Abstract
Preoperative characterization of thyroid follicular lesions is challenging. Fine-needle aspiration specimens cannot differentiate follicular carcinomas from benign follicular neoplasias. Recently, promising markers have been detected using modern molecular techniques. We conducted a retrospective study to confirm the usefulness of immunohistochemical staining for the protein markers, DDIT3, STT3A (ITM1), ARG2 and FAM129A (C1orf24) in separating benign and malignant thyroid follicular lesions. Formalin-fixed, paraffin-embedded thyroid tissue from 30 in-house cases (15 follicular carcinomas and 15 follicular adenomas), as well as 8 follicular carcinomas and 21 follicular adenomas on tissue microarray slides were stained immunohistochemically for DDIT3, STT3A, ARG2 and FAM129A expression. Control tissue consisted of thyroid parenchyma adjacent to the tumors and 11 separate cases of normal thyroid parenchyma. All in-house cases of follicular adenomas, follicular carcinomas and adjacent normal thyroid tissue showed positive immunostaining with anti-DDIT3 and anti-STT3A. Anti-ARG2 and anti-FAM129A polyclonal antibodies showed positive staining in 20 and 60% of in-house follicular adenomas, and 40 and 87% of in-house follicular carcinomas, respectively. Monoclonal anti-FAM129A demonstrated positive staining in 13 and 33% of in-house follicular adenomas and follicular carcinomas, respectively. Polyclonal anti-DDIT3, -STT3A and -FAM129A antibodies showed positive staining in all tissue microarray slides of follicular carcinoma and in 76, 85 and 81% of the follicular adenomas, respectively. Monoclonal anti-STT3A stained 81% of the follicular adenoma cores. Anti-ARG2 stained positive in 13% of follicular carcinomas and 10% of follicular adenomas on the tissue microarray slides. In conclusion, DDIT3, STT3A, ARG2 and FAM129A immunohistochemistry does not appear to be useful in the diagnosis of thyroid follicular neoplasias, as they do not reliably distinguish follicular thyroid carcinoma from follicular thyroid adenoma.
Keywords: ARG2, C1orf24, DDIT3, FAM129A, ITM1, STT3A, thyroid carcinoma
Fine-needle aspiration biopsy has become the most widely used preoperative method to diagnose thyroid nodules.1, 2, 3 Since it's introduction in Europe in the 1970s, and in the US some years later, there has been a dramatic reduction in the need for diagnostic hemi-thyroidectomies, whereas the yield of thyroid carcinomas has increased.4 For papillary carcinomas, cytology smears usually are sufficient to make a diagnosis, due to the presence of cells with nuclei characteristic for papillary carcinoma. In marked contrast, in follicular neoplasias, cytological morphology cannot reliably distinguish between benign follicular adenomas and malignant follicular carcinomas.5, 6, 7, 8, 9, 10 Indeed, a definite diagnosis of follicular carcinoma requires surgical excision for histological characterization and the examination of multiple paraffin sections for evidence of capsular or vascular invasion. The reported rate of malignancy in follicular-patterned lesions diagnosed as either atypical or indeterminate ranges between 20 and 30%,5, 8, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 and many patients are operated upon unnecessarily. The cost of these procedures is high, both economically and in patient morbidity.
Recently, a number of promising molecular and immunohistochemical methods have been described, which could improve the diagnostic accuracy of fine-needle aspiration biopsy.23, 24, 25, 26, 27, 28, 29 However, there is some inter-laboratory variability in the sensitivity and specificities obtained with the individual techniques as shown in the review by Griffith et al.30 Significantly, none of the methods has been fully validated to the extent that it is in general use in the routine diagnostic laboratory.
In 2006, Cerutti et al31 described a test that could discriminate between follicular carcinoma and follicular adenoma on both paraffin sections and cytology preparations. Their original immunohistochemical method was based on the selective staining of follicular carcinoma cells by antibodies reactive to four protein markers: DDIT3 (also known as CHOP and GADD153), STT3A (also known as ITM1), ARG2 and FAM129A (also known as C1orf24 and Niban). Overexpression of these proteins was first observed using serial analysis of gene expression procedures on a single follicular carcinoma specimen and subsequently confirmed using quantitative reverse-transcription PCR. The four proteins have diverse cellular functions including nitric oxide and polyamine metabolism (ARG2), regulation of protein translation (FAM129A), regulation of transcription as CCAAT/enhancer-binding transcription factor (DDIT3), and as component of the N-oligosaccharide transfer enzyme (STT3A). Cerutti et al31, 32 found, by using immunohistochemistry, that the combination of the four markers distinguished a variety of thyroid tumors commonly classified as indeterminate on fine-needle aspiration biopsy, with an estimated sensitivity of 100% and specificity of 85% for detecting malignancy.
The aim of this study was to verify the predictive accuracy of the new biomarkers DDIT3, STT3A, ARG2 and FAM129A in distinguishing follicular carcinoma from follicular adenoma.
Materials and methods
Tissue Sections
Formalin-fixed, paraffin-embedded thyroid tissue blocks were retrieved from the archive maintained at the Department of Pathology, The Norwegian Radium Hospital. A total of 30 cases, consisting of 15 follicular carcinomas and 15 follicular adenomas, originally submitted for diagnostic purposes, were included. The in-house cases have been diagnosed histopathologically according to World Health Organization criteria.33 Follicular neoplasias with oncocytic differentiation, and areas containing cells demonstrating oncocytic differentiation, were excluded from the evaluation. In all cases, hematoxylin and eosin-stained slides were reviewed to confirm the original histopathological diagnosis. Before embedding, the tissue specimens had been preserved in 4% buffered formalin for variable periods, but within the range of 2–5 days.
Two tissue microarrays containing either 80 samples from normal thyroid parenchyma (serial TH804) or 80 thyroid cases with pathological findings, including 8 follicular carcinomas and 21 follicular adenomas (serial TH802), were purchased from US Biomax (Rockville, USA). The product supplier stated that the tissues used to construct these arrays had been preserved in neutral phosphate-buffered formalin for a maximum of 24 h before embedding.
