Abstract
The blood–brain barrier (BBB) facilitates amyloid-β (Aβ) exchange between the blood and the brain. Here, we found that the cellular prion protein (PrPc), a putative receptor implicated in mediating Aβ neurotoxicity in Alzheimer's disease (AD), participates in Aβ transcytosis across the BBB. Using an in vitro BBB model, [125I]-Aβ1−40 transcytosis was reduced by genetic knockout of PrPc or after addition of a competing PrPc-specific antibody. Furthermore, we provide evidence that PrPc is expressed in endothelial cells and, that monomeric Aβ1−40 binds to PrPc. These observations provide new mechanistic insights into the role of PrPc in AD.
Keywords: Alzheimer's disease, amyloid-β, blood–brain barrier, cellular prion protein, transcytosis
Introduction
Alzheimer's disease (AD) is an incurable neurodegenerative disorder that is characterized by the accumulation of neurotoxic amyloid-β (Aβ) peptides in the brain and intraneuronal hyperphosphorylation of the cytoskeletal protein tau (Selkoe, 2011). Clinically, these cerebral manifestations result in memory impairment. Recent evidence suggests that the cellular prion protein (PrPc) can mediate Aβ1−42 oligomer-induced impairment of synaptic function and, furthermore, is required for spatial learning and memory deficits observed in an AD transgenic mouse model (Gimbel et al, 2010; Lauren et al, 2009). However, these results were challenged recently (Balducci et al, 2010).
Cellular prion protein is a glycosyl phosphatidylinositol (GPI)-anchored cell surface protein. Hence, it might require a transmembrane coreceptor to transduce signals into the cell that finally cause Aβ-induced neurodegenerative phenotypes (Lauren et al, 2009). To date this coreceptor has not been identified but a previous report showed that the low density lipoprotein receptor-related protein 1 (LRP1) is capable of internalizing PrPc (Taylor and Hooper, 2007). In addition, LRP1 participates in Aβ1−40 clearance across the blood–brain barrier (BBB) by means of transcytosis (Pflanzner et al, 2011; Shibata et al, 2000; Zlokovic, 2008). Interestingly, LRP1 binds Aβ1−40 with a much higher affinity than Aβ1−42, resulting in greater LRP1-dependent Aβ1−40 internalization rates in endothelial cells (ECs) (Deane et al, 2004). Based on the observation that Aβ binds to PrPc, we set out to determine if LRP1's ability to internalize PrPc has a pathophysiological role in AD pathogenesis at the BBB. By using an in vitro transwell model of the BBB consisting of primary mouse brain capillary ECs (pMBCECs), we were able to show that PrPc is required for Aβ1−40 transcytosis across the BBB.
Materials and methods
Primary Mouse Brain Capillary Endothelial Cell Transwell Transport Model
Isolation and cultivation of pMBCECs have been previously described (Pflanzner et al, 2011), and are provided as Supplementary Information.
Amyloid-β transcytosis across pMBCEC monolayers was investigated after inducing high transendothelial electrical resistance. Then, 0.1 nM [125I]-Aβ1−40 and 1 μCi/mL [14C]-inulin (both from Perkin-Elmer, Rodgau, Germany), the latter a marker for paracellular diffusion, were diluted in serum free DMEM/Ham's F12 medium (Gibco, Darmstadt, Germany) + 550 nM hydrocortisone (Sigma, Schnelldorf, Germany) + 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Lonza, Cologne, Germany) and added to the donor compartment. To inhibit Aβ binding to PrPc, mouse monoclonal anti-PrPc (Ab51.2) hybridoma supernatant was dialyzed against phosphate-buffered saline (PBS) over night at 4°C and used at a final concentration of 2.25 μg/mL. For details on the generation and specificity of monoclonal 51.2 antibody detecting PrPc, please refer to Supplementary Information. Receptor-associated protein (RAP) was expressed in bacteria as a fusion protein with GST (glutathione S-transferase) and was purified as previously described (Martin et al, 2008). RAP-GST, or GST as control, was used at 500 nM to block Aβ binding to LRP1. Amyloid-β transport was studied for 2 hours in the brain to blood and for 6 hours in the blood to brain direction. From each input and at each time point, 10 and 50 μL samples were taken from the compartment the transport was investigated to (acceptor). In all, 10μ L probes were counted on a Wallac Wizard2 2470 automatic γ-counter (Perkin-Elmer) for [125I], or on a Tri-Carb 2800 TR Liquid Scintillation Analyzer (Perkin-Elmer) for [14C]. To investigate the amount of intact [125I]-Aβ1−40 being transported, 50 μL of a 15% trichloroacetic acid solution was added to a 50 μL media sample and incubated for 10 minutes at 4°C before samples were centrifuged at 10,000 g for 10 minutes. Supernatant (free [125I]) and pellet (intact [125I]-Aβ1−40) were counted separately for [125I]. Transport of intact [125I]-Aβ1−40 across the monolayer was calculated as Aβ1−40 TQ (transcytosis quotient):
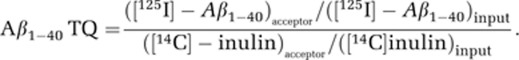 |
Results represent the mean+s.e.m. of three independent experiments in triplicate.
