Abstract
Regulator of calcineurin 1 (RCAN1) is related to the expression of human neurologic disorders such as Down syndrome, Alzheimer disease, and chromosome 21q deletion syndrome. We showed here that RCAN1-knockout mice exhibit reduced innate anxiety as indicated by the elevated-plus maze. To examine whether glucocorticoids contribute to this phenotype, we measured fecal corticosterone in male wildtype and RCAN1-knockout mice and in male and female transgenic mice with neuronal overexpression of RCAN1 (Tg-RCAN1TG). We found no difference in fecal corticosterone levels of RCAN1-knockout mice and their wildtype littermates. As expected, we found differences between sexes in fecal corticosterone levels. In addition, we found higher levels of excreted corticosterone in Tg-RCAN1TG female mice as compared with female wildtype mice. Our data indicate normal diurnal corticosterone production in RCAN1 mutant mice and do not suggest a causal role in either the cognitive or anxiety phenotypes exhibited by RCAN1-knockout mice.
Abbreviations: RCAN1, regulator of calcineurin 1
Regulator of calcineurin 1 (RCAN1; also known as MCIP1, DSCR1, and calcipressin 1) regulates the activity of the calmodulin-dependent serine–threonine calcium-dependent phosphatase calcineurin (CaN or PP2B).45 Because it is located at q21.1 on human chromosome 21, RCAN1 has been postulated to contribute to mental retardation in Down syndrome,43 neuronal degeneration and oxidative stress in Alzheimer disease,25,38,41 and in the cognitive and developmental delays associated with chromosome 21q deletion syndrome.19 We reported that loss of RCAN1 resulted in altered CaN signaling, impaired synaptic plasticity, and deficits in spatial learning.19 In addition to these cognitive deficits, RCAN1-knockout mice display reduced associative cued fear memory, which has been used as a measure of conditioned anxiety.6 As shown in the current study, mice that overexpress human RCAN1 in the brain have increased anxiety. Finally, calcineurin plays an important role in circadian behavior in both the brain and periphery.24,46 These combined results led us to investigate circadian regulation of stress-related biochemical signaling pathways in mice in which RCAN1 expression was increased or decreased genetically.
Corticosterone is a glucocorticoid that is produced in the zona fascicularis of the adrenal glands and is released under stimulation of the hypothalamic–pituitary–adrenal axis. The hypothalamic–pituitary–adrenal axis is a critical hormonal system that has a well-defined pattern of circadian activity. Many functions of the hypothalamic–pituitary–adrenal axis are influenced by stress,12,48 which can alter the homeostatic release of stress-related neuroendocrine factors and disrupt circadian patterns.2,21-23
Various neurologic rodent models have been assessed through measurement of glucocorticoid levels.15,30,32,53,57 To measure glucocorticoids and their metabolites, various body fluids or excreta can be sampled.9,34 Because blood sampling may involve increased experimental stress and anesthetics, other sampling methods have been developed. Fecal corticosterone metabolite levels can be obtained through a minimally invasive sampling procedure in rats.55 The determination of these corticosterone metabolites from fecal samples has been found to be a practical method to monitor glucocorticoid production in rodents.34
Using these studies as a guide, we measured fecal corticosterone from RCAN1-knockout mice, transgenic mice that overexpress RCAN1 in brain (Tg-RCAN1TG mice), and their wildtype littermates over a normal 24-h (12:12-h light:dark) period. Normal mice exhibit thigmotaxic behaviors and show preference for dark unexposed environments, typically entering open or exposed areas only after a period of exploration and familiarization. The elevated-plus maze measures the frequency with which an animal explores exposed areas and is considered to reflect anxiety.14 To show that mice of comparable age to those from which we evaluated fecal corticosterone show the behavior expected from our other studies, we examined elevated-plus maze behavior in RCAN1-knockout and Tg-RCAN1TG mice.
Materials and Methods
Mice and genotyping.
We bred male mice carrying the floxed-CAT-RCAN1 transgene (B6;129-Tg[CMV::CAT-RCAN1]) to female mice carrying the enolase (neuronally active)-driven Cre recombinase (B6.Cg-Tg[Eno2-cre]39Jme) to generate transgenic RCAN1-overexpressing mice (Tg-RCAN1TG) of both sexes. Breeding heterozygotic [B6;129-Rcan1tm1Eno(±)] RCAN1(±) parents resulted in the production of RCAN1 male knockout [B6;129-Rcan1tm1Eno(−/−)]mice. To this end, we used the Cre–lox expression system to selectively remove a stop codon upstream of a transgenic insert encoding the RCAN1 protein. To selectively overexpress RCAN1 in neurons, a mouse strain in which Cre recombinase was under the control of the enolase promoter was used. This promoter initiates expression of Cre in neurons at E5.5, thus enabling us to drive RCAN1 overexpression in the transgenic mice throughout development and into adulthood. Mice expressing enolase-driven Cre (Tg-RCAN1TG) overexpress RCAN1 whereas their ‘wildtype’ (that is, lacking the cre driver construct) transgene-bearing controls (Tg-RCAN1WT) express RCAN1 normally. Genotyping was performed by using primers corresponding to each genotype (for specific sequences, see references 19 and 36). All mice were approximately 6 mo of age at the time of sampling and have been shown to be reproductively active well beyond this age. The mice originated from a colony that was free from common mouse pathogens, including Sendai virus, pneumonia virus of mice, mouse hepatitis virus, mouse minute virus, mouse parvovirus types 1 and 2, Theiler mouse encephalomyelitis virus, reovirus, epizootic diarrhea of infant mice, lymphocytic choriomeningitis virus, ectromelia virus, murine adenovirus types 1 and 2, murine cytomegalovirus, Mycoplasma pulmonis, murine Helicobacter species, fur mites, and pinworms. RCAN1 (−/−) (RCAN1-knockout) mice have an exon deletion in RCAN1 that eliminates the expression of RCAN1 protein; RCAN1(+/+) (RCAN1 WT) littermates express normal levels of RCAN1; nse–Cre(±) Tg-CAT::RCAN1(±) (Tg-RCAN1TG) mice overexpress RCAN1 after activation of an enolase driver that turns on early in embryogenesis, thereby removing a floxed–chloramphenicol transferase cassette upstream of the RCAN1 transgene;36 nse-Cre (−/−) Tg-CAT::RCAN1(±) (Tg-RCAN1WT) mice lack the nse–Cre activity needed to allow RCAN1 overexpression and continue to express chloramphenicol transferase from the Tg-CAT::RCAN1 construct. To control for the presence of a genomically inserted transgene, Tg-RCAN1WT mice were used as controls to compare fecal corticosterone excretion in Tg-RCAN1TG mice. The IACUC approved all of the described experiments.
