Abstract
Purpose
Internal mammary lymph node (IMLN) metastasis is an important prognostic indicator in breast cancer. However, the necessity of internal mammary sentinel lymph node biopsy for accurate staging, for choosing adjuvant treatment, and as a prognostic indicator, has remained controversial.
Methods
From January 2001 to December 2006, 525 female breast cancer patients underwent radical surgery after preoperative lymphatic scintigraphy. We retrospectively analyzed the follow-up results, recurrences, and deaths of all patients.
Results
There was no significant difference in the clinicopathological characteristics between the axilla and the IMLN groups. The median follow-up period was 118.8 months (range, 7-122 months) in the axilla group and 107.7 months (range, 14-108 months) in the IMLN group. During the median follow-up period, the breast cancer-related death rate in the axilla group was 3.6%, which was not significantly different from that of the IMLN group (1.3%) (p=0.484). The five-year survival rates did not differ between the two groups (p=0.306). The overall recurrence rate and the locoregional recurrence rate also did not differ between the two groups (p=0.835 and p=0.582, respectively). The recurrence rate of IMLN (both ipsilateral and contralateral) metastasis was very low, accounting for 0.5% in the axilla group and 1.3% in the IMLN group (p=0.416).
Conclusion
The long-term follow-up results showed that there was no significant difference in both overall outcome and regional recurrence between the two groups. Therefore, the requirement for identification of nodal basins outside the axilla or IMLN sentinel biopsy should be reconsidered.
Keywords: Breast, Carcinoma, Internal mammary, Prognosis, Sentinel lymph node biopsy
INTRODUCTION
The internal mammary lymph nodes (IMLN) comprise the second most important regional nodal basin in breast cancer followed by the axillary lymph nodes (ALN). Metastasis to IMLN is well known as a poor prognostic indicator and has been reported in less than 30% of dissected patients. However, many clinicians have reported that surgical dissection of IMLN does not improve the outcome of breast cancer patients [1-3]. Currently, it is widely accepted that prophylactic dissection in clinically negative IMLN is not recommended as a standard surgical treatment. Moreover, because of the morbidity and uncertain benefit of surgical IMLN excision, majority of IMLN metastasis patients are treated with adjuvant therapy, such as chemotherapy or radiation therapy, as an alternative method.
Johnson et al. [4] suggested that the pathological status of the internal mammary sentinel lymph node (IMSLN) is the same as that of the axillary SLN; thus, if the pathological state of IMSLN could be predicted, IMLN may not need to be excised. Veronesi et al. [5] have suggested that selective IMSLN excision facilitated by radioactive labeled tracing method is a simple and safe technique and may provide maximum prognostic value, similar to the current use of axillary SLN biopsy. Therefore, many researchers have attempted the use of IMSLN biopsy with lymphoscintigraphy. Some reports have suggested the importance of IMSLN biopsy for accurate staging, decreasing the likelihood of the required treatment being overlooked, and as an important prognostic indicator [6-8], while others disagree on the additional surgical excision of the IMLN [9,10]. However, most studies have been limited to descriptive observations of early experiences and inconsistent results, and there is still insufficient evidence in terms of the efficacy and clinical significance of IMSLN biopsy.
The efficiency of IMSLN surgical staging elicits additional consideration, particularly in patients with early breast cancer. The purpose of this study is to investigate the clinical features of tumors with IMLN drainage and to determine the clinical significance and long-term outcomes for IMSLN in early breast cancer.
METHODS
Patients and procedures
From January 2001 to December 2006, 525 female patients with early stage breast cancer underwent lymphatic scintigraphy followed by radical surgery at Sungkyunkwan University School of Medicine. Following lymphatic scintigraphy, the ALNs were identified during surgery by tracing radioactivity, but not all hot spots in the internal mammary chain were explored. Patients with clinically positive nodes were excluded as were patients with clinically advanced (stage III or IV) tumors. Using medical records, we retrospectively analyzed patient interviews, clinical characteristics, follow-up results, recurrences, and deaths.
Lymphatic scintigraphy was performed by injecting 1 mL of Technetium 99m tin-colloid into the subareolar area. After 60-90 minutes, the frontal and lateral views were acquired. The patients then underwent surgery on the same day. A blue dye was injected into the periareolar area intraoperatively and a combination of two methods was used for axillary SLN biopsy. Axilla group was defined as patients with a hot spot on the axilla but without an uptake in the IMLN area. All patients with IMLN hot spot detected with or without uptake in the axillary region were included in the IMLN group. The staging system was based on the 7th edition of the American Joint Committee on Cancer (AJCC).
