Abstract
Purpose
This study aimed to investigate the clinical significance of chromosome 17 centromere (CEP17) multiplication (increased copy number of CEP17) related to human epidermal growth factor receptor 2 (HER2) and topoisomerase II alpha (TOP2A) status in patients with invasive breast cancer.
Methods
We constructed tissue microarrays using 594 invasive breast cancer samples and performed single-color silver-enhanced in situ hybridization (SISH) assay for HER2, TOP2A, and CEP17 to assess for copy number aberrations. The association of CEP17 multiplication with patient survival was analyzed according to HER2 and TOP2A status.
Results
Among 567 informative cases, HER2 amplification was noted in 22.8%, TOP2A amplification in 8.3% and TOP2A deletion in 11.1%. CEP17 multiplication was identified in 33.2% and was significantly associated with worse overall survival (OS) (p=0.02) and disease-free survival (DFS) (p=0.02). CEP17 multiplication correlated with patient survival in patients with normal TOP2A or non-amplified HER2 status, but the prognostic significance was lost in those with altered TOP2A or amplified HER2. On multivariate analyses, CEP17 multiplication was an independent prognostic factor for poorer OS (p=0.02) and DFS (p=0.01) in patients with normal TOP2A and non-amplified HER2.
Conclusion
CEP17 multiplication was identified as a promising prognostic marker in patients with invasive breast cancer exhibiting either non-amplified HER2 or normal TOP2A status.
Keywords: Breast neoplasms, Chromosome 17, HER2 gene, In situ hybridization, Topoisomerase II alpha
INTRODUCTION
Breast cancer is the second most prevalent cancer among newly developed cancers in Korean women and the 5-year survival rates of breast cancer have notably improved in recent years [1]. Human epidermal growth factor receptor 2 (HER2) and topoisomerase II alpha (TOP2A) have been known as predictive markers for benefit of anthracyclines [2-7]. However, for the issue of an increased copy number of the chromosome 17 centromere (CEP17) (called CEP17 multiplication in this study), its significance in breast cancer outcome and response to specific chemotherapy regimens is still uncertain, especially in patients with non-amplified HER2 or normal TOP2A status.
The TOP2A gene is located on chromosome 17q21 and the TOP2A protein is a key enzyme for DNA replication, cell cycle progression and chromosome segregation and it is a molecular target for anthracyclines [8]. TOP2A is near HER2 on chromosome 17; therefore co-amplification of both genes is not uncommon [9]. About 40-90% of TOP2A-amplified tumors showed amplification of HER2 as well, and one third of HER2-positive tumors were TOP2A-amplified [8,10]. Studies have reported that TOP2A amplification or alteration (either amplification or deletion) was associated with a favorable response to anthracycline- containing therapy [2,5,11]. However, in a recent study, CEP17 multiplication was shown to be a predictor of anthracycline benefit whereas there was no significant correlation between HER2 or TOP2A status and anthracycline benefit [12].
Polysomy indicates that the number of a particular chromosome is greater than diploid and it has been represented by ≥3 signals in fluorescent in situ hybridization (FISH) assays with a probe targeted to the centromeric area of the particular chromosome [13]. Recent studies reported that chromosome 17 polysomic cases defined by multiplication of CEP17 in FISH assays were frequently related to 17q gain involving centromeres or amplification of the centromeric region rather than whole chromosome multiplication (true chromosome 17 polysomy) [13,14]. Therefore, CEP17 multiplication by in situ hybridization does not indicate true chromosomal 17 polysomy in all cases. As HER2 gene amplification and TOP2A alteration are determined by the HER2 to CEP17 ratio and the TOP2A to CEP17 ratio by FISH or the silver-enhanced in situ hybridization (SISH) method, increased number of CEP17signals due to gain or amplification of the centromeric regions of chromosome 17 (not true polysomy) may provide misleading HER2 or TOP2A gene status assessment results [13,15]. This consideration may explain at least in part the conflicting reports about the clinical implications of TOP2A alteration or HER2 amplification [15]. CEP17 multiplication in the absence of HER2 amplification or TOP2A alteration is not a rare event, but few studies on its clinical significance related to HER2 or TOP2A status have been completed [16].
This study aimed to investigate the clinical significance of CEP17 multiplication related to TOP2A alteration and HER2 amplification in patients with invasive breast cancers by correlating CEP17 multiplication with prognostic and predictive pathologic parameters and patient survival.