Antibody Preparation
Rabbit polyclonal antisera to FAM129A (C1orf24) and STT3A (ITM1) were generated using C-terminal peptides as described by Cerutti et al.31 The peptides were custom-synthesized and conjugated to diphtheria toxoid (Mimotopes, Clayton, VIC, Australia). Immunizations were performed using the diphtheria toxoid conjugates by Eurogentec (Liège, Belgium). Monoclonal antibodies were produced by fusion of NS0 myeloma cells with splenocytes from BALB/c mice immunized with peptide–diphtheria toxoid conjugates.
Antibody titration and hybridoma screening were performed using antibody capture assays with biotinylated peptides presented on streptavidin-coated microplates (Wallac Oy, Turku, Finland). Specificity was established using ELISA, western blotting and 125I-peptide displacement assays.
Polyclonal rabbit anti-GADD 153 (DDIT3; sc-793) and anti-Arginase-2 (sc-20151) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Experimental animals were handled in accordance with national and institutional guidelines.
125I-Peptide Displacement Assays
Competitive immunoassay tracers were produced by incubating biotinylated peptides with an excess of 125I-labeled streptavidin. Iodination was performed with the indirect Iodogen-method (Pierce, Rockford, IL, USA). The test was performed by incubating tracer and antisera (dilution 1:10 000) for 3 h in the presence and absence of tissue homogenate. Antibody/antigen complexes were then isolated by the addition of sheep anti-immunoglobulin-coated magnetic beads (Invitrogen, Carlsbad, CA, USA). The suspension was incubated for 1 h with shaking, washed three times and bound tracer counted in a γ-counter.
Western Blotting
Tissue samples or cells from a follicular carcinoma line, FTC-133 (EACC, Salisbury, UK) were homogenized in ice-cold RIPA buffer (TBS, 1% NP-40, 0.25% sodium deoxycholate, 0.1% SDS) containing 5 mM EDTA, 1 mM phenylmethylsulfonylfluoride, 0.2 μM aprotinin, 1 μM leupeptin and 1 μM pepstatin A. Following clarification, soluble protein concentrations were determined using the BCA method (Pierce). Between 20 and 40 μg/lane of total protein was subjected to SDS-PAGE (Invitrogen; Novex 4–12% gradient) and subsequently transferred onto nitrocellulose. The membranes were blocked with 5% skimmed milk/TBS, followed by incubation for 1 h with the monoclonal (1:10 dilution of culture supernatant) or polyclonal (1:2000–1:5000) antibodies diluted in 5% milk/TBS. Loading controls were stained for GAPDH and α-tubulin. Detection utilized HRP-labeled goat anti-species immunoglobulin (1:6000 dilution poly-HRP, Pierce) and the SuperSignal West Pico chemiluminescent substrate (Pierce).
Immunohistochemical Staining
Immunohistochemistry was performed on 3 μm paraffin sections mounted on positively charged slides, essentially as described by Cerutti et al.31 Tissue sections were dewaxed in xylene, followed by rehydration using a graded ethanol series. Endogenous peroxidase activity was suppressed by pretreatment with 0.03% hydrogen peroxide, and antigen retrieval was achieved using buffer AR-10 (Biogenex, San Ramon, CA, USA) in a steamer for 10 min. Immunodetection was undertaken using a Dako automated tissue-staining system (S3400; Dako, Carpinteria, CA, USA), using EnVision+HRP reagents (K4007 and K4011, Dako). Tissue sections were exposed to the primary antibodies for 20–22 h at 4 °C. The optimal dilution of each antibody was determined in preliminary staining trials. Appropriate controls were prepared using (1) non-immune polyclonal rabbit antisera or mouse IgG1 myeloma immunoglobulin and (2) anti-FAM129A and anti-STT3A, preabsorbed with 1 μg/ml FAM129A antigen and 1 μg/ml STT3A antigen, respectively.
Two independent surgical pathologists (ES and AaB) performed the slide evaluation. Tissue sections were assigned to one of four groups, based on the proportion of cells showing unequivocal staining: 0, (0%); 1, (<25%); 2, (25–50%); 3, (51–75%); 4, (>75%). The scoring system used a cutoff for positive staining of >25%, which is in accordance with accepted scoring methods. Arginase-2 and STT3A staining was detected primarily in the cytoplasm of the follicular cells, FAM129A in the cytoplasmic membrane and the cytoplasm, whereas DDIT3 expression was observed both in the nucleus and the cytoplasm. For each of the in-house cases, staining of the normal thyroid tissue adjacent to the tumor was also evaluated. The inter-observer agreement was 94% for the in-house cases and 83% for the tissue microarray slides. Discordant cases were reevaluated using a multi-headed microscope to obtain a consensus. Images displayed in this article were captured using a Leica DMLB microscope (Type 020-519.511DMLB 100T; Leica Microsystems GmbH, Germany) equipped with a Nikon digital Sight DS-Fi1 camera (Nikon Corporation, Japan), and processed using JASC Paint Shop Pro software (Version 7.02, Corel Corporation, USA).
Statistics
The data were analyzed using the Statistical Package for the Social Sciences (SPSS), version 17.0.1 for Windows (SPSS, Chicago, IL, USA). The Mann–Whitney U-test was used to determine DDIT3, STT3A, ARG2 and FAM129A expression level differences between follicular adenomas and follicular carcinomas. A P-value of <0.05 was considered statistically significant. Sensitivity and specificity were calculated using standard formulae for each of the antibodies individually, using benign versus malignant histological diagnosis as the standard.
Ethics
The study was approved by the Regional Committee for Medical Research Ethics and the ‘Personvernombudet' at Rikshospitalet. Informed consent for the use of material was obtained from the patients.
