Abstract
Purpose
Sentinel lymph node biopsy (SLNB) in breast cancer patients with clinically negative axilla will ensure axillary dissection only for cases with lymph node metastasis and provide information about pathologic staging as accurate as the axillary dissection. It was shown that SLNB could be successfully performed regardless of the type of biopsy. The aim of this study was to investigate the feasibility of SLNB after excisional biopsy.
Methods
One hundred patients diagnosed with excisional biopsy or guide wire-localization and operated on with SLNB between February 2007 and March 2009 were retrospectively analyzed. SLNB was performed with 10 cc of 1% methylene blue alone or both methylene blue and 1 mCi of Tc-99m nanocolloid combination. Age, tumor localization and size, length of the biopsy incision, size of the biopsy specimen, multifocality, lymphovascular invasion, tumor grade, staining with methylene blue, localization, number and metastatic status of the lymph nodes stained, and success rate with a gamma probe were evaluated.
Results
Sentinel lymph node (SLN) could not be identified in 9 (16.9%) of patients in the methylene blue group (n=53). In the combination group (n=47), SLN could not be identified in one patient. Of 32 patients with negative SLNB, metastatic involvement was found to be present in 5 patients after axillary lymph node dissection (false negatives). The average numbers of SLNs found in the methylene blue group and combination group were 1.4 and 1.6, respectively. SLN detection and false negative rates in the methylene blue group were 83% and 15.7%, respectively. The rates for the combination group were 98% and 6.4%, respectively. None of the parameters related to patient, tumor or process were found to affect detection rates of SLN.
Conclusion
Only SLNB using a combination method is a safe and reliable technique for breast cancer patients diagnosed with excisional biopsy.
Keywords: Biopsy, Breast neoplasms, Methylene blue, Sentinel lymph node biopsy
INTRODUCTION
Axillary dissection, in the treatment of breast cancer, does not provide significant survival advantage in patients with negative axilla. However, it is useful for the assessment of prognosis and determining adjuvant therapy [1,2]. Sentinel lymph node biopsy (SLNB) in breast cancer patients with clinically negative axilla will ensure axillary dissection only for cases with lymph node metastasis and will provide information about pathologic staging as accurate as the axillary dissection, to reduce the morbidity and cost of axillary lymph node dissection (ALND). Therefore, SLNB is used as a staging procedure in patients with early-stage breast cancer instead of ALND [3,4].
Removal of the primary tumor with excisional biopsy may impair breast lymphatics. It used to be thought that success of SLNB following excisional biopsy is low [5,6]. However, studies have shown that it could be successfully performed regardless of the type of initial biopsy, the volume of excision, and the time between biopsy and SLNB [7-9]. The aim of this study was to investigate the feasibility of SLNB after excisional biopsy.
METHODS
Patients previously diagnosed with excisional biopsy or guide wire-localization and operated with SLNB between February 2007 and March 2009 were retrospectively analyzed in this study. Patients with clinically or radiologically positive axilla, treated with neoadjuvant chemotherapy and/or radiotherapy, and diagnosed with fine needle aspiration biopsy (FNAB) were excluded from the study.
SLNB was performed with 10 cc of 1% methylene blue alone or both methylene blue and 1 mCi of Tc-99m nanocolloid combination. Injections were given both subdermally around the biopsy incision and in the periareolar region. The radioactive substance was given 2-18 hours before the operation, and preoperative lymphoscintigraphy was used for all patients. A gamma probe was used for all patients labeled with radioactive iodine. Methylene blue was administered in 10 minutes before the skin incision was performed and manual massage was performed for 5 minutes. All blue and/or radioactive lymph nodes were considered as sentinel nodes. As the accuracy of this procedure is usually confirmed with axillary dissection in many studies in the literature, standard level I and II ALND was performed for all patients after SLNB.
Age, tumor localization, tumor size, length of the biopsy incision, size of the biopsy specimen, multifocality, lymphovascular invasion, tumor grade, staining with methylene blue, localization, number and metastatic status of the lymph nodes stained, and success rate with a gamma probe and metastasis were evaluated for every patient. Effects of these factors on the successful localization of SLN and false negativity were evaluated with a chi-square test using SPSS for Windows version 13.0 program (SPSS Inc., Chicago, USA). A p<0.05 value was considered as statistically significant.