METHODS
Case selection and construction of tissue microarray blocks
For this study, we collected 594 primary invasive breast cancer cases which were treated surgically at Yeungnam University Hospital, Daegu, South Korea between January 1995 and January 2004. We reviewed the slides of all cases and selected a representative tumor block per case for the construction of tissue microarrays (TMAs). A pair of 2-mm-diameter tissue cores were retrieved from each tumor block and transferred to the recipient block (Accumax™ array; ISU Abxis, Seoul, Korea). Thirteen TMA blocks were created from 594 tumor blocks. The patient age at initial diagnosis, tumor size, histological tumor grade [17], lymph node status, surgery type, adjuvant chemotherapy regimens and follow-up data were obtained from the pathology reports and patients' medical records. This study was approved by the Institutional Review Board of Yeungnam University Hospital (PCR-10-132).
Immunohistochemistry
Four-micrometer-thick TMA sections were immunostained for estrogen receptor (ER) (SP1, CONFIRM™, rabbit monoclonal; Ventana Medical Systems, Tucson, USA) and progesterone receptor (PR) (1E2, CONFIRM™, rabbit monoclonal; Ventana Medical Systems) with UltraView™ universal DAB detection kit (Ventana Medical Systems). Immunohistochemistry (IHC) was performed on the automated Benchmark® platform (Ventana Medical Systems) according to the manufacturer's recommendations. The staining results for ER and PR were considered positive if there was ≥1% positive tumor nuclei within the tumor according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guideline [18].
Single-color SISH analysis
Three tissue sections of 4 µm-thickness per case were prepared for the SISH analysis. SISH was performed using INFORM® TOP2A DNA, INFORM® HER2 DNA and Chromosome 17 (CEP17) Probes (Ventana Medical Systems) using the Ventana Benchmark® series of automated slide stainer. Probes for HER2, TOP2A, and CEP17 were labeled with dinitrophenol (DNP). The HER2 DNA probe was denatured at 95℃ for 12 minutes and hybridization was performed at 52℃ for 2 hours. After hybridization, an appropriate stringency wash (three times at 72℃) was performed. The TOP2A DNA probe was denatured at 80℃ for 12 minutes and hybridization was performed at 44℃ for 2 hours. After hybridization, an appropriate stringency wash (one time at 72℃) was performed. The CEP17 probe was denatured at 95℃ for 12 minutes and hybridization was performed at 44℃ for 2 hours. After hybridization, appropriate stringency washes were performed three times at 59℃. The HER2, TOP2A, and CEP17 DNP-labeled probes were visualized by using the rabbit anti-DNP primary antibody and the UltraView™ SISH Detection Kit, which contained a goat anti-rabbit antibody conjugated to horseradish peroxidase utilized as the chromogenic enzyme. Silver precipitation was deposited in the nuclei following the sequential addition of silver acetate, hydroquinone, and H2O2. The slides were then counterstained with Ventana hematoxylin II for interpretation by light microscopy.
The hybridization signals for HER2, TOP2A and CEP17 were counted in more than 20 non-overlapping nuclei per case. Normal HER2, TOP2A or CEP17 signals of endothelial cells, stromal fibroblasts, and lymphocytes served as the internal positive control. A discrete dot was counted as a single copy of HER2, TOP2A or CEP17. The size of these single dots was used as a reference to determine the relative number of amplified copies in cancer cell nuclei. A small cluster of multiple signals was counted as six signals and a large cluster was counted as 12 signals according to the manufacturer's instructions. The HER2/CEP17 and TOP2A/CEP17 ratios were calculated in each case. HER2 amplification was defined when the HER2/CEP17 ratio was >2.2, equivocal to HER2 amplification was defined when the HER2/CEP17 ratio was 1.8-2.2 and negative for HER2 amplification was defined when the HER2/CEP17 ratio was <1.8 [19]. We categorized equivocal cases as HER2-amplified cases when the average HER2 signal per nucleus is >6 and equivocal cases with ≤6 HER2 signal per nucleus were categorized as negative for HER2 amplification for this study. TOP2A amplification was defined when the TOP2A/CEP17 ratio was ≥2.0 and TOP2A deletion was defined when the TOP2A/ CEP17 ratio was ≤0.8. If the ratio of TOP2A/CEP17 was between 0.8 and 2.0, we considered the case as having normal TOP2A [5]. We defined CEP17 multiplication when the cases show increased copy number for CEP17 (>2 signals/nucleus) in SISH assay [6].
