Abstract
Purpose
To summarize evidence on conservative, nondialytic management of end-stage renal disease regarding 1) prognosis and 2) symptom burden and quality of life (QOL).
Methods
Medline, Cinahl, and Cochrane were searched for records indexed prior to March 1, 2011. Bibliographies of articles and abstracts from recent meetings were reviewed. Authors and nephrologists were contacted to identify additional studies. Articles were reviewed by two authors and selected if they described stage 5 chronic kidney disease (CKD) patients managed without dialysis, including one or more of the following outcomes: prognosis, symptoms, or QOL. Levels of evidence ratings were assigned using the SORT (Strength of Recommendation Taxonomy) system. Data was abstracted independently by two authors for descriptive analysis.
Results
Thirteen studies were included. In studies of prognosis, conservative management resulted in median survival of at least six months (range 6.3 to 23.4 months). Findings are mixed as to whether dialysis prolongs survival in the elderly versus conservative, nondialytic management. Any survival benefit from dialysis decreases with comorbidities, especially ischemic heart disease. Patients managed conservatively report a high symptom burden, underscoring the need for concurrent palliative care. Additional head-to-head studies are needed to compare the symptoms of age-matched dialysis patients, but preliminary studies suggest that QOL is similar.
Conclusions
Conservative management is an important alternative to discuss when counseling patients and families about dialysis. Unlike withdrawal of dialysis in which imminent death is expected, patients who decline dialysis initiation can live for months to years with appropriate supportive care.
When outpatient hemodialysis programs first began in the 1960s, strict criteria were necessary to deliver scarce resources to those patients most likely to benefit. The dialysis industry then grew exponentially, allowing for relaxation of acceptance criteria. Dialysis is currently offered to many patients who would not have been considered suitable during the early decades of outpatient dialysis.
The elderly constitute the fastest growing subset of the dialysis population.1 The number of patients aged 80 years and older starting dialysis in the United States increased from 7054 in 1996 to 13,577 in 2003.2 The current dialysis population also has more comorbid conditions. The number of end-stage renal disease patients diagnosed with coronary artery disease increased from 13.8% in 2005 to 21.0% in 2007; the incidence of cancer and chronic obstructive pulmonary disease (COPD) increased as well.1 A recent study of neuropsychiatric testing in hemodialysis patients found that 37% had severe cognitive impairment and 36% had moderate impairment.3 Finally, dialysis is increasingly offered to patients with impaired functional status. In 2007, 11.2% of dialysis patients required assistance with basic activities of daily living.1
The elderly have increased morbidity and mortality on dialysis.4 One study of dialysis patients 75 years old and older reported a one-year mortality of 46.5% with patients spending an average of 20% of days in the hospital.5 Comorbid conditions also increase mortality, independent of age.6
In response to the aging population and trends of dialyzing older and sicker patients, interest is growing in nondialytic alternatives for end-stage renal disease (ESRD). The Renal Physicians Association recently updated their practice guideline affirming the rights of patients to refuse dialysis initiation.7 The guideline specifically mentions patients with profound neurological impairment or a nonrenal terminal disease, but there may be other populations in which the burden of dialysis outweighs the potential benefit, such as the very elderly and patients with multiple comorbid diseases. The UK Renal Association recommends discussing the risks and benefits of renal replacement therapy prior to dialysis initiation with special attention to nutritional status, comorbid conditions, and functional status.8
Conservative (nondialytic) management of ESRD includes careful attention to fluid balance, treatment of anemia, and correction of acidosis and hyperkalemia. Blood pressure and calcium/phosphorus metabolism must also be managed.9 There is emerging evidence that dietary modifications may be helpful in prolonging life and decreasing symptoms10 Finally, individualized symptom management and palliative care are crucial to maximize QOL.11–13
Despite the importance of conservative management as an option for patients with ESRD, many clinicians are unfamiliar with this approach and lack the information to counsel patients and families. In addition, rigorous evidence about the actual benefits of dialysis in frail populations has not been synthesized. To assist in conversations about dialysis initiation, this systematic review will summarize current evidence regarding 1) prognosis for patients who choose not to start dialysis and 2) symptom burden and QOL with conservative management.