Coimmunoprecipitation, Sodium Dodecylsulfate-Polyacrylamide Gel, and Western Blot Analysis
For detailed information, please see Supplementary Information.
[125I]-Aβ1−40 Binding Study
Recombinant PrPc (rPrPc) was synthesized as previously described (Leliveld et al, 2008). To study Aβ binding to rPrPc, 96-well plates were coated with the indicated rPrPc concentrations in PBS over night at 4°C on a shaker. Plates were transferred to 37°C for 30 minutes and washed twice with ice-cold PBS. Unspecific binding sites were blocked with 3% bovine serum albumin (Sigma) in PBS (w/v) for 30 minutes at 37°C on a shaker and, subsequently washed thrice with ice-cold PBS. Coated wells were incubated with 0.1 nM [125I]-Aβ1−40 (Perkin-Elmer) in PBS for 30 minutes at 37°C to allow Aβ binding to rPrPc. After five washes with ice-cold PBS, proteins were dissociated by two washes with 0.2 N NaOH for 5 minutes, collected, and recovered [125I]-Aβ1−40 was counted on a Tri-Carb 2800 TR Liquid Scintillation Analyzer (Perkin-Elmer). Counts were normalized to input and expressed in pmol. Results represent the mean±s.e.m. of three independent experiments in duplicate.
Statistics
All graphs and statistical analyses were prepared using GraphPad Prism 4 software (La Jolla, CA, USA). Data were analyzed by one-way analysis of variance coupled to Newman–Keuls posttest for multiple comparison or t-test. P<0.05 was considered to be statistically significant.
Results
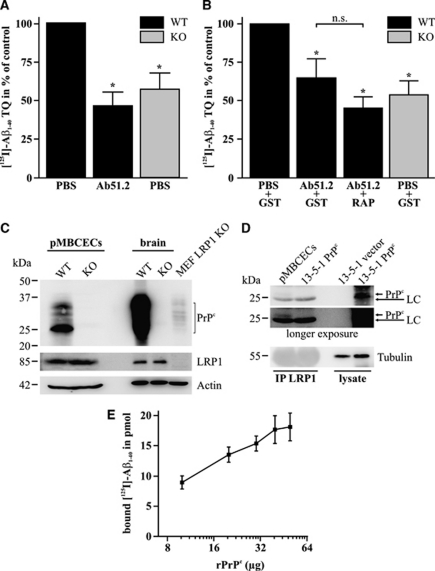
To determine whether PrPc participates in Aβ transcytosis across the BBB, we investigated the transport of [125I]-Aβ1−40 across an in vitro BBB transwell model consisting of pMBCECs. In the brain to blood (abluminal to luminal) direction, [125I]-Aβ1−40 transcytosis was significantly reduced by the addition of a mouse monoclonal antibody against PrPc (Ab51.2; for details see Supplementary Information) to levels observed in pMBCECs generated from PrPc KO (knockout) animals (Figure 1A). Similar results were obtained when [125I]-Aβ1−40 transcytosis was analyzed in the blood to brain direction (Figure 1B). LRP1 has been shown to mediate Aβ transport across the BBB (Pflanzner et al, 2011). However, we observed only a nonsignificantly different decrease in Aβ transport rates when LRP1-mediated Aβ transport was additionally inhibited by RAP (synthesized as GST fusion protein) (Figure 1B). To strengthen the participation of PrPc in Aβ transcytosis across the BBB, we analyzed protein expression in pMBCECs. Immunoblotting revealed robust expression levels of PrPc in pMBCECs derived from wild-type (WT) animals, whereas LRP1 levels were comparable between pMBCECs prepared from WT and PrPc KO animals (Figure 1C). Moreover, we were able to demonstrate that LRP1 coimmunoprecipitates with the most abundant 27 kDa PrPc isoform present in pMBCECs (Figures 1C and 1D). Previous results demonstrated that PrPc binds Aβ1−42 oligomers and mediates oligomer-induced neuronal toxicity (Lauren et al, 2009); however, these findings are controversially discussed (Balducci et al, 2010). Since we observed that PrPc has a role in the transcytosis of monomeric Aβ1−40 across the BBB, we set out to investigate whether PrPc indeed binds Aβ1−40 monomers. By using rPrPc, we provide evidence that rPrPc binds monomeric [125I]-Aβ1−40 in the low picomolar range (Figure 1E).