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, USA). Chi-square test and Fisher's exact test were used to determine correlations between CEP17 multiplication and clinicopathological parameters. Overall survival (OS) and disease-free survival (DFS) for the groups defined by TOP2A or HER2 status and CEP17 multiplication were plotted using Kaplan-Meier survival curves analyzed by the log-rank test. We obtained the hazard ratios and associated 95% confidence intervals using the Cox univariate model to compare groups defined by clinicopathological parameters. Multivariate analysis was carried out using Cox's regression. A p-value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics
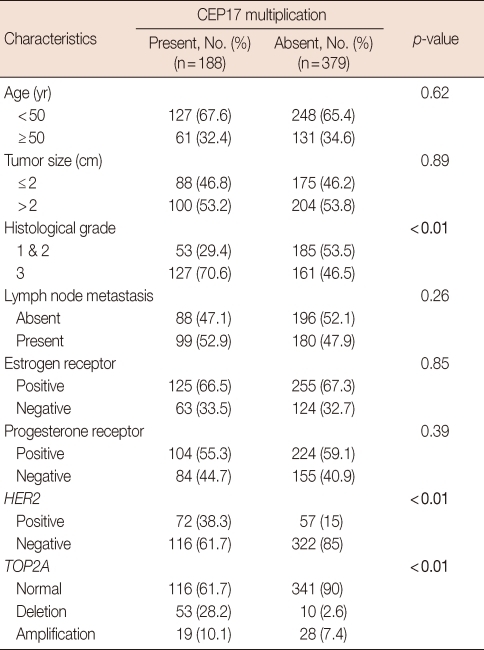
Of 594 invasive breast cancer samples, we obtained immunohistochemical and SISH results from 567 cases due to noninformative cores by acquisition tissue cores from non-neoplastic areas or loss of cores while performing immunohistochemical or SISH analysis. Among 567 patients, 174 underwent breast conserving surgery and 393 underwent mastectomy. Patient ages ranged from 20 to 85 years (mean, 47.1 years). The histological types included invasive ductal carcinoma, not otherwise specified (513 cases, 90.5%); invasive lobular carcinoma (20 cases, 3.5%); invasive micropapillary carcinoma (14 cases, 2.5%); mucinous carcinoma (9 cases, 1.6%); medullary carcinoma (4 cases, 0.7%); invasive tubular carcinoma (4 cases, 0.7%); invasive papillary carcinoma (2 cases, 0.4%); and invasive cribriform carcinoma (1 case, 0.2%). Tumor sizes varied from 0.5 to 11 cm (mean, 2.5 cm).
Among 567 patients, 263 (46.4%) were pT1, 280 (49.4%) were pT2 and 24 (4.2%) were pT3. At the time of surgery, 279 (49.2%) patients had positive lymph nodes. The histological grade was available in 526 cases; 92 (16.2%) were grade 1, 146 (25.7%) were grade 2, and 288 (50.8%) were grade 3. For adjuvant chemotherapy, 346 patients (61%) received anthracycline-based chemotherapy including combined 5-fluorouracil (5-FU), epirubicin and cyclophosphamide (CEF); combined 5-FU, doxorubicin and cyclophosphamide; combined doxorubicin and cyclophosphamide; or combined epirubicin and taxol. Another 142 patients (25%) received chemotherapeutic regimens including combined cyclophosphamide, methotrexate and 5-FU (CMF); taxol alone; oral 5-FU alone; or oral furtulon alone. The remaining 79 patients had no chemotherapy. The mean follow-up period was 87.4 months (range, 7-170 months). Patient characteristics are summarized in Table 1.
Table 1.
Characteristics of cases
*Mean (range).
IHC and SISH results
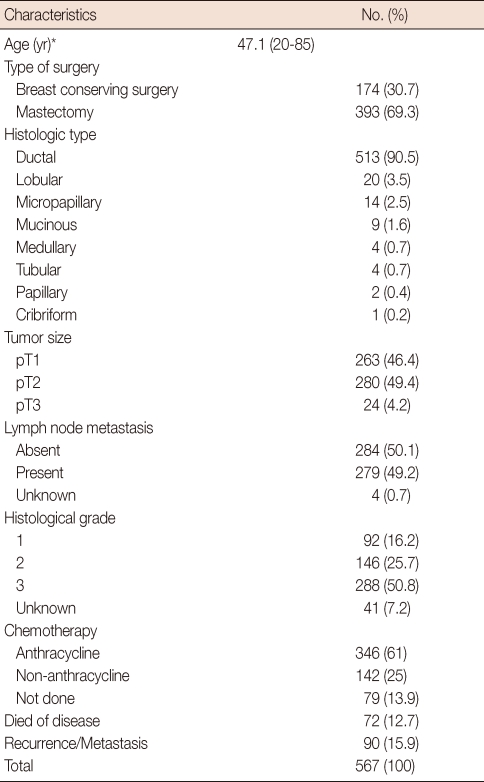
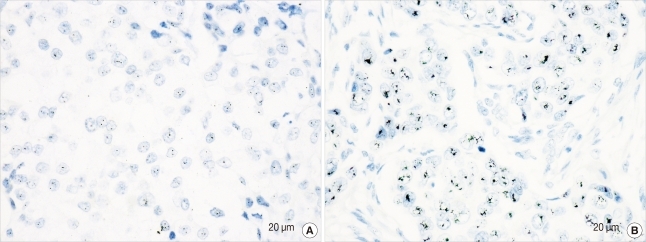
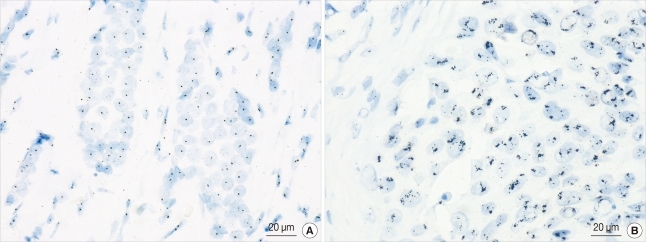
We interpreted the staining results in both cores to obtain a representative result for each parameter. Among 567 informative cases, ER was positive in 380 (67%) and PR was positive in 328 (57.8%) cases. A total of 129 (22.8%) cases had HER2 gene amplification (Figure 1). For the TOP2A gene, 457 (80.6%) had normal TOP2A, 47 (8.3%) had TOP2A amplification and 63 (11.1%) had TOP2A deletion (Figure 2). Multiplication of CEP17 was identified in 188 (33.2%) cases (Figure 3).