RESULTS
One hundred patients were evaluated in this study. Mean age of the patients was 49.8 years (range, 27-74 years). Eighty patients with palpable tumors were diagnosed with excisional biopsy and 20 with excisional biopsy directed by guide wire localization. Median length of the biopsy incision was 3 cm, median length of the long axis of the biopsy specimen was 4 cm, and average tumor size was 2.9 cm. Multifocal tumors were reported in 11 patients. Tumors of 52 patients were located in the upper lateral quadrant, 22 patients in the lower lateral quadrant, and 8 patients in the central region. The average time period between excisional biopsy and definitive surgery was 18.5 days (range, 9-27 days). There were no allergic reactions, dermal necrosis or fibrosis related to the methylene blue or radiocolloid.
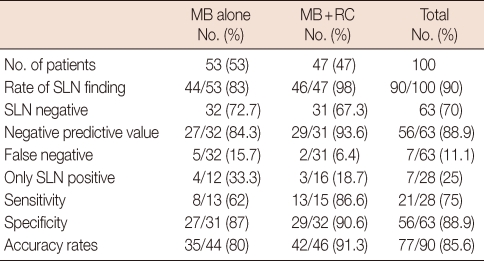
Fifty-three patients were given 10 cc of 1% methylene blue alone and the remaining 47 were given methylene blue and 1 mCi of Tc-99m nanocolloid. SLNs could not be identified in 9 patients in the group where only methylene blue was administered (16.9%). Of the 32 patients with a negative SLNB, metastatic involvement was found in 5 patients after ALND (false negativity). In the combination group, SLN could not be identified in 1 patient. In this group, metastatic lymph nodes were detected in 2 patients after ALND out of 31 patients where SLN was negative. In the combination group, there was no staining with methylene blue in 3 patients. A SLN was found with the help of methylene blue in 2 of 3 patients that SLN could not be defined with gamma probe. SLN could not be visualised in only one of the lymphoscintigraphies. The average numbers of SLNs found in the methylene blue group and combination group were 1.4 and 1.6, respectively. Multiple SLNs were found in 51% of the cases in the combination group and in 49% of the cases in the methylene blue group. Numbers of lymph nodes in groups with methylene blue and gamma probe are shown in Table 1. Unusual localization of a SLN was level 2 in one patient, and mammaria interna in one patient. SLN detection and false negative rates in the methylene blue group were 83% and 15.7%, respectively. These rates for the combination group were 98% and 6.4%, respectively (Table 2). Sensitivity and specificity rates and negative predictive values for the methylene blue and combination techniques were given in Table 2.
Table 1.
The number of SLNs stained with methylene blue and detected with gamma probe
SLN=sentinel lymph node; LN=lymph node.
Table 2.
Accuracy of SLNB with different techniques
SLNB=sentinel lymph node biopsy; MB=methylene blue; RC=radiocolloid; SLN=sentinel lymph node.
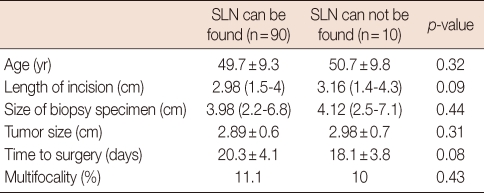
None of the parameters related to patient, tumor or process was found to affect the detection rate of SLN (Table 3).
Table 3.
The results of the parameters that statistically analyzed
SLN=sentinel lymph node.
DISCUSSION
The triple test consisting of physical examination, mammography, and percutaneous biopsy is the gold standard for the diagnosis of breast lesions [10]. In case of suspicion of the diagnosis in the tests, the diagnosis should be confirmed with excisional biopsy. Since the facilities for percutaneous biopsy of non-palpable lesions are not available in every center, excisional biopsy after guide-wire localization is still used for the diagnosis of these lesions. But the excisional biopsy impairs the lymphatic drainage between the tumor and axilla. As SLNB gained popularity, debate on the reliability of SLNB after excisional biopsy started [11].
Many studies on reliability of SLNB following excisional biopsy by performing SLNB and ALND simultaneously and comparing it with percutaneous biopsy have been reported [9,12]. In a study comparing excisional biopsy and FNAB, the SLN detection rate and false negativity rate were not significantly different [9]. In another study, median numbers of SLNs were 1.6 in patients with excisional biopsy and 1.7 in patients with FNAB and SLN detection rates were similar [7]. It was also emphasized that the time period between the excisional biopsy and SLNB had no effect on the localization of SLNs [13]. In accordance with this, the false negative rate was 8.1% with FNAB and 15.3% with excisional biopsy in the NSABP-32 study [14].