Methods
Medline, Cinahl, and the Cochrane Library were searched for records in any language indexed between the beginning of the database and March 1, 2011. Searches combined the following terms: (end-stage renal disease OR end-stage renal failure OR stage 5 CKD OR advanced CKD) AND (nondialytic OR conservative management OR palliative care). “Palliative care” is a Medical Subject Headings (MeSH) term; the other search terms were entered as keywords. For consistency, the same search strategy was used in each database. Given the difficulty in translating our particular research questions into concise search terms, extensive additional strategies were pursued to capture any articles that might have been missed in the database searches. Bibliographies of identified articles were reviewed. Authors of included articles and several academic nephrologists were contacted. Finally, abstracts were reviewed from the most recent meetings of the American Society of Nephrology, the World Congress of Nephrology, the Renal Association, and the British Renal Society.
One author (NO) reviewed the initial citations based on title and abstract alone, excluding duplicate studies and studies without relevance. Two authors (NO and PK) reviewed all remaining articles. Articles were selected if they described patients with stage 5 or end-stage CKD, at least some of whom were managed without dialysis. Additionally, articles were selected only if they reported original research and included one or more of the following outcomes: prognosis, symptoms, or QOL. Studies of acute renal failure were excluded. Review articles, practice guidelines, and editorials were also excluded. Any discrepancy between authors was resolved by discussion and consensus.
Articles were assessed for quality using the SORT system.14 This system was chosen because it includes extensive guidelines for cohort and cross-sectional studies, the most common research designs among included articles. It assigns level of evidence 1, 2, or 3 based on study design and specific validity criteria; level of evidence 1 is the highest rating.
Data was then abstracted independently by two authors (NO and PK). The data abstraction was cross-checked by both authors, and any differences were resolved by discussion and consensus. Meta-analysis was not performed due to wide variability in study populations and different methods of measuring outcomes. The articles were therefore analyzed descriptively with an emphasis on trends.
Results
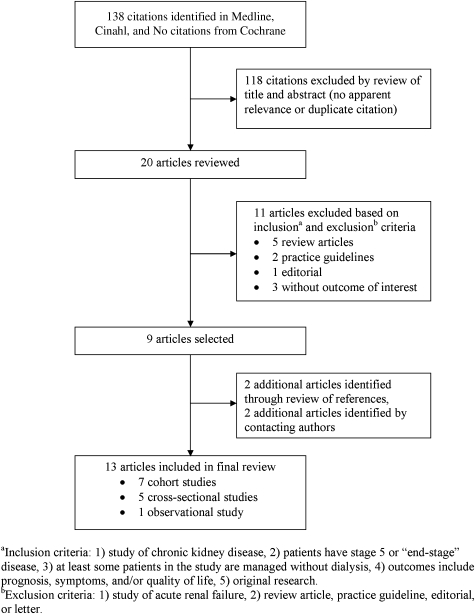
A total of 138 citations were obtained through the initial literature search (90 from Medline, 48 from Cinahl, and none from Cochrane). After exclusion of duplicate studies (n=29) and studies without relevance (n=89), 20 citations remained (Figure 1). Nine of these citations met inclusion and exclusion criteria for the final systematic review. A hand search of bibliographies yielded two additional articles. Two more articles were found through contact with authors and academic nephrologists. Review of records from recent nephrology meetings failed to identify any relevant abstracts. A total of thirteen articles were included in the final analysis (Table 1).
FIG. 1.
Study inclusion and exclusion flow diagram.
Table 1.
Studies Included in Systematic Review
| Reference | Study design | Study follow-up | Level of evidencea |
|---|---|---|---|
| Carson et al., 200921 | Prospective cohort study of patients with GFR<30 and age ≥70 receiving either CM or dialysis | 95.0% | 1 |
| Chanda et al., 201017 | Retrospective cohort study of patients with GFR<15 receiving either CM or dialysis | n/a | 2 |
| Ellam et al., 200919 | Retrospective cohort study of patients with GFR<15 receiving CM | n/a | 2 |
| Joly et al., 200320 | Prospective cohort study of patients with CrCl<10 and age ≥80 receiving either CM or dialysis | 100% | 1 |
| Murtagh et al., 200718 | Retrospective cohort study of patients with GFR<15 and age >75 receiving either CM or dialysis | n/a | 2 |
| Smith et al., 200315 | Prospective cohort study of patients with GFR<15 recommended for CM by multidisciplinary team | 100% | 1 |
| Wong et al., 200716 | Prospective cohort study of patients with GFR<30 receiving CM | 100% | 1 |
| DeBiase et al., 200829 | Observational study of patients with age >75 and GFR<15 recommended for CM | n/a | 2 |
| Murphy et al., 200925 | Cross-sectional survey of patients with GFR<30 receiving CM | n/a | 2 |
| Murtagh et al., 200722 | Cross-sectional survey of patients with GFR<15 receiving CM | n/a | 2 |
| Murtagh et al., 201023 | Longitudinal survey of patients with GFR<15 receiving CM, data presented from the month prior to death | n/a | 2 |
| Saini et al., 200624 | Cross-sectional survey of patients with GFR<15 receiving CM; survey also administered to comparison group of patients with terminal malignancy | n/a | 2 |
| Yong et al., 200928 | Cross-sectional survey of patients with GFR<15 receiving either CM or dialysis | n/a | 2 |
GFR, glomerular filtration rate; CM, conservative management; CrCl, creatinine clearance; n/a, not applicable.
