Abstract
Purpose
The aims of our study were to assess the correlation between serum HER2 and clinicopathologic factors, the effect of serum HER2 on survival rate, and the effect of changes in serum HER2 levels between pre- and post-adjuvant chemotherapy on survival rate.
Methods
The study subjects, 200 women with breast cancer, were a subset of patients operated on between January 2005 and December 2006. We evaluated changes in serum HER2 levels between pre- and post-adjuvant chemotherapy.
Results
Being estrogen receptor (ER) negative was also correlated with high serum HER2 (p=0.017). The number of patients with changes in serum HER2 (>20% increased level during the follow-up period) was correlated with advanced T-stage (p=0.010), advanced American Joint Committee on Cancer (AJCC) stage (p=0.015) and poor histologic grade (p=0.001). Univariate analysis for prognostic factors associated with disease-free survival (DFS) revealed that the difference in DFS between those with serum HER2 level <15 ng/mL and those with levels ≥15 ng/mL was statistically significant (p=0.0129) and the changes in serum HER2 levels were also statistically significant (p=0.001). Prognostic factors associated with overall survival revealed that the changes in serum HER2 levels between pre- and post-adjuvant chemotherapy were statistically significant (p=0.0012).
Conclusion
Serum HER2 level is associated with a more advanced degree of axillary lymph node involvement and associated with ER negativity. And Changes in serum HER2 levels are associated with more advanced AJCC staging and histologic tumor grade. There are significant associations between serum HER2 level, changes in serum HER2 levels and 5-year DFS.
Keywords: Breast neoplasms, Prognosis, Serum HER2
INTRODUCTION
HER2 is overexpressed or amplified in 15% to 25% of breast cancers. Determination of HER2 tumor status offers clinically useful information. HER2 is composed of a cytoplasmic domain with tyrosine kinase activity, a transmembrane domain and an extracellular domain (ECD) [1,2]. The HER2 ECD may be cleaved and shed from the surface of breast cancer cells and serum HER2 levels can be detected by enzyme-linked immunosorbent assays (ELISAs) without any significant cross-reactivity with other members of the HER receptor family [1,3]. Many reports have associated increased serum HER2 concentration with earlier disease recurrence and shortened overall survival (OS), decreased response to chemotherapy and hormonal therapy, and prediction of response to trastuzumab based treatments [1-4]. The association between tissue HER2 status and serum HER2 is controversial, as some studies have identified a positive correlation, while others have not. The aims of our study were to assess 1) the correlation between serum HER2 and clinicopathologic factors, 2) the effect of serum HER2 on survival rate, and 3) the effect of changes in serum HER2 levels between pre-adjuvant chemotherapy and post-adjuvant chemotherapy on survival rate.
METHODS
Patients and specimens
For this study, we included 200 patients with primary breast cancer who were treated at Kosin University Hospital between January 2005 and December 2006. This retrospective trial was conducted in patients with a histological diagnosis of stage I-III breast cancer treated with conservative surgery or mastectomy. Tumor staging followed the tumor-node-metastasis (TNM)-American Joint Committee on Cancer (AJCC) classification and the p-TNM was obtained after classical pathological examination. Patients with metastatic disease and with other previous tumors were excluded from this study. Immunohistochemical screening for estrogen receptors (ER), progesterone receptors (PR), and HER2 status was performed on formalin-fixed paraffin embedded tissue blocks of the primary tumor in the Pathology Department of the Kosin University Hospital. Recorded clinical and pathological features for each patient include age, histologic grade, Ki-67, ER, and PR status, AJCC stage, surgical treatment and medical adjuvant therapy. Follow-up, including a clinical examination (every 6 months for the first 2 years, every 1 year for the next 3 years), mammography, bone scan and chest X-ray were carried out in all patients. Recurrence was defined as the first documented evidence of new disease manifestation in the locoregional area, in the contralateral breast, in distant sites, or in a combination of these. The study was reviewed and approved by the Institutional Review Board (No. 11-104) and informed consent was obtained from all patients.
