Abstract
Zapotin, a tetramethoxyflavone, is a natural compound with a wide spectrum of activities in neoplastic cells. Protein kinase C epsilon (PKCε) has been shown to be oncogenic, with the ability to increase cell migration, invasion and survival of tumor cells. Here we report that zapotin inhibits cell proliferation. In wild-type HeLa cells with basal endogenous expression of PKCε, the IC50 was found to be 17.9 ± 1.6 μM. In HeLa cells overexpressing doxycycline-inducible constitutively active PKCε (HeLaPKCεA/E), the IC50 was 7.6 ± 1.3 μM, suggesting that PKCε enhances the anti-proliferative effect of zapotin. Moreover, we found that zapotin selectively activated PKCε in comparison with other PKC family members, but attenuated doxycycline-induced PKCε expression. As a result of zapotin treatment for 6, 12 and 24 h, the doxycycline-induced levels of the two differently phosphorylated PKCε forms (87 kDa and 95 kDa) were decreased. Migration assays revealed that increasing concentrations of zapotin (from 3.5 to 15 μM) decreased migration of HeLaPKCεA/E cells. Furthermore, zapotin significantly increased the fraction of apoptotic cells in doxycycline-induced (HeLaPKCεA/E) cells after 24 h and decreased the levels of Bcl-2, c-Jun, c-Fos. This was accompanied by a degradation of PARP-1. In summary, activation of PKCε and down-modulation of the induced PKCε level by zapotin were associated with decreased migration and increased apoptosis. These observations are consistent with the previously reported chemopreventive and chemotherapeutic action of zapotin.
Keywords: Zapotin, Protein kinase C epsilon, Migration, Cell cycle, Apoptosis
1. Introduction
Flavonoids belong to the family of polyphenolic compounds that are common components of human diet. Epidemiological studies have shown that consumption of flavonoids is associated with a low risk of cardiovascular diseases and cancer. Flavones, a group of flavonoids, are neuroprotective, cardioprotective and chemopreventive agents acting as antioxidants and modulators of protein kinases and lipid-dependent signaling pathways (Duraj et al., 2005). The flavone zapotin (5,6,2′,6′-tetramethoxyflavone) was first identified in the tropical fruit zapote blanco (Casimiroa edulis) (Murillo et al., 2007), later isolated from Sargentia gregii (Meyer et al., 1985) and extracted from the leaves of Primula veris (Budzianowski et al., 2005). In a previous study it was demonstrated that zapotin prevented colon carcinogenesis (Murillo et al., 2007). In human promyelocytic HL-60 leukemia cells, zapotin induced both, cell differentiation and apoptosis (Mata-Greenwood et al., 2001). Moreover, zapotin inhibited 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ornithine decarboxylase activity in human T24 bladder carcinoma cells and TPA-induced nuclear factor kappa B (NF-κB) activity in human HepG2 hepatocellular carcinoma cells (Maiti et al., 2007). However, any influence of zapotin on protein kinase pathways has not been reported so far. In our work, we focused on the effects of zapotin on protein kinase C (PKC) which is a family of ten isozymes comprising (i) the conventional PKCs α, βΙ, βΙΙ, γ, (ii), the novel δ, ε, θ, η, and (iii) the atypical λ/ι (mouse/human) and ζ. PKC isozymes play important roles in the activation of signal transduction pathways leading to synaptic transmissions, the activation of ion fluxes, secretion, proliferation, cell cycle control, ischemic preconditioning, differentiation and tumorigenesis. PKC has become of major interest as a target for therapeutic intervention in a range of different diseases such as allergy, asthma, rheumatoid arthritis, transplantation, AIDS, Alzheimer's disease, multiple sclerosis, hypertension, cardiac hypertrophy, ischemic insult, atherosclerosis, diabetes and cancer (Goekjian and Jirousek, 1999). The exact functions of the different PKC isozymes are not known at present. Here we investigated the influence of zapotin on the ten PKC isoenzymes and found that it selectively activates PKCε.
2. Materials and methods
2.1. Isolation of zapotin
The leaves of Primula veris L. (cowslip) were collected from the field (A) and maintained by Dr. Maria Morozowska at the Department of Botany, Poznań University of Life Sciences, Poznań, Poland; in vitro cultures (B) were obtained by Dr. Maria Wesołowska at the Department of Pharmaceutical Botany and Plant Biotechnology, as described by Budzianowski et al. (2005). The dried leaves of each collection (A and B, each 80.0 g) were separately extracted with chloroform under reflux (7 × 600 ml/h). The concentrated extracts (A: 8.0 g, B: 6.3 g) were separately chromatographed over cellulose CF-11 columns (Whatman, Maidstone, UK), eluted with a methanol–water mixture (ratio 7:3, v/v), and subsequently eluted from polyamide columns (Roth, Karlsruhe, Germany) with methanol to yield crude lipophilic flavone fractions (A: 3.4 g, B: 2.3 g). Portions of each of those fractions (A: 1.5 g, B: 1.8 g) were combined and separated on a silica gel column (Merck, Darmstadt, Germany) eluted with hexane, hexane–ethyl acetate mixtures (ratio 9:1, 8:2, 7:3, 6:4, v/v) and ethyl acetate to give fractions containing various mixtures of lipophilic flavones. The fractions containing zapotin were separated by preparative thin-layer chromatography on polyamide 6 (Macherey-Nagel, Düren, Germany) using acetic acid–water mixture (ratio 3:7, v/v) and on silica gel using hexane–ethyl acetate mixture (ratio 7:3, v/v) to give crude zapotin. This was purified by two-step column chromatography on Sephadex LH20 (Amersham-Pharmacia, Dübendorf, Switzerland) using methanol of HPLC grade and a mixture of redistilled ethanol and double distilled water (ratio 4:1, v/v) to yield a pure compound as white crystals from methanol (97 mg). For the experiments, a 40 mM stock solution in DMSO was used.
