, single, and dual treatment conditions using 8,037 animals. On average, one treatment improved infarct size (or neurological score) by 20% (or 12%) compared with control, and the inclusion of a second intervention improved efficacy by an additional 18% (or 26%). The addition of a second therapy to thrombolysis may extend the time window from 4 hours 20 minutes to as much as 8 hours in animal models of stroke, although experiments testing time windows beyond 6 hours are needed to confirm this prediction.
As the efficacy of the primary therapy increases, the marginal benefits gained from the addition of a second therapy falls, and at higher levels of primary protection, negative effects may be encountered with use of a second therapy. Combination therapy appears to plateau when the efficacy of the primary therapy reaches 60%. These are of course global estimates and at higher efficacies, the small number of findings and wide CIs may mask important effects for particular combinations. For instance, in individual experiments, tPA was highly effective when combined with other treatments, notably: eliprodil (Lekieffre et al, 1997), hypothermia (Meden et al, 1994), dizocilpine (Sereghy et al, 1993), YM872 (Suzuki et al, 2003), and heparin (Carter et al, 1992). Further, the positive relationship between the efficacy of the primary and combination treatments and the inverse relationship between additive and combination efficacy will also reflect regression toward the mean. However, the importance of the efficacy of the first therapy in the overall therapeutic effect also suggests the need to build combination therapy on a solid therapeutic foundation.
The results also have implications for the design and interpretation of animal models. Surgical therapy with craniectomy—a treatment of most benefit to patients with malignant infarct rather than small strokes—was remarkably successful in reducing brain damage in animals, although the number of experiments was small. Further, animal studies typically use anesthetics. The substantial additional efficacy brought by anesthetic agents (e.g., ketamine and isoflurane) raises questions about the importance of this confounding factor—common to most animal studies but absent from clinical stroke—and also, about whether this protective mechanism may be exploited clinically to reduce brain damage.
Our analysis was observational rather than experimental and should therefore be viewed as hypothesis generating (Thompson and Higgins, 2002). However, we reduced the possibility of multiple ‘significant' findings by prespecifying analyses (Alder et al, 2006; Thompson and Higgins, 2002). Results can be swayed by outlying studies, but we conducted sensitivity analyses with these excluded and our conclusions were not substantially different.
Owing to heterogeneity in the data, the scope of the paper was restricted in terms of end points; however, end points other than neurological score and infarct volume may be relevant to the treatment under investigation and to the external validity of animal models. In a nod to heterogeneity, random effects analyses were performed and individual treatments were listed so that readers may make a more detailed study of treatments of interest. Analysis of specific factors affecting ceiling effects might provide greater insight into the relationship between the combination therapies and potential ceiling effects. This analysis is currently being undertaken.
Publication bias clouds the interpretation of meta-analyses (Sena et al, 2010). Here, estimates of efficacy on infarct were revised downward by 10.7% to 28.7%, although neurological score effects remained the same. Techniques to correct for bias are not without critics, and there are many reasons for funnel plot asymmetry other than publication bias (e.g., poor methodological quality of smaller studies, true heterogeneity, other selection biases, chance) (Higgins and Green, 2006). Nevertheless, the similarity of the bias-adjusted estimate (28.7%) to the overall effect found in a broad sweep of neuroprotectives (O'Collins et al, 2006) provides further evidence that there may be a substantial positive ‘offset' in preclinical neuroprotection publications.
Because this meta-analysis is not based on direct comparisons of different combination treatments under identical conditions and because the range of testing is likely to be low (O'Collins et al, 2009), it is not possible to recommend one particular approach to combination therapy. Such considerations are further constrained by the impact of additional therapies given in clinical trials as part of standard care (Lees, 2002), and in animal experiments as part of periprocedural treatments such as anesthetics (Danton and Dietrich, 2004).
Combining data are important if we are to critically examine not just combination therapy, but the validity and utility of animal models themselves. However, as demonstrated by the NXY059 animal data (Macleod et al, 2008; Savitz, 2007), careful analysis of individual experiments is critical. Both approaches can coexist and both approaches are important.
Conclusion
Combining two treatments brings additional benefits for the treatment of acute ischemic stroke in animals, although findings are constrained by potential publication bias and heterogeneity. We have yet to discover a magic bullet for acute stroke, but there is room for some optimism in the development of combination therapy as a viable therapeutic strategy.