Serum HER2 assays
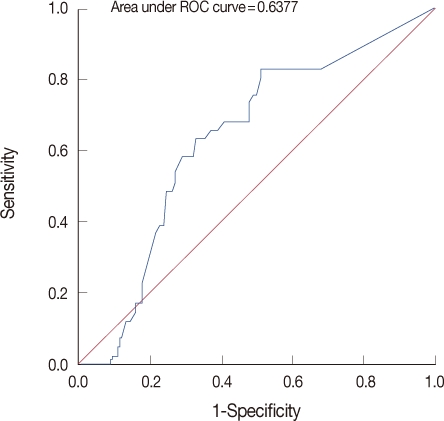
The ADVIA Centaur HER2 assay™ (Siemens, Tarrytown, USA), which is available as both a manual microtiter plate and as an automated platform, was approved by the U.S. Food and Drug Administration (FDA) in 2000. It uses two monoclonal antibodies against two independent epitopes of the serum HER2 protein (NG-3 and TA-1), The assay is calibrated using dilutions of serum HER2 control. The ECD calibrator is a recombinant protein secreted by HER2 ECD-transfected mouse NIH 3T3 cells. Quality control was ensured by assaying the two levels of control sera supplied with the kit in each series. Mean standard deviation (SD) and coefficient of variation (CV) for the controls were 15.7 vs. 0.75 ng/mL (CV, 4.5%) and 112.9 vs. 4.99 ng/mL (CV, 4.4%), respectively. This automated assay for serum HER2 was demonstrated to be accurate, precise, resistant to interferences and reliable for longitudinal monitoring; the upper limit of normal was defined as 15 ng/mL [4,5]. Receiver operating curve (ROC) analysis was performed to determine the optimal serum HER2 cutoff level. From that ROC analysis, a significant serum HER2 decline was defined as a decrease >20% at the follow-up visit (Figure 1). This definition of a significant change was similar to that derived from the FDA cleared cutoff value for non trastuzumab therapies. We measured serum HER2 levels between pre-adjuvant chemotherapy and post-adjuvant chemotherapy and then analysed the percentage of changes of serum HER2 levels (<20% vs. ≥20%). Further data analysis was performed by comparing the group of patients who achieved this significant decline in serum HER2 level (>20%) with patients who did not achieve this decline in serum HER2.
Figure 1.
This grape illustrates the area under the receiver operating characteristic (ROC) curve for changes in serum HER2 levels and patient response to chemotherapy (n=200 patients).
Other histopathological features assays
Histopathological features such as hormone receptor status and HER2 status on immunohistochemistry were all analyzed at the Institute of Pathology at the Kosin University Hospital. Expressions of p53, ER, Ki-67, and HER2 were determined immunohistochemically on paraffin sections using antibodies against ER, Ki-67, HER2, and p53. Histologic grading was performed using the criteria of Bloom and Richardson [1,3]. Lymphatic vascular invasion (LVI) was defined as the presence of tumor emboli in peritumoral lymphatic spaces, capillaries or postcapillary venules. ER status and PR status were taken as positive if more than 10% of tumor cells showed staining. An immunohistochemical score of 3+ or fluorescence in situ hybridization (FISH)+ for HER2 was accepted as HER2 positivity.
Statistical analysis
Disease-free survival (DFS) was defined as the time from surgery to first appearance of disease or death from any cause. Survival curves were estimated using the Kaplan-Meier method. Statistical tests were performed using the SPSS version 12.0 statistical software package for Windows (SPSS Inc., Chicago, USA). The survival function was calculated from the time of the onset of disease to the occurrence of death. Survival data were censored on December 31, 2009, which was the date on which the survival data were correlated with the death registry for the last time or 5 years after the onset of the disease. Kaplan-Meier estimates are presented for the survival function, and differences in survival were analyzed using the log-rank test. Associations between specific histopathological and clinical survival estimates and curves were established using the Kaplan-Meier method and differences in observed survival distribution among patient subgroups were tested with a two-sided log-rank test. All survival rates are presented with their standard errors. We used Pearson's correlation to determine the association of pairs of explanatory variables and differences in qualitative variables were evaluated by a chi-square test, where necessary. All p-values were two-sided and a p-value of less than 0.05 was considered to indicate a statistically significant difference.