2.2. NMR identification of zapotin
The identity of the isolated zapotin sample was determined by 1H (400 MHz) and 13C (100 MHz) NMR spectra recorded for the solution in deuterated chloroform (CDCl3) and compared to the spectral data previously published (Budzianowski et al., 2005). The purity of zapotin was determined to be 95% from the integrals observed in the 1H NMR spectrum. Those integrals indicated 18 protons for zapotin and 2 protons for an impurity, which was visible as a broad singlet at 1.78 ppm and hence considered to correspond to water crystallization.
2.3. Cell culture
The HeLaPKCεA/E subline was derived from parental HeLa wild-type cells (HeLaWT; human epitheloid cervix carcinoma cells; ATCC No. CCL-2) by transfection with a pUHD 172-1-neo vector carrying a tetracycline/doxycycline-inducible Tet-on vector (Clontech, Palo Alto, CA) containing a constitutively active rat PKCε (PKCεA/E, Ala159 is replaced by Glu) (Garczarczyk et al., 2009). In this cell line the expression of PKCεA/E can be induced with 2 μg/ml doxycyline. This mutated form of PKCε is constitutively active without activators such as TPA. Cells were cultured in RPMI 1640 medium supplemented with 2 mM glutamine (Sigma Chemicals, Munich, Germany), 10% Tet-approved fetal bovine serum (Clontech, Mountain View, USA), 100 μg/ml geneticin and 100 μg/ml hygromycin (both from Roche Diagnostics, Mannheim, Germany). HeLaWT cells were cultured in RPMI 1640 medium with 10% fetal bovine serum and 50 μg/ml gentamicin (Sigma Chemicals, Munich, Germany).
2.4. Cell proliferation assay
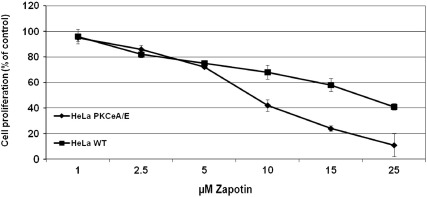
Zapotin cytotoxicity was assessed as previously described (Rubis et al., 2008) using the MTT Proliferation Assay (Cell Proliferation Kit, Roche Diagnostics, Mannheim, Germany). Briefly, ~ 3000 HeLaWT or HeLaPKCεA/E cells/well were seeded in 96-well microplates and exposed to 1–25 μM of zapotin for 72 h. Cell viability was quantified using a Labsystems Multiscan RC spectrophotometer. IC50 values were calculated with CalcuSyn (Biosoft, Cambridge, UK) and standard deviation with Excel software. The final concentration of DMSO in the medium of controls and zapotin-treated cells was 0.1% and this did not show any effect on cell proliferation. The mean of 3 experiments, each in duplicate (+/− S.D.), is indicated in Fig. 2.
Fig. 2.
Influence of zapotin on cell viability. Cell proliferation was measured with the MTT assay. HeLaWT and HeLaPKCεA/E cells were seeded at a density of ~ 3000 cells/well in a 96-well microplate and exposed for 72 h to 1, 2.5, 5, 10, 15 and 25 μM zapotin. Cell proliferation was quantified spectrophotometrically. The final concentration of DMSO in the medium of controls and zapotin-treated cells was 0.1%.
2.5. Western blot analysis
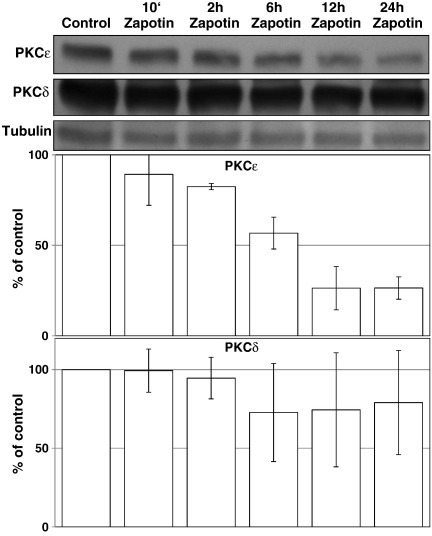
HeLaPKCεA/E cells were treated with 7.5, 15, 30 μM zapotin or 2 μg/ml doxycycline, or a combination of both compounds. Whole cell extracts were prepared using lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1% Triton X-100, 100 mM PMSF, 25 μg/ml Na3VO4, 25 μg/ml NaF, 25 μg/ml leupeptin and 25 μg/ml aprotinin) as previously described (Garczarczyk et al., 2009). Protein concentration was measured with the Bradford assay (Sigma, Munich, Germany) and 30 μg (or 60 μg for phospho PKCε detection) of each extract was loaded onto SDS-PAGE ready gels (BioRad, Hercules, CA). Western blotting was performed by a standard procedure using PVDF membrane (Pierce Biotechnology, Rockford, USA). The following antibodies were used for detection: anti-PKCε, anti-pPKCε(Ser729), anti-PKCδ, anti-Bcl-2, anti-c-Jun, anti-c-Fos, anti-PARP-1, anti-NF-κB, anti-pNF-κB(Ser536), anti-actin, anti-GAPDH, anti-tubulin (all from Santa Cruz Biotechnology); 1 μg/ml of each primary antibody was used in the blotting solution. The proteins were visualized using SuperSignal® West Pico Chemiluminescent Substrate and a CL-X Posure™ film (Pierce Biotechnology, Rockford, USA). The optical density (Arbitrary Units) of the bands was measured using LabWorks software (UVP, Upland, CA). In Figs. 4 and 5, representatives of two experiments are shown.
Fig. 4.
Influence of zapotin on the expression of doxycycline-induced PKCε. The nearest homologue of PKCε, PKCδ was employed as control. The expression of PKCε was induced with doxycycline for 24 h. Zapotin was added together with doxycycline at the start of the 24 h-treatment, and 12 h, 6 h, 2 h and 10 min before the end of the experiment. PKCδ was detected under the same conditions. Western blot analysis was performed as described in Materials and methods. The results are shown as relative to loading control, tubulin.