Breast conserving surgery was performed if possible and margin was evaluated by frozen biopsy at operation and confirmed by final pathology. The primary end point was overall survival from the time of the operation until death due to breast cancer-related causes. Locoregional (LR) recurrence was defined as recurrences in the ipsilateral breast, chest wall, axillary, supraclavicular, or internal mammary lymph nodes, or in the skin of the chest wall or axilla. Breast cancer development in the contralateral breast was not included in the LR area. Disease-free survival was defined as the period between the day of the breast cancer surgery until LR or distant recurrences of breast cancer.
Statistical analysis
A comparative analysis of the two groups was performed. Baseline clinical and histological variables between each group was assessed with SPSS for Windows version 18.0 (SPSS Inc., Chicago, USA) using the Pearson chi-square test. A mean age comparison was done with the Kruskal-Wallis test. Multivariate analyses were conducted using logistic regression and the Cox regression method. Cumulative overall survival (OS) rate and disease-free survival (DFS) rate were estimated by the Kaplan-Meier method. The log rank test was used for comparison of survival outcome between the subgroups according to ALN metastasis and radiotherapy. Any local recurrence of distant metastasis was included in the analysis. The cut-off value for statistical significance was set at 0.05. The χ2 test was used to determine the differences in the clinicopathological features between the two groups.
RESULTS
Among the 525 early stage breast cancer patients, no uptake in either the axilla or IMLN was observed in 55 cases (10.4%) and 11 cases (2.1%) were found to have uptake in only IMLN. Axillary SLN was detected in all cases. Among the 470 patients exhibiting uptake (excluding the 55 patients in whom there was no uptake), 393 were included in the axillary uptake only group (74.9%) and 77 patients (14.7%) were included in the IMLN uptake group.
Comparison of clinicopathological characteristics
There were no significant differences in the median age and body mass index between the two groups (p=0.189 and p=0.433, respectively). Tumor location was subdivided into inner and outer, upper and lower. Multifocal lesions, central locations, and subareolar locations were categorized as "other." In both groups, tumors were most frequently located in the outer half and upper half. The type of operation did not differ between groups. Breast-conserving surgery was more frequent in both groups (p=0.594). The results of postoperative final pathological staging revealed that stage 1 was most frequent in both groups, with 198 cases (50.4%) in the axillary uptake only group and 40 cases (51.9%) in the IMLN uptake group. Also, there was no significant difference in the distribution of tumor, node, metastasis (TNM) stages between the two groups. ALN metastasis was the most frequent pattern in the axillary uptake only group (23.4%), but this observation was not statistically significant. Molecular profiles including hormone receptor (HR) and HER2 overexpression were also not significantly different between the two groups. Post-operative adjuvant therapies, including radiation, chemo, and hormonal, was similar in both groups (Table 1).
Table 1.
Clinicopathologic features of the breast cancer patients
IMLN=internal mammary lymph node; BMI=body mass index; PM=partial mastectomy; TM=total mastectomy; DCIS=ductal carcinoma in situ; IDC=invasive ductal carcinoma; TNM=tumor, node, metastasis; ALN=axillary lymph node; HR=hormone receptor.
*Median (range); †Kruskal-Wallis test; ‡Fisher's exact test; §Others include multifocal lesions, central location, subareolar location, borderline location; ∥Others include lobular, mucinous, tubular, papillary micropapillary cribriform, medullary, mixed, metaplastic subtypes.
DFS and OS analysis
Among the 470 patients, 15 (3.2%) died of breast cancer during a median follow-up period of 119.2 months (range, 7-124 months), and breast cancer-related relapse occurred in 46 (9.8%) patients during a median follow-up period of 109.8 months (range, 7-122 months). LR recurrence was found in 25 patients (5.3%). Recurrence in the IM lymphatic chain was found in 3 cases (0.6%). One of these patients underwent partial mastectomy, while the other two underwent total mastectomy. Of these three patients, the one who underwent total mastectomy and SLN biopsy was stage I (T1N0), the one who underwent lumpectomy was stage II (T2N1), and the third patient who underwent total mastectomy was stage II (T1N1). The former 2 patients were included in the axillary uptake group and the latter patient was included in the IMLN uptake group.
