Abstract
The genus Passiflora provides a remarkable example of floral complexity and diversity. The extreme variation of Passiflora flower morphologies allowed a wide range of interactions with pollinators to evolve. We used the analysis of expressed sequence tags (ESTs) as an approach for the characterization of genes expressed during Passiflora reproductive development. Analyzing the Passiflora floral EST database (named PASSIOMA), we found sequences showing significant sequence similarity to genes known to be involved in reproductive development such as MADS-box genes. Some of these sequences were studied using RT-PCR and in situ hybridization confirming their expression during Passiflora flower development. The detection of these novel sequences can contribute to the development of EST-based markers for important agronomic traits as well as to the establishment of genomic tools to study the naturally occurring floral diversity among Passiflora species.
1. Introduction
The genus Passiflora comprises almost 600 species of vines, lianas, and small trees, and its diversity reaches a maximum in Central and South America [1, 2]. To the genus Passiflora belongs the passionfruit (Passiflora edulis Deg.) and other species producing ornamental flowers known collectively as “passionflowers.” Passionflowers are appreciated exactly due to a remarkable range of floral complexity and diversity. The flowers of Passiflora exhibits several unique floral features, including multiple series of brightly colored coronal filaments, diverse operculum morphology, an androgynophore, and elaborate floral nectary structures (Figure 1). The evolution of this extreme variation of flower morphologies is believed to be the result of interactions with a wide range of pollinators [2, 3]. Therefore, this genus is specially suited to any study on the evolution of pollination syndromes, especially those aiming to elucidate the molecular mechanisms underlying these adaptative steps.
Figure 1.
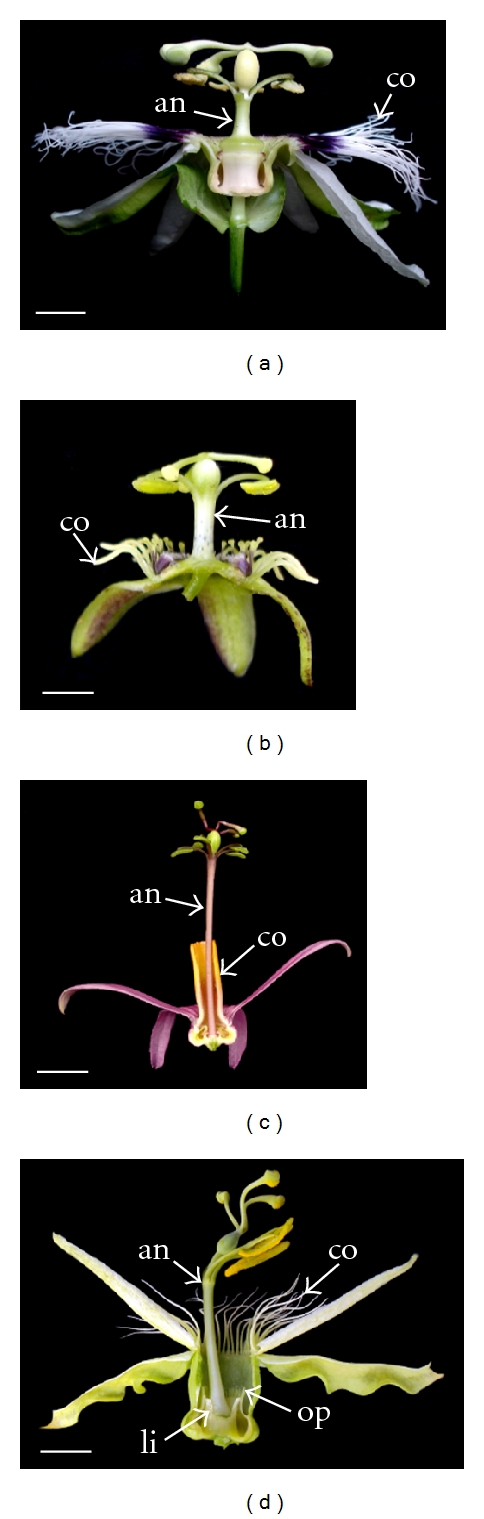
Longitudinal sections of Passiflora spp. flowers. (a) a large insect- (bumblebee) pollinated flower (P. edulis); (b) a small insect- (wasp) pollinated flower (P. suberosa); (c) a hummingbird-pollinated flower (P. tulae); (d) a bat-pollinated flower (P. setacea). co: corona; an: androgynophore; li: limen; op: operculum. Bars: (a), (c), and (d): 1 cm; (b): 0.2 cm.
Accordingly, one of the major challenges of current plant biology is to understand the genetic basis and molecular mechanisms of all naturally occurring developmental variation. This analysis has begun to benefit from the ever growing number of plant genomes readily available in public databases and from the availability of genomic tools aimed to identify gene functions and the mechanistic basis of phenotypes in model plant species such as Arabidopsis thaliana. Among these tools, expressed sequence tags (ESTs) have played significant roles in accelerating gene discovery in plants including those involved in flower development and evolution [4–7].
The main goal of our work is to understand the molecular mechanisms of the divergence of floral features among Passiflora species, with the aim of elucidating the role of pollinating agents in shaping the genomic shifts that lead to the actual patterns of diversification of different floral forms and structures observed in the genus. With that aim, we characterized a set of cDNA libraries from developing reproductive tissues of two divergent Passiflora species: Passiflora suberosa L. and P. edulis Sims. These two species were chosen due to their contrasting phenotypic characteristics: P. edulis produces the commercial passionfruit, with large juicy scented fruits and complete flowers while P. suberosa produces small fruits and small flowers lacking petals. Producing ESTs from these contrasting species might help to better study their reproductive characteristics in the future.
2. Material and Methods
Reproductive meristems and flower buds at different developmental stages of P. edulis and P. suberosa were collected from plants cultivated at the experimental fields at the Department of Plant Biology, IB/UNICAMP at Campinas, SP, Brazil. The samples to be used in in situ hybridization were fixed in 4% paraformaldehyde for 24 h at 4°C and dehydrated in an ethanol series. The samples for RNA extraction were immediately frozen in liquid nitrogen immediately after collection and stored at −80°C until use.
2.1. Construction of cDNA Libraries
Total RNA samples were obtained from floral buds at different developmental stages from P. edulis and P. suberosa frozen in liquid nitrogen and extracted with Trizol (Invitrogen) following the manufacturer instructions. mRNA samples were purified using Oligotex-dT (QIAGEN) resin. One to 5 μg mRNA were used in cDNA synthesis and further cloning of cDNA fragments into pSPORT1 vector with the SuperScript Plasmid System for cDNA Synthesis and Cloning kit (Invitrogen), according to the manufacturer instructions. Ultracompetent Escherichia coli DH10B cells (Invitrogen) were electroporated (25 μF; 200 Ω; 1,8 kV) with the resulting constructs, and possible transformants were plated on LB medium supplemented with ampicillin, IPTG, and X-Gal. Individual positive clones were transferred to 96-well plates containing liquid CG medium (Circle Growth, BIO 101) supplemented with. Replicates of these plates were used either for plasmid extraction and sequencing or to make glycerol stocks for library storage at −80°C.