Based on Strength of Recommendation Taxonomy (SORT) criteria: level of evidence 1=good-quality, patient-oriented evidence; level of evidence 2=limited-quality, patient-oriented evidence; level of evidence 3=other evidence.
Prognosis
Seven articles were identified describing prognosis with conservative, nondialytic management of ESRD (Table 2). All seven articles were cohort studies, reflecting the difficulty of randomizing patients to dialysis or nondialytic management. Four of these were prospective cohort studies with good follow-up; these studies were given a level of evidence 1 rating. The remaining three studies were given a level of evidence 2 rating.
Table 2.
Prognosis with conservative, nondialytic management of end-stage renal disease
| Reference | Conservative management group | Dialysis group | Results |
|---|---|---|---|
| Carson et al., 200921 | median age 83.0 13.8% diabetes mean age-adjusted CCI score 7.4 n=29 |
median age 75.0 29.5% diabetes mean age adjusted CCI score 7.2 n=173 |
median survival from first known date of GFR ≤10.8a: 13.9 months (range 2–44) with CM 37.8 months (range 0–106) with dialysis p<0.01 |
| Chanda et al., 201017 | mean age 77.5 68.4% over age 75 35.5% diabetes 49.7% high comorbidity n=155 |
mean age 58.5 11.2% over age 75 34.3% diabetes 17.3% high comorbidity n=689 |
median survival from first known date of GFR<15: 21.2 months with CMb 67.1 months with dialysisb p<0.001 |
| Ellam et al., 200919 | median age 80 38% diabetes 32% ischemic heart disease n=69 |
None | median survival from first known date of GFR<15: 21 months (range 1–100) with CM |
| Joly et al., 200320 | mean age 84.1 51.4% late referral to nephrology 21.6% diabetes 48.6% ischemic heart disease 43.3% socially isolated n=37 |
mean age 83.2 28.9% late referral to nephrology 6.5% diabetes 42.5% ischemic heart disease 14.7% socially isolated n=107 |
median survival from first day of dialysis or decision not to perform dialysis: 8.9 months (95% CI 4–10) with CM 28.9 months (95% CI 24–38) with dialysis p<0.0001 |
| Murtagh et al., 200718 | median age 83 23.4% diabetes n=77 |
median age 79.6 25.0% diabetes n=52 |
median survival from first known date of GFR<15: 18.0 months (range 0.1–73.1) with CM 19.6 months (range 2.2–84.2) with dialysis |
| Smith et al., 200315 |
n=34c |
n=10c | median survival from proposed date of dialysis initiation: 6.3 months (range 0–46) with CM 8.3 months (range 2–20) with dialysis |
| Wong et al., 200716 | median age 79 mean GFR 12 28% diabetes 34% ischemic heart disease n=73 |
None | median survival from decision not to perform dialysis: 23.4 months with CMb |
CCI, Charlson Comorbidity Index; GFR, glomerular filtration rate; CM, conservative management.
Mean GFR at time of dialysis initiation was 10.8 in the dialysis group, so this was used as the “threshold GFR” from which survival was measured.
Range/confidence interval not reported.
Demographic variables were only reported for conservative management and dialysis groups combined: mean age 71, mean modified Karnofsky Performance Scale score 55, 27% diabetes.
Median survival with conservative management ranged from 6.3 months15 to 23.4 months.16 The study reporting the shortest survival (Smith et al.) included only patients for whom nondialytic management was recommended by an interdisciplinary team. These patients were poor candidates for dialysis due to multiple comorbidities or impaired functional status, factors that would also decrease their life expectancy. In contrast, the study reporting the longest survival (Wong et al.) enrolled patients with the youngest median age.