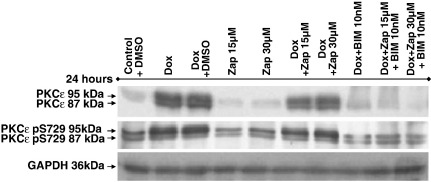
Fig. 5.
Influence of zapotin on PKCε expression in HeLaPKCεA/E cells. Western blot analysis was performed with 30 μg of protein extract separated in SDS-PAGE and detected with anti-PKCε antibody and with a PKCε phospho-Ser729 antibody. For densitometric analysis of the two PKC isoforms, GAPDH was employed as internal standard. Dox 24 h, 2 μg/ml doxycycline; Zap 24 h, 15 or 30 μM zapotin; (Dox + Zap) 24 h, concomitant treatment with 2 μg/ml doxycycline and 15 or 30 μM zapotin for 24 h; Dox 24 h + Zap 24 h, doxycycline 2 μg/ml for 24 h followed by a wash and subsequent treatment with 15 or 30 μM zapotin for another 24 h. BIM, bisindolylmaleimide.
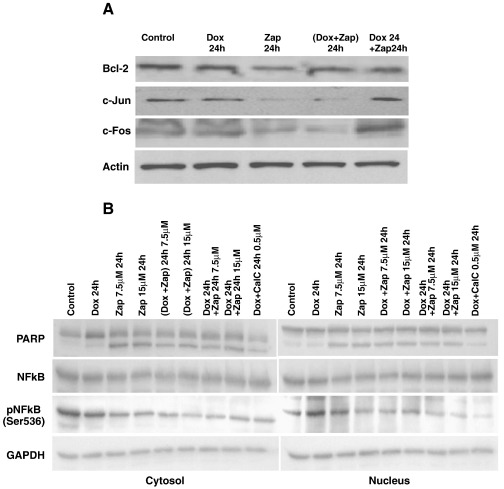
To prepare the nuclear and cytosolic fractions, cells were homogenized in ice-cold lysis buffer (10 mM Hepes (pH 7.4), 1.5 mM MgCl2, 10 mM KCl and 5 mg/ml aprotinin and 5 mg/ml leupeptin) for 5 min as described by Brodsky et al. (2010). The extract was centrifuged at 2400 g for 15 min, and the supernatants were centrifuged for 45 min at 14,000 g. The cytosolic extract was stored at 4 °C. The nuclear pellet was resuspended and incubation for 45 min in lysis buffer containing 20 mM Hepes (pH 7,4) 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA and 5 mg/ml aprotinin, and 5 mg/ml leupeptin. The nuclear lysates were centrifuged for 45 min at 14,000 g and the supernatant containing the soluble nuclear proteins was taken for further experiments. Protein concentrations were measured with the Bradford assay, and 40 μg protein from each extract was loaded onto 7–12% SDS-PAGE gels (BioRad, Hercules, CA). In Fig. 8, the scans of one representative experiment (out of three) are shown.
Fig. 8.
Effect of zapotin on expression levels of proteins involved in apoptosis. A) HeLaPKCεA/E cells were grown in the presence of 2 μg/ml doxycycline, 7.5 or 15 μM zapotin, or a combination of both for 24 h; 30 μg of cell lysate was loaded onto the gels. (Dox + Zap) 24 h, concomitantly 2 μg/ml doxycycline and 15 μM zapotin for 24 h; Dox 24 h + Zap 24 h, doxycycline 2 μg/ml for 24 h followed by a wash and subsequent treatment with 15 μM zapotin for another 24 h. B) PARP-1, NFκB and NFκB-phospho-Ser536 were detected. Legend is as described in A.
2.6. PKC activity
PKC assays were performed with 150 ng of each recombinant PKC isozyme (Proqinase, Freiburg, Germany) in 100 μl of 20 mM Tris–HCl pH 7.5, 20 mM MgCl2, 1 mM CaCl2, 50 μM substrate peptide (PKCα-19–31, RFARKGSLRQKNV; NeoMPS, Strasbourg, France), 10 μM phosphatidylserine, 1 μM TPA (Sigma, Munich, Germany), 40 μM ATP and 1 μCi γ-33P-ATP (NEG602H, PerkinElmer, Waltham, MA). Zapotin was dissolved in DMSO. In untreated controls (set as 100%), the same volume of DMSO was added as in the zapotin-treated samples. After 10 min incubation at 30 °C, 50 μl of the reaction mix was transferred to a phosphocellulose disk (Whatman, Dassel, Germany), washed three times with 1.5% phosphoric acid and twice with distilled water. Subsequently, the disks were transferred into scintillation vials, and 3 ml of Ultima Gold (PerkinElmer, Waltham, MA) was added for determining the 33P incorporation in a liquid scintillation counter. The data shown represents means of at least three independent experiments, each in triplicates (+/− S.D.).
2.7. Cell migration analysis
For cell migration and motility, a scratch migration assay described by Cha et al. (1996) was employed. In a 60 mm tissue culture dish with logarithmically growing HeLaPKCεA/E cells, a migration gap of approximately 1 mm was created by introducing a ‘scratch’ to the adherent layer of cultured cells using a sterile Gilson 200 μl pipette tip. The scratch was administered by hand with sufficient pressure to remove adherent cells from the polystyrene substrate, but without causing a physical damage to the polystyrene surface. The dish was washed with PBS to remove the detached cells and further incubated for 12 h. This was followed by a wash with phosphate buffered saline. Cells were then treated with zapotin (3.75–15 μM) and/or doxycycline (2 μg/ml) or calphostin C (500 nM) for the indicated times. After microscopic observation, photographs were taken (Axiovert 40 CFL, Zeiss, Göttingen, Germany). A representative of three experiments is shown.
2.8. Cell cycle analysis
After culturing for 16 h, cells were treated with zapotin (15 μM) and/or doxycycline (2 μg/ml), and after a further incubation for 24 h, cell cycle analysis was performed. Cells were collected using 0.25% trypsin (Sigma, Munich, Germany) and cell pellets were resuspended in 100 μl of phosphate buffered saline containing 250 μg/ml propidium iodide and 25 μl ribonuclease A (10 mg/ml; Sigma, Munich, Germany). After incubation in the dark at room temperature for 1 h, flow cytometry analysis was performed (FacSCAN, Becton-Dickinson, Franklin Lakes, NJ). The mean of three experiments in duplicate +/− S.D. is shown.