2.2. Extraction of Plasmid DNA and EST Sequencing
Plasmid DNA extractions were performed according to a modified alcaline lysis method described elsewhere (http://www.protocol-online.org/prot/Protocols/Isolation-of-Plasmid-DNA-3922.html). We used the DYENAMIC kit (GE Biosciences) for sequencing reactions. Sequencing reaction products were precipitated with 95% ethanol, 1/10 reaction volume 3 M sodium acetate, and 1 g·L−1 glycogen. The precipitate was washed with 75% ethanol, speedvac-dryed, and loaded into a 3100 Genetic Analyser (Applied Biosystems).
2.3. Bioinformatics and Sequence Analysis
We used phred [8] to determine sequence quality. The protocols for removing low-quality sequences, ribosomal sequences, and other possible contaminants as well as poly-A tails and vector sequences were described elsewhere [9]. We used CAP3 [10] for sequence clusterization. Different BLAST algorithms [11] (http://www.ncbi.nlm.nih.gov/BLAST/) were used to compare reads and the obtained clusters to publicly available sequences at the NCBI database [12]. To assemble all sequence data and their analysis, a relational database was created using in-house Pearl programming (dos Santos et al., unpublished data). This relational database allows the search of unique reads or clusters of contiguous sequences and their BLAST matches. The code used to name sequences and clusters was the same used within the frame of the SUCEST database [13]. For instance, for the sequence named PACEPE3001A01.g, “PA” designated the sequencing project, named “PASSIOMA”; “CE” referred to the sequencing lab (in this case, the Molecular and Cellular Biology Lab, CENA/USP, Brazil); “PE3” indicated the library, in this case a library made with P. edulis floral buds with 1 cm in length; “001” referred to plate number and “A01” to the clone position within a 96-well plate. Finally, “.g” indicated the T7 sequencing primer (alternatively, “.b” indicated that a SP6 primer was used). Clusters arbitrarily received the code of the first sequence to be included in the cluster. All sequences were automatically annotated according to their category, following the Gene Ontology Consortium (http://www.geneontology.org/) and the instructions of Telles and da Silva. [9]. Searches within the relational database can be performed using either key words or a local BLAST tool. Multiple sequence alignments of selected sequences and available putative homologs from Arabidopsis and/or other plant species were performed using CLUSTALX (http://www.clustal.org/). Distance trees were obtained from neighbor-joining matrices, with Bootstrap calculated from 1000 replicates and visualized with TreeView (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). Parsimony trees were obtained using hand-corrected sequence alignments with MEGA software (http://www.megasoftware.net).
2.4. Gene Expression Analysis
2.4.1. RT-PCR
Total RNA samples, extracted as described above were treated with DNaseI at 37°C for 15 min. Tem micrograms of total RNA were used in a Superscript II (Invitrogen) reverse transcriptase reaction with oligo (dT)20 following the instructions of the manufacturer. Normalized cDNA samples were used as templates in PCR reactions using gene-specific primers (Supplementary Table 1 available online at doi: 10.1155/2011/510549) and under the following conditions: 3 min of initial denaturation at 94°C; 35 cycles of 94°C for 30 s, 60°C for 45 s, 72°C for 1 min and a final extension at 72°C for 10 min. Reactions using primers for constitutive genes were used as positive controls (see Supplementary Table 1 and Supplementary Figure 1) and reactions containing RNA samples without DNAse treatment were used as negative controls. We have also tested performing the PCR for only 20 or 25 cycles (see Supplementary Figure 2). The PCR products were separated by gel electrophoresis, and the results were documented and analyzed.
2.5. In Situ Hybridization
After RT-PCR validation, the expression patterns of selected genes (Supplementary Table 1) were assessed by in situ hybridization. Nonradioactive probes were labeled with digoxygenin (DIG-dUTP) following the instructions of the manufacturer (Roche). The prehybridization and hybridization conditions were described elsewhere [14, 15]. Apices of reproductive shoots and flowers buds a different developmental stages were fixed and dehydrated as described above; embedded in paraffin, sectioned (8 μm), and attached to glass slides previously coated with organosilane (2% solution in acetone). Prior to hybridization, the paraffin was removed from the sections by quickly washing the slides in xylol. Hybridization signal was visualized as using anti-DIG antibodies conjugated to alkaline phosphatase and a NBT/BCIP solution with levamissole (Pierce) as a substrate. Hybridized slides were observed and documented in a Zeiss Axioskop microscope.
3. Results and Discussion
3.1. Characterization of cDNA Libraries from Developing Passiflora Flowers
The data of the PASSIOMA Project can be accessed through an internet interface (passioma.ib.unicamp.br), and a login can be obtained upon contacting the authors. We produced 10,272 high-quality Passiflora sequences (frap/Fred >20 and >300 valid nucleotides) from 6 libraries (Table 1). All libraries contributed equally in terms of number of sequences. About half of the sequences (5,109) were from P. Suberosa, and the other half (5,163) were produced from P. edulis (Table 2). Insert amplification of 100 random clones from each library revealed inserts ranging between 500 bp and 2500 bp, with an average size of 1400 bp (Table 2). About 53% of all obtained sequences were not included in contigs and, therefore, are singletons (Table 2.). The high number of singletons reflects the low redundancy of the libraries. The Passiflora ESTs were annotated according to their BLAST matches and to the gene ontology (GO) [16, 17], defining functional categories to the sequences (Figure 2). The primary BLAST matches revealed three major groups of assembled Passiflora EST sequences with varying potential to predict their cellular function. Sequences belonging to the first group, matched sequences of known proteins with strong and nominal similarity, and are therefore likely to be transcripts of genes with similar functions (this group corresponded to 68% of all sequences). The function of the BLAST match was used to assign putative roles to this group. The second class was formed by 15% of the assembled Passiflora EST sequences and this group matched to “unknown protein,” “hypothetical protein,” or “putative protein,” with no indication of the function of the gene product (Figure 2). Most of the unknown proteins came from ESTs from other plant species that had been entered into the GenBank nonredundant (nr) database. The third group consisted of sequences with no matches in the GenBank nr database, and they were put into an “unable to classify” category (Figure 2); they may represent untranslated mRNAs, as well as novel Passiflora-specific genes. Shoemaker et al. [18] demonstrated that 13% of the soybean ESTs returned no matches after BLASTX search on trimmed sequences against the GenBank nonredundant database.
Table 1.