The following factors were identified by at least one study as predictive of prolonged survival with conservative management: female gender,17 lower comorbidity score16,18 albumin >35g/l19 and referral to a nephrologist before reaching stage 5 CKD.19
Five of the prognosis studies included a comparison group of patients on dialysis. Two of these studies (Murtagh et al. and Smith et al.) found little or no survival benefit with dialysis versus conservative management in elderly patients.15,18 In the study by Murtagh et al., the modest survival benefit found with dialysis in the overall study population (median survival 19.6 versus 18.0 months) decreased significantly with increasing comorbidities and disappeared completely with ischemic heart disease.18 In the study by Smith et al., the difference in survival between groups was not statistically significant.15
The remaining three prognosis studies reported significant survival benefits with dialysis.17,20,21 Of note, there were differences between study groups in all three studies. The study by Joly et al. found a large survival benefit with dialysis (median survival 28.9 months versus 8.9 months with conservative management, p<0.0001)20 Patients in the dialysis group had a lower incidence of diabetes than patients in the conservative management group (6.5% versus 21.6%, p=0.008). Additionally, many more patients in the conservative management group were socially isolated (43.3% versus 14.7%, p=0.003) or late presenters to nephrology. The study by Carson et al. also reported a statistically significant survival benefit with dialysis (median survival 37.8 months versus 13.9 months with conservative management, p<0.01).21 In this study, dialysis patients were younger (median age 75 versus 83, p=0.000001), but comorbidities were similar between groups.
In the study by Chanda et al., dialysis patients lived longer than patients managed conservatively (median survival 67.1 months versus 21.2 months, p<0.001).17 Conservative management patients were much older (median age 77.4 versus 58.5, p<0.001) and had more comorbidities (49.7% versus 17.3% high comorbidity score, p<0.001). These differences between groups complicate interpretation of the overall survival data. The authors performed further analysis for the subset of patients over 75 years of age; after adjustment for age, comorbidity score, and diabetes, the survival benefit from dialysis was not statistically significant.17
Symptoms and QOL
Six articles were identified describing symptom burden and/or QOL (Table 3). Cross-sectional survey was the most common study design used, and all articles received a level of evidence 2 rating. Three studies22–24 utilized the Memorial Symptom Assessment Scale Short Form (MSAS-SF) to collect data on the prevalence of various symptoms; one study25 used a modified version of the Patient Outcome Scale Symptom Module (POSs). Both the MSAS-SF and the POSs have been validated for use in palliative care populations.26,27 One study28 surveyed patients using a list of symptoms generated from the investigators' clinical experience. Only three articles addressed QOL24,28,29 standardized tools included the Short Form 36 Health Survey and the Euroqol-5Q questionnaire, both of which have been previously validated.30,31 One study by DeBiase et al. also included semistructured interviews of both patients and caregivers.29
Table 3.
Symptoms and Quality of Life with Conservative, Nondialytic Management of End-stage Renal Disease
| Reference | CM group | Comparison group | Symptoms in CM group | Other outcomes |
|---|---|---|---|---|
| DeBiase et al., 200829 | mean age 81.5 mean GFR 11.2 mean number of comorbidities 5.7 n=11 |
mean age 79.4 mean GFR 9.0 mean number of comorbidities 2.6 n=5 (dialysis patients) |
not reported |
similar quality of life between groupsa |
| Murphy et al., 200925 | mean age 82 mean GFR 12.75 n=55 |
none | weakness 75% poor mobility 75% poor appetite 58% pain 56% pruritus 56% dyspnea 49% |
mean number of symptoms 6.8 (range 1–14) |
| Murtagh et al., 200722 | mean age 82 mean GFR 11.2 n=66 |
none | lack of energy 76% pruritus 74% drowsiness 65% dyspnea 61% edema 58% pain 53% dry mouth 50% muscle cramps 50% restless legs 48% lack of appetite 47% poor concentration 44% dry skin 42% sleep disturbances 41% |
mean number of symptoms 11.58 (range 0–22) |
| Murtagh et al., 201023 | mean age 80.9 mean GFR 11.0 n=49 |
none | lack of energy 86% pruritus 84% drowsiness 82% dyspnea 80% poor concentration 76% pain 73% poor appetite 71% swelling 71% dry mouth 69% constipation 65% nausea 59% |
median number of symptoms 16.6 (range 6–27) |
| Saini et al., 200624 | median age 67 median GFR 11.4 median KPS 90 n=11 |