Housing conditions.
Mice were housed in an AAALAC-accredited facility and in accordance with The Guide for the Care and Use of Laboratory Animals.60 The mice were housed on irradiated corncob bedding (1/8-in.; Bed O'Cobs, The Andersons, Maumee, OH) in a caging system (Innorack, Innovive, San Diego, CA) with 40 air changes hourly. Mouse cages were not changed for 6 d prior to the sampling cycle. Mice were provided water ad libitum by using prefilled water bottles (Aquavive, Innovive, San Diego, CA). The imported, nonvendor source mice were placed on ad libitum irradiated fenbendazole (150 ppm)-medicated chow (Lab Diet 5001, Test Diet, Richmond, IN) as a routine quarantine precaution; this practice has been shown not to adversely affect the vast majority of research, including behavioral studies.42,61 All mice were provided nesting pads (Nestlets, Ancare, Bellmore, NY) in each cage as enrichment. The mice were maintained under a 12:12-h light:dark cycle, at 68 to 74 °F (20.0 to 23.3 °C) and 30% to 70% relative humidity, and with 10 to 15 fresh room air changes hourly.
Fecal sample collection and analysis.
Individual fecal samples were collected from 40 RCAN1 transgenic, transgenic control, knockout, and wildtype mice by using gentle manipulation. Mice were picked up by the base of the tail and placed on a wire cage grid, while the hindquarters were lifted slightly to allow fecal pellets to be collected directly into a container. If a mouse did not produce a sample within 5 min of gentle abdominal palpation, the mouse was placed into an unused, sterile, bedded, closed cage until the sample was produced (less than 15 min spent alone). The mouse was returned to the original homecage after collection of 1 or 2 fecal pellets. Samples were collected every 4 h over a 24-h cycle, for a total of 6 time points.9 All samples were collected in microcentrifuge tubes (Eppendorf , Hauppauge, NY) and stored in a freezer at 0 °C until shipping on the day of the last sample collection to the diagnostic lab for analysis. Samples were analyzed by the Yerkes National Primate Research Center (Biomarkers Core Lab, Emory University, Atlanta, GA) using a commercially prepared kit (TKRC1, lot 394 [expiration date, 31 August 2010], Coat-A-Count Rat Corticosterone, Siemens Healthcare Diagnostics, Tarrytown, NY). The normal assay range reported by the diagnostic laboratory is 1.21 to 483.76 ng corticosterone per tube, with conversion to nanograms per gram of dried fecal mass of individual sample.
Immunohistochemistry.
Mice were euthanized by using cervical dislocation prior to tissue harvest. Soluble protein extracts were prepared by homogenizing the cortical tissue samples in ice-cold buffer (50 mM Tris-HCl [pH 7.5], 150 mM KCl, 1 mM DTT, 1 mM EDTA, 1× complete protease inhibitor cocktail III [Sigma–Aldrich, St Louis, MO], 1× phosphatase inhibitor cocktail I [Sigma–Aldrich]). Proteins were resolved on SDS–polyacrylamide gels, and immunoblotting was performed by using standard techniques. Protein concentrations were determined by absorbance reading at 562 nm (Synergy 2 Plate Reader, Biotek, Winooski, VT). Total protein (20 μg) was combined with 6× SDS–PAGE buffer (final SDS concentration, 1%). Samples were heated at 95 °C for 5 min and snap-chilled before loading. Proteins were separated on Novex 4% to 12% gradient Tris-Bis gels (Invitrogen, Carlsbad, CA) then transferred to PVDF blots using conventional methodology. Blots were blocked in 0.2% I-Block (Tropix, Foster City, CA) and then incubated overnight with primary antibodies at 4 °C. RCAN1 antibody (prepared in-house) was diluted 1:1000; GAPDH antibody (Novus Biologicals, Littleton, CO) was diluted 1:5000; Antirabbit antibodies tagged with horseradish peroxidase (Promega, Madison, WI) were diluted 1:5000. All antibodies used were diluted in 0.2% I-Block (Tropix). Bands were resolved by using secondary antibodies conjugated with horseradish peroxidase and visualized by using chemiluminescence (ECL+, GE Healthcare, Piscataway, NJ) on an imaging system (Kodak 4000MM or GE LAS4000, Kodak, Atlanta, GA). All chemiluminescent signals were obtained in the linear range of detection as confirmed by time course of exposures.