RESULTS
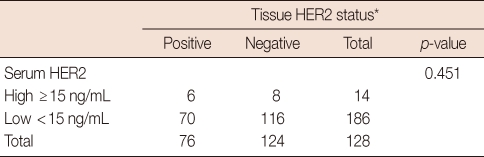
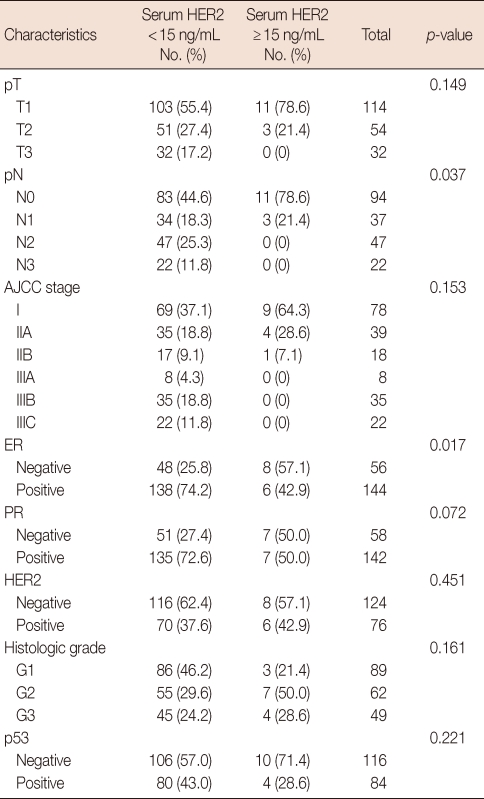
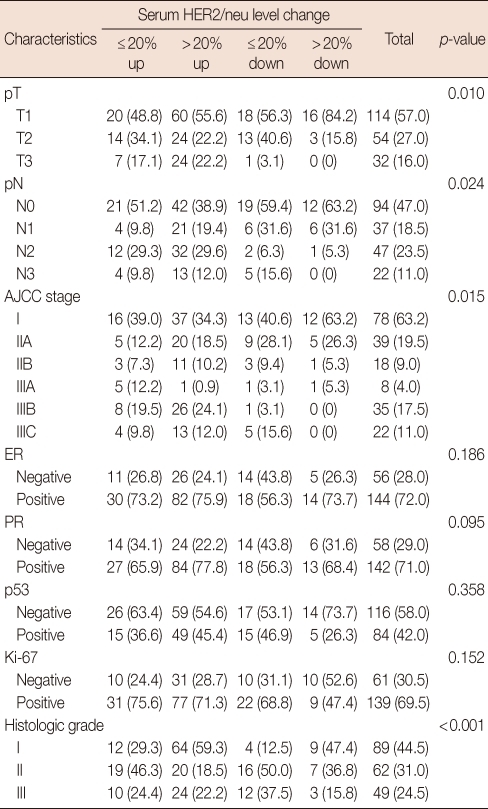
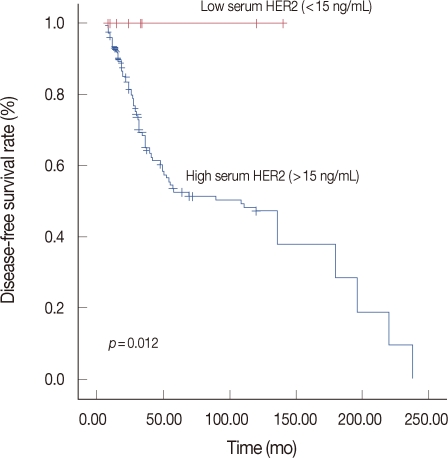
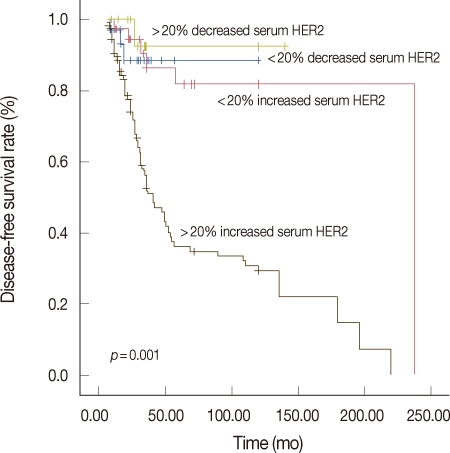
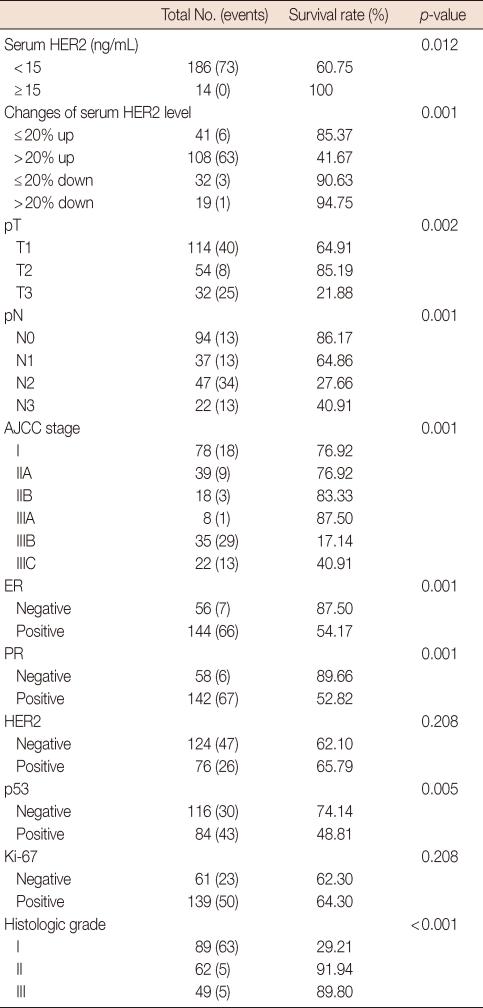
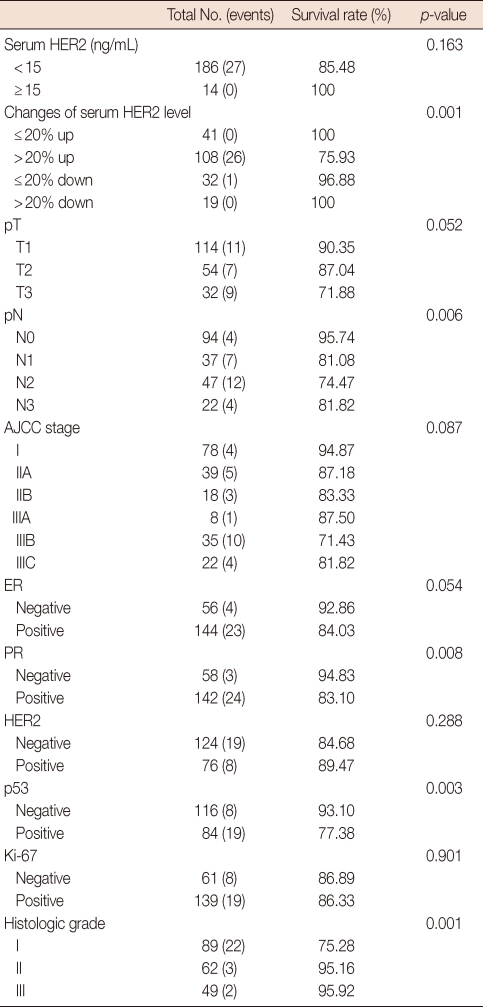
The main clinicopathological characteristics of the patients in our series are summarized in Table 1. Mean age was 50 years. T1 stage was reported in 114 patients (57.0%). N0 stage was reported in 94 patients (47.0%). All patients underwent surgery: conservative surgery was carried out in 159 patients (79.6%) and mastectomy in 41 patients (20.4%). Radiotherapy was delivered to 169 patients (84.5%). Adjuvant chemotherapy was administered to 183 patients (91.7%): 24 patients (13.1%) received combination chemotherapy with cyclophosphamide, methotrexate and fluorouracil, 129 patients (70.5%) anthracycline-based therapy, 3 patients (1.4%) taxane-based therapy and 30 patients (15.0%) anthracycline- and taxane-based regimens. Endocrine therapy was administered to 144 of 200 patients (72.0%). With a cutoff value of 15 ng/mL, 14 of the 200 patients (7.0%) had serum HER2 levels ≥15 ng/mL (high levels) and 186 patients (93.0%) had serum HER2 levels <15 ng/mL (low levels). Cancer tissue HER2 status was positive (3+ by immunohistochemistry [IHC] or 2+ by IHC with FISH amplification) in 76 patients (38.0%) and negative in 