median age 63 median GFR 81.3 median KPS 80 n=11 (patients with terminal malignancyb) |
lack of energy 100% dyspnea 82% difficulty sleeping 82% swelling 73% pain 64% numbness/tingling 64% food taste changes 55% pruritus 55% lack of appetite 55% changes in skin 55% |
median number of symptoms: 17 (range 11–24) in CM group; 15 (range 5–23) in malignancy group similar quality of life between groupsc |
| Yong et al., 200928 | mean age 73.1 mean CCI score 8.5 n=45 |
mean age 58.2 mean CCI score 6.1 n=134 (dialysis patients) |
cold aversion 78% fatigue 69% pruritus 58% lower torso weakness 58% difficulty sleeping 49% pain 49% dyspnea 47% |
mean number of symptoms±SD: 8.2±3.9 with CM 9.3±4.7 with dialysis similar quality of life between groupsa |
CM, conservative management; GFR,glomerular filtration rate; KPS, Karnofsky Performance Scale; SD, standard deviation; CCI, Charlson Comorbidity Index.
Quality of life measured by Short-Form 36 Health Survey questionnaire.
3 GI cancers, 2 breast cancers, 3 lung cancers, 1 skin cancer, 1 CNS cancer, 1 mesothelioma.
Quality of life measured by Euroqol EQ-5Q questionnaire.
All of the patients undergoing conservative management reported significant symptom burden, with the average number of symptoms varying from 6.8 to 17. The most common symptoms included weakness, lack of energy, poor appetite, pruritus, drowsiness, dyspnea, pain, edema, and difficulty sleeping; symptoms were relatively consistent across studies. Symptom burden and severity increased in the month prior to death.23
Three of the studies of symptoms and QOL included a comparison group. The study by Saini et al. compared conservatively managed ESRD patients to a comparison group of patients with terminal malignancy.24 The median number of symptoms was similar between groups, emphasizing the high symptom burden in ESRD. Additionally, both groups had similarly impaired QOL.
The study by Yong et al. directly compared dialysis and conservative management patients, reporting similar symptom burden and QOL between groups.28 In the study by DeBiase et al., elderly patients undergoing conservative management were compared to a group of patients who initiated dialysis despite a physician's recommendation for nondialytic management.29 Patients in the dialysis group were slightly younger and had fewer comorbid conditions than patients in the conservative management group. Despite these differences, QOL was similar between groups as determined by standardized questionnaires and interviews.
Discussion
This systematic review demonstrates that conservative, nondialytic management of ESRD is a viable option in certain patients. For the elderly and patients with multiple comorbid conditions, dialysis does not always offer a survival advantage. Five prognosis studies in this review included both conservative management and dialysis patients; three of these studies found a statistically significant survival benefit with dialysis, but the other two found no difference. Patients with multiple comorbid conditions, especially ischemic heart disease, were the least likely to experience a survival benefit.
Future research could develop clinical tools to predict which patients will survive longer with dialysis versus conservative management. Several models already exist to predict survival on dialysis,32–34 but similar models have not yet been developed for conservative management. In the meantime, physicians must rely on clinical judgment and the preliminary results presented in this review. If dialysis is not expected to prolong life due to extremely advanced age or comorbid conditions, patients and families should receive counseling to ensure that their expectations are realistic.35
Even when dialysis can be expected to prolong survival, the burdens of dialysis (cost, infections, vascular access issues, fluctuating blood pressure) deserve careful consideration. The prognosis study by Carson et al. included additional analyses of hospitalization rates and location of death21 Dialysis patients spent a greater proportion of days in the hospital compared with conservative management patients (25 versus 16 days per patient per year). Conservative management patients were four times more likely to die at home or in a hospice (OR 4.15; 95% CI 1.67 to 10.25). Routine outpatient hemodialysis is also extremely time consuming. Thus, while patients may live longer with dialysis, they can expect to spend a significant proportion of that time in a medical setting. Patients and families differ in how they prioritize prolonging life versus maximizing time at home; these preferences are important to elicit when discussing dialysis initiation.