Figure 1.
Cellular prion protein (PrPc) enables amyloid-β (Aβ) endocytosis and transcytosis across the blood–brain barrier. (A, B) [125I]-Aβ1−40 transcytosis across primary mouse brain capillary endothelial cells (pMBCECs) from PrPc wild-type (WT) and knockout (KO) mice was investigated in the presence of [14C]-inulin, a marker for paracellular diffusion, to determine the transcytosis quotient (TQ). Mouse monoclonal anti-PrPc antibody (Ab51.2) decreases the [125I]-Aβ1−40 TQ in the brain to blood (A) and blood to brain (B) direction to levels observed in PrPc KO pMBCECs. Simultaneous incubation of Ab51.2 and receptor-associated protein (RAP), a ligand binding inhibitor for lipoprotein receptors, did not further decrease Aβ transcytosis below pMBCECs treated with Ab51.2 alone or PrPc KO monolayers. Results represent mean+s.e.m. of three independent experiments in triplicate. *Statistically significant difference compared with control (P<0.05; one-way analysis of variance), n.s., not significant. (C) PrPc expression was analyzed in pMBCECs by immunoblotting. Brain homogenates and low density lipoprotein receptor-related protein 1 (LRP1)-deficient mouse embryonic fibroblast (MEF) cell lysate were included as controls. Mouse monoclonal anti-PrPc antibody (W226) detects strong expression levels in pMBCECs derived from WT animals, whereas LRP1-specific rabbit polyclonal 1704 antibody does not show any differences in LRP1 expression between both genotypes. Actin was used as loading control. (D) LRP1 was immunoprecipitated from pMBCECs and LRP1-deficient Chinese hamster ovary 13-5-1 cells transiently transfected with PrPc using 1704 antibody. Immunoblotting with W226 antibody shows that PrPc coimmunoprecipitates with LRP1 in pMBCECs. Tubulin was used as loading control, antibody light chain (LC). (E) Increasing concentrations of immobilized recombinant PrPc (rPrPc) were incubated with monomeric [125I]-Aβ1−40. Results represent mean±s.e.m. of three independent experiments in duplicate.
Discussion
The BBB has an important role in the pathogenesis of AD by governing bidirectional transcytosis of neurotoxic Aβ, thereby, maintaining an Aβ equilibrium that is likely critical to prevent accumulation and aggregation of Aβ species in the brain. Several transmembrane receptors and transporters have been identified to have a role in Aβ transport mechanisms at the BBB (Pflanzner et al, 2010; Zlokovic, 2008), emphasizing the complexity and necessity of these exchanges.