124 patients (62.1%). The bivariate distributions of patients with tissue HER2 status and baseline serum HER2 levels are shown in Table 2. We observed high serum HER2 levels in 7.8% (6 of 76) of patients with tissue HER2-positive status versus only 6.4% (8 of 124) of patients with tissue HER2-negative status. There was no significant association between high serum HER2 levels and tissue HER2 status (p=0.451). But high serum HER2 levels were significantly associated with lymph node involvement (p=0.037) and ER negativity (p=0.017) (Table 3). No significant relationship was found between serum HER2 levels and other variables, such as age, tumor size, PR, and p53. We observed serum HER2 with more than 20% increased modulation in 54.0% (108 of 200) versus only 9.5% (19 of 200) of patients with more than 20% decreased modulation. Serum HER2 with more than 20% increased modulation was significantly associated with advanced AJCC stage (Table 4). At a median follow-up of 3.14 years (range, 2.85-3.38 years). Univariate analysis for prognostic factors associated with DFS revealed that the difference in factors between serum HER2 level <15 ng/mL and ≥15 ng/mL was statistically significant (p=0.012) (Figure 2). Changes in serum HER2 levels were also significant (p=0.001) (Figure 3). Differences in serum HER2 were significantly different between tumor groups (T1, T2, T3, and T4) (p=0.002) and between lymph node stage groups (N0, N1, N2, and N3) (p<0.001) (Table 5). Univariate analysis for prognostic factors associated with OS revealed that the changes in serum HER2 level were significant (p=0.001). Lymph node stage groups (N0, N1, N2, and N3) were also significant (p=0.006) (Table 6).
Table 1.
Patients and tumor characteristics
AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor.
*High level, serum HER2 ≥15 ng/mL; Low level, serum HER2 <15 ng/mL.
Table 2.
Association between tissue HER2 status and serum HER2 levels
*3+ by immunohistochemical staining (IHC) or 2+ by IHC with fluorescent in situ hybridization.
Table 3.
Relationship between serum HER2 levels and clinicopathological variables
AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor.
Table 4.
Relationship between the changes in serum HER2 levels and clinicopathological variables
Values represent number (%).
AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor.
Figure 2.
Kaplan-Meier estimates for disease-free survival according to serum HER2 levels.
Figure 3.
Kaplan-Meier estimates for disease-free survival according to changes in serum HER2 levels.
Table 5.
Univariate analysis for variable considered for DFS
DFS=disease-free survival; AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor.
Table 6.
Univariate analysis for variable considered for OS
OS=overall survival; AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor.