There are several limitations to the conclusions from this systematic review. As already mentioned, the literature in this field is widely dispersed and difficult to target with database search strategies. We believe that we overcame this limitation through multiple other methodologies to locate articles, but it is possible that a study was missed.
The prognosis studies exhibit significant variability in inclusion criteria, resulting in heterogeneous study populations. In addition, different starting points were used in the measurement of survival (decision not to initiate dialysis, proposed date of first dialysis, first measurement of glomerular filtration rate (GFR) ≤10.8, and first measurement of GFR < 15). These factors contribute to the wide variability in reported survival. While this variability makes it more difficult to counsel individual patients about what to expect with conservative management, this systematic review provides at least a starting point for discussion.
The results of this review may not be generalizable to nursing home residents, a growing subset of the population; patients in the included studies were recruited from ambulatory clinics and were cognitively intact. Nursing home residents have especially poor outcomes on dialysis with a mortality rate of 58% in the first year.36 Pre-dialysis functional status is maintained in only 13% of nursing home residents after one year on dialysis.36 Comparable data for conservative management is needed to fully inform discussions about dialysis initiation in this population.
It is clear that conservatively managed patients have many symptoms. Unfortunately, none of the symptom studies conducted a head-to-head comparison of conservative management versus dialysis. It is possible that patients on dialysis have similarly high symptom burden; additional research is needed to address this question. In the meantime, patients considering conservative management should be informed of the high incidence of various symptoms and then reassured that aggressive symptom management will be part of their care. A recent longitudinal cohort study found that functional status with nondialytic management remains relatively constant until the last month of life.37 A second study of conservative management found an increase in symptom distress and health related concerns in the last two months of life.38
The findings presented on QOL are limited and preliminary. The included studies compare small groups of patients, and the results are not stratified by age or comorbidities to allow more precise determination of which patients benefit from dialysis in terms of QOL. Larger head-to-head studies would allow qualitative and quantitative QOL analyses to guide patient decision making. Ideally, comparative data about both survival and QOL would be presented when counseling patients about dialysis versus conservative management.
New evidence suggests that delayed initiation may also be a safe alternative in patients who ultimately choose dialysis. A recent trial randomized 828 patients to dialysis initiation when their GFR reached 10.0 to 14.0 ml per minute (early start) or when their GFR reached 5.0 to 7.0 ml per minute (late start).39 During a median follow-up period of 3.59 years, there was no significant difference between groups in mortality or the frequency of adverse events. Initiating dialysis later in the disease course would give patients and physicians additional time to determine whether dialysis is the best treatment option.
For patients who opt for conservative management, guidelines are needed to determine the best clinical practices in nondialytic management. Most nephrologists currently extrapolate fluid and electrolyte management from earlier-stage CKD. Several centers are starting to develop specialized renal palliative care teams to provide concurrent renal care and symptom management for patients who decline or discontinue dialysis.40–42 These multidisciplinary teams typically include nurses, social workers, and physicians from both nephrology and palliative care. Preliminary data suggests that they are successful in managing symptoms40 and providing for advanced care planning and family support.42 ESRD represents a growing opportunity to offer palliative care to a nonmalignant disease with extensive end-of-life care needs.
Finally, the results of this review demonstrate that failure to initiate dialysis is fundamentally different from the withdrawal of dialysis in which imminent death is expected. Patients can live for months or even years after deciding not to start dialysis. Patients and clinicians who are familiar with dialysis withdrawal may assume that failure to initiate dialysis is analogous to stopping dialysis. Educational efforts targeting patients, primary care physicians, and the renal community are needed to raise awareness about conservative management as an acceptable alternative.