2.9. Statistical analysis
The data shown are means from at least three separate experiments unless specified otherwise. Statistical analysis was performed by one-way ANOVA (GraphPad Prism, San Diego, CA). P < 0.05 was considered as significant difference.
3. Results
HeLaWT cells were treated with zapotin (Fig. 1) for 72 h and the IC50 value was found to be 17.9 ± 1.6 μM. In HeLaPKCεA/E cells overexpressing PKCεA/E following induction with doxycycline, the IC50 was 7.6 ± 1.3 μM (Fig. 2). Thus, zapotin exhibited a significantly (P < 0.05) higher cytotoxic effect in cells expressing PKCεA/E. The difference between the IC50 values in HeLaWT and doxycycline-induced HeLaPKCεA/E cells indicated that PKCε signaling is involved in zapotin-induced cytotoxicity.
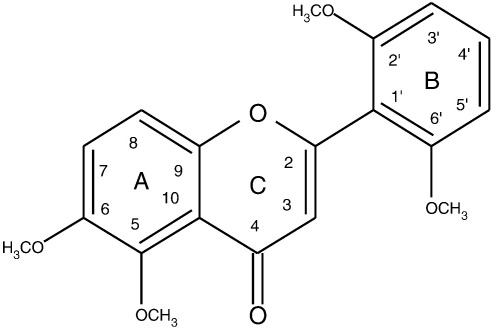
Fig. 1.
Chemical structure of zapotin (5,6,2′,6′-tetramethoxyflavone).
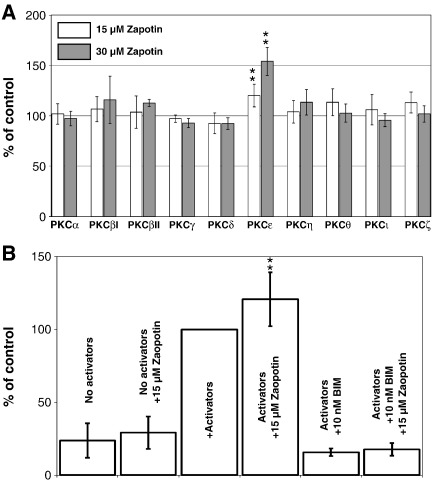
In order to determine whether zapotin exhibits any influence on PKC family members, an in vitro assay with all the ten recombinant PKC isozymes was performed. As shown in Fig. 3A, zapotin was a selective activator of PKCε. The activation of recombinant PKCε was dose-dependent and significant as calculated by the Student's t-test (15 μM zapotin/control P = 0.0001, 30 μM zapotin vs control P = 0.0009). Other PKC isozymes were not affected significantly by zapotin (Fig. 3A). Even if the PKC activators phosphatidylserine and diacylglycerol were omitted, or the PKC inhibitor bisindolylmaleimide I (BIM) was added, also a trend to activate PKCε was observed (Fig. 3B).
Fig. 3.
Effect of zapotin on PKC isozymes in vitro. A) 150 ng of the different recombinant PKC isozymes were incubated in presence of calcium, TPA and phosphatidylserine with a substrate peptide as described in Materials and methods. Controls without zapotin were set as 100% for each PKC isozyme. **15 μM zapotin/control P = 0.0001 and 30 μM zapotin/control P = 0.0009. B) Effect of zapotin on PKCε. Activators were phosphatidylserine and diacylglycerol as indicated in Materials and methods. BIM, bisindolylmaleimide. **, P = 0.003.
Usually, PKC family members are down-modulated after activation. Therefore, we investigated the influence of zapotin on PKCε expression in HeLaPKCεA/E cells. As shown in Fig. 4, doxycycline-induced expression of PKCεA/E was down-modulated by zapotin. In order to determine whether the down-modulation by zapotin is specific for PKCε, its closest homologue, PKCδ was investigated. As shown in Fig. 4, PKCδ was down-modulated to a lesser extent by zapotin, illustrating a preference of this compound for PKCε. The most pronounced down-modulation of PKCεA/E was observed after 24 h. Therefore, for the following experiments this period of time was employed. PKCε with the molecular mass of 87 kDa is phosphorylated at Thr566 and Ser703, and PKCε with the molecular mass of 95 kDa is additionally phosphorylated at Ser729 (England et al., 2001; Garczarczyk et al., 2009). Immunodetection in a gel which allows separation of both forms revealed that in HeLaPKCεA/E cells doxycycline significantly induced both PKCε isoforms by over 2-fold after 24 h treatment (Fig. 5). Simultaneous treatment with doxycycline and zapotin reduced the doxycycline-inducing effect of both differently phosphorylated forms (Fig. 5, (Dox + Zap 24 h), ~ 30%, P < 0.05).
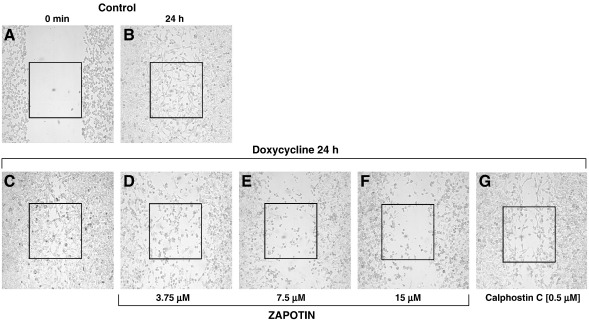
It has been shown previously that PKCε is involved in cell migration and invasion (England et al., 2001; Garczarczyk et al., 2009). Therefore, the influence of zapotin on cell migration of PKCεA/E cells was investigated. After a scratch made in a monolayer (Fig. 6A), doxycycline-induced PKCεA/E overexpression led to increased migration (Fig. 6C) compared to untreated control cells (Fig. 6B). Increasing doses of zapotin (3.75, 7.5 and 15 μM) attenuated the enhanced migration of doxycycline-induced cells overexpressing PKCεA/E (Fig. 6D, E and F, respectively). The general PKC inhibitor calphostin C was used as a control (Fig. 6G).