Figure 1.
Silver-enhanced in situ hybridization results for HER2. (A) Negative for HER2 amplification. The average number of HER2 signals per nucleus was 1.9 and the HER2/CEP17 ratio was 0.95 (×400). (B) Positive for HER2 amplification. The average number of HER2 signals per nucleus was 20 and the HER2/CEP17 ratio was 9.62 (×400).
Figure 2.
Silver-enhanced in situ hybridization results for TOP2A. (A) Deletion of TOP2A. The majority of tumor cells have single signal for TOP2A (×400). The average number of TOP2A signals per nucleus was 1.2 and the TOP2A/CEP17 ratio was 0.7. (B) Amplification of the TOP2A gene. Hybridization signals are conglomerated (×400). The average number of TOP2A signals per nucleus was 20 and the TOP2A/CEP17 ratio was 17.4.
Figure 3.
Silver-enhanced in situ hybridization results for CEP17. (A) Normal CEP17 signals. The majority of cells have one or two hybridization signals (×400). The average number of CEP17 signals per nucleus was 1.65. (B) CEP17 multiplication. The average number of CEP17 signals per nucleus was 3.4 in this case (×400).
Association between HER2 and TOP2A status
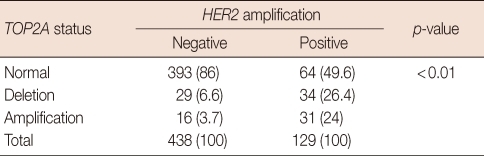
TOP2A alteration was more frequent in patients with amplified HER2 than in those with non-amplified HER2. Of 129 HER2-amplified tumors, TOP2A deletion and amplification were observed in 34 (26.4%) and 31 (24%), respectively. In contrast, of 438 cases with non-amplified HER2, TOP2A deletion and amplification were observed in 29 (6.6%) and 16 (3.7%), respectively (Table 2).
Table 2.
Correlation between HER2 status and TOP2A status
HER2=human epidermal growth factor receptor 2; TOP2A=topoisomerase II alpha.
Association of CEP17 multiplication with tumor characteristics and HER2 or TOP2A status
Multiplication of CEP17 was associated with high histological grade (p<0.01), HER2 amplification (p<0.01), and TOP2A alteration (p<0.01). HER2-amplified tumors were twice as likely to have CEP17 multiplication as were those without HER2 amplification (55.8% [72/129] vs. 26.5% [116/438]). TOP2A-amplified and -deleted tumors were also significantly more likely to have CEP17 multiplication than were those that show normal TOP2A status (40.4% [19/47] and 84.1% [53/63] vs. 25.4% [116/457], respectively) (Table 3).
Table 3.
Association of CEP17 multiplication with clinicopathological features
CEP17=chromosome 17 centromere; HER2=human epidermal growth factor receptor 2; TOP2A=topoisomerase II alpha.
Association of TOP2A or HER2 status and CEP17 multiplication with patient survival
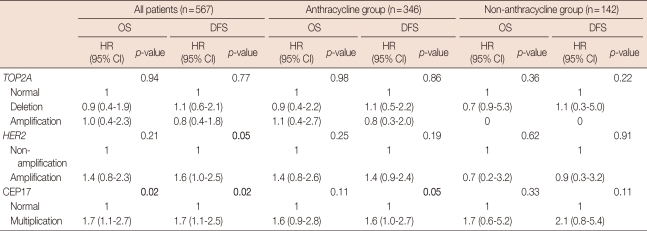
When TOP2A status was compared with patient survival, there was no statistically significant difference of OS and DFS between the TOP2A-amplified, TOP2A-deleted and TOP2A-normal groups. The patients with amplified HER2 showed poorer DFS than those with non-amplified HER2, but the difference was not statistically significant for OS. Multiplication of CEP17 was associated with a poor prognosis in all patients, but the survival difference was lost in subgroups by the chemotherapy regimen (Table 4).