Results
Production and Characterization of Antibodies
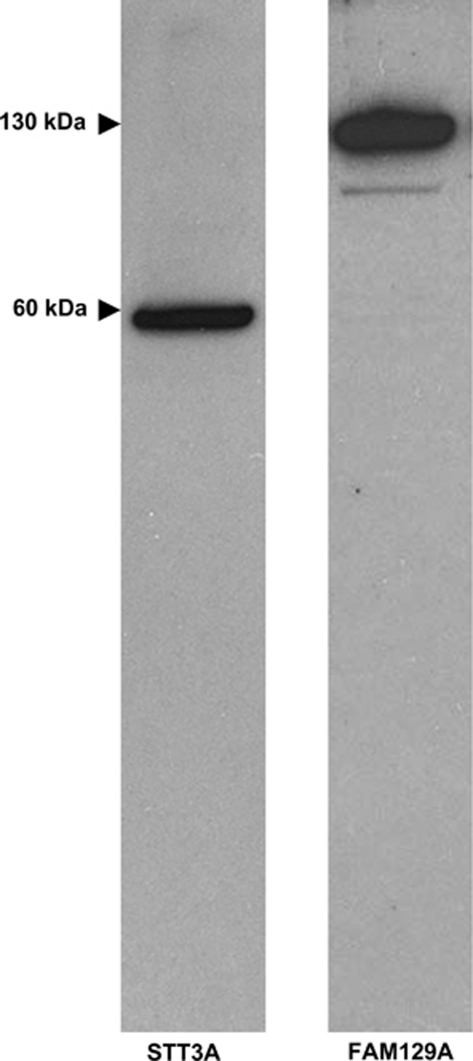
Antibodies to STT3A and FAM129A are not commercially available and were produced in-house. Rabbits were hyper-immunized with the appropriate C-terminal peptide and antibody titers followed by antibody-capture ELISA. After one primary injection and three booster doses, antisera with titers >1:10 000 were obtained. The sera were screened by radioimmunoassay displacement analysis using follicular carcinoma, follicular adenoma and normal tissue lysates. Sera that demonstrated more efficient peptide displacement with follicular carcinoma lysates compared with follicular adenoma or normal cell extracts were selected (data not shown). The specificity of the anti-FAM129A and anti-STT3A antibodies determined by western blotting of total cell lysates of the FTC-133 cell line detected proteins with the expected molecular weight of approximately 130 and 60 kDa, respectively (Figure 1).
Figure 1.
Specificity of the anti-FAM129A and anti-STT3A antibodies as determined by western blotting. Total cell lysates of the FTC-133 cell line were subjected to SDS-PAGE using 4–12% gradient gels. After transfer, the nitrocellulose membranes were stained with the primary antibody followed by a HRP-goat anti-rabbit secondary reagent. Detection utilized a chemoluminescent substrate.
Monoclonal antibodies were also generated using splenocytes from mice immunized with the peptide conjugates. Approximately, 1000 parental hybridomas/peptide were screened for reactivity by antibody-capture assay using biotinylated peptides. Secondary screening was undertaken by immunocytochemistry using the FTC-133 cell line. Two hybridomas were selected, producing antibodies to FAM129A (clone E253) and STT3A (clone E239).
The specificity of the anti-FAM129A and anti-STT3A was determined by preabsorption of the antiserum with blocking peptides. Absorbed polyclonal and monoclonal anti-FAM129A antibodies and the monoclonal anti-STT3A antibody gave negative immunostaining. A faint immunoreaction was observed with preabsorbed polyclonal anti-STT3A antibody (data not shown). However, this background was easily distinguishable from a positive staining reaction.
Immunohistochemistry
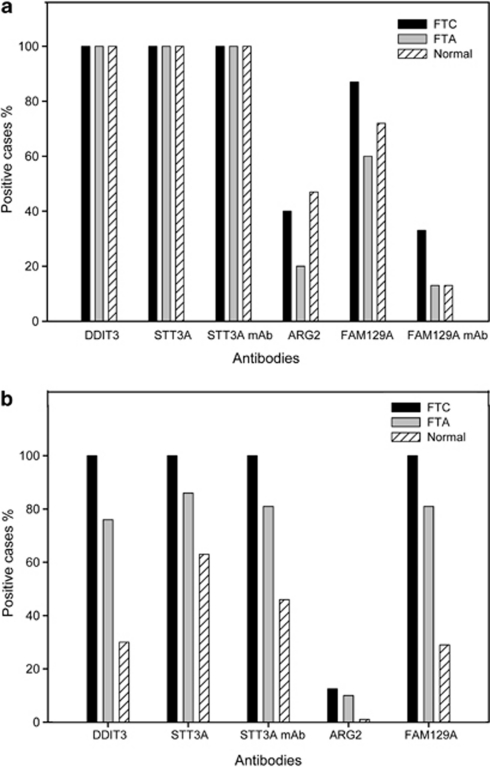
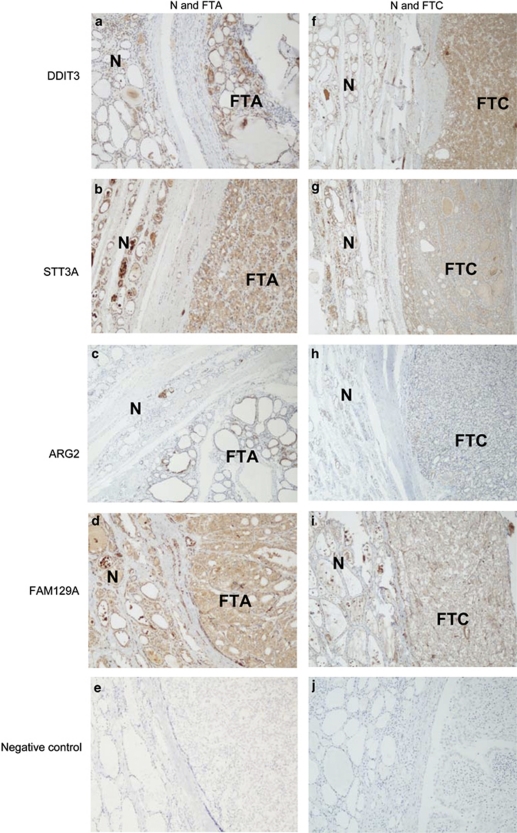
Paraffin sections from 30 in-house cases were screened for the expression of DDIT3, STT3A, ARG2 and FAM129A, using a panel of polyclonal and monoclonal antibodies (Figure 2a). Positive staining for anti-DDIT3 and anti-STT3A was observed in all the cases of follicular carcinoma and follicular adenoma. The normal tissue adjacent to the tumors was also stained. Comparable binding was obtained with polyclonal or monoclonal anti-STT3A antibodies. ARG2 was detected in 40% of follicular carcinoma cases, 20% of the follicular adenomas and 47% of the normal thyroid tissues adjacent to the tumors. The polyclonal antibody to FAM129A demonstrated positive staining of follicular cells in 87% of the follicular carcinoma cases, 60% of the follicular adenomas and 72% of the adjacent normal tissues. In contrast, the anti-FAM129A monoclonal antibody stained 33% of follicular carcinoma cases, and 13% of both the follicular adenomas and the adjacent normal tissues. The