Acknowledgments
The authors extend their thanks and appreciation to the following researchers: Ana Antonic and Candace Loy for ordering several papers used in this analysis; Dr Emily Sena for her advice on STATA syntax; Dr Leonid Churilov for his comments on adjusted sample sizes; Dr Neil Spratt, Dr Peter Batchelor, and Dennis Young for their comments on combination neuroprotection.
DWH, GAD, and MRM have been the recipients of industry and government awards for research into neuroprotection. VOC was the recipient of an APA scholarship. While no author has been involved in studies included in this paper, they have an interest in developing effective combination therapy for acute ischemic stroke.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Adams HP, Adams RJ, Brott T, Del Zoppo GJ, Furlan AJ, Goldstein LB, Grubb RL, Higashida R, Kidwell CS, Kwiatkowski TG, Marler JR, Hademenos GJ. Guidelines for the early management of patients with ischemic stroke. A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–1108. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Alder N, Fenty J, Warren F, Sutton AJ, Rushton L, Jones DR, Abrams KR. Meta-analysis of mortality and cancer incidence among workers in the synthetic rubber-producing industry. Am J Epidemiol. 2006;164:405–420. doi: 10.1093/aje/kwj252. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:452–457. doi: 10.1097/00004647-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Bochelen D, Rudin M, Sauter A. Calcineurin inhibitors FK506 and SDZ ASM 981 alleviate the outcome of focal cerebral ischemic/reperfusion injury. J Pharmacol Exp Ther. 1999;288:653–659. [PubMed] [Google Scholar]
- Brimble MA, Levi MS. A review of agents patented for their neuroprotective properties. Recent Patents on CNS Drug Discovery. 2006;1:139–146. doi: 10.2174/157488906777452758. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Cruickshank JK, Dominiczak AF, MacGregor GA, Poulter NR, Russell GI, Thom S, Williams B. Better blood pressure control: how to combine drugs. J Human Hypertension. 2003;17:81–86. doi: 10.1038/sj.jhh.1001511. [DOI] [PubMed] [Google Scholar]
- Byrne BE. Drug interactions: a review and update. Endodontic Topics. 2003;4:9–21. [PubMed] [Google Scholar]
- Carter LP, Guthkelch AN, Orozco J, Temeltas O. Influence of tissue plasminogen-activator and heparin on cerebral-ischemia in a rabbit model. Stroke. 1992;23:883–888. doi: 10.1161/01.str.23.6.883. [DOI] [PubMed] [Google Scholar]
- Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn BW. Advances in the combination of radiation therapy and chemotherapy against cancer. Drug News Perspect. 2004;17:469–475. [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. The search for neuroprotective strategies in stroke. AJNR Am J Neuroradiol. 2004;25:181–194. [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res ed) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Ad Hoc Consensus Group: Neuroprotection as initial therapy in acute stroke. Third Report of an Ad Hoc Consensus Group Meeting Cerebrovasc Dis. 1998. pp. 59–72. [DOI] [PubMed]
- Fang PC, Barbay S, Plautz EJ, Hoover E, Strittmatter SM, Nudo RJ. Combination of NEP 1–40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544–549. doi: 10.1161/STROKEAHA.109.572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003;34:1539–1546. doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- Fisher M, Albers GW, Donnan GA, Furlan AJ, Grotta JC, Kidwell CS, Sacco RL, Wechsler LR. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry roundtable. Stroke. 2005;36:1808–1813. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- Fisher M, Hanley DF, Howard G, Jauch EC, Warach S. Recommendations from the STAIR V meeting on acute stroke trials, technology and outcomes. Stroke. 2007;38:245–248. doi: 10.1161/01.STR.0000255951.37434.aa. [DOI] [PubMed] [Google Scholar]