The cDNA libraries obtained from Passiflora reproductive tissues.
| Library | Source | Passiflora species |
|---|---|---|
| PE1 | Apices (about 1 cm) from adult plants at the reproductive state | P. edulis |
| PE2 | Developing flower buds (0.2 to 0.5 cm) | P. edulis |
| PE3 | Developing flower buds (0.5 to 1 cm) | P. edulis |
| PS1 | Apices (about 1 cm) from adult plants at the reproductive state | P. suberosa |
| PS2 | Developing flower buds (0.1 to 0.5 cm) | P. suberosa |
| PS3 | Developing flower buds (0.5 cm to preanthesis) | P. suberosa |
Table 2.
Characterization of the cDNA libraries obtained from Passiflora reproductive tissues at different developmental stages.
| P. edulis | P. suberosa | |
|---|---|---|
| Number of valid ESTs | 5,163 | 5,109 |
| Clusters | 597 | 588 |
| Singletons | 2,731 | 2,743 |
| Cluster composition a | 3 | 2 |
| Mean insert size (bp) | 1,386 | 1,423 |
| Mean ORF size (bp) | 482 | 463 |
| Novelty (%) b | 86.45 | 89.65 |
a: Mean number of ESTs in each cluster.
b: Mean proportion of a novel singleton or cluster formed for each new sequence introduced in the database (considering all libraries).
Figure 2.
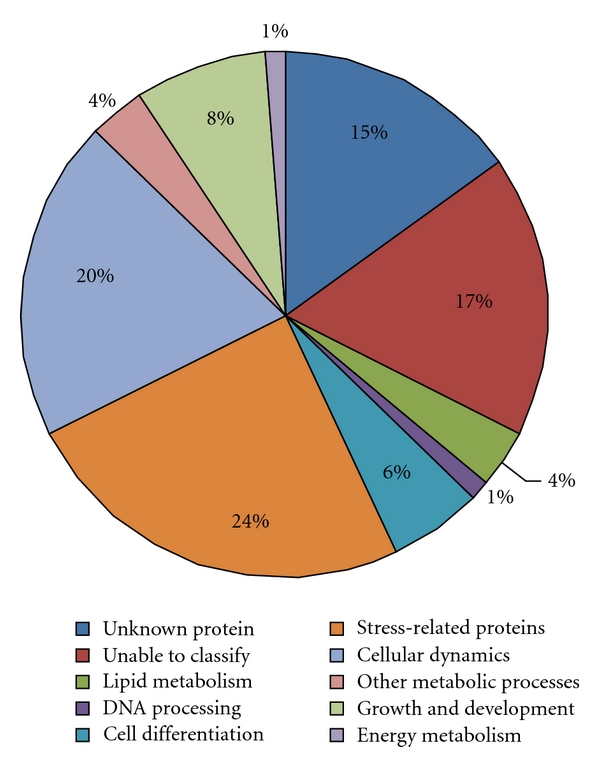
Relative frequency of Passiflora EST classes (all libraries combined) according to their identity and involvement in biological processes.
The comparison of our data with the literature describing the analysis of normalized and nonnormalized libraries of floral tissues showed similar indices of novel gene discovery [6, 7, 19–26].
As the PASSIOMA libraries were not normalized, we assumed that the most abundant transcripts found in the Passiflora reproductive tissues would be represented by the contig containing the largest numbers of ESTs. These contigs encode proteins generally related to stresses such as glucanases, catalases, peroxidases, jasmonate-induced proteins, and thaumatins. These results confirm previous reports that transcripts related to plant defense and stress are highly expressed during floral development [19–21]. Additionally, transcripts potentially encoding linamarases and other proteins involved in the biosynthesis of cyanogenic glycosides were generally included in clusters with up to 35 ESTs each, indicating that genes related to the biosynthesis of protecting substances are highly activated during Passiflora reproductive development.
Traditionally, transcripts encoding transcription factors and homeotic genes are considered less abundant and generally are poorly represented in EST collections [6, 21–26]. However, we detected in the PASSIOMA database a number of sequences with significant similarity to transcription factors belonging to families known to play an important role during reproductive development in model species, such as the MADS-box gene family (Figure 3). Therefore, we selected some of these sequences to perform phylogenetic analyses (Figure 3) and further investigate their expression patterns using RT-PCR and in situ hybridization (Figures 4–6).
Figure 3.
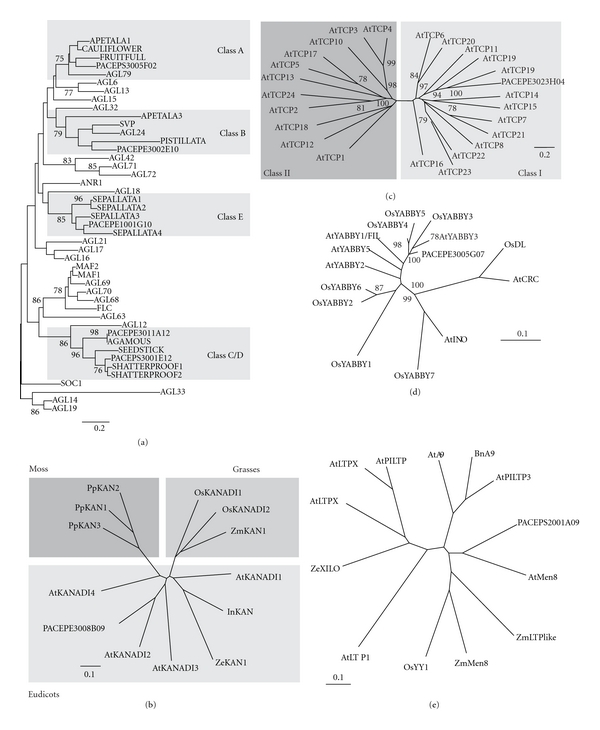
Phylogenetic analysis of selected Passiflora sequences. (a) sequence comparisons among Arabidopsis and Passiflora MADS-box proteins; (b) sequence comparisons among Passiflora KANADI protein and other plant counterparts; (c) sequence comparisons among Arabidopsis and Passiflora TCP proteins; (d) Sequence comparisons among Arabidopsis, rice and Passiflora YABBY proteins; (e) sequence comparisons among Passiflora Men8/9 protein and other plant counterparts. Bootstrap values above 75% are shown. Bars indicate substitution rates.
Figure 4.