immunohistochemical staining with the four antibodies of in-house follicular adenoma and follicular carcinoma cases, and the adjacent non-neoplastic thyroid tissue and two negative controls is shown in Figure 3. The observed staining of normal tissue was not due to leaking from the adjacent follicular adenoma or follicular carcinoma, as we found antibody binding in a panel of 11 thyroid specimens that displayed normal morphology. With this normal tissue panel, binding of the polyclonal DDIT3, STT3A and ARG2 antibodies was seen in all 11 individuals. Staining with the FAM129A polyclonal antibody was found in nine cases, whereas the monoclonal anti-FAM129A and anti-STT3A stained seven and nine cases, respectively.
Figure 2.
Frequency of binding of the four antibodies to follicular adenoma, follicular carcinoma and adjacent normal thyroid tissue in in-house cases (a), and in follicular adenoma, follicular carcinoma and separate cases of normal thyroid tissue in tissue microarray cases (b). FTA=follicular thyroid adenoma, FTC=follicular thyroid carcinoma, N=normal thyroid tissue.
Figure 3.
Immunohistochemical detection of DDIT3, STT3A, ARG2 and FAM129A in follicular adenoma and the adjacent normal thyroid tissue (a–d; × 50) and follicular carcinoma and the adjacent normal thyroid tissue (f–i; × 50) in in-house cases. Negative controls are shown in panels e and j. FTA=follicular thyroid adenoma, FTC=follicular thyroid carcinoma, N=normal thyroid tissue.
In preliminary trials, we tried to ameliorate what we thought was the ‘artifactual' staining of follicular adenoma and normal thyroid parenchyma, using a range of techniques. These included alternative antigen-retrieval buffers (citrate, Tris-Cl, citraconic anhydride), peroxidase blocking (H2O2, H2O2/methanol, H2O2/sodium azide), protein blocking reagent (normal serum, casein, fish gelatine, different BSA batches) and pre-treatment of the primary antibodies with thiol reagents. In each case, we failed to block the staining of follicular adenoma and normal thyroid epithelium (data not shown).
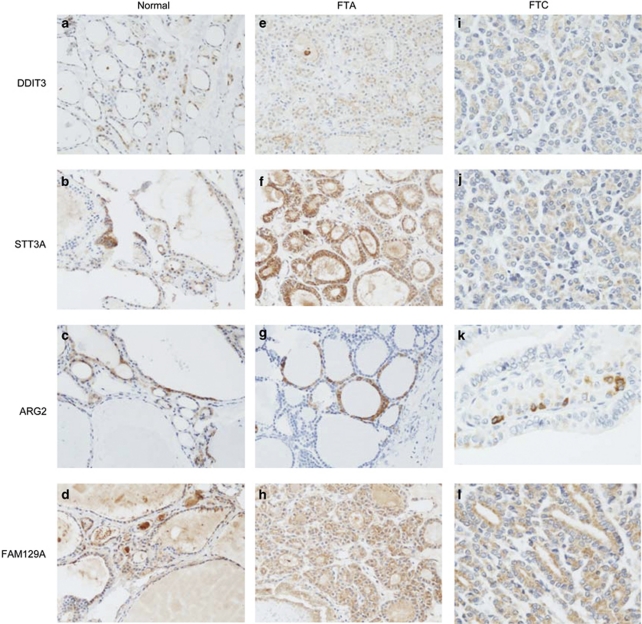
On the tissue microarray slides (Figure 2b), expression of DDIT3, STT3A and FAM129A was found in all the follicular carcinoma tissue cores and in 76, 85 and 81% follicular adenoma cores, respectively. Only 13% follicular carcinoma samples and 10% of the follicular adenoma cores showed positive staining with anti-ARG2. The tissue microarray slides containing normal thyroid tissue (which did not originate from the same individuals as the tumor cases) showed positive staining in 30, 63 and 29%, of the cores with polyclonal anti-DDIT3, -STT3A and -FAM129A, respectively. Expression of ARG2 was detectable in only 1% of individuals. The monoclonal antibody to STT3A stained 46% of the tissue cores. Immunostaining with the four antibodies of tissue microarray cores from follicular adenoma and follicular carcinoma cases, and separate tissue microarray cores from normal thyroid tissue is shown in Figure 4.
Figure 4.
Immunohistochemical detection of DDIT3, STT3A, ARG2 and FAM129A in separate cases of normal thyroid tissue (a–d; × 200), follicular adenoma (e–h; × 200) and follicular carcinoma (i–l; × 400) on tissue microarray slides. FTA=follicular thyroid adenoma, FTC=follicular thyroid carcinoma.