The PrPc belongs to the family of GPI-anchored cell surface proteins. Its role in mediating Aβ oligomer-induced neurotoxicity is not fully resolved due to the lack of intracellular motifs that enable signal transduction into the cell. Hence, it has been suggested that PrPc requires a transmembrane coreceptor to elicit, for example, synaptic dysfunction on Aβ oligomer binding to PrPc (Lauren et al, 2009). To date, the low density LRP1 is the only transmembrane protein that has been shown to mediate PrPc internalization through clathrin-coated pits through interactions between the extracellular domains of both receptors (Shyng et al, 1994; Taylor and Hooper, 2007). LRP1 endocytosis is facilitated through its cytosolic NPxYxxL motif (Li et al, 2000; Pflanzner et al, 2011), a highly conserved domain that also functions in signal transduction (Martin et al, 2008).
Genetic evidence supports that LRP1 mediates Aβ1−40 transcytosis across the BBB (Pflanzner et al, 2011). Due to the fact that LRP1 is required for PrPc endocytosis (Taylor and Hooper, 2007), we investigated whether PrPc is involved in Aβ1−40 transport across the BBB. Strikingly, on genetic deletion of PrPc, we found that Aβ transcytosis in the brain to blood direction was reduced by ∼50% in our primary in vitro BBB model. In addition, a monoclonal antibody against PrPc diminished Aβ transport to levels observed in PrPc-deficient endothelial monolayers (Figure 1A), indicating that the binding of monomeric Aβ1−40 to PrPc is required for Aβ transcytosis across the BBB. To emphasize this novel role of PrPc, we analyzed the presence of PrPc in our primary ECs and tested whether monomeric Aβ1−40 binds to rPrPc. In contrast to ECs that were derived from PrPc KO animals, we found robust PrPc expression levels in freshly isolated ECs, whereas LRP1 levels were comparable between both genotypes (Figure 1C). This excluded the possibility that differences in Aβ transport rates are due to altered LRP1 expression levels. Although previous efforts have focused on Aβ1−42 oligomer binding to PrPc (Lauren et al, 2009), recent evidence supports that low molecular weight Aβ1−42 species also bind to PrPc (Calella et al, 2010). In agreement with our transport studies, we demonstrate that Aβ1−40 monomers bind to rPrPc in the low picomolar range (Figure 1E). Therefore, the presence of PrPc in ECs, its binding capacity for monomeric Aβ1−40, and the role in Aβ1−40 transcytosis across the BBB highlights an additional mechanism by which PrPc could influence AD pathogenesis.
Since PrPc does not contain intracellular motifs that facilitate receptor internalization, we investigated whether PrPc facilitated Aβ1−40 transport across the BBB might be coupled to LRP1-mediated Aβ1−40 transcytosis. We have previously shown that inhibition of ligand binding to LRP1 with RAP or knockin mutation of the NPxYxxL endocytosis motif reduces LRP1-mediated Aβ1−40 transcytosis across the BBB by ∼50% (Pflanzner et al, 2011). Assuming that Aβ transcytosis by LRP1 and PrPc occur independent of each other, RAP treatment should have a clear additional effect on Aβ transcytosis compared with PrPc antibody alone. However, RAP did not further decrease Aβ transport compared with anti-PrPc alone (Figure 1B). In line with this observation and LRP1's ability to internalize PrPc (Taylor and Hooper, 2007), we therefore hypothesize that Aβ1−40 transcytosis across the BBB could occur through a mechanism in which both receptors cooperate to (1) increase the amount of available Aβ-binding sites and (2) compensate for the lack of a functional endocytosis motif within PrPc, to enhance transcytosis efficiency. RAP treatment has been shown to interfere with both LRP1-mediated Aβ transcytosis and PrPc endocytosis through clathrin-coated pits in separate studies (Pflanzner et al, 2011; Taylor and Hooper, 2007). Taken together, these findings suggest that the role of PrPc in Aβ transcytosis across the BBB is potentially linked to LRP1. This is further emphasized by the fact that LRP1 coimmunoprecipitates with 27 kDa PrPc in vivo (Figure 1D), an isoform that is present on the cell surface although it is not glycosylated (Korth et al, 2000). Transcytosis of cell surface proteins including LRP1 depends on the presence of tyrosine-based motifs (Anderson et al, 2005; Donoso et al, 2009). Although these motifs are absent in some GPI-anchored proteins such as PrPc, many GPI-anchored proteins are initially basolaterally sorted and then transcytosed to the apical membrane in polarized cells (Polishchuk et al, 2004). Cellular prion protein has been detected in early endosomes (Magalhaes et al, 2002), whereas one route of PrPc internalization has been demonstrated to be dependent on extracellular interactions with the endocytic receptor LRP1 (Taylor and Hooper, 2007). Here, we provide evidence that PrPc facilitates Aβ transcytosis across the BBB, conceivably in concert with LRP1, highlighting a novel role for PrPc in AD pathogenesis.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Parts of this work were supported by the German Bundesministerium für Bildung und Forschung (BMBF) (01EW1009 and 01GI1004D to CUP) and by a grant from the EU-FP7 PRIORITY to CK.