Estourgie et al. [15] examined the lymphatic drainage patterns of 25 patients with lymphoscintigraphy before and after excisional biopsy. There were inconsistent results in 17 patients. The original SLN could not be visualized in 7 patients and new hot spots were found in 4 patients.
Excisional biopsy may alter the lymphatics drainage tracts [15]. But high accuracy rates for SLNB after excisional biopsy are still reported [16]. It is possible that the modified lymphatic tracts are still draining to the original SLN. Lymphatic anatomy of the breast parenchyma is divided into two groups that are linked to each other: cutaneous lymphatics (superficial lymphatics) and parenchymal lypmhatics (deep lymphatics). There are two lymphatic networks: subepithelial plexus and subdermal plexus. The subepithelial lymphatic plexus in the dermis has no valves and is linked to the subepithelial plexus throughout the body. Therefore, theoretically it can easily bypass the biopsy incision made on the skin. The subepithelial plexus is linked to the subdermal lymphatic labyrinth via vertical lymphatics. The lymphatic flow within the subdermal lymphatics is unidirectional, since these lymphatics do not have valves. The lymphatics under the areola receives its lymphatics from the areola and nipple and communicates with other plexuses. The lymph flows through the lactiferous ducts from the subareolar plexus to perilobular and subcutaneous lymphatics unidirectionally to regional lymph nodes. Theoretically, injections to periareolar region must reach the original SLN even with excisional biopsies in other regions of the breast. This can explain high SLN detection rates that are not affected by tumor size, localization and the amount of tissue removed by biopsy [12].
If drainage occurs to another lymph node, this lymph node can be in close proximity to the original SLN and both lymph nodes can have similar histopathological characteristics. Additionally, removal of more than one SLN can solve this problem. It has been shown that, while the false negative rate of a single SLN was 14.3%, this rate fell to 4.3% with multiple SLNs [9]. Success of the combined technique is also attributed to the removal of more SLNs.
The combination technique and the methylene blue dye technique had been compared after FNAB and core breast biopsies [17-19], but, we could not find any literature in English about the use of methylene blue alone after excisional breast biopsy. In these studies, it was reported that the success rate of the combination technique was higher than methylene blue dye alone (100% vs. 86%) [17-20].
In another study, no difference between the success rates of the methylene blue and lymhazurin dyes had been reported [21]. But in all these studies, the false negative rates with methylene blue dye was not higher than 7%. The high false negative rate reported in our series (15.7%) makes this technique less confident. A single use of methylene blue dye isn't reliable in SLNB after excisional breast biopsy.
In our series, with the use of a combined technique, the detection rate of SLNs, negative predictive value, false negativity, sensitivity, specificity, and accuracy rates are consistent with the literature and are not different from results obtained with percutaneous biopsy. We conclude that, only SLNB using a combination method is safe and reliable for breast cancer patients diagnosed with excisional biopsy.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Greco M, Agresti R, Cascinelli N, Casalini P, Giovanazzi R, Maucione A, et al. Breast cancer patients treated without axillary surgery: clinical implications and biologic analysis. Ann Surg. 2000;232:1–7. doi: 10.1097/00000658-200007000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nos C, Fréneaux P, Guilbert S, Falcou MC, Salmon RJ, Clough KB. Sentinel lymph node detection for breast cancer: which patients are best suited for the patent blue dye only method of identification? Ann Surg Oncol. 2001;8:438–443. doi: 10.1007/s10434-001-0438-1. [DOI] [PubMed] [Google Scholar]
- 4.Chen SL, Iddings DM, Scheri RP, Bilchik AJ. Lymphatic mapping and sentinel node analysis: current concepts and applications. CA Cancer J Clin. 2006;56:292–309. doi: 10.3322/canjclin.56.5.292. [DOI] [PubMed] [Google Scholar]