Fig. 6.
Influence of zapotin on cell migration. HeLaPKCεA/E cells were incubated with doxycycline, zapotin or calphostin C on 60-mm dishes for 24 h. Migration of HeLaPKCεA/E cells into a scratch of logarithmic growing cells made with a pipette tip was assessed microscopically.
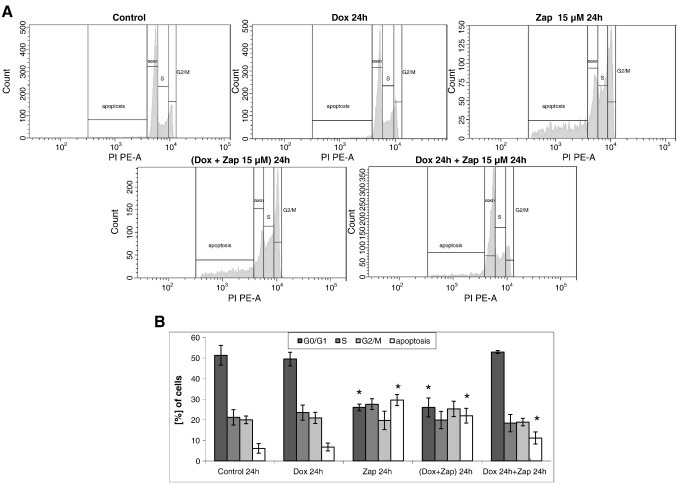
Next we tested the effects of zapotin on the cell cycle and on apoptosis. HeLaPKCεE/A cells with and without doxycycline-induced PKCεA/E expression showed very similar cell cycle profiles with approximately 5% apoptotic cells (Fig. 7). HeLaPKCεE/A cells treated only with zapotin (15 μM) for 24 h revealed a significant decrease (P < 0.05) in the number of G0/G1 cells (> 25% decrease compared to controls) and a significant increase (P < 0.05) in apoptotic cells (up to 30%). This effect was very similar to that observed in the analysis of cells treated simultaneously with doxycycline and zapotin (Dox + Zap 24 h). When cells were treated with doxycycline for 24 h followed by treatment with zapotin for another 24 h, no significant alteration in the cell cycle profile was observed compared to untreated controls or cells with doxycycline-induced PKCεA/E expression. These results were confirmed by the assessment of PCNA accumulation. This S-phase-specific marker was not altered in any of the investigated samples (data not shown). However, a significant increase (P < 0.05) of apoptotic cells up to over 11% was observed after concomitant treatment with doxycycline and zapotin (Fig. 7).
Fig. 7.
Cell cycle analysis. HeLaPKCεA/E cells were incubated in the presence or absence of 2 μg/ml doxycycline and/or 15 μM zapotin for 24 h. Cells were collected (using trypsin) and the cell pellet was resuspended in propidium iodide/ribonuclease and incubated for 1 h, followed by flow cytometry analysis. *, P < 0.05, compared to the controls.
As zapotin increased the percentage of apoptotic cells (Fig. 7), proteins involved in apoptosis were investigated. Induction of PKCεA/E with doxycycline did not significantly alter the level of Bcl-2 protein (Fig. 8A). Zapotin alone (Zap 24 h, 15 μM) caused a significant (P < 0.05) decrease in the level of Bcl-2 by almost 40% compared to control cells. This effect was weaker but still significant in cells incubated simultaneously with doxycycline and zapotin (Dox + Zap; 24 h) (approximately 20%) (P < 0.05). An intermediate effect was observed when cells were treated with zapotin and doxycycline for 24 h (30% decrease compared to control cells) (Fig. 8A). Poly [ADP-ribose] polymerase 1 also known as NAD+ ADP-ribosyltransferase 1 (PARP-1), is a substrate of caspase 3 (Chow et al., 2008). The proteolytic degradation of PARP-1 by caspase 3 is a hallmark of apoptosis. The analysis of PARP-1 (Fig. 8B) revealed no change after doxycycline treatment. However, all treatment protocols with zapotin (zapotin alone, doxycycline and zapotin for 24 h, doxycycline 24 h and subsequently zapotin for 24 h) led to PARP-1 degradation. Doxycycline + calphostin C were employed as a control (Fig. 8B).
The analysis of c-Jun showed that treatment of PKCεA/E cells with zapotin led to a significant decrease (over 90% decrease relative to control, P < 0.05) (Fig. 8A). A similar effect was observed after treatment with a combination of the two compounds ((Dox + Zap) 24 h). However, when cells were successively incubated with the two compounds (Dox 24 h + Zap 24 h), no change in c-Jun was found. A similar profile was observed with c-Fos. A downregulation of protein expression by over 50% in cells treated with zapotin alone or with jointly both compounds (Dox + Zap; 24 h) was found. When cells were treated first with doxycycline for 24 h and thereafter with zapotin for another 24 h (Dox 24 h + Zap 24 h), a significant (P < 0.05) increase (almost 80% compared to control) of c-Fos protein expression was demonstrated (Fig. 8A).
The transcription factor NF-κB is involved in inhibition of apoptosis. Upon activation, an IκB kinase/NF-κB complex is split and NFκB is translocated to the nucleus to activate a series of genes. In an alternative pathway it is activated by phosphorylation. As shown in Fig. 8B, NF-κB levels were not altered or translocated to the nucleus significantly under zapotin treatment. However, the phosphorylated form of NF-κB was reduced after each of the zapotin treatments, indicating less inhibition of apoptosis.