- Fujiwara N, Murata Y, Arai K, Egi Y, Lu J, Wu O, Singhal AB, Lo EH. Combination therapy with normobaric oxygen (NBO) plus thrombolysis in experimental ischemic stroke. BMC Neurosci. 2009;10:79. doi: 10.1186/1471-2202-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S.2006(eds).Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006] Chichester, UK: John Wiley & Sons Ltd [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR. Management of acute stroke. Lancet Neurol. 2002;1:41–50. doi: 10.1016/s1474-4422(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Lekieffre D, Benavides J, Scatton B, Nowicki JP. Neuroprotection afforded by a combination of eliprodil and a thrombolytic agent, rt-PA, in a rat thromboembolic stroke model. Brain Res. 1997;776:88–95. doi: 10.1016/s0006-8993(97)00992-x. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu Q, He SQ, Simpkins JW, Yang SH. Combination therapy of 17 beta-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. J Pharmacol Exp Ther. 2010;332:1006–1012. doi: 10.1124/jpet.109.160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res. 2005a;38:35–41. doi: 10.1111/j.1600-079X.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab. 2005b;25:713–721. doi: 10.1038/sj.jcbfm.9600064. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39:2824–2829. doi: 10.1161/STROKEAHA.108.515957. [DOI] [PubMed] [Google Scholar]
- Maeda M, Furuichi Y, Noto T, Matsuoka N, Mutoh S, Yoneda Y. Tacrolimus (FK506) suppresses rt-PA-induced hemorrhagic transformation in a rat thrombotic ischemia stroke model. Brain Res. 2009;1254:99–108. doi: 10.1016/j.brainres.2008.11.080. [DOI] [PubMed] [Google Scholar]
- Meden P, Overgaard K, Pedersen H, Boysen G. Effect of hypothermia and delayed thrombolysis in a rat embolic stroke model. Acta Neurol Scand. 1994;90:91–98. doi: 10.1111/j.1600-0404.1994.tb02686.x. [DOI] [PubMed] [Google Scholar]
- Moleac 2007WIPO: (WO/2007/106049) Combination therapy for treatment of patients with neurological disorders and cerebral infarction . http://www.wipo.int/pctdb/en/
- Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurokey A/S.2007WIPO: (WO 2008/040361) Use of a combination of hypothermia inducing drugs . http://www.wipo.int/pctdb/en/
- Novartis 2007WIPO: (WO/2007/041362) 2-AMINO-7,8-DIHYDRO-6H-PYRIDO[4,3-D] PYRIMIDIN-5-ONES . http://www.wipo.int/pctdb/en/
- O'Collins VE, Donnan GA, Macleod MR, Howells DW. Scope of preclinical testing versus quality control within experiments. Stroke. 2009;40:e497. doi: 10.1161/STROKEAHA.109.550335. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Ramanath VS, Eagle KA. Evidence-based medical therapy of patients with acute coronary syndromes. Am J Cardiovasc Drugs. 2007;7:95–116. doi: 10.2165/00129784-200707020-00002. [DOI] [PubMed] [Google Scholar]
- Rogalewski A, Schneider A, Ringelstein EB, Schabitz WR. Toward a multimodal neuroprotective treatment of stroke. Stroke. 2006;37:1129–1136. doi: 10.1161/01.STR.0000209330.73175.34. [DOI] [PubMed] [Google Scholar]
- Saver JL, Kalafut M. Combination therapies and the theoretical limits of evidence-based medicine. Neuroepidemiology. 2001;20:57–64. doi: 10.1159/000054762. [DOI] [PubMed] [Google Scholar]
- Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205:20–25. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereghy T, Overgaard K, Boysen G. Neuroprotection by excitatory amino acid antagonist augments the benefit of thrombolysis in embolic stroke in rats. Stroke. 1993;24:1702–1708. doi: 10.1161/01.str.24.11.1702. [DOI] [PubMed] [Google Scholar]
- Stata Technical Bulletin (STB) 42. College Station, TX: Texas A & M University; 1998. STB-42. [Google Scholar]
- Suzuki M, Sasamata M, Miyata K. Neuroprotective effects of YM872 coadministered with t-PA in a rat embolic stroke model. Brain Res. 2003;959:169–172. doi: 10.1016/s0006-8993(02)03759-9. [DOI] [PubMed] [Google Scholar]
- Teichner A, Ovadia H, Lavie G, Leker RR. Combination of dexanabinol and tempol in focal cerebral ischemia: is there a ceiling effect. Exp Neurol. 2003;182:353–360. doi: 10.1016/s0014-4886(03)00083-9. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted. Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–2708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Chen T-Y, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Buller B, Jiang J, Jiang YT, Zhao DP, Liu XS, Morris D, Chopp M. Combination treatment With VELCADE and low-dose tissue plasminogen activator provides potent neuroprotection in aged rats after embolic focal ischemia. Stroke. 2010;41:1001–1007. doi: 10.1161/STROKEAHA.109.577288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Behavioral and histological effects of chronic antipsychotic and antidepressant drug treatment in aged rats with focal ischemic brain injury. Behav Brain Res. 2005;158:211–220. doi: 10.1016/j.bbr.2004.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.