DISCUSSION
HER2 is a 185 kDa protein composed of three domain; a cytoplasmic domain, a transmembrane domain and an ECD [1-3]. The ECD of HER2 can be cleaved from the surface of breast cancer cells by matrix metalloproteases and released into the serum, where it is detectable using an ELISA [4-6]. Before the FDA guideline were promulgated, a variety of different assays and cutoffs were used. These made it difficult to reach robust conclusions from the date derived. But the currently approved cutoff for an elevated serum HER2 is greater than 15 ng/mL, which results in a positive test in approximately 5% of healthy controls [7-9]. So we also defied the 15 ng/mL upper limit of normal. Increased levels of serum HER2 (15 ng/mL) can be detected in approximately 15% to 30% of unselected presurgical breast cancer samples and in up to 45% of patients with metastatic breast cancer, suggesting that serum HER2 levels may be reflective of a more aggressive tumor type [10-13]. According to our study, increased serum HER2 was detected in approximately 7%. A relatively low rate of increased serum HER2 may be attributable to variability in tissue metalloproteinases (TMPs), which generate proteolytic cleavage of full length HER2. ECD production is increased by TMP activators and is decreased by TMP inhibitors in vitro. Increased levels of serum ECD have also been correlated with higher relapse rates, and patients in whom ECD levels exceed the upper limit of normal (ULN) for prolonged periods have been shown to have a poor prognosis [2,14]. Serum HER2 levels have been proposed as a method of determining the HER2 status of breast cancers and potential eligibility for trastuzumab treatment [3,15]. Several studies demonstrate a decrease in serum HER2 regardless of response, but there is a consensus that increasing serum HER2 levels indicates progressive disease. Most, but not all, studies reported that serum HER2 decreases to a greater degree among responders than non-responders. There have been efforts to establish algorithms to relate the magnitude of the decrease with the benefit from treatment. One study showed that those with a greater than 20% decrease in serum HER2 have a greater benefit from trastuzumab-based therapy than those with a less than 20% decrease. Also, in our study, a significant serum HER2 decline was defined as a decrease >20% at the follow-up visit. This definition of a significant change was similar to that derived from the FDA cleared cutoff value for non-trastuzumab therapies [7-9]. Further data analysis was performed by comparing the group of patients who achieved this significant decline in serum HER2 level (>20%) with patients who did not achieve this decline in serum HER2. However, not all patients with HER2-positive breast cancer have elevated serum HER2 levels and, in a series of 55 patients, levels above the ULN were detected in one third of patients with HER2-negative disease [16-18]. Therefore, tissue-based methods such as IHC and FISH or chromogenic in situ hybridization should still be used to determine HER2 status, as these involve direct assessment of HER2 in the tumor [19,20]. According to our study, the sensitivity of serum HER2 is 7.8%. The variation in sensitivity may be attributable in part to the cutoffs and to the fact that, in some studies, it is unclear whether the serum was collected pre- or postoperatively. At 90-95%, specificity was better, but it suggests that some women with HER2 negative tumors secrete detectable amounts of serum HER2. According to our study, the specificity was also better (93.6%), but it suggests that eight patients (6.4%) secrete detectable amounts of serum HER2. Overall these results demonstrate that serum HER2 is not a reliable determinant of the HER2 status of a tumor. It is likely that the secretion of ECD is influenced by factors such as tumor vascularization or the activity of TMPs. A number of studies found that serum HER2 levels is likely to be markedly influenced by tumor load/size. The utility of serum HER2 levels at baseline and during therapy as a potential marker of tumor response or progression is currently a matter of debate. In a study of 43 patients with metastatic breast cancer, elevated pretreatment serum levels of ECD were associated with poor clinical responsiveness to hormone therapy and chemotherapy [21,22]. Conversely, in another study of patients with HER2-positive metastatic breast cancer, 19 of 55 patients with elevated serum HER2 levels at baseline demonstrated significantly higher response rates to trastuzumab-based treatments [23-25]. Trastuzumab reduces the cleavage of serum HER2 by metalloproteases, thus reducing serum HER2, and it has been suggested that early changes in serum HER2 may predict both subsequent responses to trastuzumab and progression-free survival [26-28]. It was also suggested, following a study of 210 patients, that increases in domains: a cytoplasmic domain and a transmembrane of serum HER2 might indicate disease recurrence or metastasis after adjuvant treatment [29,30]. It is important to note that the patient numbers in most of these trials were relatively small and, therefore, provide insubstantial evidence on which to base treatment decisions. Also the preliminary results we present were obtained from a relatively small number of patients. So this issue should be clarified with larger prospective studies. Also, we did not do biopsies, so we were not able to determine whether a change in the level of serum HER2 took place in the metastasis of patients with a high level serum HER2 and a negative tissue HER2 status. According to our study, ER-negativity was associated with high serum HER2. And AJCC N0 stage was associated with low serum HER2. But serum HER2 was not associated with advanced staging. So serum HER2 can't be thought of as an advanced AJCC TNM stage. And serum HER2 changes with more than 20% elevation were associated with advanced AJCC TNM staging. And there is a significant association between serum HER2 and 5-year DFS not including the OS rate. And there is a significant association between serum HER2 level changes of more than 20% and 5-year DFS rate, including the OS rate. Tumor size was also an important factor affecting 5-year DFS rate. But there are several limitations to our study. The primary limitation of our study is the small sample size; as a consequence, it had low statistical power to detect associations. In addition, we had limited evidence of an association between changes in serum HER2 levels and survival rate. To the best of our knowledge, this is one of a few studies to show an association between changes in serum HER2 levels after adjuvant chemotherapy and survival rate. To more powerfully demonstrate the results observed in our study, further prospective functional studies are needed to validate our findings.