Elevated-plus maze.
The elevated-plus maze consisted of 4 white, equally spaced arms, 39 cm in height and 33.9 cm from the center of the apparatus. Two opposing arms were each enclosed by white walls extending 15.3 cm above the surface, and 2 arms were open. Individual mice, the same age as used for corticosterone testing, were placed in the center of the maze to start and their activity videorecorded (LTC0335, Bosch, Farmington Hills, MI) on computer for 5 min. The animals’ movements were captured and analyzed by using Ethovision XT software (Noldus, Wageningen, Netherlands). The apparatus was cleaned thoroughly with 70% isopropanol before each mouse was tested. The dependent variable was time spent in the exposed compared with enclosed arms and center. Illumination levels during testing were maintained at a constant 195 lx.
Statistical analysis.
To assess differences in fecal corticosterone over the 24-h testing period, 2-way repeated-measure ANOVA was used, with time of day and genotype as dependent variables. To assess the overall corticosterone differences between genotypes, one-way ANOVA or Mann–Whitney and posthoc t tests were carried out (Tukey least significant difference procedure). For elevated-plus maze behavior experiments, Student t test were applied to the behavior data as appropriate. All statistical tests are 2-sided at a significance level of 0.05. Data on graphs are given as mean ± 1 SD. Outliers were identified by using standard Gibbs sampling. All data were analyzed by using SPSS software (SSPS, Somers, NY).
Results
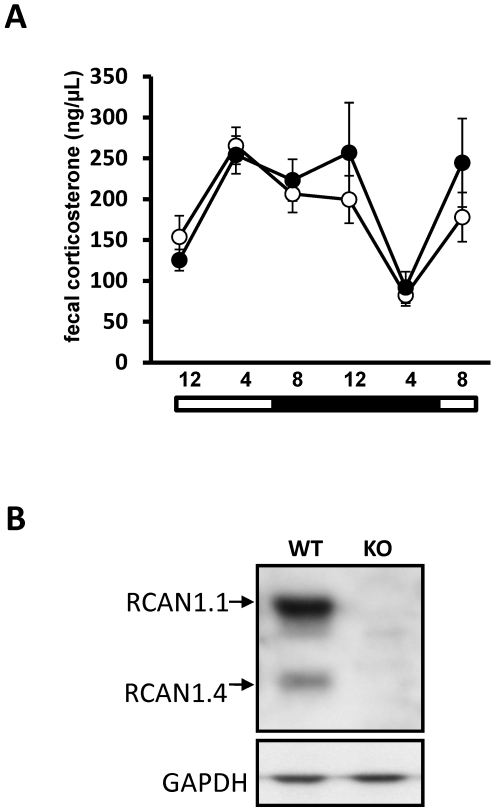
Feces were collected from RCAN1-knockout and wildtype mice 6 times over a 24-h time period for measurement of corticosterone. Wildtype mice excreted corticosterone in a cyclic pattern over the collection period (Figure 1 A), with a peak at the 2330 time point and a nadir at the 0430 time point, consistent with previous studies.9 RCAN1-knockout mice excreted corticosterone in an amount and pattern indistinguishable from that of controls (P > 0.05, repeated-measures ANOVA; Figure 1 A). After completing the fecal collection procedure, we confirmed the absence of RCAN1 in RCAN1-knockout mice by using protein isolated from brain containing the suprachiasmatic nucleus by Western blotting (Figure 1 B).
Figure 1.
(A) Fecal corticosterone metabolite concentrations (mean ± SEM) in RCAN1 wildtype (n = 11; open circles) and knockout (n = 10; filled circles) mice over a 24-h collection period. No difference in average levels of fecal corticosterone is detected between the genotypes at any time point. In addition, the pattern of fecal corticosterone concentration is maintained between genotypes. (B) RCAN1 is absent from the brains of RCAN1-knockout mouse mutants. In the rodent brain, RCAN1 is expressed as 2 isoforms, RCAN1.1 (approximately 38 kDa) and RCAN1.4 (approximately 28 kDa); GAPDH is included as a loading control. KO, knockout; WT, wildtype.