The median follow-up period was 118.8 months (range, 7-122 months) in the axillary uptake group and 107.7 months (range, 14-108 months) in the IMLN group. During these follow-up periods, the breast cancer-related death rate in the axillary uptake only group was 3.6%, which was not statistically different from that in the IMLN group (1.3%) (p=0.484). The 5-year rate also did not differ between the two groups (p=0.306). The overall recurrence rate and the LR-specific recurrence rate did not differ between the two groups (p=0.835 and p=0.582, respectively). The 5-year DFS rate and LR-specific DFS rate were not different between the two groups (p=0.962 and p=0.723, respectively) (Table 2). In particular, the recurrence rate of IMLN (both ipsilateral and contralateral) was very low, with a rate of 0.5% in the axillary uptake only group and 1.3% in the IMLN uptake group (p=0.416).
Table 2.
Breast cancer specific death rate and recurrence rate
5YSR=5 year survival; IMLN=internal mammary lymph node; LR=locoregional.
*Median (range); †Log-rank test.
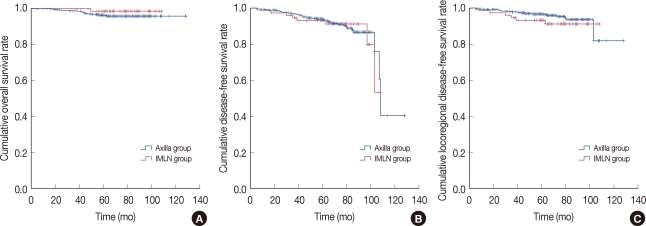
Survival analysis using the Kaplan-Meier survival curve also produced similar results (p=0.306). Survival analysis using the Kaplan-Meier survival curve also did not show any difference between the two groups for DFS or LR recurrence specific DFS (p=0 .962 and p=0.723, respectively) (Figure 1).
Figure 1.
Survival analysis of the axillary uptake group and the internal mammary lymph node (IMLN) uptake group using a Kaplan-Meier survival curve. (A) Breast cancer-specific overall survival outcome (p=0.306). (B) Breast cancer-specific disease-free survival outcome (p=0.962). (C) Locoregional disease-free survival outcome (p=0.723).
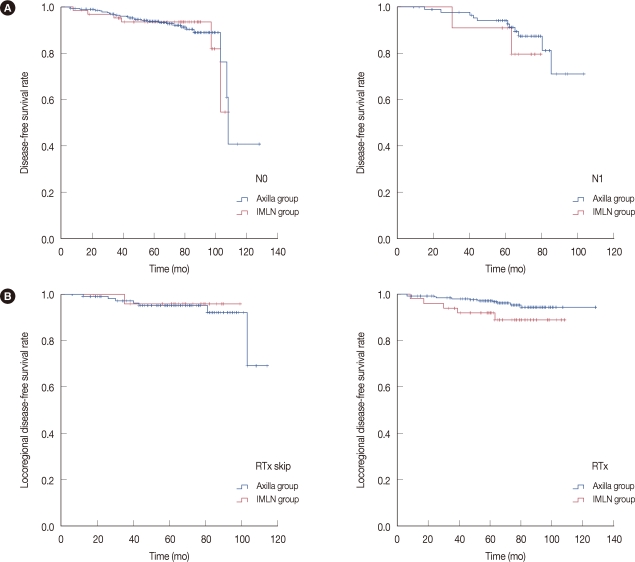
A detailed analysis showed that ALN metastasis was not associated with the outcome of IMLN uptake patients, in either overall DFS outcome or LR-specific DFS outcome (Figure 2A and 2B). Moreover, radiation did not affect overall or LR-DFS outcome in IMLN uptake patients (Figure 2B).
Figure 2.
Subgroup analysis of survival outcome between the axillary uptake group and the internal mammary lymph node (IMLN) uptake group using the Kaplan-Meier survival curve. (A) Disease-free survival outcome according to axillary lymph nodes metastasis (p=0.932 and p=0.631, respectively). (B) Locoregional disease-free survival of IMLN patients according to radiation therapy (p=0.893, and p=0.705, respectively).