Footnotes
This article was granted by Kosin University College of Medicine 2011.
The authors declare that they have no competing interests.
References
- 1.Ludovini V, Gori S, Colozza M, Pistola L, Rulli E, Floriani I, et al. Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol. 2008;19:883–890. doi: 10.1093/annonc/mdm585. [DOI] [PubMed] [Google Scholar]
- 2.Kang SH, Cho J, Ha JS, Kwon SY. Evaluation of serum HER-2/neu extracelluar domain in breast cancer patients: correlation with tissue HER-2/neu status and clinicopathological factors. J Korean Surg Soc. 2010;78:271–276. [Google Scholar]
- 3.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Min WK, Park EH, Lim WS, Choi SL, Son BH, et al. Correlation between the Her-2/neu status as determined by immunohistochemical analysis and the serum Her-2/neu concentration as determined by the use of ADVIA Cencaur(R) automated immunoassay in breast cancer patients. J Breast Cancer. 2008;11:116–124. [Google Scholar]
- 5.Park NK, Woo HD, Sohn DM, Kim SY, Lim CW, Choi TY, et al. The correlation of serum HER-2/neu and CA15-3 in patients with metastatic breast cancer. J Breast Cancer. 2008;11:18–24. [Google Scholar]
- 6.Kim JW, Kim SY, Lee HS, Woo HD, Son DM, Lim CW, et al. Establishment for reference range of serum HER-2/neu in Korean healthy women. J Breast Cancer. 2006;9:301–308. [Google Scholar]
- 7.Muller V, Witzel I, Pantel K, Krenkel S, Lück HJ, Neumann R, et al. Prognostic and predictive impact of soluble epidermal growth factor receptor (sEGFR) protein in the serum of patients treated with chemotherapy for metastatic breast cancer. Anticancer Res. 2006;26:1479–1487. [PubMed] [Google Scholar]
- 8.Payne RC, Allard JW, Anderson-Mauser L, Humphreys JD, Tenney DY, Morris DL. Automated assay for HER-2/neu in serum. Clin Chem. 2000;46:175–182. [PubMed] [Google Scholar]
- 9.Cook GB, Neaman IE, Goldblatt JL, Cambetas DR, Hussain M, Lüftner D, et al. Clinical utility of serum HER-2/neu testing on the Bayer Immuno 1 automated system in breast cancer. Anticancer Res. 2001;21:1465–1470. [PubMed] [Google Scholar]
- 10.Lennon S, Barton C, Banken L, Gianni L, Marty M, Baselga J, et al. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. J Clin Oncol. 2009;27:1685–1693. doi: 10.1200/JCO.2008.16.8351. [DOI] [PubMed] [Google Scholar]
- 11.Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin Oncol. 1999;26(4 Suppl 12):51–59. [PubMed] [Google Scholar]
- 12.Yaziji H, Goldstein LC, Barry TS, Werling R, Hwang H, Ellis GK, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA. 2004;291:1972–1977. doi: 10.1001/jama.291.16.1972. [DOI] [PubMed] [Google Scholar]
- 13.Buchholz TA, Tu X, Ang KK, Esteva FJ, Kuerer HM, Pusztai L, et al. Epidermal growth factor receptor expression correlates with poor survival in patients who have breast carcinoma treated with doxorubicin-based neoadjuvant chemotherapy. Cancer. 2005;104:676–681. doi: 10.1002/cncr.21217. [DOI] [PubMed] [Google Scholar]
- 14.Skasko E, Paszko Z, Mazur S. A new look at the prognostic value of the estrogen, progesterone and epidermal growth factor receptors in breast cancer tissue. Neoplasma. 2005;52:10–17. [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002;71:67–75. doi: 10.1023/a:1013397232011. [DOI] [PubMed] [Google Scholar]