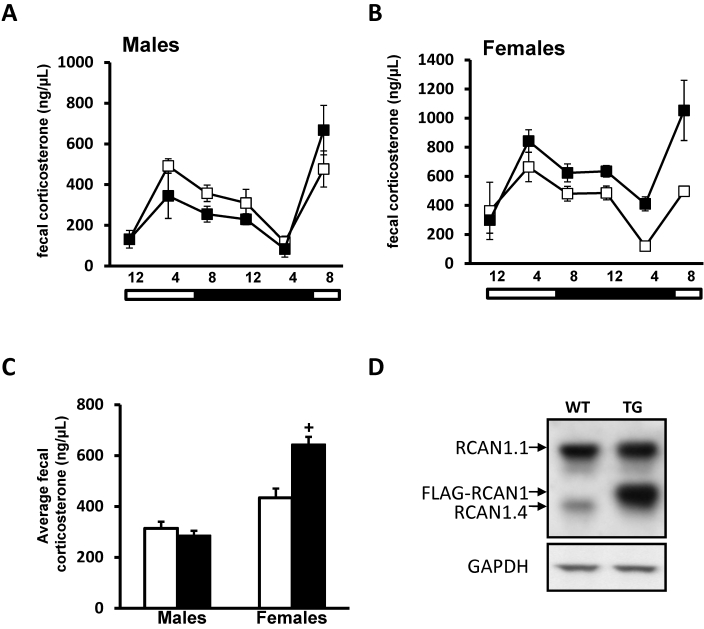
We next examined whether overexpression of RCAN1 in neurons could alter levels or patterns of fecal corticosterone. Patterns of fecal corticosterone in male mice overexpressing RCAN (Tg-RCAN1TG) were indistinguishable from those of controls (repeated-measures ANOVA; Figure 2 A). Corticosterone levels excreted in feces over a 24-h period in male Tg-RCAN1WT mice (control for RCAN1 overexpressors; 314 ± 47 ng/µL) were slightly but significantly (P = 0.0249, one-way ANOVA) elevated compared with those of male RCAN1 WT mice (control for RCAN1-knockout mice; 227 ± 19 ng/µL). Given that the strains used as controls in the current study have different genetic backgrounds,3,8 this finding perhaps is not surprising. However, the differences between male mice within each experimental group, as defined by genetic background, were not significant (RCAN1-knockout compared with RCAN1 wildtype mice, P = 0.3475; Tg-RCAN1WT compared with Tg-RCAN1TG mice, P = 0.7130; one-way ANOVA). Because RCAN1 expression can be regulated by estrogen signaling,40 we also investigated whether neuronal overexpression of RCAN1 would produce different patterns of corticosterone signaling in mice depending on their sex.10,58 We examined fecal corticosterone in female RCAN1-overexpressing and control mice over 24-h period. Similar to the results from RCAN1-knockout and male RCAN1-overexpressing mice, female RCAN1-overexpressors demonstrated a circadian pattern of fecal corticosterone that was indistinguishable from that of wildtype female controls (P > 0.05, repeated-measures ANOVA; Figure 2 B). In addition, overall levels of fecal corticosterone were approximately 70% higher in female Tg-RCAN1TG compared with control mice (745 ± 117 ng/µL compared with 434 ± 52 ng/µL, P = 0.0197 [one-way ANOVA]; Figure 2 C). After completion of the fecal collection procedure, we confirmed the overexpression of RCAN1 (tagged with FLAG epitope) in the brain (Figure 2 D).
Figure 2.
Fecal corticosterone metabolite concentrations (mean ± SEM) in transgenic mutant mice overexpressing RCAN1 in the forebrain. (A) Wildtype (n = 7; open squares) and RCAN1-overexpressing (n = 5; filled squares) male mice show identical patterns of fecal corticosterone exretion over a 24-h collection period. (B) Wildtype (n = 6; open squares) and RCAN1-overexpressing (n = 4; filled squares) female mice show identical patterns of fecal corticosterone shedding over a 24-h collection period. Female mice of either genotype display significantly (P < 0.05 for both sex and time) elevated corticosterone levels compared with male mice of the same genotype. (C) Transgenic female RCAN1 mice display approximately 70% greater (+, P = 0.0021) levels of average corticosterone expression over the 24-h collection period than do wildtype female mice. Bars at the bottoms of panels A through C indicate the lights-on (open) and lights-off (filled) portions of the photoperiod. (D) RCAN1 expression in the brains of genetically manipulated RCAN mouse mutants. RCAN1 is expressed as 2 isoforms in the rodent brain, RCAN1.1 (approximately 38 kDa) and RCAN1.4 (approximately 28 kDa). Overexpression of a FLAG-tagged isoform of RCAN1.1 by using the nse–CRE driver line (TG); note the band runs slightly higher than endogenous 1.4 because of the presence of the FLAG epitope. RCAN1 overexpression is strongly evident compared with that in a non-nse–CRE-expressing control (WT). GAPDH is included as a loading control.
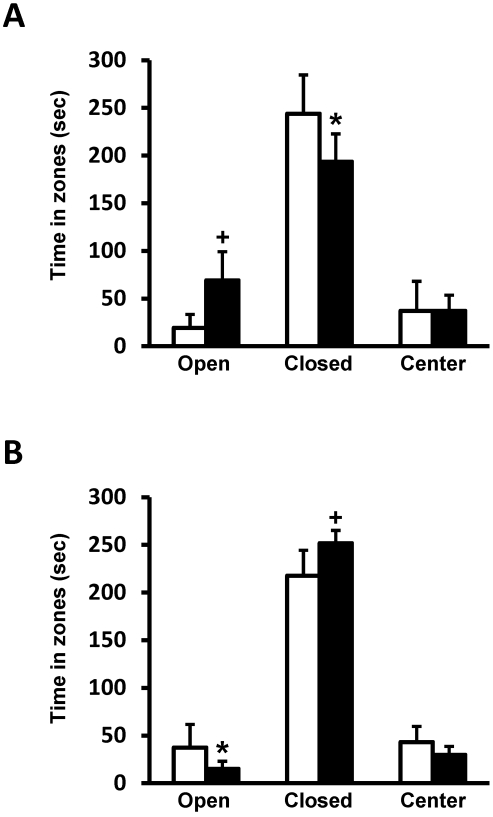
RCAN1-knockout mice spent more time in the open areas of the elevated-plus maze, (P = 0.006) and less time in the closed areas (P = 0.044) compared with wildtype controls (Figure 3 A), indicating less anxiety in RCAN1-knockout mice. By comparison, RCAN1-overexpressing mice spent significantly more time in the closed portion (P = 0.006) and less time exploring the open arms (P = 0.028) than did wildtype controls, thereby indicating increased anxiety in RCAN1-overexpressors (Figure 3 B). These differences in elevated-plus maze performance are not explained by locomotor differences because both experimental and control genotypes travelled equivalent total distances during testing (data not shown).