4. Discussion
Plants represent a rich source of pharmacologically active compounds. Flavones, representing the flavonoid family, have potent anticancer properties. They have also been shown to exert chemopreventive effects (Cuendet et al., 2008; Gupta et al., 2002; Lepley et al., 2006; Lim et al., 2007; Murakami et al., 2000) by modulating proliferation and differentiation and inducing apoptosis in colon (Pan et al., 2002), breast (Ullmannova and Popescu, 2007) and prostate cancer cells (Gupta et al., 2002; Lepley et al., 2006). An increased number of methoxyl groups in the basic skeleton of the aglycone of flavones, especially in the A ring (Fig. 1), results in an increased antiproliferative activity (Li et al., 2007; Walle, 2007). Moreover, inhibition of proliferation by flavones was observed selectively in cancer cells (Walle, 2007). A high antiproliferative potential of the polymethoxyflavones tangeretin and nobiletin is well documented in various types of cancers (Li et al., 2007; Morley et al., 2007). Cytotoxic activity of the tetramethoxyflavone zapotin, isolated from Casimiroa edulis was demonstrated in T24 and HepG2 cells (Maiti et al., 2007). The present study showed that the IC50 value for inhibition of proliferation was lower in cells overexpressing constitutively active PKCε than in HeLaWT cells (7.6 vs. 17.9 μM). PKCε is known to play a crucial role in neoplastic transformation (Hofmann, 2004; Jobbagy et al., 1999). Overexpression of PKCε has been observed in cancer of the prostate, lung, breast, liver and thyroid gland. PKCε has been reported to be associated with tumor promotion along the Ras/Raf/MAPK phosphorylation cascade (Bae et al., 2007; Basu and Sivaprasad, 2007; England et al., 2001). It was reported that in MCF7 breast cancer cells, overexpression of PKCε mediates an antiapoptotic effect partly by preventing activation and translocation of Bax to the mitochondria (Lu et al., 2007). PKCε downregulation may thus represent one anticancer strategy. As shown in Fig. 3, PKCε is activated by zapotin which leads to a down-modulation of its protein expression (compared to untreated controls) after 6 h and 12 h (Fig. 4).
PKC is a family of serine/threonine-specific protein kinases with at least ten different isozymes. The exact functions of the different PKC isozymes are not yet clear. Selective activators or inhibitors of these PKC isozymes could be helpful to elucidate their functions and also for pharmaceutical purposes. Most inhibitors of kinases interact with the ATP-binding site that is well conserved among kinase families. This poses a serious hurdle for the development of isozyme-selective inhibitors. Several activators such as TPA or bryostatin nonspecifically activate a series of PKC isozymes. Ingenol is a PKCε and δ isozyme-selective activator (Armstrong and Ganote, 1994). 8-[2-(2-pentyl-cyclopropylmethyl)-cyclopropyl]-octanoic acid (DCP-LA), a linoleic acid derivative, activates PKCε and to a lesser extent PKCγ (Kanno et al., 2006). Bisphosphatidic acid was found to be a strong activator of PKCε, and a weak one of PKCα (Limatola et al., 1994). Benzyladriamycin-14-valerate was shown to be cardioprotective through PKCε activation (Hofmann et al., 2007). 12(S)-HETE activated PKCε, whereas α and θ were not affected. (Mikule et al., 2003). As our experiments show, zapotin can be used to selectively activate PKCε (Fig. 3) and down-modulate its protein expression (Figs. 4 and 5). Other activators of PKC, such as TPA or bryostatin, also lead to down-modulation of PKC isozymes.
Zapotin induced a dose-dependent decrease in migration of HeLaPKCεA/E cells (Fig. 6). Significant inhibition of migration was observed at relatively low concentrations of zapotin (starting with 3.75 μM) that showed only a slight cytotoxic effect (approximately 20% compared to untreated controls, Fig. 2). Furthermore, zapotin significantly decreased the percentage of cells in the G1 phase of the cell cycle and increased the amount of apoptotic cells (up to about 30%). Elevated expression of PKCεA/E following doxycycline treatment decreased the apoptotic effect of zapotin, demonstrating pro-survival effects of this PKC isozyme (Fig. 7). Previous studies revealed flavones as inducers of apoptosis with cleavage of PARP-1 (Chen et al., 2004; Singh et al., 2006). We found that each of the different treatments with zapotin induced cleavage of PARP-1, illustrating activation of the pro-apoptotic caspase 3. The degradation of PARP-1 was accompanied by increased apoptosis in HeLaPKCεA/E cells (Figs. 7, 8).
Overexpression of Bcl-2 was observed in most of human neoplastic cells and was found in 40–80% cases of breast (Aggarwal et al., 2007), prostate (McDonnell et al., 1997), lung (Ohmura et al., 2000) and neuroendocrine tumors (Brambilla et al., 1996). It was suggested that a flavonoid-mediated effect could contribute to the decrease of Bcl-2 in neoplastic cells (Shim et al., 2007; Shukla and Gupta, 2004). The decrease in Bcl-2 following zapotin treatment might contribute to the pro-apoptotic effect of zapotin in HeLaPKCεA/E cells. Zapotin was also shown to inhibit TPA-induced ornithine decarboxylase activity in human bladder carcinoma cells and TPA-induced NF-κB activity in human hepatocellular carcinoma cells (Maiti et al., 2007). Our study revealed that zapotin treatment reduced the level of phosphorylated NF-κB slightly (Fig. 8B). Since NF-κB plays an important role as an apoptosis inhibitor and Bcl-2 inducer (Gupta et al., 2002; Kundu et al., 2006; Shim et al., 2007; Shishodia and Aggarwal, 2002), the downregulation of Bcl-2 in zapotin-treated cells (Fig. 8E) is consistent with zapotin-mediated apoptosis induction.
In conclusion, our results show that, under zapotin treatment, cell proliferation in PKCε-overexpressing HeLaPKCεA/E cells was decreased to a higher extent than in HeLaWT cells. Zapotin selectively activated and down-modulated PKCε which seems to be involved in cancer metastasis. Furthermore, zapotin increased the percentage of apoptotic cells and decreased cell migration, the percentage of cells in G1 and the protein levels of Bcl-2, c-Jun, c-Fos and the level of phosphorylated NFκB. It led to a degradation of PARP-1. These observations give an indication of the mechanism of the chemopreventive and chemotherapeutic anticancer activities of zapotin reported previously.