- 17.Carney WP, Neumann R, Lipton A, Leitzel K, Ali S, Price CP. Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast cancer. Clin Chem. 2003;49:1579–1598. doi: 10.1373/49.10.1579. [DOI] [PubMed] [Google Scholar]
- 18.Ali SM, Leitzel K, Chinchilli VM, Engle L, Demers L, Harvey HA, et al. Relationship of serum HER-2/neu and serum CA 15-3 in patients with metastatic breast cancer. Clin Chem. 2002;48:1314–1320. [PubMed] [Google Scholar]
- 19.Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 20.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 21.Bewick M, Chadderton T, Conlon M, Lafrenie R, Morris D, Stewart D, et al. Expression of C-erbB-2/HER-2 in patients with metastatic breast cancer undergoing high-dose chemotherapy and autologous blood stem cell support. Bone Marrow Transplant. 1999;24:377–384. doi: 10.1038/sj.bmt.1701907. [DOI] [PubMed] [Google Scholar]
- 22.Fehm T, Jäger W, Krämer S, Sohn C, Solomayer E, Wallwiener D, et al. Prognostic significance of serum HER2 and CA 15-3 at the time of diagnosis of metastatic breast cancer. Anticancer Res. 2004;24:1987–1992. [PubMed] [Google Scholar]
- 23.Harris LN, Liotcheva V, Broadwater G, Ramirez MJ, Maimonis P, Anderson S, et al. Comparison of methods of measuring HER-2 in metastatic breast cancer patients treated with high-dose chemotherapy. J Clin Oncol. 2001;19:1698–1706. doi: 10.1200/JCO.2001.19.6.1698. [DOI] [PubMed] [Google Scholar]
- 24.Müller V, Witzel I, Lück HJ, Köhler G, von Minckwitz G, Möbus V, et al. Prognostic and predictive impact of the HER-2/neu extracellular domain (ECD) in the serum of patients treated with chemotherapy for metastatic breast cancer. Breast Cancer Res Treat. 2004;86:9–18. doi: 10.1023/B:BREA.0000032919.83803.48. [DOI] [PubMed] [Google Scholar]
- 25.Lipton A, Ali SM, Leitzel K, Demers L, Chinchilli V, Engle L, et al. Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol. 2002;20:1467–1472. doi: 10.1200/JCO.2002.20.6.1467. [DOI] [PubMed] [Google Scholar]
- 26.Esteva FJ, Cheli CD, Fritsche H, Fornier M, Slamon D, Thiel RP, et al. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7:R436–R443. doi: 10.1186/bcr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasparini G, Sarmiento R, Amici S, Longo R, Gattuso D, Zancan M, et al. Gefitinib (ZD1839) combined with weekly epirubicin in patients with metastatic breast cancer: a phase I study with biological correlate. Ann Oncol. 2005;16:1867–1873. doi: 10.1093/annonc/mdi393. [DOI] [PubMed] [Google Scholar]
- 28.Lafky JM, Baron AT, Cora EM, Hillman DW, Suman VJ, Perez EA, et al. Serum soluble epidermal growth factor receptor concentrations decrease in postmenopausal metastatic breast cancer patients treated with letrozole. Cancer Res. 2005;65:3059–3062. doi: 10.1158/0008-5472.CAN-05-0067. [DOI] [PubMed] [Google Scholar]
- 29.Hudelist G, Köstler WJ, Gschwantler-Kaulich D, Czerwenka K, Kubista E, Müller R, et al. Serum EGFR levels and efficacy of trastuzumab-based therapy in patients with metastatic breast cancer. Eur J Cancer. 2006;42:186–192. doi: 10.1016/j.ejca.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–4434. [PubMed] [Google Scholar]