Figure 3.
(A) Compared with wildtype controls (n = 5; open squares), RCAN1-knockout mice (n = 7; closed squares) spend significantly more time (mean ± SEM) exploring the open arms (+, P = 0.006) and less time in the closed portion (*, P = 0.044) of the elevated-plus maze, demonstrating decreased anxiety. (B) Compared with wildtype controls (n = 8; open squares), Tg-RCAN1TG mice (n = 8; closed squares) spend significantly more time (mean ± SEM) in the closed portion (+, P = 0.006) and less time exploring the open arms (*, P = 0.028), demonstrating an increased level of anxiety.
Discussion
The RCAN1 gene is located on chromosome 21 in humans and its corresponding homolog is located on chromosome 16 in mice. RCAN1 is implicated in cognitive and emotional deficits manifested in several neurologic disorders including Alzheimer disease and Down syndrome and has recently been shown to be involved in the display of innate anxiety in rodents.56 In the current study, we hypothesized that glucocorticoid (corticosterone) signaling would be disrupted in RCAN1 mutant mice, and this abnormality might explain the observed cognitive and anxiety-related phenotypes in this model system. When we assayed fecal corticosterone sampled over several times during a 24-h period, we found no difference in corticosterone levels between wildtype and RCAN1-knockout mice. Because RCAN1 is overexpressed in the brains of patients with Alzheimer disease or Down syndrome, we also examined whether corticosterone signaling was disrupted when RCAN1 was overexpressed in the neurons of mice.54 We found no difference in the diurnal pattern of fecal corticosterone in either male or female transgenic mice overexpressing RCAN1, although transgenic RCAN1 female mice had higher overall levels of fecal corticosterone than did controls. Together these data indicate that corticosterone signaling is normal in RCAN1-knockout mice and that disruption of inherent corticosterone signaling likely does not explain the anxiety or cognitive phenotypes expressed in this strain.
The paraventricular nucleus triggers the stress response and regulates changes that lead to adrenal secretion of glucocorticoids. Adrenal steroidal hormones influence many facets of an organism's homeostasis, including responses to environmental perturbations. In addition to regulating energy storage in response to the master circadian regulator in the suprachiasmatic nucleus,9,33,44 adrenal glucocorticoids protect the body during and after the stress response (here defined as the cumulative physiologic reactions triggered by unpredictable events), including exposure to unknown environments and anxiogenic stimuli. Stress-induced functions of glucocorticoids include: increasing available glucose; improving cardiovascular tone; and inhibiting gastrointestinal, reproductive, and immune systems.31 The circadian corticosterone rhythm is altered in several pathologic states, including major depressive disorder,29,47 Alzheimer disease,56 and sleep deprivation,52 and during normal aging.27,50,59 We previously reported that mice deficient for RCAN1 manifested severe deficits in spatial memory and reduced conditioned associative-fear memory.19 Behavioral tests such as the elevated-plus maze, open-field, and light-dark tests have been used as measures of anxiety.6 We found in the current study that RCAN1-knockout mice (age, 6 mo and older) display greatly reduced anxiety compared with that of control mice. One possible explanation for these observed learning deficits and reduced expression of anxiety is altered glucocorticoid function due to the loss of RCAN1. However, the results of the current study likely dispel this explanation. RCAN1 protein is overexpressed in human patients with Down syndrome,16 most of whom have been codiagnosed with neurobehavioral disorders, including anxiety.7,11,35
Other mouse strains modeling neurodevelopmental and neurodegenerative disorders display altered glucocorticoid signaling. Levels of glucocorticoids and 3α-hydroxy-5α-pregnan-20-one are altered among BTBR mice, an animal model of autism, compared with C57/J mice. This model exhibits enhanced anxiety in the elevated-plus maze test after tail suspension.5 In Fmr1-knockout mice, a model of fragile X mental retardation, elevated serum corticosterone levels in response to a single stressful event, 30 min of acute restraint, show a protracted return to baseline, indicating impaired negative corticosterone feedback.30 In a mouse strain mutant for amyloid precursor protein, a model of Alzheimer disease, continuous noninvasive monitoring of corticosterone concentrations over a 4-mo period revealed adrenocortical hyperactivity in this model. This hyperactivity started very early (from day 30) in male mice and later (around day 90) in female mice. These changes in the activity of the hypothalamic–pituitary–adrenal axis are thought to be linked to amyloid-β–associated pathologic alterations in the hippocampus, causing degenerations in the negative feedback regulation of the axis and leading to hypersecretion of glucocorticoid. Therefore, the development of adrenocortical hyperactivity might be a key element in the understanding of Alzheimer disease.56
The excretion of corticosterone varies markedly between sexes, and most female laboratory rodents have larger adrenal glands, and therefore greater steroid production, than do males. Most of the corticosterone produced is excreted via the feces in both male and female mice, with no difference between the sexes in the time course of corticosterone excretion in urine and feces.58 In our current study, Tg-RCAN1TG female mice excreted higher levels of corticosterone than did their male counterparts (Figure 3 A and B). This result is in agreement with sex-based corticosterone analyses from previous studies.17 Even though the phase of the female estrous cycle may cause differences in excreted fecal corticosterone levels,9,58 the sex-associated differences we observed probably are not influenced strongly by estrus, because all female mice were group-housed in same-sex cages since their arrival at our facility, 3 wk prior to sampling. In addition, we observed higher overall fecal corticosterone excreted by female Tg-RCAN1TG mice compared with controls. This finding is interesting for 2 reasons. First, RCAN1 was overexpressed only in the forebrains of these mice and not in the adrenal glands, where the majority of corticosterone is secreted. Second, we did not observe this increase in male Tg-RCAN1TG mice. These data point to the possible existence of an RCAN1-mediated positive feedback loop leading to enhanced corticosterone biosynthesis in female mice. Interestingly, the RCAN1.1 isoform of RCAN1 is regulated by estrogen.40 Given that estrogen negatively regulates calcineurin activity,40 female mice that overexpress RCAN1 in the brain may be more susceptible to RCAN1-mediated signaling feedback that promotes excessive estrogen–corticosterone biosynthesis. Although we observed differences in total corticosterone levels between the 2 strains, we conclude that RCAN1 overexpression itself did not affect the total levels or pattern of shed corticosterone in male mice.