- 5.Tafra L, Lannin DR, Swanson MS, Van Eyk JJ, Verbanac KM, Chua AN, et al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233:51–59. doi: 10.1097/00000658-200101000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman SM, Krag DN, McNally RK, Moor BB, Weaver DL, Klein P. Limitation in gamma probe localization of the sentinel node in breast cancer patients with large excisional biopsy. J Am Coll Surg. 1999;188:248–254. doi: 10.1016/s1072-7515(98)00306-8. [DOI] [PubMed] [Google Scholar]
- 7.Ruano R, Ramos M, Garcia-Talavera JR, Serrano E, De Arriba A, Gonzalez-Orus J, et al. Staging the axilla with selective sentinel node biopsy in patients with previous excision of non-palpable and palpable breast cancer. Eur J Nucl Med Mol Imaging. 2008;35:1299–1304. doi: 10.1007/s00259-008-0730-4. [DOI] [PubMed] [Google Scholar]
- 8.Chagpar AB, Scoggins CR, Sahoo S, Martin RC, 2nd, Carlson DJ, Laidley AL, et al. Biopsy type does not influence sentinel lymph node status. Am J Surg. 2005;190:551–556. doi: 10.1016/j.amjsurg.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Wong SL, Edwards MJ, Chao C, Tuttle TM, Noyes RD, Carlson DJ, et al. The effect of prior breast biopsy method and concurrent definitive breast procedure on success and accuracy of sentinel lymph node biopsy. Ann Surg Oncol. 2002;9:272–277. doi: 10.1007/BF02573065. [DOI] [PubMed] [Google Scholar]
- 10.Rutgers EJ EUSOMA Consensus Group. Quality control in the locoregional treatment of breast cancer. Eur J Cancer. 2001;37:447–453. doi: 10.1016/s0959-8049(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 11.Sakorafas GH. Breast cancer surgery: historical evolution, current status and future perspectives. Acta Oncol. 2001;40:5–18. doi: 10.1080/028418601750070984. [DOI] [PubMed] [Google Scholar]
- 12.Haigh PI, Hansen NM, Qi K, Giuliano AE. Biopsy method and excision volume do not affect success rate of subsequent sentinel lymph node dissection in breast cancer. Ann Surg Oncol. 2000;7:21–27. doi: 10.1007/s10434-000-0021-1. [DOI] [PubMed] [Google Scholar]
- 13.Cody HS, 3rd, Fey J, Akhurst T, Fazzari M, Mazumdar M, Yeung H, et al. Complementarity of blue dye and isotope in sentinel node localization for breast cancer: univariate and multivariate analysis of 966 procedures. Ann Surg Oncol. 2001;8:13–19. doi: 10.1007/s10434-001-0013-9. [DOI] [PubMed] [Google Scholar]
- 14.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 15.Estourgie SH, Valdés Olmos RA, Nieweg OE, Hoefnagel CA, Rutgers EJ, Kroon BB. Excision biopsy of breast lesions changes the pattern of lymphatic drainage. Br J Surg. 2007;94:1088–1091. doi: 10.1002/bjs.5763. [DOI] [PubMed] [Google Scholar]
- 16.McMasters KM, Tuttle TM, Carlson DJ, Brown CM, Noyes RD, Glaser RL, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560–2566. doi: 10.1200/JCO.2000.18.13.2560. [DOI] [PubMed] [Google Scholar]
- 17.O'Hea BJ, Hill AD, El-Shirbiny AM, Yeh SD, Rosen PP, Coit DG, et al. Sentinel lymph node biopsy in breast cancer: initial experience at Memorial Sloan-Kettering Cancer Center. J Am Coll Surg. 1998;186:423–427. doi: 10.1016/s1072-7515(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 18.Derossis AM, Fey J, Yeung H, Yeh SD, Heerdt AS, Petrek J, et al. A trend analysis of the relative value of blue dye and isotope localization in 2,000 consecutive cases of sentinel node biopsy for breast cancer. J Am Coll Surg. 2001;193:473–478. doi: 10.1016/s1072-7515(01)01038-9. [DOI] [PubMed] [Google Scholar]
- 19.Hung WK, Chan CM, Ying M, Chong SF, Mak KL, Yip AW. Randomized clinical trial comparing blue dye with combined dye and isotope for sentinel lymph node biopsy in breast cancer. Br J Surg. 2005;92:1494–1497. doi: 10.1002/bjs.5211. [DOI] [PubMed] [Google Scholar]
- 20.Varghese P, Abdel-Rahman AT, Akberali S, Mostafa A, Gattuso JM, Carpenter R. Methylene blue dye--a safe and effective alternative for sentinel lymph node localization. Breast J. 2008;14:61–67. doi: 10.1111/j.1524-4741.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 21.Blessing WD, Stolier AJ, Teng SC, Bolton JS, Fuhrman GM. A comparison of methylene blue and lymphazurin in breast cancer sentinel node mapping. Am J Surg. 2002;184:341–345. doi: 10.1016/s0002-9610(02)00948-0. [DOI] [PubMed] [Google Scholar]