DISCUSSION
With the recent use of preoperative lymphoscintigraphy and improved techniques, IMSLN biopsy has become clinically feasible and applicable with greatly decreased morbidity compared to IMLN dissection. Different techniques for IMSLN biopsy are used at different institutes and are associated with different detection rates [11]. The amount of radioisotope injection, time interval between the injection, and the lymphoscintigraphy, or the type of operation may have an effect on the IMLN detection rate. In general, IMSLN drainage is found in up to approximately 25% of patients during preoperative lymphoscintigraphy [4,8,9]. The IMLN is usually visualized simultaneously with ALN, and the incidence of isolated lymphatic drainage to IMLN has been estimated to be 1-8% [6,8,10,12,13]. Comparable to previous reports, approximately 10% of our patients showed IMLN uptake on lymphoscintigraphy, and isolated IMLN drainage was found in 2.1% of patients. Few studies have presented a strong association between tumor location and lymphatic drainage to the internal mammary chain and tumor aggressiveness [1,4]. In this study, tumor location was not associated with stronger internal mammary drainage, but IMLN uptake tended to have a slightly decreased association with axillary metastasis.
The IMLN metastasis rate with successful IMSLN biopsy has been reported to be approximately 14%, a success rate that is lower than the actual detection rate [4,8,9]. Overall, the incidence of IMLN metastasis among breast cancer patients has been estimated to be less than 3% [9,14]. Moreover, due to advances in SLN biopsy techniques along with the surgeon's experience, the success rate may increase over time; however, complications are currently encountered in approximately 4.6-8% of patients, which is slightly higher than the metastasis rate in IMSLN [8,9,14]. IMSLN excision is associated with technical issues such as a higher morbidity rate, a lower metastasis rate in IMSLN than complication incidence, and limited surgeon experience and technical skills. In this study, only one patient underwent an intraoperative IMSLN biopsy, which was negative on frozen section analysis and permanent section analysis, with no biopsy-related morbidity.
It is not feasible to directly compare IMSLN biopsy with resection of all IMLN in the same patients because the uptake of IMLN in lymphoscintigraphy does not represent metastasis in the IMLN [9]. In the literature, less than 1% of node-positive breast cancer patients who underwent mastectomy and chemotherapy, but not radiation therapy, developed IM recurrence [15]. In this study, all 3 IMLN recurrent patients (0.6%) underwent chemo-radiation therapy. van Rijk et al. [16] explained that the failure of extra-axillary SLN biopsy is the cause of extra-axillary nodal recurrence of breast cancer. These authors also found that all IMLN metastasis developed 24, 46, and 63 months after surgery. Even though we assumed that IMLN uptake in patients indicated metastasis or micrometastases at the time of the diagnosis, the recurrence rate did not differ between the two groups. IMLN recurrence was equally rare in both the axillary uptake group and the IMLN uptake group, with a relatively long time interval between diagnosis and recurrence.
IMLN metastasis is an important prognostic factor in breast cancer patients [1,17 19]. However, several studies have demonstrated that surgical dissection of IMLN is not associated with increasing rate of survival. Several trials have failed to prove the advantage of surgical dissection of IMLN, and others have demonstrated that the reduction of IMLN recurrence by calculation does not improve survival outcome in early breast cancer patients [5,20]. This study also suggests that predominant drainage to the IMLN area is not associated with IMLN involvement and long-term outcome, even in the theoretical overestimation of IMLN containing micro or macro-metastasis at the time of diagnosis. In particular, early stage breast cancer patients are not appropriate candidates for IMSLN biopsy.
Although, IMLN metastasis without ALN metastasis is rarely found in early stage breast cancer [21], ALN positivity is known to be a risk factor for IMLN metastasis [22]. In this study, the presence of ALN metastasis did not affect the outcome for DFS in either group. ALN metastasis was less likely to occur in patients with IMLN drainage, although this trend was not statistically significant. Veronesi et al. [5] reported that the annual death rate of axillary node negative/IMLN-positive patients was not significantly different from that of axillary node positive/IMLN-negative patients. Others have also presented comparable significance for IMLN metastasis compared to axillary metastases, showing that the prognosis of patients with disease limited to axillary or IMLN is intermediate between that of patients with negative nodes and those with IM and ALN metastases [18]. Further, radiation therapy did not affect the DFS outcome in the present study. Previously published randomized controlled trials reported that post-mastectomy radiation therapy resulted in a significant improvement in survival [23,24]. Veronesi et al. [25] have also reported that IMLN radiation may have an effective role in the local control according to results on a large series. On the other hand, some reports have shown that irradiation in the IMLN shows no long-term survival benefits [2,26,27]. Trials are still ongoing to address this issue. Adjuvant treatment may play an important role when there is IMLN metastasis.