Acknowledgment
The authors wish to thank Mariusz Kaczmarek (Department of Clinical Immunology, Poznan University of Medical Sciences, Poland) for the helpful suggestions for flow cytometry analysis. This work was supported by the Austrian Science Fund (FWF): P15039-B12, 19878-B12, by the Austrian National Bank (grant no. 7068) and by research grant 502-01-03318432-02496 from the Poznan University of Medical Sciences.
References
- Aggarwal H., Lubana P.S., Jain D.K., Mathur R.K. Estimation of Bcl-2 protein in carcinoma of the breast and its clinical correlation in locally advanced breast cancer. J. Cancer Res. Ther. 2007;3:207–211. doi: 10.4103/0973-1482.38995. [DOI] [PubMed] [Google Scholar]
- Armstrong S., Ganote C.E. Preconditioning of isolated rabbit cardiomyocytes: effects of glycolytic blockade, phorbol esters, and ischaemia. Cardiovasc. Res. 1994;28:1700–1706. doi: 10.1093/cvr/28.11.1700. [DOI] [PubMed] [Google Scholar]
- Bae K., Wang H., Jiang G., Chen M.G., Lu L., Xiao L. Protein kinase C epsilon is overexpressed in primary human non-small cell lung cancers and functionally required for proliferation of non-small cell lung cancer cells in a p21/Cip1-dependent manner. Cancer Res. 2007;67:6053–6063. doi: 10.1158/0008-5472.CAN-06-4037. [DOI] [PubMed] [Google Scholar]
- Basu A., Sivaprasad U. Protein kinase C epsilon makes the life and death decision. Cell. Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla E., Negoescu A., Gazzeri S., Lantuejoul S., Moro D., Brambilla C., Coll J.L. Apoptosis-related factors p53, Bcl-2, and Bax in neuroendocrine lung tumors. Am. J. Pathol. 1996;149:1941–1952. [PMC free article] [PubMed] [Google Scholar]
- Brodsky M., Halpert G., Albeck M., Sredni B. The anti-inflammatory effects of the tellurium redox modulating compound, AS101, are associated with regulation of NFkB signaling pathway and nitric oxide induction in macrophages. J. Inflamm. 2010;7:3. doi: 10.1186/1476-9255-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzianowski J., Morozowska M., Wesołowska M. Lipophilic flavones of Primula veris L. from field cultivation and in vitro cultures. Phytochemistry. 2005;66:1033–1039. doi: 10.1016/j.phytochem.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cha D., O'Brien P., O'Toole E.A., Woodley D.T., Hudson L.G. Enhanced modulation of keratinocyte motility by transforming growth factor-alpha (TGF-alpha) relative to epidermal growth factor (EGF) J. Invest. Dermatol. 1996;106:590–597. doi: 10.1111/1523-1747.ep12345083. [DOI] [PubMed] [Google Scholar]
- Chen C., Chow M., Huang W., Lin Y., Chang Y. Flavonoids inhibit tumor necrosis factor-α-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-κB: structure–activity relationships. Mol. Pharmacol. 2004;66:683–693. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Chow J.M., Huang G.C., Shen S.C., Wu C.Y., Lin C.W., Chen Y.C. Differential apoptotic effect of wogonin and nor-wogonin via stimulation of ROS production in human leukemia cells. J. Cell. Biochem. 2008;103:1394–1404. doi: 10.1002/jcb.21528. [DOI] [PubMed] [Google Scholar]
- Cuendet M., Oteham C.P., Maiti A., Craig B.A., Cushman M., Moon R.C., Pezzuto J.M. Zapotin prevents mouse skin tumorigenesis during the stages of initiation and promotion. Anticancer. Res. 2008;28:3705–3709. [PubMed] [Google Scholar]
- Duraj J., Zazrivcova K., Bodo J., Sulikova M., Sedlak J. Flavonoid quercetin, but not apigenin or luteolin, induced apoptosis in human myeloid leukemia cells and their resistant variants. Neoplasma. 2005;52:273–279. [PubMed] [Google Scholar]
- England K., Watson J., Beale G., Warner M., Cross J., Rumsby M. Signalling pathways regulating the dephosphorylation of Ser729 in the hydrophobic domain of protein kinase C upon cell passage. J. Biol. Chem. 2001;276:10437–10442. doi: 10.1074/jbc.M009421200. [DOI] [PubMed] [Google Scholar]
- Garczarczyk D., Toton E., Biedermann V., Rosivatz E., Rechfeld F., Rybczynska M., Hofmann J. Signal transduction of constitutively active protein kinase C epsilon. Cell. Signal. 2009;21:745–752. doi: 10.1016/j.cellsig.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Goekjian P.G., Jirousek M.R. Protein kinase C in the treatment of disease: signal transduction pathways, inhibitors, and agents in development. Curr. Med. Chem. 1999;6:877–903. [PubMed] [Google Scholar]
- Gupta S., Afaq F., Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- Hofmann J. Protein kinase C isozymes as potential targets for anticancer therapy. Curr. Cancer Drug. Targets. 2004;4:125–146. doi: 10.2174/1568009043481579. [DOI] [PubMed] [Google Scholar]
- Hofmann P.A., Israel M., Koseki Y., Laskin J., Gray J., Janik A., Sweatman T.W., Lothstein L. N-Benzyladriamycin-14-valerate (AD 198): a non-cardiotoxic anthracycline that is cardioprotective through PKC-epsilon activation. J. Pharmacol. Exp. Ther. 2007;323:658–664. doi: 10.1124/jpet.107.126110. [DOI] [PubMed] [Google Scholar]
- Jobbagy Z., Olah Z., Petrovics G., Eiden M.V., Leverett B.D., Dean N.M. Up-regulation of the Pit-2 phosphate transporter/retrovirus receptor by protein kinase C epsilon. J. Biol. Chem. 1999;274:7067–7071. doi: 10.1074/jbc.274.11.7067. [DOI] [PubMed] [Google Scholar]