Mann–Whitney test was performed on the scoring data from both in-house slides and tissue microarray slides. The evaluation of difference in sum of ranks between follicular carcinoma and follicular adenoma cases revealed no significant P-values for the in-house cases, and for the tissue microarray cases, a P-value of 0.04 for DDIT3, and a non-significant value for STT3A polyclonal of P=0.37, for STT3Amonoclonal of P=0.24, for ARG2 of P=0.28 and for FAM129A of P=0.08. Defining sensitivity as the proportion of follicular carcinoma patients who expressed a marker, and specificity as the proportion of patients with follicular adenoma, who did not express the marker,31 the sensitivity and specificity of staining with each of the antibodies were calculated, and a summary of the calculated sensitivity and specificity of staining with each of the antibodies for the in-house and tissue microarray slides is shown in Table 1a and b , respectively.
Table 1. Sensitivity and specificity of immunohistochemistry with CI; sensitivity is defined as the proportion of follicular carcinoma patients who expressed the marker in tumor, and specificity as the proportion of patients with follicular adenoma, who did not express the marker31.
| Sensitivity | 95% CI | Specificity | 95% CI | |
|---|---|---|---|---|
| (a) In-house cases | ||||
| DDIT3 | 1.00 | — | 0 | — |
| STT3Ap | 1.00 | — | 0 | — |
| STT3Am | 1.00 | — | 0 | — |
| ARG2 | 0.40 | 0.29–0.64 | 0.80 | 0.55–0.93 |
| FAM129Ap | 0.87 | 0.62–0.96 | 0.40 | 0.20–0.64 |
| FAM129Am | 0.33 | 0.15–0.58 | 0.87 | 0.62–0.96 |
| (b) Tissue microarray cases | ||||
| DDIT3 | 1.00 | — | 0.24 | 0.11–0.45 |
| STT3Ap | 1.00 | — | 0.14 | 0.05–0.35 |
| STT3Am | 1.00 | — | 0.19 | 0.08–0.40 |
| ARG2 | 0.12 | 0.02–0.47 | 0.90 | 0.71–0.97 |
| FAM129Ap | 1.00 | — | 0.19 | 0.08–0.40 |
Abbreviation: CI, confidence intervals.
Tissue Distribution of DDIT3, STT3A, ARG2 and FAM129A by Western Blotting
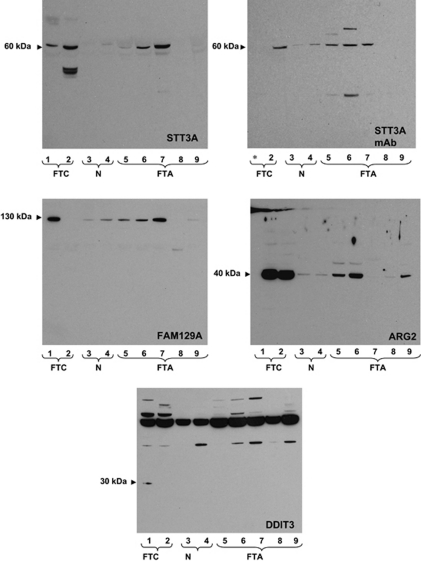
The results from immunoblotting studies are shown in Figure 5. Tissue extracts from two carcinomas and five adenomas were used. For one follicular carcinoma (lane 2) and one follicular adenoma (lane 5), tissue extracts from the non-involved lobe were also available for testing (sample lane 3 and lane 4, respectively). Our polyclonal and monoclonal antibodies to STT3A showed high specificity and detected a protein with the expected molecular weight of approximately 60 kDa. Immunoblots performed using the polyclonal anti-STT3A or the corresponding monoclonal antibody showed good concordance. With both reagents, STT3A protein was found in all follicular carcinoma cases and in three out of five of the follicular adenoma tissue lysates. Low, but detectable STT3A expression was also found in normal thyroid tissue. The polyclonal anti-FAM129A was highly specific, detecting a protein with an apparent Mw of about 130 kDa. FAM129A was highly expressed in one of the follicular carcinoma lysates, four out of five follicular adenoma cases and one normal tissue extract. In contrast to the anti-STT3A monoclonal antibody, the anti-FAM129A monoclonal antibody performed very poorly in western blotting and was not characterized (data not shown). The commercial anti-ARG2 reacted with a protein of 40 kDa with good specificity. The protein was highly expressed in both follicular carcinomas and three out of five follicular adenoma lysates. Low levels of ARG2 were found in all the tissue samples. Surprisingly, the commercial anti-DDIT3 proved highly non-specific, showing binding to many proteins within the 50–200 kDa range. This antibody did, however, detect a 30 kDa protein, presumably mature DDIT3, in one of the follicular carcinoma, but none of the follicular adenoma tumor lysates.
Figure 5.
Determination of ARG2, FAM129A, DDIT3 and STT3A tissue expression by western blotting. Lysates prepared from follicular carcinoma (lane 1, 2), normal thyroid parenchyma (lane 3, 4) and follicular adenoma (lane 5–9) tissue were subjected to western blotting as described in the legend to Figure 1. Normal tissues 2 and 3 were obtained from the uninvolved thyroid lobe of patent 2 and 5, respectively. *Sample not run due to insufficient lysate.
Discussion
Fine-needle aspiration biopsy of the thyroid is the most commonly used preoperative method to diagnose thyroid tumors. Although fine-needle aspiration biopsy can reduce the number of diagnostic thyroidectomies by identifying clearly benign lesions, it cannot reliably distinguish between follicular adenoma and follicular carcinoma. Many authors have attempted to identify molecular markers able to predict follicular carcinoma, but with conflicting results.30 The discordances may be a result of the biased selection of material, the limited number of cases examined, or the use of very different molecular and immunohistochemical methods. Among the most promising markers in the recent literature are DDIT3, STT3A, ARG2 and FAM129A that, according to Cerutti et al,31, 32 reliably identify follicular carcinoma. However, the utility of these markers has not been confirmed by other laboratories, and they are not widely used in diagnostic practice.