Biopsy results confirming the pathological state of IMLN for patients included in this study was absent. Therefore, a direct comparison analysis between IMSLN metastasis and pathologically confirmed IMSLN tumor-free patients was not possible. Discrepancy and possible bias are the main limitations of the current study. However, this is the largest observational analysis and the first review of IMLN detected by lymphoscintigraphy in terms of long-term outcome and prognostic significance. Subset analysis of the breast tumor, the treatment of which may benefit from IMSLN biopsy in early or advanced stage breast cancer, is open for future discussion.
The long-term follow-up results for early stage breast cancer patients showed that there was no significant difference in the survival outcome of patients with or without prominent IMLN drainage. Recurrence in a LR area including the internal mammary lymphatic chain was very low and did not differ between the two groups. ALN metastasis or additional radiation therapy did not affect the DFS outcome of breast cancer patients with internal mammary lymphatic drainage. Surgical exploration may also increase the risk of the interfering adjuvant treatment schedule without any advantage. Therefore, solid based clinical research may be required to clarify the necessity of IMSLN biopsy. Moreover, in a clinical setting, the authors suggest that IMSLN biopsy can be omitted in selected cases of early stage breast cancer without ALN metastasis.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Veronesi U, Cascinelli N, Greco M, Bufalino R, Morabito A, Galluzzo D, et al. Prognosis of breast cancer patients after mastectomy and dissection of internal mammary nodes. Ann Surg. 1985;202:702–707. doi: 10.1097/00000658-198512000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Redmond C, Fisher ER, Bauer M, Wolmark N, Wickerham DL, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 3.Lacour J, Le M, Caceres E, Koszarowski T, Veronesi U, Hill C. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer. 1983;51:1941–1943. doi: 10.1002/1097-0142(19830515)51:10<1941::aid-cncr2820511032>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Johnson N, Soot L, Nelson J, Franzini MD, Vea H, Gruner S, et al. Sentinel node biopsy and internal mammary lymphatic mapping in breast cancer. Am J Surg. 2000;179:386–388. doi: 10.1016/s0002-9610(00)00365-2. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Marubini E, Mariani L, Valagussa P, Zucali R. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. Eur J Cancer. 1999;35:1320–1325. doi: 10.1016/s0959-8049(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 6.Carcoforo P, Sortini D, Feggi L, Feo CV, Soliani G, Panareo S, et al. Clinical and therapeutic importance of sentinel node biopsy of the internal mammary chain in patients with breast cancer: a single-center study with long-term follow-up. Ann Surg Oncol. 2006;13:1338–1343. doi: 10.1245/s10434-006-9062-4. [DOI] [PubMed] [Google Scholar]
- 7.Yao MS, Kurland BF, Smith AH, Schubert EK, Dunnwald LK, Byrd DR, et al. Internal mammary nodal chain drainage is a prognostic indicator in axillary node-positive breast cancer. Ann Surg Oncol. 2007;14:2985–2993. doi: 10.1245/s10434-007-9473-x. [DOI] [PubMed] [Google Scholar]
- 8.van der Ent FW, Kengen RA, van der Pol HA, Povel JA, Stroeken HJ, Hoofwijk AG. Halsted revisited: internal mammary sentinel lymph node biopsy in breast cancer. Ann Surg. 2001;234:79–84. doi: 10.1097/00000658-200107000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrús B, Vidal-Sicart S, Velasco M, Zanón G, Fernández PL, Muñoz M, et al. Incidence of internal mammary node metastases after a sentinel lymph node technique in breast cancer and its implication in the radiotherapy plan. Int J Radiat Oncol Biol Phys. 2004;60:715–721. doi: 10.1016/j.ijrobp.