Table 4.
Association of TOP2A, HER2, and CEP17 multiplication with patient survival
CEP17=chromosome 17 centromere; HER2=human epidermal growth factor receptor 2; TOP2A=topoisomerase II alpha; OS=overall survival; DFS=disease-free survival; HR=hazard ratio; CI=confidence interval.
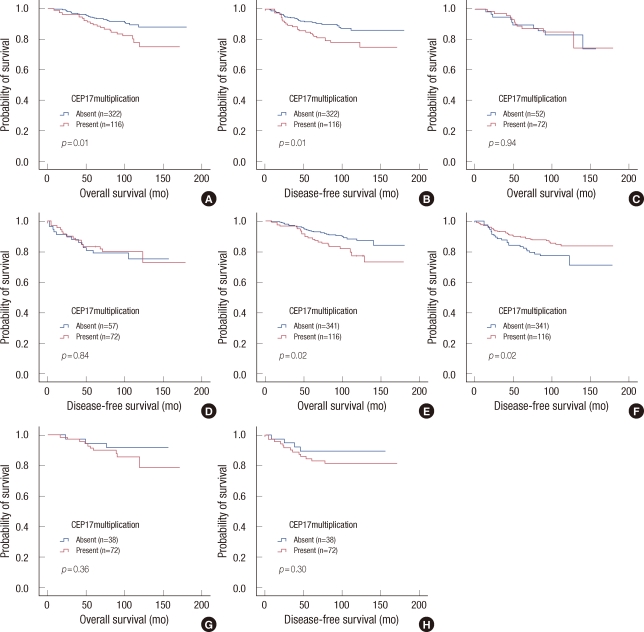
In patients with non-amplified HER2, CEP17 multiplication was associated with worse OS (p=0.01) and DFS (p=0.01). However, CEP17 multiplication did not correlate with survival in patients with amplified HER2. In patients with normal TOP2A status, CEP17 multiplication was significantly associated with worse OS (p=0.02) and DFS (p=0.02), but it was not associated with either OS or DFS in patients with TOP2A alteration (Figure 4).
Figure 4.
Kaplan-Meier curves of overall survival and disease-free survival according to CEP17 copy number. (A, B) Survival curves of patients with tumors exhibiting non-amplified HER2. (C, D) Survival curves in patients with tumors exhibiting amplified HER2 status. (E, F) Survival curves of patients with tumors exhibiting normal TOP2A. (G, H) Survival curves of patients with tumors exhibiting altered TOP2A status.
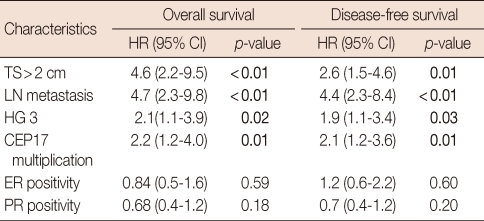
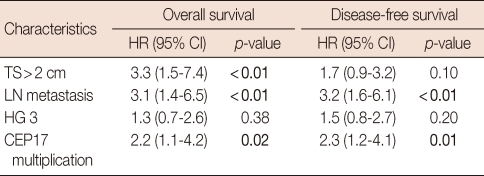
In patients with both non-amplified HER2 and normal TOP2A status (n=393), tumor size, lymph node status, histological grade and CEP17 multiplication correlated with OS and DFS in univariate analyses (Table 5). The prognostic significance of CEP17 multiplication was also observed in patients treated with anthracyclines (n=221) (OS, p=0.03; DFS, p=0.01). The survival differences in both OS and DFS according to CEP17 multiplication were apparent, but not statistically significant in patients treated with non-anthracyclines (n=104) (OS, p=0.30; DFS, p=0.10). In multivariate analyses, CEP17 multiplication was an independent prognostic factor for poor OS (p=0.02) and DFS (p=0.01) together with large tumor size and lymph node metastasis in patients with both normal TOP2A and non-amplified HER2 status regardless of treatment type (Table 6).
Table 5.
Effect of tumor characteristics on disease-free and overall survivals in patients with tumors exhibiting normal TOP2A and HER2 status (n=393)
TOP2A=topoisomerase II alpha; HER2=human epidermal growth factor receptor 2; HR=hazard ratio; CI=confidence interval; TS=tumor size; LN=lymph node; HG=histological grade; CEP17=chromosome 17 centromere; ER=estrogen receptor; PR=progesterone receptor.