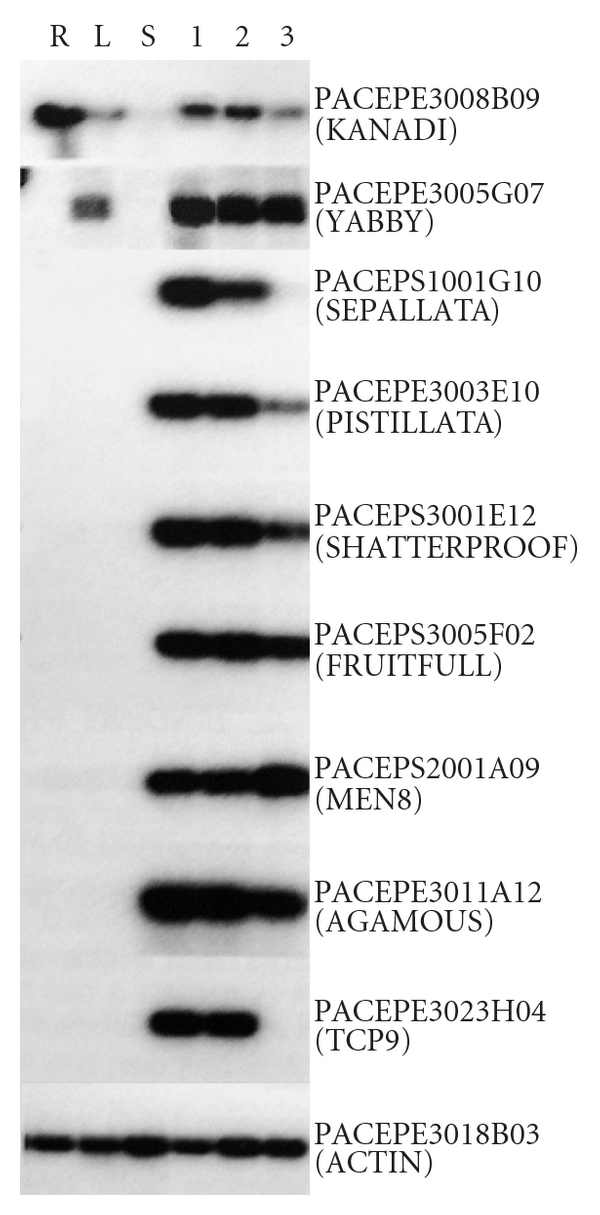
Expression patterns of Passiflora putative orthologs to genes involved in floral development. The ACTIN putative ortholog was used as a control. RT-PCR reactions were performed using as a template cDNA from roots (R), leaves (L), shoot (S), floral buds smaller than 0.5 cm (1), floral buds from 0.5 to 1.0 cm (2), and floral buds from 1.0 to 3 cm (3).
Figure 6.
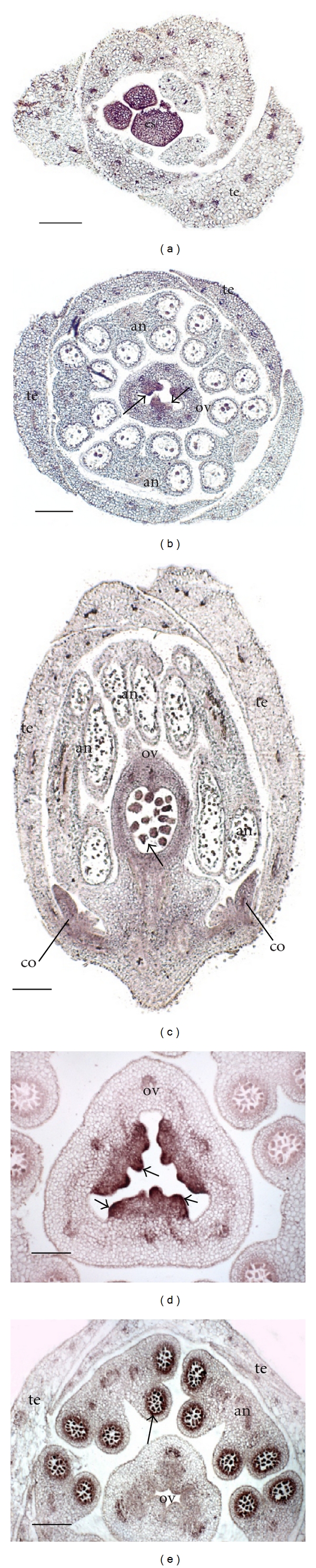
In situ expression patterns of putative P. suberosa genes involved in flower development. The hybridization signal is observed as a pink-red precipitate. (a, b, d, and e) show transversal sections though 5 mm long P. suberosa floral buds. (c) shows a longitudinal section. (a and b): the hybridization signal of PACEPS3005F02, a putative FRUITFULL ortholog, can be detected in the differentiating stigma (a) and in the ovule primordia (b, arrows). (b) is a lower section of the same floral bud shown in (a, c, and d): the expression of PACEPS3001E12, a putative SHATTERPROOF ortholog, is concentrated in the ovary and ovule primordia (arrow), but is also detected in the corona primordia. (d): detail of early stages of ovule primordia (arrows) showing PACEPS3001E12 expression. (e): the hybridization signal of PACEPS2001A09, a putative MEN8 ortholog, is restricted to the tapetal cells (arrow) in developing P. suberosa anthers. an: anther; co: corona primordia; ov: ovary; te: tepal primordium. Bars: (a, b, and c): 200 μm; (d): 80 μm; (e): 150 μm.
3.2. Validating Passiflora ESTs via Gene Expression Analysis
The efficiency of the EST approach to gene discovery in Passiflora could be illustrated by finding Passiflora EST sequences showing significant similarity to genes known to be involved in the reproductive processes in model plants such as Arabidopsis (Figure 3). Complementary evidences that the observed sequence similarity may reflect conservation of function might be obtained with the comparative study of their expression patterns during Passiflora flower development. Thus, we investigated the expression patterns of a small sample of sequences showing high-sequence similarity to known Arabidopsis genes, reported to be involved with key aspects of flower development.
3.3. RT-PCR
Both P. edulis and P. suberosa sequences were expressed in a pattern that was very similar to their putative Arabidopsis orthologs, indicating a potential conservation of function. We observed two main expression patterns: sequences preferentially expressed in reproductive tissues and sequences exclusively expressed in reproductive tissues (Figure 4). Belonging to the first group are the P. edulis putative orthologs to the Arabidopsis KANADI and YABBY genes (Figures 3 and 4).
The products of the Arabidopsis YABBY and KANADI genes interact to establish and maintain the abaxial-adaxial polarity of all plant organs, including the reproductive ones [27, 28]. We observed the expression of the P. edulis putative ortholog to KANADI (PACEPE3008B09) in roots and leaves, besides in young floral buds (Figure 4). The transcripts of the P. edulis putative ortholog to YABBY (PACEPE3005G07) were detected in leaves and also in developing floral buds. The coexpression of PACEPE3008B09 and PACEPE3005G07 raises the possibility that the product of these sequences also interact as observed for their Arabidopsis counterparts.