In this study, we tested the ability of immunohistochemical staining for DDIT3, STT3A, ARG2 and FAM129A, to distinguish follicular carcinoma from follicular adenoma. Our intention was to optimize the previously described methodology before establishing it in our diagnostic routine. Surprisingly, and discordant to the results of Cerutti et al,31 we found, as shown in Figure 2a, the percentage of our in-house cases with positive staining of follicular adenoma and follicular carcinoma to be identical (100%) for two of the antibodies examined (DDIT3 and STT3A), and with no significant difference in percentage of cases of follicular adenoma and follicular carcinoma with staining for ARG2 and FAM129A. Furthermore, the percentage of cases showing staining of the adjacent benign thyroid cells was not significantly different from the percentage of tumor cases showing staining. When examining the tissue microarray cases (Figure 2b), we found no significant difference in percentage of positive follicular adenoma and follicular carcinoma cases for three of the four antibodies examined (STT3A, ARG2 and FAM129A), and only for DDIT3, we did find the difference in percentage of positively stained cases of follicular adenoma and follicular carcinoma to be of weak significance. It is unlikely that the positive DDIT3, STT3A, ARG2 and FAM129A immunostaining of follicular adenoma and normal tissues was due to differences in methodology. We used the same antigen retrieval and staining protocols as originally described by Cerutti et al,31, 32 and extensively optimalized the dilutions of the primary antibodies used. Furthermore, staining was performed using the same commercially available antibodies against ARG2 and DDIT3 utilized by Cerutti et al,31 and in-house generated reagents were found to have high specificity to their cognate antigen in western blots. However, the anti-FAM129A monoclonal antibody performed poorly in immunohistochemistry and western blotting, probably reflecting its low avidity.
We additionally stained multiple cores of follicular adenoma, follicular carcinoma and normal thyroid parenchyma on commercially constructed tissue microarray slides. The tissue microarray results agreed with those obtained using wax sections prepared in-house, and positive follicular cell staining was found in cores containing normal thyroid tissue. The P-value of 0.04 for DDIT3 was found examining a small number of follicular carcinoma (n=8) and follicular adenoma (n=21) tissue microarray specimens. This finding does not fit with the observations made of the in-house cases demonstrating positive staining in all follicular adenomas (n=15) and all follicular carcinomas (n=15). Additionally, some quantitative differences were seen when comparing staining with anti-ARG2 and anti-FAM129A polyclonal antibodies on fresh sections with that on tissue microarray slides. These were most probably related to variable degrees of epitope degradation during the prolonged storage of the commercial tissue microarrays.34 However, as the tissues used to construct these arrays were fixed, embedded and sectioned by an independent laboratory, they provide an appropriate control for the variables associated with paraffin block preparation and handling.
Because of the surprising disparity between our immunohistochemical results and those described previously,31, 32 we determined the expression of ARG2, FAM129A, DDIT3 and STT3A in follicular adenoma and follicular carcinoma biopsy specimens, using western blotting. In contrast to immunohistochemistry, western blotting can evaluate protein expression, antibody specificity and provide a Mr of the antibody-binding moiety and hence, a presumptive identity. With the exception of the commercial anti-DDIT3 antibody, the antibody reagents demonstrated good specificity and reacted with antigens of the expected size in follicular adenoma and follicular carcinoma lysates. In agreement with our observations using immunohistochemistry, a sizable proportion of the follicular adenomas displayed significant expression of ARG2, FAM129A and STT3A. Although anti-DDIT3 (Santa Cruz Biotechnology) detected a protein of the expected size in one follicular carcinoma lysate, it displayed marked non-specificity. In a recent paper by Haataja et al,35 three out of seven commercially available DDIT3 antibodies (including the antibody from Santa Cruz Biotechnology that we used) gave false results by western blotting and immunocytochemistry. This non-specificity may explain why we observed positive immunohistochemical staining in all freshly cut follicular adenoma and normal tissue sections. Although Cerutti et al31, 32 used the same antibody, they did not report on whether they characterized each of their purchased batches.
Considering the urgent need for a technique that can reliably discriminate between follicular carcinoma and follicular adenoma, it is surprising that a search of the literature indicates that we are the first to attempt to replicate a method originally described in 2004.32 Matsumoto et al36 observed binding of antibodies to FAM129A to thyroid tumors with oxyphilic cytoplasm, to oxyphilic follicular adenoma and to oxyphilic variants of follicular carcinoma. However, the authors also noted FAM129A expression in 25% of follicular non-oxyphilic follicular adenoma and in only 50% of the non-oxyphilic follicular carcinomas. This study would therefore also suggest that FAM129A expression discriminates poorly between follicular carcinoma and follicular adenoma. In a recent reverse-transcription PCR study by Patel et al37 on fine-needle aspiration biopsy specimens, a marked downregulation of FAM129A was found in follicular carcinoma, follicular variant of papillary thyroid carcinoma and oxyphilic follicular carcinomas, compared with matched-normal tissue. The findings by Matsumoto et al36 and Patel et al37 are discordant to those presented by Cerutti et al38 in a recent validation study, which confirmed their previous findings of overexpression of FAM129A in all follicular carcinomas. Bryson et al39 found significant differences in DDIT3 expression in a tissue panel comprising 62 follicular adenomas and 62 follicular carcinomas. However, they determined a sensitivity of 82% with a specificity of only 21%, which is in agreement with our results. A study by Netea-Maier et al40 using different gel electrophoresis found no variance in the abundance of ARG2, FAM129A, DDIT3 and STT3A between follicular carcinoma and follicular adenoma. The authors concluded that the differences in mRNA levels between follicular carcinoma and follicular adenoma as reported by Cerutti et al32 are of limited value, because mRNA expression frequently does not reflect protein abundance. Similarly, a study performing global gene expression analysis of follicular thyroid tumors found no difference in ARG2, DDIT3 and STT3A expression between follicular adenoma and follicular carcinoma.41 In a meta-analysis and meta-review by Griffith et al42 on 21 published thyroid cancer gene expression studies, including the study by Cerutti et al,32 ARG2, DDIT3, STT3A and FAM129A are not among the 39 markers with overlap of three or more candidate markers for the cancer versus non-cancer group.