Acknowledgments
We thank David Slawson, MD, at the University of Virginia for his helpful review of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2009. USRDS 2009 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. [Google Scholar]
- 2.Kurella M. Covinsky KE. Collins AJ. Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146:177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 3.Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Advances in Chronic Kidney Disease. 2008;15:123–132. doi: 10.1053/j.ackd.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamping DL. Constantinovici N. Roderick P. Normand C. Henderson L. Harris S. Brown E. Gruen R. Victor C. Clinical outcomes, quality of life, and costs in the North Thames dialysis study of elderly people on dialysis: A prospective cohort study. Lancet. 2000;356:1543–1450. doi: 10.1016/S0140-6736(00)03123-8. [DOI] [PubMed] [Google Scholar]
- 5.Munshi SK. Vijayakumar N. Taub NA. Bhullar H. Lo TC. Warwick G. Outcome of renal replacement therapy in the very elderly. Nephrol Dial Transplant. 2001;16:128–133. doi: 10.1093/ndt/16.1.128. [DOI] [PubMed] [Google Scholar]
- 6.Khan IH. Catto GR. Edward N. Fleming LW. Henderson IS. MacLeod AM. Influence of coexisting disease on survival on renal-replacement therapy. Lancet. 1993;341:415–418. doi: 10.1016/0140-6736(93)93003-j. [DOI] [PubMed] [Google Scholar]
- 7.Rockville, MD: The Renal Physicians Association; 2010. Shared decision-making in the appropriate initiation of and withdrawal from dialysis. [Google Scholar]
- 8.United Kingdom: UK Renal Association; 2009. Planning, initiating, withdrawal of renal replacement therapy. [Google Scholar]
- 9.Moncrief JW. Decherd JF. Conservative management of end-stage renal failure. Tex Med. 1975;71:74–79. [PubMed] [Google Scholar]
- 10.Brunori G. Viola BF. Parrinello G. De Biase V. Como G. Franco V. Garibotto G. Zubani R. Cancarini GC. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: A prospective randomized multicenter controlled study. Am J Kidney Dis. 2007;49:569–580. doi: 10.1053/j.ajkd.2007.02.278. [DOI] [PubMed] [Google Scholar]
- 11.O'Hare AM. The management of older adults with a low eGFR: Moving toward an individualized approach. Am J Kidney Dis. 2009;53:925–927. doi: 10.1053/j.ajkd.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fassett RG. Robertson IK. Mace R. Youl L. Challenor S. Bull R. Palliative care in end-stage kidney disease. Nephrology. 2011;16:4–12. doi: 10.1111/j.1440-1797.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 13.Murtagh FEM. Sheerin N. Conservative management of end stage renal disease. In: Chambers J, editor; Brown E, editor; Germain MJ, editor. Suportive care for the renal patient. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- 14.Ebell MH. Siwek J. Weiss BD. Woole SH. Susman J. Ewigman B. Bowman M. Strength of Recommendation Taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548–556. [PubMed] [Google Scholar]
- 15.Smith C. Da Silva-Gane M. Chandna S. Warwicker P. Greenwood R. Farrington K. Choosing not to dialyse: Evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron. 2003;95:40–46. doi: 10.1159/000073708. [DOI] [PubMed] [Google Scholar]
- 16.Wong CF. McCarthy M. Howse ML. Williams PS. Factors affecting survival in advanced chronic kidney disease patients who choose not to receive dialysis. Ren Fail. 2007;29:653–659. doi: 10.1080/08860220701459634. [DOI] [PubMed] [Google Scholar]
- 17.Chanda SM. Da Silva-Gane M. Marshall C. Warwicker P. Greenwood RN. Farrington K. Survival of elderly patients with stage 5 CKD: Comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2010 Nov 22; doi: 10.1093/ndt/gfq630. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murtagh FE. Marsh JE. Donohoe P. Ekbal NJ. Sheerin NS. Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephr Dial Transplant. 2007;22:1955–1962. doi: 10.1093/ndt/gfm153. [DOI] [PubMed] [Google Scholar]
- 19.Ellam T. El-Kossi M. Prasanth KC. El-Nahas M. Khwaja A. Conservatively managed patients with stage 5 chronic kidney disease– outcomes from a single center experience. QJM. 2009;102:547–554. doi: 10.1093/qjmed/hcp068. [DOI] [PubMed] [Google Scholar]
- 20.Joly D. Anglicheau D. Alberti C. Nguyen AT. Touam M. Grunfeld JP. Jungers P. Octogenarians reaching end-stage renal disease: Cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14:1012–1021. doi: 10.1097/01.asn.0000054493.04151.80. [DOI] [PubMed] [Google Scholar]