The second class of gene expression pattern observed include those Passiflora sequences expressed only in floral organs. Within this class are the Passiflora putative orthologs to the Arabidopsis MADS-box genes SEPALLATA (SEP), PISTILLATA (PI), AGAMOUS (AG), SHATTERPROOF (SHP), and FRUITFULL (FUL).
The proteins encoded by these MADS-box genes belong to a family of transcription factors highly conserved in eukaryotes [29]. The Arabidopsis MADS-box family has 107 members, indicating the relevance of this transcription factor-encoding genes to plants [30]. The biological function of most genes of the family is unknown, but three classes of MADS-box genes (named A, B, and C) are responsible to the establishment of the identities of all floral organs according to the classical ABC Model of flower development proposed by [31]. According to this model, the expression of A class MADS-box gene APETALA1 (AP1) in the periphery of the floral meristem determines the formation of sepals; the combined expression of AP1 and B class genes APETALA3 (AP3) and PISTILLATA (PI) in a ring of cells corresponding to the second whorl determines the differentiation of petals; the coexpression of PI, AP3, and C class gene AGAMOUS (AG) in the third whorl establishes the formation of stamens and, finally, the expression of AG alone in the center of the floral meristem determines the differentiation of carpels [31]. Later on, four E class paralogous genes SEPALLATA were added to the model, where they determine the “floral” identity of all ABC-expressing organs [32, 33]. All the ABCE genes are expressed exclusively in floral tissues in Arabidopsis. Accordingly, their Passiflora putative orthologs (Figure 3) were also expressed only during flower development (Figure 4).
The Arabidopsis SHATTERPROOF (SHP) and FRUITFULL (FUL) genes also belong to the MADS-box gene family, and they are involved in Arabidopsis carpel and fruit development [34–36]. The transcripts of their Passiflora putative orthologs were found only during floral development (Figure 4). This expression pattern was also observed for orthologs of these genes in other plant species [34–37].
The homologs of MEN8 from Arabidopsis and Brassica oleracea are expressed exclusively in floral tissues, more specifically during anther development [38]. The MEN8 gene encodes a lipid transfer protein, apparently involved with the deposition of pollenkit on the surface of developing pollen grains [38]. In agreement to this putative function, the Passiflora MEN8 putative ortholog (PACEPS2001A09, see Figure 3) was also expressed only in floral tissues (Figure 4).
There are 24 members in the Arabidopsis TCP family of transcription factors, organized in two subfamilies [39]. The members of subfamily I, which include TCP9, generally show flower-specific expression, even in monocots such as rice [40]. The TCP genes are involved with the maintenance of cellular proliferation within the floral meristem and with plant organ growth. Therefore, tcp mutants frequently are affected in the size and shape of floral organs [39, 41]. According to its possible role during early floral development, the transcripts of the putative Passiflora ortholog of TCP9 (Figure 3) were only detected during early flower development (Figure 4).
3.4. In Situ Hybridization
In order to corroborate and complement the RT-PCR results, we performed in situ hybridization experiments. The presence of transcripts was investigated in histological sections of reproductive apices and floral buds of P. edulis (Figure 5) and P. suberosa (Figure 6) at different developmental stages.
Figure 5.
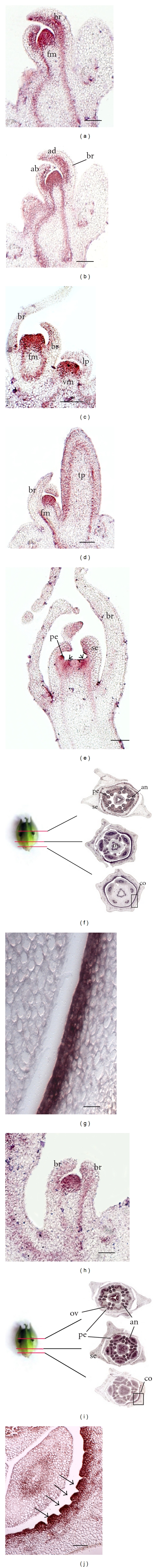
In situ expression patterns of putative P. edulis genes involved in flower development. The hybridization signal is observed as a pink-red precipitate. (a–e) and (h) show longitudinal sections though early stages of P. edulis flower meristems. (f, g, i, and j) show transversal sections of 4 mm long P. edulis flower buds. (a) the hybridization signal of PACEPE3008B09, a putative KANADI ortholog, can be detected throughout the floral meristem and in the adaxial side of the bracts. As floral organ primordia are formed, the expression is confined to the adaxial side (not shown). (b) the expression of PACEPE3005G07, a putative YABBY ortholog, is restricted to the floral meristem dome and to the adaxial side of all floral organ primordia. (c) the hybridization signal of PACEPE3023H04, a putative TCP9 ortholog, is restricted to the dome of both floral and axillary vegetative meristems. (d) transcripts of PACEPE3007D03, a putative SEPALLATA ortholog, are detected not only in the floral meristem, but also in the tendril primordium. (e) the hybridization signal of PACEPE3002E10, a putative PISTILLATA ortholog, is restricted to the group of cells in whorls 3 (petal primordia) and 4 (stamen primordia, arrowheads), but later in development (f, g), it is also detected in the group of cells that originate the corona filaments (g) is a magnification of the insert shown in the lower right corner in (f). (h) transcripts of PACEPE3011A12, a putative AGAMOUS ortholog, are detected in the floral meristem dome early in development, but later in development (i, j), it is also detected in the group of cells that originate the corona filaments ((j) is a magnification of the insert shown in the lower right corner in (i), arrows point to primordia of the corona filaments). ab: abaxial side; ad: adaxial side; an: anther; br: bract primordium; co: corona primordia; fm: floral meristem; lp: leaf primordium; ov: ovary; pe: petal primordium; se: sepal primordium; tp: tendril primordium; vm: vegetative meristem. Bars: (a–e and h): 200 μm; (g): 20 μm; (j): 60 μm.
The RT-PCR results for the P. edulis putative ortholog of KAN (PACEPE3008B09) indicated that it is predominantly expressed in reproductive tissues, but, in conformity to the predicted function of the Arabidopsis KAN, it was also expressed in vegetative tissues. The hybridization signal of PACEPE3008B09 was detected throughout the P. edulis floral meristem and in the adaxial side of the bracts (Figure 5(a)). Its expression was consistently conserved in the adaxial side of other floral organ primordia during their early developmental stages (data not shown). Arabidopsis mutants to two of the KAN paralogous genes show a substitution of abaxial cell types to adaxial ones and the ectopic expression of KAN in leaf or floral organ primordia results in the abaxialization of the tissues as well as defects in vascular differentiation and abnormalities on the expansion of laminar organs such as sepals and petals [28]. These phenotypes suggest that the KAN genes perform an important role in the determination of adaxial-abaxial organ polarity.