Our insight into gene expression in tumor cells has increased rapidly with the help of DNA and protein microarray analyses. However, the interpretation of the acquired data is challenging, and is subject to several potential pitfalls. The importance of a close collaboration between the investigators performing the microarrays and experienced biostatisticians is crucial.43 Morphology is still the gold standard when evaluating thyroid neoplasms. Indeed, for the majority of thyroid tumors, the identification of “abnormal” tissue on histological preparations will probably long remain a prerequisite before performing immunohistochemistry or molecular analysis. Unfortunately, as this study demonstrates, the search for the ideal marker capable of discriminating between follicular carcinoma and follicular adenoma must continue.
In conclusion, in this study, we determined whether screening for the expression of the biomarkers DDIT3, STT3A, ARG2 and FAM129A, is able to distinguish between follicular thyroid adenoma and carcinoma. Regrettably, using both immunohistochemistry and western blotting, we demonstrate that these markers are not useful for this purpose. The reasons for the discordance between our results and those of Cerutti et al31, 32 are, at present, and despite considerable focus on quality assurance of all steps, unknown. In our hands, the method is not usable for screening thyroid nodules in patient populations. The failure of this study to replicate a previously published method highlights the need for extensive validation studies to determine if a previously advanced classifier has clinical utility in an independent set of cases.
Acknowledgments
We deeply appreciate and thank Ms Ellen Hellesylt for her great work in the tissue processing and staining. This study was supported by Grant from the Norwegian Cancer Society and South-Eastern Norway Regional Health Authority, project no 2009008.
The authors declare no conflict of interest.
References
- Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118:282–289. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- Suen KC. Fine-needle aspiration biopsy of the thyroid. CMAJ. 2002;167:491–495. [PMC free article] [PubMed] [Google Scholar]
- Hamberger B, Gharib H, Melton LJ, III, et al. Fine-needle aspiration biopsy of thyroid nodules. Impact on thyroid practice and cost of care. Am J Med. 1982;73:381–384. [PubMed] [Google Scholar]
- Fadda G, Rabitti C, Minimo C, et al. Morphologic and planimetric diagnosis of follicular thyroid lesions on fine needle aspiration cytology. Anal Quant Cytol Histol. 1995;17:247–256. [PubMed] [Google Scholar]
- Fadda G, Balsamo G, Fiorino MC, et al. Follicular thyroid lesions and risk of malignancy: a new diagnostic classification on fine-needle aspiration cytology. J Exp Clin Cancer Res. 1998;17:103–107. [PubMed] [Google Scholar]
- Baloch ZW, Fleisher S, Livolsi VA, et al. Diagnosis of ‘follicular neoplasm': a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002;26:41–44. doi: 10.1002/dc.10043. [DOI] [PubMed] [Google Scholar]
- Yang GC, Liebeskind D, Messina AV. Should cytopathologists stop reporting follicular neoplasms on fine-needle aspiration of the thyroid. Cancer. 2003;99:69–74. doi: 10.1002/cncr.10957. [DOI] [PubMed] [Google Scholar]
- Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–516. doi: 10.1002/cncr.23116. [DOI] [PubMed] [Google Scholar]
- Schlinkert RT, van Heerden JA, Goellner JR, et al. Factors that predict malignant thyroid lesions when fine-needle aspiration is ‘suspicious for follicular neoplasm. Mayo Clin Proc. 1997;72:913–916. doi: 10.1016/S0025-6196(11)63360-0. [DOI] [PubMed] [Google Scholar]
- Goodell WM, Saboorian MH, Ashfaq R. Fine-needle aspiration diagnosis of the follicular variant of papillary carcinoma. Cancer. 1998;84:349–354. doi: 10.1002/(sici)1097-0142(19981225)84:6<349::aid-cncr6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998;8:377–383. doi: 10.1089/thy.1998.8.377. [DOI] [PubMed] [Google Scholar]
- Carpi A, Menchini FF, Ferrari E, et al. Aspiration needle biopsy in preoperative selection of thyroid nodules defined at fine-needle aspiration as microfollicular lesions. Am J Clin Oncol. 1999;22:65–69. doi: 10.1097/00000421-199902000-00016. [DOI] [PubMed] [Google Scholar]
- Raber W, Kaserer K, Niederle B, et al. Risk factors for malignancy of thyroid nodules initially identified as follicular neoplasia by fine-needle aspiration: results of a prospective study of one hundred twenty patients. Thyroid. 2000;10:709–712. doi: 10.1089/10507250050137806. [DOI] [PubMed] [Google Scholar]
- Renshaw AA. Follicular lesions of the thyroid. Am J Clin Pathol. 2001;115:782–785. [PubMed] [Google Scholar]
- Matesa N, Tabain I, Dabelic N, et al. Diagnostic relevance of fine needle aspiration cytology for follicular lesions of the thyroid: retrospective study. Croat Med J. 2002;43:606–609. [PubMed] [Google Scholar]
- Stelow EB, Bardales RH, Crary GS, et al. Interobserver variability in thyroid fine-needle aspiration interpretation of lesions showing predominantly colloid and follicular groups. Am J Clin Pathol. 2005;124:239–244. doi: 10.1309/P5MF-MA7U-MFUL-2KWT. [DOI] [PubMed] [Google Scholar]