Fecal corticosterone levels may vary between studies due to differences in collection methods, storage conditions, or procedures.26 The metabolism and excretion of steroids differ markedly between sexes and species.37 Noninvasive methods permit repeated sampling of the same subject without affecting its behavior or its endocrine status. Stress can have disruptive physiologic effects in captive animals and in numerous human disorders.1,4,20,39,49,62 Because the pattern of corticosterone secretion we observed matched that previously reported for rats and other mouse strains,9,18,28,31 our data probably were not affected by serial handling and sample acquisition over the 24-h collection period.
Well-defined circadian rhythms of plasma corticosterone—those with peak levels 5 to 10 times higher than trough levels—occur in most vertebrate species.9,13,58 Usually, the peak of hormone secretion occurs toward the end of the dark period in primates and other diurnal animals, whereas in primarily nocturnal animals such as most rodents and cats, corticosterone peaks toward the end of the light period,51 consistent with what we observed in the current study. Therefore, one should sample corticosterone at the same time of day if repeated measurements are made on different days or if different groups of animals are compared. Fecal corticosterone metabolite levels have been used as a noninvasive estimate of circadian glucocorticoid production in rats, illustrating a 7- to 9-h time shift from the plasma corticosterone while reflecting the circulating corticosterone levels. Our data recapitulate this observation.55 In our study of RCAN1 mutant mice, all groups showed circadian patterns typical of a nocturnal species, indicating that the RCAN1-knockout neurologic phenotype is not due to alterations in the diurnal secretion of corticosterone.
We found that all groups of RCAN1 mutant mice tested displayed similar patterns of circadian-mediated corticosterone excretion. RCAN1-knockout mice showed no difference in total fecal corticosterone compared with wildtype controls. In addition, patterns of circadian corticosterone shedding were similar between female Tg-RCAN1WT and their wildtype controls, but corticosterone levels were higher in the female transgenic mice. These findings support the idea that the learning and memory deficits and reduced innate anxiety observed in RCAN1-knockout mice do not likely result from perturbation of circadian regulation of glucocorticoid secretion.
Acknowledgments
We thank the Yerkes National Primate Research Center (Emory University, Biomarkers Core Lab, Atlanta, GA) for processing the samples on a fee-for-service basis.
References
- 1.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. 2009. Clinical review. The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94:2692–2701 [DOI] [PubMed] [Google Scholar]
- 2.Anderson SM, Saviolakis GA, Bauman RA, Chu KY, Ghosh S, Kant GJ. 1996. Effects of chronic stress on food acquisition, plasma hormones, and the estrous cycle of female rats. Physiol Behav 60:325–329 [DOI] [PubMed] [Google Scholar]
- 3.Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. 2001. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci 115:443–454 [PubMed] [Google Scholar]
- 4.Bayazit V. 2009. Evaluation of cortisol and stress in captive animals. Austral J Basic Appl Sci 3:1022–1031 [Google Scholar]
- 5.Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. 2009. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res 197:462–465 [DOI] [PubMed] [Google Scholar]
- 6.Bouwknecht JA, Paylor R. 2008. Pitfalls in the interpretation of genetic and pharmacological effects on anxiety-like behaviour in rodents. Behav Pharmacol 19:385–402 [DOI] [PubMed] [Google Scholar]
- 7.Capone G, Goyal P, Ares W, Lannigan E. 2006. Neurobehavioral disorders in children, adolescents, and young adults with Down syndrome. Am J Med Genet C Semin Med Genet 142C:158–172 [DOI] [PubMed] [Google Scholar]
- 8.Carter RN, Paterson JM, Tworowska U, Stenvers DJ, Mullins JJ, Seckl JR, Holmes MC. 2009. Hypothalamic–pituitary–adrenal axis abnormalities in response to deletion of 11β-HSD1 is strain-dependent. J Neuroendocrinol 21:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK. 2005. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrinol 184:153–163 [DOI] [PubMed] [Google Scholar]
- 10.Chelini MO, Otta E, Yamakita C, Palme R. 2010. Sex differences in the excretion of fecal glucocorticoid metabolites in the Syrian hamster. J Comp Physiol B 180:919–925 [DOI] [PubMed] [Google Scholar]
- 11.Clark D, Wilson GN. 2003. Behavioral assessment of children with Down syndrome using the Reiss psychopathology scale. Am J Med Genet A 118A:210–216 [DOI] [PubMed] [Google Scholar]
- 12.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. 1998. Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301 [DOI] [PubMed] [Google Scholar]