The asymmetric distribution of KAN transcripts in organ primordia is related to the differential activation of PHANTASTICA (PHAN), PHABULOSA (PHAB), PHAVOLUTA (PHAV) as well as the YABBY genes [27, 28]. We did not detect any sequences in the PASSIOMA database showing significant similarity to PHAN, PHAB, or PHAV, but we found the sequence PACEPE3005G07, which shows high similarity to the Arabidopsis YABBY1/FILAMENTOUS FLOWER (YAB1/FIL). The Arabidopsis YABBY family of transcription factors has 6 members differentially expressed during plant development [27, 42]. The current models predict that the YABBY transcription factors promote the identity of abaxial cells in all plant lateral organs, in association with the KANADI gene products [27, 28]. In Arabidopsis, YAB1/FIL is expressed in the embryonic root and in all shoot meristem products after germination. In the aerial organs, its expression is restricted to the adaxial side [42, 43], thus co-expressing with KAN. Accordingly, PACEPE3005G07 transcripts were detected in the same floral tissues as the putative ortholog of KAN (PACEPE3008B09). PACEPE3005G07 hybridization signal was detected uniformly in the shoot apical meristem as well as in the early tendril primordia and in the adaxial side of leaf primordia (data not shown). During early floral meristems development, PACEPE3005G07 transcripts were detected in the adaxial side of floral organ primordia (Figure 5(b)). Further investigation is needed in order to clarify if the products of the co-expressing putative KAN and YAB Passiflora orthologs interact, as their Arabidopsis counterparts do.
The Arabidopsis TCP family of transcription factors has 24 members organized into two subfamilies [39, 41]. TCP9 belongs to subfamily I. The members of this subfamily are specifically expressed in lateral meristems and floral tissues and are involved in the maintenance of cell proliferation, generally associated to the control of size and shape of floral organs [39, 41]. The expression of the Passiflora putative ortholog of TCP9, PACEPE3023H04, agreeing with its expected function in controlling cell proliferation, is concentrated both in the vegetative lateral meristem and floral meristems (Figure 5(c)). The members of the TCP family are highly conserved among angiosperms and one of the maize homologs, TEOSINTHE BRANCHED1, is important to the determination of plant architecture in modern corn cultivars [44]. Future experiments involving transgenic P. edulis plants, in which PACEPE3023H04 expression is modulated, may shed light into his function during Passiflora development.
The Arabidopsis SEPALLATA (SEP) genes act redundantly to specify all floral whorls, in combination with the ABC-class MADS-box genes [45]. Our in situ hybridization results indicated that PACEPE1001G10, the putative P. edulis ortholog of SEP3 was expressed in the developing tendril primordia as well as during early flower meristem development, being excluded from the floral bract primordia (Figure 5(d)). PACEPE1001G10 transcripts were not detected in the shoot apical meristem or in the lateral vegetative meristems (data not shown). The expression of a putative Passiflora ortholog of SEP in tendril primordia is somewhat surprising, but Carmona et al. [46] reported the expression of floral MADS-box genes in developing grape tendril primordia, suggesting that grape tendrils are modified flowers. Interestingly, it was recently suggested that Passiflora tendrils might be modified flowers as well [47]. Additionally, SEP orthologs in other plant species seem to have acquired diverse roles in inflorescence architecture specification and during fruit maturation [48].
The transcripts of PACEPE3002E10, the putative P. edulis ortholog of the Arabidopsis PISTILLATA gene, were detected in the developing floral meristem, in cellular domains corresponding to the forming petal and sepal whorls, even before the corresponding primordia were formed (Figure 5(e)). The presence of PACEPE3002E10 transcripts in the second and third whorls of floral organs was maintained until late during flower bud development (Figure 5(f)). Accordingly, the Arabidopsis B class MADS-box genes AP3 and PI are expressed early during floral meristem development in a ring of cells that will give rise to petal and stamen primordia [49]. Interestingly, PACEPE3002E10 transcripts were also detected in the group of cells from which the corona filaments will develop (Figures 5(f) and 5(g)), raising the possibility that B class MADS-box genes might be involved in the determination of corona identity.
In the classical ABC model [31], AG is the only Arabidopsis C class gene. Its expression is important to the determination of stamen (when coexpressed with B class genes) and carpel identities (when expressed in the center of the floral meristem). Generally, the mutations in the AG locus promote the production of indeterminate flowers made of successive whorls of sepals and petals [31]. The Arabidopsis AG expression is restricted to the floral meristematic dome and in posterior stages, AG transcripts can be found only in the two innermost floral whorls [50, 51]. In agreement with its putative role as a potential AG ortholog, PACEPE3011A12 transcripts were detected in the central region of early floral meristems (Figure 5(h)) and later found in developing stamen and carpel primordia (Figure 5(i)). Additionally, PACEPE3002E10 transcripts were also detected in the developing primordia of corona filaments (Figures 5(i) and 5(j)), indicating a possible coexpression with the B class putative ortholog PACEPE3002E10, suggesting that a combination of B and C classes MADS-box genes might be involved in corona development. Further research is necessary to confirm this possibility.
During Arabidopsis flower development, FUL transcripts are detected in the early stages of carpel development. FUL transcripts continue to be detected throughout carpel development and later accumulate in ovule primordia [35, 52, 53]. The transcripts of PACEPS3005F02, the putative P. suberosa ortholog of FUL, were detected in P. suberosa carpel tissues upon their differentiation (data not show). Later in development, hybridization signal was detected in differentiating stigmatic tissues (Figure 6(a)) and in the ovary, where it was concentrated in the ovule primordia (Figure 6(b)). Among the proposed roles for the Arabidopsis FUL gene product is the coordination of cell-cell interactions that leads to the differentiation of a dehiscence zone in the mature fruit [36]. Since P. suberosa fruits are indehiscent, a detailed analysis of PACEPS3005F02 expression patterns is necessary to suggest a putative function for this gene in Passiflora reproductive development. Nevertheless, putative orthologs of FUL and SHP were described to be expressed during fruit development of peach (Prunus persica) [37]. Similar to Passiflora, peach also has indehiscent fleshy fruits, and, in this species, it was suggested that these MADS-box genes might be related to specific peach fruit features such as the differentiation of the split junction between the nut and the fruit flesh, an important characteristic to the industrial processing of peach fruits [37]. Therefore, there might be interesting features associated to the function of the PACEPS3005F02 product, which may be of relevant interest to the passionfruit juice industry. This gene product is of special interest, since its interaction partner in Arabidopsis, SHP, also has a potential ortholog in P. suberosa, PACEPS3001E12.