Supplementary Material
References
- Anderson E, Maday S, Sfakianos J, Hull M, Winckler B, Sheff D, Folsch H, Mellman I. Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways. J Cell Biol. 2005;170:595–605. doi: 10.1083/jcb.200506051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci USA. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, Mansuy IM, Aguzzi A. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol Med. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Donoso M, Cancino J, Lee J, van Kerkhof P, Retamal C, Bu G, Gonzalez A, Caceres A, Marzolo MP. Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways. Mol Biol Cell. 2009;20:481–497. doi: 10.1091/mbc.E08-08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth C, Kaneko K, Prusiner SB. Expression of unglycosylated mutated prion protein facilitates PrP(Sc) formation in neuroblastoma cells infected with different prion strains. J Gen Virol. 2000;81:2555–2563. doi: 10.1099/0022-1317-81-10-2555. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliveld SR, Stitz L, Korth C. Expansion of the octarepeat domain alters the misfolding pathway but not the folding pathway of the prion protein. Biochemistry. 2008;47:6267–6278. doi: 10.1021/bi800253c. [DOI] [PubMed] [Google Scholar]
- Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Silva JA, Lee KS, Martins VR, Prado VF, Ferguson SS, Gomez MV, Brentani RR, Prado MA. Endocytic intermediates involved with the intracellular trafficking of a fluorescent cellular prion protein. J Biol Chem. 2002;277:33311–33318. doi: 10.1074/jbc.M203661200. [DOI] [PubMed] [Google Scholar]
- Martin AM, Kuhlmann C, Trossbach S, Jaeger S, Waldron E, Roebroek A, Luhmann HJ, Laatsch A, Weggen S, Lessmann V, Pietrzik CU. The functional role of the second NPXY motif of the LRP1 beta-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors. J Biol Chem. 2008;283:12004–12013. doi: 10.1074/jbc.M707607200. [DOI] [PubMed] [Google Scholar]
- Pflanzner T, Janko MC, Andre-Dohmen B, Reuss S, Weggen S, Roebroek AJ, Kuhlmann CR, Pietrzik CU. LRP1 mediates bidirectional transcytosis of amyloid-beta across the blood-brain barrier. Neurobiol Aging. 2011;32:2323 e1–2323 e11. doi: 10.1016/j.neurobiolaging.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Pflanzner T, Kuhlmann CR, Pietrzik CU. Blood-brain-barrier models for the investigation of transporter- and receptor-mediated amyloid-beta clearance in Alzheimer's disease. Curr Alzheimer Res. 2010;7:578–590. doi: 10.2174/156720510793499066. [DOI] [PubMed] [Google Scholar]
- Polishchuk R, Di Pentima A, Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ.2011Alzheimer's disease Cold Spring Harb Perspect Bioladvance online publication, 1 July 2011; 3pii: a004457; doi: 10.1101/cshperspect.a004457Review [DOI] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Heuser JE, Harris DA. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J Cell Biol. 1994;125:1239–1250. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Hooper NM. The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem J. 2007;402:17–23. doi: 10.1042/BJ20061736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.