Table 6.
Multivariate analysis of factors associated with disease-free and overall survivals in patients with tumors exhibiting normal TOP2A and HER2 status
TOP2A=topoisomerase II alpha; HER2=human epidermal growth factor receptor 2; HR=hazard ratio; CI=confidence interval; TS=tumor size; LN=lymph node; HG=histological grade; CEP17=chromosome 17 centromere.
DISCUSSION
HER2 gene amplification or HER2 protein overexpression has been considered predictive of a favorable response to anthracycline chemotherapy [20-22]. However, recent studies indicated that such an association between HER2 and anthracycline is indirect and could be mediated through TOP2A [4-6]. TOP2A aberrations were initially reported in HER2-amplified tumors [2]. The proximity of TOP2A and HER2 genes in chromosome 17 has led to the conception of co-amplification of a whole amplicon containing both genes [9]. TOP2A amplification and deletion have been observed with variable frequencies in other studies. TOP2A amplification was noted in 24.3-54% of HER2-positive tumors and 0-6.4% of HER2-negative tumors, whereas TOP2A deletion was observed in 8.1-35% of HER2-positive tumors and 0-11.7% of HER2-negative tumors [9].
The results of the present study corresponded well with those of earlier studies. TOP2A amplification was observed in 24% of HER2-amplified tumors and 3.7% of HER2-non-amplified tumors. TOP2A deletion was identified in 26.4% of HER2-amplified tumors and 6.6% of HER2-non-amplified tumors. The discrepant prevalence of TOP2A alterations according to HER2 status can result from technical differences in measuring alterations such as cutoffs used for defining amplification or deletion. We used the same criteria to define TOP2A alterations as O'Malley et al. [5] did and evaluated TOP2A status using a SISH method. SISH has been introduced as an alternative to FISH in evaluating HER2 gene status in recent years and several studies have reported good concordance between SISH and FISH results in breast cancers [23-26]. Because SISH visualizes hybridization signals as light opaque silver instead of fluorescent spots, interpretation of SISH results can be performed on a conventional light microscope. Therefore, SISH would be more appropriate for pathology laboratories using a large number of samples.
To the best of our knowledge, this is the first study to use the SISH method to evaluate TOP2A status. In our earlier study, we obtained a concordance rate of 93.7% between SISH and FISH for TOP2A status in 206 invasive breast cancer samples (unpublished data). The ASCO/CAP developed a guideline to improve the accuracy of HER2 testing in breast cancers [19]. As in the case of HER2 testing, standardization of the test method and criteria for defining TOP2A alterations is necessary to discuss clinical implications of TOP2A alterations. In addition, SISH method needs to be validated for TOP2A testing.
The prognostic and predictive value of TOP2A alterations remains controversial. In the current study, TOP2A amplification, deletion or combined alterations were not predictive of DFS or OS. An earlier in vitro study suggested that the sensitivity to TOP2A inhibitors is dependent on the expression levels of TOP2A protein and that TOP2A gene amplification leads to better response to anthracycline [10]. Cardoso et al. [3] measured TOP2A protein expression and TOP2A gene amplification using immunohistochemistry and FISH and found that TOP2A expression, but not TOP2A gene amplification, was correlated with response to anthracyclines. Knoop et al. [4] reported that patients with TOP2A amplification had an increased DFS and OS in the subgroup treated with CEF compared to the subgroup treated with CMF. O'Malley et al. [5] reported that TOP2A status was not associated with clinical outcomes but that treatment with CEF was significantly superior to treatment with CMF in patients whose tumors showed TOP2A alterations (either amplification or deletion). Mukherjee et al. [7] reported that TOP2A protein expression strongly correlated with pathological complete response to neoadjuvant anthracyclines in locally advanced primary breast cancers.
Bartlett et al. [12] reported that there was no significant interaction between anthracycline benefit and HER2 or TOP2A alteration but that CEP17 duplication (average CEP17 signals/cell >1.86) was a predictive biomarker of anthracycline benefit. One of the most interesting findings to us in their study to us was that CEP17 duplication was significantly associated with OS and relapse-free survival regardless of chemotherapy regimen. We also found that CEP17 multiplication (average CEP17 signals/cell >2) was associated with poor OS and DFS irrespective of treatment regimen. CEP17 multiplication unrelated to HER2 amplification or TOP2A alteration was an independent prognostic factor for poor clinical outcome in multivariate analysis, but its prognostic significance disappeared in patients with TOP2A alteration or HER2 amplification. We were not able to compare OS and DFS between the anthracycline and non-anthracycline groups according to HER2 and TOP2A status and CEP17 multiplication due to the limited number of study subjects who received non-anthracyclines and the imbalance of clinicopathological factors between the two groups.