PACEPS3001E12 expression was detected early during P. suberosa carpel development (data not show). Later in development, PACEPS3001E12 transcripts were detected in the ovary tissues, including the ovary wall and ovule primordia (Figures 6(c) and 6(d)). Additionally, a hybridization signal was detected in the developing corona filaments (Figure 6(c)). The expression in the corona filaments is intriguing, and further analysis will be necessary to uncover the role of PACEPS3001E12 transcripts during corona development. Generally, only one ortholog of the Arabidopsis paralogous genes SHATTERPROOF 1 and 2 (SHP1 and SHP2) is found in angiosperms [37]. Therefore, it is probable that the Arabidopsis SHP1/SHP2 duplication is recent and is present only within Brassicaceae [36]. In Arabidopsis, SHP1/SHP2 are widely expressed in the medial tissues of the gynoecium, including the replum, the valve margins, septum and ovule primordia. Later in development, their expression is excluded from the replum tissues, where SHP1/SHP2 are downregulated by FUL [36].
4. Conclusions and Perspectives
The sequencing and characterization of Passiflora ESTs can contribute to establish a consistent genomic basis for the analysis of the molecular regulation of floral organ development. The use of this approach was very fruitful in revealing sequences potentially involved in different aspects of floral development such as the determination of floral organ identity (with the characterization of putative Passiflora orthologs of MADS-box genes), floral organ symmetry (with the characterization of putative Passiflora orthologs of KAN and YAB genes) and the differentiation of specialized tissues within a given organ (with the characterization of a putative Passiflora ortholog of MEN8). Additionally, the EST sequences of the PASSIOMA database might provide useful resources for the development of EST-based markers for important agronomic traits as well as to the establishment of genomic tools to study the naturally occurring floral diversity among Passiflora species. The manipulation of the expression patterns of these candidate genes in transgenic Passiflora plants sounds as an obvious continuation of our studies and experiments with this aim are underway in our group.
Supplementary Material
Supplementary Figure 1: describes RT-PCR reactions performed using primers for constitutive genes such as 25S and GADPH.
Supplementary Figure 2: describes RT-PCR reactions performed using primers for the PePISTILLATA gene.
Supplementary Table 1: contains primer sequences used for RT-PCR.
Supplementary Table 2: contains the list of accession numbers for the sequences used in the phylogenetic analyses.
Acknowledgments
The authors thank Renato Vincentini dos Santos (CEBEMEG/UNICAMP, Brazil) for formatting the PASSIOMA database, Siu Mui Tsai, and Fabiana Cannavan (CENA/USP, Brazil) for the use of the sequencing facility. They also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for funding.
References
- 1.MacDougal JM, Feuillet C. Systematics. In: Ulmer T, MacDougal JM, editors. Passiflora: Passion Flowers of the World. Cambridge, UK: Timber Press; 2004. pp. 27–31. [Google Scholar]
- 2.Ulmer T, MacDougal JM. Passiflora, Passion Flowers of the World. Cambridge, UK: Timber Press; 2004. [Google Scholar]
- 3.Krosnick SE, Freudenstein JE. Monophyly and floral character homology of old world Passiflora (Subgenus Decaloba: Supersection Disemma) Systematic Botany. 2005;30(1):139–152. [Google Scholar]
- 4.Dornelas MC, Rodriguez APM. A genomic approach to elucidating grass flower development. Genetics and Molecular Biology. 2001;24(1–4):69–76. [Google Scholar]
- 5.de Oliveira Dias BF, Simões-Araújo JL, Russo CAM, Margis R, Alves-Ferreira M. Unravelling MADS-box gene family in eucalyptus spp.: a starting point to an understanding of their developmental role in trees. Genetics and Molecular Biology. 2005;28(3):501–510. [Google Scholar]
- 6.Laitinen RA, Immanen J, Auvinen P, et al. Analysis of the floral transcriptome uncovers new regulators of organ determination and gene families related to flower organ differentiation in Gerbera hybrida (Asteraceae) Genome Research. 2005;15(4):475–486. doi: 10.1101/gr.3043705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dornelas MC, Camargo RLB, Figueiredo LHM, Takita MA. A genetic framework for flowering-time pathways in citrus spp. Genetics and Molecular Biology. 2007;30(3):769–779. [Google Scholar]
- 8.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Research. 1998;8(3):175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 9.Telles GP, da Silva FR. Trimming and clustering sugarcane ESTs. Genetics and Molecular Biology. 2001;24(1–4):14–24. [Google Scholar]
- 10.Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Research. 1999;9(9):868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL. GenBank. Nucleic Acids Research. 2002;30(1):17–20. doi: 10.1093/nar/30.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vettore AL, da Silva FR, Kemper EL, Arruda P. The libraries that made SUCEST. Genetics and Molecular Biology. 2001;24(1–4):1–7. [Google Scholar]
- 14.Dornelas MC, Wittich P, Von Recklinghausen I, Van Lammeren A, Kreis M. Characterization of three novel members of the Arabidopsis SHAGGY-related protein kinase (ASK) multigene family. Plant Molecular Biology. 1999;39(1):137–147. doi: 10.1023/a:1006102812280. [DOI] [PubMed] [Google Scholar]
- 15.Dornelas MC, van Lammeren AAM, Kreis M. Arabidopsis thaliana SHAGGY-related protein kinases (AtSK11 and 12) function in perianth and gynoecium development. Plant Journal. 2000;21(5):419–429. doi: 10.1046/j.1365-313x.2000.00691.x. [DOI] [PubMed] [Google Scholar]
- 16.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology .The gene ontology consortium. Nature Genetics. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris MA, Clark J, Ireland A, et al. The gene oncology (GO) database and informatics resource. Nucleic Acids Research. 2004;32:258–261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoemaker R, Keim P, Vodkin L, et al. A compilation of soybean ESTs: generation and analysis. Genome. 2002;45(2):329–338. doi: 10.1139/g01-150. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez P, Paniego N, Lew S, Hopp HE, Heinz RA. Differential representation of sunflower ESTs in enriched organ-specific cDNA libraries in a small scale sequencing project. BMC Genomics. 2003;4(1):40–49. doi: 10.1186/1471-2164-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W, Wang Y, Bowers C, Ma H. Isolation, sequence analysis, and expression studies of florally expressed cDNAs in Arabidopsis . Plant Molecular Biology. 2003;53(4):545–563. doi: 10.1023/B:PLAN.0000019063.18097.62. [DOI] [PubMed] [Google Scholar]
- 21.Albert VA, Soltis DE, Carlson JE, et al. Floral gene resources from basal angiosperms for comparative genomics research. BMC Plant Biology. 2005;5, article 5 doi: 10.1186/1471-2229-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YH, Tsai YJ, Huang JZ, Chen FC. Transcription analysis of peloric mutants of phalaenopsis orchids derived from tissue culture. Cell Research. 2005;15(8):639–657. doi: 10.1038/sj.cr.7290334. [DOI] [PubMed] [Google Scholar]