- Kanno T., Yamamoto H., Yaguchi T., Hi R., Mukasa T., Fujikawa H., Nagata T., Yamamoto S., Tanaka A., Nishizaki G. The linoleic acid derivative DCP-LA selectively activates PKC-epsilon, possibly binding to the phosphatidylerine binding site. J. Lipid Res. 2006;47:1146–1156. doi: 10.1194/jlr.M500329-JLR200. [DOI] [PubMed] [Google Scholar]
- Kundu J.K., Shin Y.K., Kim S., Surh Y. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-κB and AP-1 as prime targets. Biochem. Pharmacol. 2006;72:1506–1515. doi: 10.1016/j.bcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Lepley D.M., Li B., Birt D.F., Pelling J.C. The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis. 2006;17:2367–2375. doi: 10.1093/carcin/17.11.2367. [DOI] [PubMed] [Google Scholar]
- Li S., Pan M., Lai C., Lo C., Dushenkov S., Ho C. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorg. Med. Chem. 2007;15:3381–3389. doi: 10.1016/j.bmc.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Lim D.Y., Jeong Y., Tyner A.L., Park J.H.Y. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G66–G75. doi: 10.1152/ajpgi.00248.2006. [DOI] [PubMed] [Google Scholar]
- Limatola C., Schaap D., Moolenaar W.H., van Blitterswijk W.J. Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids. Biochem. J. 1994;304:1001–1008. doi: 10.1042/bj3041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Sivaprasad U., Huang J., Shanker E., Morrow S., Basu A. Protein kinase C epsilon protects MCF-7 cells from TNF-mediated cells death by inhibiting Bax translocation. Apoptosis. 2007;10:1893–1900. doi: 10.1007/s10495-007-0111-7. [DOI] [PubMed] [Google Scholar]
- Maiti A., Cuendet M., Kondratyuk T., Croy V.L., Pezzuto J.M., Cushman M. Synthesis and cancer chemopreventive activity of zapotin, a natural product from Casimiroa edulis. J. Med. Chem. 2007;50:350–355. doi: 10.1021/jm060915+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Greenwood E., Ito A., Westenburg H., Cui B., Mehta R.G., Kinghorn A.D. Discovery of novel inducers of cellular differentiation using HL-60 promyelocytic cells. Anticancer. Res. 2001;21:1763–1770. [PubMed] [Google Scholar]
- McDonnell T.J., Navone N.M., Troncoso P., Pisters L.L., Conti C., von Eschenbach A.C., Brisbay S., Logothetis C.J. Expression of bcl-2 oncoprotein and p53 protein accumulation in bone marrow metastases of androgen independent prostate cancer. J. Urol. 1997;157:569–574. [PubMed] [Google Scholar]
- Meyer B.N., Wall M.E., Wani M.C., Taylor H.L. Flavones, coumarins, and an alkaloid from Sargentia greggii. J. Nat. Prod. 1985;48:952–956. doi: 10.1021/np50042a012. [DOI] [PubMed] [Google Scholar]
- Mikule K., Sunpaweravong S., Gatlin J.C., Pfenninger K.H. Eicosanoid activation of protein kinase C epsilon: involvement in growth cone repellent signaling. J. Biol. Chem. 2003;278:21168–21177. doi: 10.1074/jbc.M211828200. [DOI] [PubMed] [Google Scholar]
- Morley K.L., Ferguson P.J., Koropatnick J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007;25:1168–1178. doi: 10.1016/j.canlet.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Murakami A., Nakamura Y., Torikai K., Tanaka T., Koshiba T., Koshimizu K., Kuwahara S., Takahashi Y., Ogawa K., Yano M., Tokuda H., Nishino H., Mimaki Y., Sashida Y., Kitanaka S., Ohigashi H. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000;60:5059–5066. [PubMed] [Google Scholar]
- Murillo G., Hirschelman W.H., Ito A., Moriarty R.M., Kinghorn A.D., Pezzuto J.M., Mehta R.G. Zapotin, a phytochemical present in a Mexican fruit, prevents colon carcinogenesis. Nutr. Cancer. 2007;57:28–37. doi: 10.1080/01635580701268097. [DOI] [PubMed] [Google Scholar]
- Ohmura Y., Aoe M., Andou A., Shimizu N. Telomerase activity and Bcl-2 expression in non-small cell lung cancer. Clin. Cancer Res. 2000;6:2980–2987. [PubMed] [Google Scholar]
- Pan M., Chen W., Lin-Shiau S., Ho C., Lin J. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677–1684. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- Rubis B., Paszel A., Kaczmarek M., Rudzinska M., Jelen H., Rybczynska M. Beneficial or harmful influence of phytosterols on human cells? Br. J. Nutr. 2008;100:1183–1191. doi: 10.1017/S0007114508981423. [DOI] [PubMed] [Google Scholar]
- Shim H.Y., Park J.H., Paik H.D., Nah S.Y., Kim D.S.H.L., Han Y.S. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol. Cells. 2007;24:95–104. [PubMed] [Google Scholar]
- Shishodia S., Aggarwal B.B. Nuclear Factor-κB activation: a question of life or death. J. Biochem. Mol. Biol. 2002;35:28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- Shukla S., Gupta S. Supression of constitutive and tumor necrosis factor α-induced nuclear factor (NF)-κB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-κB-responsive genes. Clin. Cancer Res. 2004;10:3169–3178. doi: 10.1158/1078-0432.ccr-03-0586. [DOI] [PubMed] [Google Scholar]
- Singh R.P., Dhanalakshmi S., Mohan S., Agarwal C., Agarwal R. Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-κB in mouse epidermal JB6 cells. Mol. Cancer Ther. 2006;5:1145–1153. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- Ullmannova V., Popescu N.C. Inhibition of cell proliferation, induction of apoptosis, reactivation of DLC1, and modulation of other gene expression by dietary flavone in breast cancer lines. Cancer Detect. Prev. 2007;31:110–118. doi: 10.1016/j.cdp.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin. Cancer Biol. 2007;17:354–362. doi: 10.1016/j.semcancer.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]