Although true chromosome 17 polysomy is a rare event, CEP17 multiplication is not uncommon in breast cancers [27, 28]. To date, an aberrant copy number of CEP17 in FISH analyses has been described as chromosome 17 polysomy or aneusomy. Marchiò et al. [13] reported that only one of 18 CEP17 polysomic cases (increased copy number of CEP17 by FISH) was true chromosome 17 polysomy by microarray-based comparative genomic hybridization and FISH for HER2 (17q12), CEP17, SMS (17p11.2), and RARA (17q21.2). Another 17 polysomic cases showed a gain of 17q with involvement of the centromere, 17q gain sparing the centromeric region, or amplification of the centromeric region rather than true chromosome 17 polysomy. For this reason, we described an increased copy number of CEP17 signals in SISH analysis as CEP17 multiplication instead of using the traditional term, chromosome 17 polysomy. The prognostic value of an aberrant copy number of CEP17 (described as chromosome 17 polysomy or aneusomy in previous reports) has been reported in a limited number of studies.
Krishnamurti et al. [16] reported that HER2-unamplified chromosome 17 polysomy (CEP17 signals/cell ≥3) was associated with several adverse prognostic indicators such as a higher nuclear grade, mitotic activity, Nottingham score, histological grade, tumor stage, and estrogen receptor negativity. However, they did not correlate chromosome 17 polysomy with clinical outcome due to the small number of cases with HER2-unamplified chromosome 17 polysomy. Watters et al. [27] reported that aneusomy 17 (CEP17 signals/cell <1.35 or >1.86) was associated with high grade, ER negativity, and Nottingham prognostic index >5.4, but was not associated with survival by univariate analysis. As seen in the studies of TOP2A alteration, the criteria defining chromosome 17 polysomy varied in different studies [28]. However, it needs to be standardized for the assessment of clinical significance of CEP17 multiplication in further studies.
The current study did not elucidate the exact mechanism underlying the association between CEP17 multiplication unrelated to HER2 amplification and TOP2A alteration and poor prognosis of breast cancer patients. The adverse clinical outcome could be secondary to activation of unknown oncogenes that reside in the locus of chromosome 17 which is close to the CEP17 region and is frequently involved in subchromosomal duplication or amplification (represented by CEP17 multiplication on SISH analysis).
In conclusion, CEP17 multiplication was associated with worse OS and DFS in patients with invasive breast cancers exhibiting either non-amplified HER2 or normal TOP2A status. Validation in a larger population is needed to provide confirmatory evidence for the adoption of CEP17 status as a promising prognostic biomarker in routine clinical practice of breast cancers. Further studies should be performed to examine molecular mechanism underlying the association between CEP17 multiplication and adverse clinical outcome.
ACKNOWLEDGMENTS
The authors thank E.H. Lee for her technical assistance.
Footnotes
This research was supported by the Yeungnam University research grants in 2010.
The authors declare that they have no competing interests.
References
- 1.Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010;25:1113–1121. doi: 10.3346/jkms.2010.25.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Järvinen TA, Tanner M, Rantanen V, Bärlund M, Borg A, Grénman S, et al. Amplification and deletion of topoisomerase IIalpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol. 2000;156:839–847. doi: 10.1016/s0002-9440(10)64952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, Durbecq V, Larsimont D, Paesmans M, Leroy JY, Rouas G, et al. Correlation between complete response to anthracycline-based chemotherapy and topoisomerase II-alpha gene amplification and protein overexpression in locally advanced/metastatic breast cancer. Int J Oncol. 2004;24:201–209. [PubMed] [Google Scholar]
- 4.Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, et al. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol. 2005;23:7483–7490. doi: 10.1200/JCO.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 5.O'Malley FP, Chia S, Tu D, Shepherd LE, Levine MN, Bramwell VH, et al. Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst. 2009;101:644–650. doi: 10.1093/jnci/djp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konecny GE, Pauletti G, Untch M, Wang HJ, Möbus V, Kuhn W, et al. Association between HER2, TOP2A, and response to anthracycline-based preoperative chemotherapy in high-risk primary breast cancer. Breast Cancer Res Treat. 2010;120:481–489. doi: 10.1007/s10549-010-0744-z. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee A, Shehata M, Moseley P, Rakha E, Ellis I, Chan S. Topo2alpha protein expression predicts response to anthracycline combination neo-adjuvant chemotherapy in locally advanced primary breast cancer. Br J Cancer. 2010;103:1794–1800. doi: 10.1038/sj.bjc.6605960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Järvinen TA, Tanner M, Bärlund M, Borg A, Isola J. Characterization of topoisomerase II alpha gene amplification and deletion in breast cancer. Genes Chromosomes Cancer. 1999;26:142–150. [PubMed] [Google Scholar]