- 23.Hecht V, Foucher F, Ferrandiz C, et al. Conservation of Arabidopsis flowering genes in model legumes. Plant Physiology. 2005;137(4):1420–1434. doi: 10.1104/pp.104.057018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegarty MJ, Jones JM, Wilson ID, et al. Development of anonymous cDNA microarrays to study changes to the senecio floral transcriptome during hybrid speciation. Molecular Ecology. 2005;14(8):2493–2510. doi: 10.1111/j.1365-294x.2005.02608.x. [DOI] [PubMed] [Google Scholar]
- 25.Jouannic S, Argout X, Lechauve F, et al. Analysis of expressed sequence tags from oil palm (Elaeis guineensis) FEBS Letters. 2005;579(12):2709–2714. doi: 10.1016/j.febslet.2005.03.093. [DOI] [PubMed] [Google Scholar]
- 26.Wang XJ, Cao XL, Hong Y. Isolation and characterization of flower-specific transcripts in Acacia mangium . Tree Physiology. 2005;25(2):167–178. doi: 10.1093/treephys/25.2.167. [DOI] [PubMed] [Google Scholar]
- 27.Bowman JL. Axial patterning in leaves and other lateral organs. Current Opinion in Genetics and Development. 2000;10(4):399–404. doi: 10.1016/s0959-437x(00)00103-9. [DOI] [PubMed] [Google Scholar]
- 28.Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Current Biology. 2001;11(16):1251–1260. doi: 10.1016/s0960-9822(01)00392-x. [DOI] [PubMed] [Google Scholar]
- 29.Purugganan MD, Rounsley SD, Schmidt RJ, Yanofsky MF. Molecular evolution of flower development: diversification of the plant MADS-box regulatory gene family. Genetics. 1995;140(1):345–356. doi: 10.1093/genetics/140.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Folter S, Immink RGH, Kieffer M, et al. Comprehensive interaction map of the Arabidopsis MADS box transcription factors. Plant Cell. 2005;17(5):1424–1433. doi: 10.1105/tpc.105.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353(6339):31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 32.Ferrándiz C, Liljegren SJ, Yanofsky MF. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science. 2000;289(5478):436–438. doi: 10.1126/science.289.5478.436. [DOI] [PubMed] [Google Scholar]
- 33.Pelaz S, Tapia-López R, Alvarez-Buylla ER, Yanofsky MF. Conversion of leaves into petals in Arabidopsis . Current Biology. 2001;11(3):182–184. doi: 10.1016/s0960-9822(01)00024-0. [DOI] [PubMed] [Google Scholar]
- 34.Gu Q, Ferrándiz C, Yanofsky MF, Martienssen R. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development. 1998;125(8):1509–1517. doi: 10.1242/dev.125.8.1509. [DOI] [PubMed] [Google Scholar]
- 35.Ferrándiz C, Pelaz S, Yanofsky MF. Control of carpel and fruit development in Arabidopsis . Annual Review of Biochemistry. 1999;68:321–354. doi: 10.1146/annurev.biochem.68.1.321. [DOI] [PubMed] [Google Scholar]
- 36.Dinneny JR, Yanofsky MF. Floral development: an ABC gene chips in downstream. Current Biology. 2004;14(19):R840–R841. doi: 10.1016/j.cub.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Tani E, Polidoros AN, Tsaftaris AS. Characterization and expression analysis of FRUITFULL- and SHATTERPROOF-like genes from peach (Prunus persica) and their role in split-pit formation. Tree Physiology. 2007;27(5):649–659. doi: 10.1093/treephys/27.5.649. [DOI] [PubMed] [Google Scholar]
- 38.Rubinelli P, Hu Y, Ma H. Identification, sequence analysis and expression studies of novel anther-specific genes of Arabidopsis thaliana. Plant Molecular Biology. 1998;37(4):607–619. doi: 10.1023/a:1005964431302. [DOI] [PubMed] [Google Scholar]
- 39.Aguilar-Martínez JA, Poza-Carrión C, Cubas P. Arabidopsis branched1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007;19(2):458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosugi S, Ohashi Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. Plant Cell. 1997;9(9):1607–1619. doi: 10.1105/tpc.9.9.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cubas P, Lauter N, Doebley J, Coen E. The TCP domain: a motif found in proteins regulating plant growth and development. Plant Journal. 1999;18(2):215–222. doi: 10.1046/j.1365-313x.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 42.Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis . Development. 1999;126(18):4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- 43.Sawa S, Watanabe K, Goto K, Kanaya E, Morita EH, Okada K. Filamentous flower, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes and Development. 1999;13(9):1079–1088. doi: 10.1101/gad.13.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukens L, Doebley J. Molecular evolution of the teosinte branched gene among maize and related grasses. Molecular Biology and Evolution. 2001;18(4):627–638. doi: 10.1093/oxfordjournals.molbev.a003843. [DOI] [PubMed] [Google Scholar]
- 45.Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATTA MADS-box genes. Nature. 2000;405(6783):200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- 46.Carmona MJ, Cubas P, Martínez-Zapater JM. VFL, the grapevine FLORICAULA/LEAFY ortholog, is expressed in meristematic regions independently of their fate. Plant Physiology. 2002;130(1):68–77. doi: 10.1104/pp.002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nave N, Katz E, Chayut N, Gazit S, Samach A. Flower development in the passion fruit Passiflora edulis requires a photoperiod-induced systemic graft-transmissible signal. Plant, Cell and Environment. 2010;33(12):2065–2083. doi: 10.1111/j.1365-3040.2010.02206.x. [DOI] [PubMed] [Google Scholar]
- 48.Malcomber ST, Kellogg EA. SEPALLATA gene diversification: Brave new whorls. Trends in Plant Science. 2005;10(9):427–435. doi: 10.1016/j.tplants.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Krizek BA, Meyerowitz EM. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development. 1996;122(1):11–22. doi: 10.1242/dev.122.1.11. [DOI] [PubMed] [Google Scholar]
- 50.Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990;346(6279):35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- 51.Mandel MA, Yanofsky MF. A gene triggering flower formation in Arabidopsis . Nature. 1995;377(6549):522–524. doi: 10.1038/377522a0. [DOI] [PubMed] [Google Scholar]
- 52.Parenicová L, de Folter S, Kieffer M, et al. Molecular and phylogenetic analyses of the complete MADS-Box transcription factor family in Arabidopsis : new openings to the MADS world. Plant Cell. 2003;15(7):1538–1551. doi: 10.1105/tpc.011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giovannoni JJ. Genetic regulation of fruit development and ripening. Plant Cell. 2004;16, supplement:S170–S180. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: describes RT-PCR reactions performed using primers for constitutive genes such as 25S and GADPH.
Supplementary Figure 2: describes RT-PCR reactions performed using primers for the PePISTILLATA gene.
Supplementary Table 1: contains primer sequences used for RT-PCR.
Supplementary Table 2: contains the list of accession numbers for the sequences used in the phylogenetic analyses.