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Madsen E, Gobardhan P, Bongers V, Albregts M, Burgmans J, De Hooge P, et al. The impact on post-surgical treatment of sentinel lymph node biopsy of internal mammary lymph nodes in patients with breast cancer. Ann Surg Oncol. 2007;14:1486–1492. doi: 10.1245/s10434-006-9230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimazu K, Tamaki Y, Taguchi T, Motomura K, Inaji H, Koyama H, et al. Lymphoscintigraphic visualization of internal mammary nodes with subtumoral injection of radiocolloid in patients with breast cancer. Ann Surg. 2003;237:390–398. doi: 10.1097/01.SLA.0000055226.89022.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrd DR, Dunnwald LK, Mankoff DA, Anderson BO, Moe RE, Yeung RS, et al. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol. 2001;8:234–240. doi: 10.1007/s10434-001-0234-y. [DOI] [PubMed] [Google Scholar]
- 13.Shahar KH, Buchholz TA, Delpassand E, Sahin AA, Ross MI, Ames FC, et al. Lower and central tumor location correlates with lymphoscintigraphy drainage to the internal mammary lymph nodes in breast carcinoma. Cancer. 2005;103:1323–1329. doi: 10.1002/cncr.20914. [DOI] [PubMed] [Google Scholar]
- 14.Bourre JC, Payan R, Collomb D, Gallazzini-Crepin C, Calizzano A, Desruet MD, et al. Can the sentinel lymph node technique affect decisions to offer internal mammary chain irradiation? Eur J Nucl Med Mol Imaging. 2009;36:758–764. doi: 10.1007/s00259-008-1034-4. [DOI] [PubMed] [Google Scholar]
- 15.Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 16.van Rijk MC, Nieweg OE, Valdés Olmos RA, Rutgers EJ, Hoefnagel CA, Kroon BB. Non-axillary breast cancer recurrences after sentinel node biopsy. J Surg Oncol. 2005;92:292–298. doi: 10.1002/jso.20407. [DOI] [PubMed] [Google Scholar]
- 17.Cody HS, 3rd, Urban JA. Internal mammary node status: a major prognosticator in axillary node-negative breast cancer. Ann Surg Oncol. 1995;2:32–37. doi: 10.1007/BF02303699. [DOI] [PubMed] [Google Scholar]
- 18.Klauber-DeMore N, Bevilacqua JL, Van Zee KJ, Borgen P, Cody HS., 3rd Comprehensive review of the management of internal mammary lymph node metastases in breast cancer. J Am Coll Surg. 2001;193:547–555. doi: 10.1016/s1072-7515(01)01040-7. [DOI] [PubMed] [Google Scholar]
- 19.Sugg SL, Ferguson DJ, Posner MC, Heimann R. Should internal mammary nodes be sampled in the sentinel lymph node era? Ann Surg Oncol. 2000;7:188–192. doi: 10.1007/BF02523652. [DOI] [PubMed] [Google Scholar]
- 20.Cody HS., 3rd Clinical significance and management of extra-axillary sentinel lymph nodes: worthwhile or irrelevant? Surg Oncol Clin N Am. 2010;19:507–517. doi: 10.1016/j.soc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay SC, Cassidy N, Meade S. Clinically node-negative breast cancer, internal mammary lymph nodes, and sentinel lymph node biopsy. Clin Nucl Med. 2008;33:391–393. doi: 10.1097/RLU.0b013e318170d569. [DOI] [PubMed] [Google Scholar]
- 22.Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management: a systematic review. J Clin Oncol. 2008;26:4981–4989. doi: 10.1200/JCO.2008.17.4862. [DOI] [PubMed] [Google Scholar]
- 23.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Danish Breast Cancer Cooperative Group 82b Trial. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 24.Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 25.Veronesi U, Arnone P, Veronesi P, Galimberti V, Luini A, Rotmensz N, et al. The value of radiotherapy on metastatic internal mammary nodes in breast cancer. Results on a large series. Ann Oncol. 2008;19:1553–1560. doi: 10.1093/annonc/mdn183. [DOI] [PubMed] [Google Scholar]
- 26.Palmer MK, Ribeiro GG. Thirty-four year follow up of patients with breast cancer in clinical trial of postoperative radiotherapy. Br Med J (Clin Res Ed) 1985;291:1088–1091. doi: 10.1136/bmj.291.6502.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veronesi U, Zucali R, Luini A. Local control and survival in early breast cancer: the Milan trial. Int J Radiat Oncol Biol Phys. 1986;12:717–720. doi: 10.1016/0360-3016(86)90027-1. [DOI] [PubMed] [Google Scholar]