- 9.Slamon DJ, Press MF. Alterations in the TOP2A and HER2 genes: association with adjuvant anthracycline sensitivity in human breast cancers. J Natl Cancer Inst. 2009;101:615–618. doi: 10.1093/jnci/djp092. [DOI] [PubMed] [Google Scholar]
- 10.Järvinen TA, Liu ET. HER-2/neu and topoisomerase IIalpha in breast cancer. Breast Cancer Res Treat. 2003;78:299–311. doi: 10.1023/a:1023077507295. [DOI] [PubMed] [Google Scholar]
- 11.Burgess DJ, Doles J, Zender L, Xue W, Ma B, McCombie WR, et al. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc Natl Acad Sci U S A. 2008;105:9053–9058. doi: 10.1073/pnas.0803513105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett JM, Munro AF, Dunn JA, McConkey C, Jordan S, Twelves CJ, et al. Predictive markers of anthracycline benefit: a prospectively planned analysis of the UK National Epirubicin Adjuvant Trial (NEAT/BR9601) Lancet Oncol. 2010;11:266–274. doi: 10.1016/S1470-2045(10)70006-1. [DOI] [PubMed] [Google Scholar]
- 13.Marchiò C, Lambros MB, Gugliotta P, Di Cantogno LV, Botta C, Pasini B, et al. Does chromosome 17 centromere copy number predict polysomy in breast cancer? A fluorescence in situ hybridization and microarray-based CGH analysis. J Pathol. 2009;219:16–24. doi: 10.1002/path.2574. [DOI] [PubMed] [Google Scholar]
- 14.Yeh IT, Martin MA, Robetorye RS, Bolla AR, McCaskill C, Shah RK, et al. Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod Pathol. 2009;22:1169–1175. doi: 10.1038/modpathol.2009.78. [DOI] [PubMed] [Google Scholar]
- 15.Viale G. Be precise! The need to consider the mechanisms for CEP17 copy number changes in breast cancer. J Pathol. 2009;219:1–2. doi: 10.1002/path.2593. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamurti U, Hammers JL, Atem FD, Storto PD, Silverman JF. Poor prognostic significance of unamplified chromosome 17 polysomy in invasive breast carcinoma. Mod Pathol. 2009;22:1044–1048. doi: 10.1038/modpathol.2009.61. [DOI] [PubMed] [Google Scholar]
- 17.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 18.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 21.Gennari A, Sormani MP, Pronzato P, Puntoni M, Colozza M, Pfeffer U, et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst. 2008;100:14–20. doi: 10.1093/jnci/djm252. [DOI] [PubMed] [Google Scholar]
- 22.Dhesy-Thind B, Pritchard KI, Messersmith H, O'Malley F, Elavathil L, Trudeau M. HER2/neu in systemic therapy for women with breast cancer: a systematic review. Breast Cancer Res Treat. 2008;109:209–229. doi: 10.1007/s10549-007-9656-y. [DOI] [PubMed] [Google Scholar]
- 23.Dietel M, Ellis IO, Höfler H, Kreipe H, Moch H, Dankof A, et al. Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007;451:19–25. doi: 10.1007/s00428-007-0424-5. [DOI] [PubMed] [Google Scholar]
- 24.Kang J, Kwon GY, Lee YH, Gong G. Comparison of silver-enhanced in situ hybridization and fluorescence in situ hybridization for HER2 gene status in breast carcinomas. J Breast Cancer. 2009;12:235–240. [Google Scholar]
- 25.Kim TJ, Kim TE, Jung ES, Yim HW, Song BJ, Jung SS, et al. The comparison of automated silver in situ hybridization and fluorescence in situ hybridization for evaluating HER2 gene amplification in breast carcinoma. J Breast Cancer. 2009;12:295–301. [Google Scholar]
- 26.Sung WJ, Park SJ, Gu MJ, Bae YK. Automated silver-enhanced in situ hybridization for evaluation of HER2 gene status in breast carcinoma: comparison with fluorescence in situ hybridization and immunohistochemistry. Korean J Pathol. 2010;44:28–34. [Google Scholar]
- 27.Watters AD, Going JJ, Cooke TG, Bartlett JM. Chromosome 17 aneusomy is associated with poor prognostic factors in invasive breast carcinoma. Breast Cancer Res Treat. 2003;77:109–114. doi: 10.1023/a:1021399923825. [DOI] [PubMed] [Google Scholar]
- 28.Reinholz MM, Bruzek AK, Visscher DW, Lingle WL, Schroeder MJ, Perez EA, et al. Breast cancer and aneusomy 17: implications for carcinogenesis and therapeutic response. Lancet Oncol. 2009;10:267–277. doi: 10.1016/S1470-2045(09)70063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]