- 13.Eriksson E, Royo F, Lyberg K, Carlsson HE, Hau J. 2004. Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats. Exp Physiol 89:427–433 [DOI] [PubMed] [Google Scholar]
- 14.File SE, Mabbutt PS, Hitchcott PK. 1990. Characterisation of the phenomenon of ‘one-trial tolerance’ to the anxiolytic effect of chlordiazepoxide in the elevated-plus maze. Psychopharmacology (Berl) 102:98–101 [DOI] [PubMed] [Google Scholar]
- 15.Frye CA, Llaneza DC. 2010. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav 100:264–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuentes JJ, Genesca L, Kingsbury TJ, Cunningham KW, Perez-Riba M, Estivill X, de la Luna S. 2000. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet 9:1681–1690 [DOI] [PubMed] [Google Scholar]
- 17.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. 1994. Gonadal steroid hormone receptors and sex differences in the hypothalamo–pituitary–adrenal axis. Horm Behav 28:464–476 [DOI] [PubMed] [Google Scholar]
- 18.Harper JM, Austad SN. 2000. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool 73:12–22 [DOI] [PubMed] [Google Scholar]
- 19.Hoeffer CA, Dey A, Sachan N, Wong H, Patterson RJ, Shelton JM, Richardson JA, Klann E, Rothermel BA. 2007. The Down syndrome critical region protein RCAN1 regulates long-term potentiation and memory via inhibition of phosphatase signaling. J Neurosci 27:13161–13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 21.Janssen SF, Twickler TB, Jublanc C, Cramer MJ, Bruckert E. 2008. Patients with the metabolic syndrome and a disturbed cortisol balance display more microalbuminuria. Diab Vasc Dis Res 5:54–58 [DOI] [PubMed] [Google Scholar]
- 22.Kant GJ, Bauman RA, Anderson SM, Mougey EH. 1992. Effects of controllable versus uncontrollable chronic stress on stress-responsive plasma hormones. Physiol Behav 51:1285–1288 [DOI] [PubMed] [Google Scholar]
- 23.Kant GJ, Bauman RA, Feaster SR, Anderson SM, Saviolakis GA, Garcia GE. 2001. The combined effects of pyridostigmine and chronic stress on brain cortical and blood acetylcholinesterase, corticosterone, prolactin and alternation performance in rats. Pharmacol Biochem Behav 70:209–218 [DOI] [PubMed] [Google Scholar]
- 24.Kant GJ, Pastel RH, Bauman RA, Meininger GR, Maughan KR, Robinson TN, 3rd, Wright WL, Covington PS. 1995. Effects of chronic stress on sleep in rats. Physiol Behav 57:359–365 [DOI] [PubMed] [Google Scholar]
- 25.Katz ME, Simonetta SH, Ralph MR, Golombek DA. 2008. Immunosuppressant calcineurin inhibitors phase shift circadian rhythms and inhibit circadian responses to light. Pharmacol Biochem Behav 90:763–768 [DOI] [PubMed] [Google Scholar]
- 26.Keating DJ, Chen C, Pritchard MA. 2006. Alzheimer's disease and endocytic dysfunction: clues from the Down syndrome-related proteins, DSCR1 and ITSN1. Ageing Res Rev 5:388–401 [DOI] [PubMed] [Google Scholar]
- 27.Keay JM, Singh J, Gaunt MC, Kaur T. 2006. Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species: a literature review. J Zoo Wildl Med 37:234–244 [DOI] [PubMed] [Google Scholar]
- 28.Kern W, Dodt C, Born J, Fehm HL. 1996. Changes in cortisol and growth hormone secretion during nocturnal sleep in the course of aging. J Gerontol A Biol Sci Med Sci 51: M3–M9 [DOI] [PubMed] [Google Scholar]
- 29.Li C, Jiang Z, Tang S, Zeng Y. 2007. Influence of enclosure size and animal density on fecal cortisol concentration and aggression in Pere David's deer stags. Gen Comp Endocrinol 151:202–209 [DOI] [PubMed] [Google Scholar]
- 30.Linkowski P, Van Cauter E, Leclercq R, Desmedt D, Brasseur M, Golstein J, Copinschi G, Mendlewicz J. 1985. ACTH, cortisol, and growth hormone 24-hour profiles in major depressive illness. Acta Psychiatr Belg 85:615–623 [PubMed] [Google Scholar]
- 31.Markham JA, Beckel-Mitchener AC, Estrada CM, Greenough WT. 2006. Corticosterone response to acute stress in a mouse model of fragile X syndrome. Psychoneuroendocrinology 31:781–785 [DOI] [PubMed] [Google Scholar]
- 32.Mateo JM, Cavigelli SA. 2005. A validation of extraction methods for noninvasive sampling of glucocorticoids in free-living ground squirrels. Physiol Biochem Zool 78:1069–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. 2006. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 103:18267–18272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore RY, Eichler VB. 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42:201–206 [DOI] [PubMed] [Google Scholar]
- 35.Mostl E, Palme R. 2002. Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74 [DOI] [PubMed] [Google Scholar]
- 36.Myers BA, Pueschel SM. 1991. Psychiatric disorders in persons with Down syndrome. J Nerv Ment Dis 179:609–613 [DOI] [PubMed] [Google Scholar]
- 37.Oh M, Rybkin II, Copeland V, Czubryt MP, Shelton JM, van Rooij E, Richardson JA, Hill JA, De Windt LJ, Bassel-Duby R, Olson EN, Rothermel BA. 2005. Calcineurin is necessary for the maintenance but not embryonic development of slow muscle fibers. Mol Cell Biol 25:6629–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palme R. 2005. Measuring fecal steroids: guidelines for practical application. Ann N Y Acad Sci 1046:75–80 [DOI] [PubMed] [Google Scholar]