- Deveci MS, Deveci G, Livolsi VA, et al. Fine-needle aspiration of follicular lesions of the thyroid. Diagnosis and follow-Up. Cytojournal. 2006;3:9. doi: 10.1186/1742-6413-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijovic T, Rochon L, Gologan O, et al. Fine-needle aspiration biopsies in the management of indeterminate follicular and Hurthle cell thyroid lesions. Otolaryngol Head Neck Surg. 2009;140:715–719. doi: 10.1016/j.otohns.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Layfield LJ, Morton MJ, Cramer HM, et al. Implications of the proposed thyroid fine-needle aspiration category of ‘follicular lesion of undetermined significance': A five-year multi-institutional analysis. Diagn Cytopathol. 2009;37:710–714. doi: 10.1002/dc.21093. [DOI] [PubMed] [Google Scholar]
- Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009;117:195–202. doi: 10.1002/cncy.20029. [DOI] [PubMed] [Google Scholar]
- Barden CB, Shister KW, Zhu B, et al. Classification of follicular thyroid tumors by molecular signature: results of gene profiling. Clin Cancer Res. 2003;9:1792–1800. [PubMed] [Google Scholar]
- Aldred MA, Huang Y, Liyanarachchi S, et al. Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. J Clin Oncol. 2004;22:3531–3539. doi: 10.1200/JCO.2004.08.127. [DOI] [PubMed] [Google Scholar]
- Finley DJ, Zhu B, Barden CB, et al. Discrimination of benign and malignant thyroid nodules by molecular profiling. Ann Surg. 2004;240:425–436. doi: 10.1097/01.sla.0000137128.64978.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz CC, Gallagher LA, Finley DJ, et al. Molecular analysis of minimally invasive follicular carcinomas by gene profiling. Surgery. 2005;138:1042–1048. doi: 10.1016/j.surg.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Fryknas M, Wickenberg-Bolin U, Goransson H, et al. Molecular markers for discrimination of benign and malignant follicular thyroid tumors. Tumour Biol. 2006;27:211–220. doi: 10.1159/000093056. [DOI] [PubMed] [Google Scholar]
- Pagedar NA, Chen DH, Wasman JK, et al. Molecular classification of thyroid nodules by cytology. Laryngoscope. 2008;118:692–696. doi: 10.1097/MLG.0b013e31815ed0ff. [DOI] [PubMed] [Google Scholar]
- Wiseman SM, Melck A, Masoudi H, et al. Molecular phenotyping of thyroid tumors identifies a marker panel for differentiated thyroid cancer diagnosis. Ann Surg Oncol. 2008;15:2811–2826. doi: 10.1245/s10434-008-0034-8. [DOI] [PubMed] [Google Scholar]
- Griffith OL, Chiu CG, Gown AM, et al. Biomarker panel diagnosis of thyroid cancer: a critical review. Expert Rev Anticancer Ther. 2008;8:1399–1413. doi: 10.1586/14737140.8.9.1399. [DOI] [PubMed] [Google Scholar]
- Cerutti JM, Latini FR, Nakabashi C, et al. Diagnosis of suspicious thyroid nodules using four protein biomarkers. Clin Cancer Res. 2006;12:3311–3318. doi: 10.1158/1078-0432.CCR-05-2226. [DOI] [PubMed] [Google Scholar]
- Cerutti JM, Delcelo R, Amadei MJ, et al. A preoperative diagnostic test that distinguishes benign from malignant thyroid carcinoma based on gene expression. J Clin Invest. 2004;113:1234–1242. doi: 10.1172/JCI19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLellis RA, Lloyd RV, Heitz PU, et al. (eds).WHO Classification of Tumors. Pathology and Genetics. Tumours of Endocrine Organs IARC Press: Lyon, France; 2004 [Google Scholar]
- DiVito KA, Charette LA, Rimm DL, et al. Long-term preservation of antigenicity on tissue microarrays. Lab Invest. 2004;84:1071–1078. doi: 10.1038/labinvest.3700131. [DOI] [PubMed] [Google Scholar]
- Haataja L, Gurlo T, Huang CJ, et al. Many commercially available antibodies for detection of CHOP expression as a marker of endoplasmic reticulum stress fail specificity evaluation. Cell Biochem Biophys. 2008;51:105–107. doi: 10.1007/s12013-008-9019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto F, Fujii H, Abe M, et al. A novel tumor marker, Niban, is expressed in subsets of thyroid tumors and Hashimoto's thyroiditis. Hum Pathol. 2006;37:1592–1600. doi: 10.1016/j.humpath.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Patel MR, Stadler ME, Deal AM, et al. STT3A, C1orf24, TFF3: Putative Markers for Characterization of Follicular Thyroid Neoplasms From Fine-Needle Aspirates. Laryngoscope. 2011;121:983–989. doi: 10.1002/lary.21736. [DOI] [PubMed] [Google Scholar]
- Cerutti JM, Oler G, Delcelo R, et al. PVALB, a new Hurthle adenoma diagnostic marker identified through gene expression. J Clin Endocrinol Metab. 2011;96:E151–E160. doi: 10.1210/jc.2010-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson PC, Shores CG, Hart C, et al. Immunohistochemical distinction of follicular thyroid adenomas and follicular carcinomas. Arch Otolaryngol Head Neck Surg. 2008;134:581–586. doi: 10.1001/archotol.134.6.581. [DOI] [PubMed] [Google Scholar]
- Netea-Maier RT, Hunsucker SW, Hoevenaars BM, et al. Discovery and validation of protein abundance differences between follicular thyroid neoplasms. Cancer Res. 2008;68:1572–1580. doi: 10.1158/0008-5472.CAN-07-5020. [DOI] [PubMed] [Google Scholar]
- Hinsch N, Frank M, Doring C, et al. QPRT: a potential marker for follicular thyroid carcinoma including minimal invasive variant; a gene expression, RNA and immunohistochemical study. BMC Cancer. 2009;9:93. doi: 10.1186/1471-2407-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OL, Melck A, Jones SJ, et al. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J Clin Oncol. 2006;24:5043–5051. doi: 10.1200/JCO.2006.06.7330. [DOI] [PubMed] [Google Scholar]
- Simon R, Radmacher MD, Dobbin K, et al. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J Natl Cancer Inst. 2003;95:14–18. doi: 10.1093/jnci/95.1.14. [DOI] [PubMed] [Google Scholar]