- 39.Park J, Oh Y, Chung KC. 2009. Two key genes closely implicated with the neuropathological characteristics in Down syndrome: DYRK1A and RCAN1. BMB Rep 42: 6–15 [DOI] [PubMed] [Google Scholar]
- 40.Pasquali R, Gagliardi L, Vicennati V, Gambineri A, Colitta D, Ceroni L, Casimirri F. 1999. ACTH and cortisol response to combined corticotropin releasing hormone-arginine vasopressin stimulation in obese males and its relationship to body weight, fat distribution, and parameters of the metabolic syndrome. Int J Obes Relat Metab Disord 23:419–424 [DOI] [PubMed] [Google Scholar]
- 41.Pedram A, Razandi M, Aitkenhead M, Levin ER. 2005. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 280:26339–26348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porta S, Serra SA, Huch M, Valverde MA, Llorens F, Estivill X, Arbones ML, Marti E. 2007. RCAN1 (DSCR1) increases neuronal susceptibility to oxidative stress: a potential pathogenic process in neurodegeneration. Hum Mol Genet 16:1039–1050 [DOI] [PubMed] [Google Scholar]
- 43.Pritchett KR, Johnston NA. 2002. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp Top Lab Anim Sci 41:36–46 [PubMed] [Google Scholar]
- 44.Rachidi M, Lopes C. 2008. Mental retardation and associated neurological dysfunctions in Down syndrome: a consequence of dysregulation in critical chromosome 21 genes and associated molecular pathways. Eur J Paediatr Neurol 12:168–182 [DOI] [PubMed] [Google Scholar]
- 45.Reppert SM, Weaver DR. 2001. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63:647–676 [DOI] [PubMed] [Google Scholar]
- 46.Rothermel BA, Vega RB, Williams RS. 2003. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med 13:15–21 [DOI] [PubMed] [Google Scholar]
- 47.Sachan N, Dey A, Rotter D, Grinsfelder DB, Battiprolu PK, Sikder D, Copeland V, Oh M, Bush E, Shelton JM, Bibb JA, Hill JA, Rothermel BA. 2011. Sustained hemodynamic stress disrupts normal circadian rhythms in calcineurin-dependent signaling and protein phosphorylation in the heart. Circ Res 108:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, Gallagher TF. 1973. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry 28:19–24 [DOI] [PubMed] [Google Scholar]
- 49.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89 [DOI] [PubMed] [Google Scholar]
- 50.Sen Y, Aygun D, Yilmaz E, Ayar A. 2008. Children and adolescents with obesity and the metabolic syndrome have high circulating cortisol levels. Neuroendocrinol Lett 29:141–145 [PubMed] [Google Scholar]
- 51.Sherman B, Wysham C, Pfohl B. 1985. Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab 61:439–443 [DOI] [PubMed] [Google Scholar]
- 52.Smale L, Nunez AA, Schwartz MD. 2008. Rhythms in a diurnal brain. Biol Rhythm Res 39:305–318 [Google Scholar]
- 53.Spiegel K, Leproult R, Van Cauter E. 1999. Impact of sleep debt on metabolic and endocrine function. Lancet 354:1435–1439 [DOI] [PubMed] [Google Scholar]
- 54.Stranahan AM, Lee K, Pistell PJ, Nelson CM, Readal N, Miller MG, Spangler EL, Ingram DK, Mattson MP. 2008. Accelerated cognitive aging in diabetic rats is prevented by lowering corticosterone levels. Neurobiol Learn Mem 90:479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun X, Wu Y, Chen B, Zhang Z, Zhou W, Tong Y, Yuan J, Xia K, Gronemeyer H, Flavell RA, Song W. 2011. Regulator of calcineurin 1 (RCAN1) facilitates neuronal apoptosis through caspase-3 activation. J Biol Chem 286:9049–9062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thanos PK, Cavigelli SA, Michaelides M, Olvet DM, Patel U, Diep MN, Volkow ND. 2009. A noninvasive method for detecting the metabolic stress response in rodents: characterization and disruption of the circadian corticosterone rhythm. Physiol Res 58:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Touma C, Ambree O, Gortz N, Keyvani K, Lewejohann L, Palme R, Paulus W, Schwarze-Eicker K, Sachser N. 2004. Age- and sex-dependent development of adrenocortical hyperactivity in a transgenic mouse model of Alzheimer's disease. Neurobiol Aging 25:893–904 [DOI] [PubMed] [Google Scholar]
- 58.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 59.Touma C, Sachser N, Mostl E, Palme R. 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278 [DOI] [PubMed] [Google Scholar]
- 60.Van Cauter E, Leproult R, Kupfer DJ. 1996. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81:2468–2473 [DOI] [PubMed] [Google Scholar]
- 61.Villar D, Cray C, Zaias J, Altman NH. 2007. Biologic effects of fenbendazole in rats and mice: a review. J Am Assoc Lab Anim Sci 46:8–15 [PubMed] [Google Scholar]
- 62.Vogelzangs N, Beekman AT, Dik MG, Bremmer MA, Comijs HC, Hoogendijk WJ, Deeg DJ, Penninx BW. 2009. Late-life depression, cortisol, and the metabolic syndrome. Am J Geriatr Psychiatry 17:716–721 [DOI] [PubMed] [Google Scholar]