- 21.Carson RC. Juszczak M. Davenport A. Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4:1611–1609. doi: 10.2215/CJN.00510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murtagh FE. Addington-Hall JM. Edmonds PM. Donohoe P. Carey I. Jenkins K. Dip PG. Higgison IJ. Symptoms in advanced renal disease: A cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med. 2007;10:1266–1276. doi: 10.1089/jpm.2007.0017. [DOI] [PubMed] [Google Scholar]
- 23.Murtagh FE. Addington-Hall J. Edmonds P. Donohoe P. Carey I. Jenkins K. Dip PG. Higgison IJ. Symptoms in the month before death for stage 5 chronic kidney disease patients managed without dialysis. J Pain Symptom Manage. 2010;40:342–352. doi: 10.1016/j.jpainsymman.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Saini T. Murtagh FE. Dupont PJ. McKinnon PM. Hatfield P. Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20:631–636. doi: 10.1177/0269216306070236. [DOI] [PubMed] [Google Scholar]
- 25.Murphy EL. Murtagh FE. Carey I. Sheerin NS. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: Use of a short patient-completed assessment tool. Nephron. 2009;111:74–80. doi: 10.1159/000183177. [DOI] [PubMed] [Google Scholar]
- 26.Chang VT. Hwang SS. Feuerman M. Kasimis BS. Howard HT. The Memorial Symptom Assessment Scale Short Form (MSAS-SF) Cancer. 2000;89:1162–1171. doi: 10.1002/1097-0142(20000901)89:5<1162::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Hearn J. Higginson IJ. Development and validation of a core outcome measure for palliative care: The palliative care outcome scale. Quality in Health Care. 1999;8:219–227. doi: 10.1136/qshc.8.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yong DS. Kwok AO. Wong DM. Suen MH. Chen WT. Tse DM. Symptom burden and quality of life in end-stage renal disease: A study of 179 patients on dialysis and palliative care. Palliat Med. 2009;23:111–119. doi: 10.1177/0269216308101099. [DOI] [PubMed] [Google Scholar]
- 29.De Biase V. Tobaldini O. Boaretti C. Abaterusso C. Pertica N. Loschiavo C. Trabucco G. Lupo A. Gembaro G. Prolonged conservative treatment for frail elderly patients with end-stage renal disease: The Verona experience. Nephrol Dial Transplant. 2008;23:1313–1317. doi: 10.1093/ndt/gfm772. [DOI] [PubMed] [Google Scholar]
- 30.Wight JP. Edwards L. Brazier J. Walters S. Payne JN. Brown CB. The SF36 as an outcome measure of services for end stage renal failure. Quality in Health Care. 1998;7:209–221. doi: 10.1136/qshc.7.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brazier J. Jones N. Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res. 1993;2:169–180. doi: 10.1007/BF00435221. [DOI] [PubMed] [Google Scholar]
- 32.Quinn RR. Laupacis A. Hux JE. Oliver MJ. Austin PC. Predicting the risk of 1-year mortality in incident dialysis patients: Accounting for case-mix severity using administrative data. Med Care. 2011;49:257–266. doi: 10.1097/MLR.0b013e318202aa0b. [DOI] [PubMed] [Google Scholar]
- 33.Mauri JM. Cleries M. Vela E. Design and validation of a model to predict early mortality in haemodialysis patients. Nephrol Dial Transplant. 2008;23:1690–1696. doi: 10.1093/ndt/gfm728. [DOI] [PubMed] [Google Scholar]
- 34.Couchard C. Labeeuw M. Moranne O. Allot V. Esnault V. Frimat L. Stengel B. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24:1553–1561. doi: 10.1093/ndt/gfn698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Germain MJ. Davison SN. Moss AH. When enough is enough: The nephrologist's responsibility in ordering dialysis treatments. Am J Kidney Dis. 2011;58:135–143. doi: 10.1053/j.ajkd.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Kurella Tamura M. Covinsky KE. Chertow GM. Yaffe K. Landefeld S. McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murtagh FE. Addington-Hall JM. Higginson IJ. End-stage renal disease: A new trajectory of functional decline in the last year of life. J Am Geriatr Soc. 2011;59:304–308. doi: 10.1111/j.1532-5415.2010.03248.x. [DOI] [PubMed] [Google Scholar]
- 38.Murtagh FE. Sheerin NS. Addington-Hall J. Higginson IJ. Trajectories of illness in stage 5 chronic kidney disease: A longitudinal study of patient symptoms in the last year of life. Clin J Am Soc Nephrol. 2011;6:1580–1590. doi: 10.2215/CJN.09021010. [DOI] [PubMed] [Google Scholar]
- 39.Cooper BA. Branley P. Bulfone L. Collins JF. Craig JC. Fraenkel MB. Harris A. Johnson DW. Kesselhut J. Li JJ. Luxton G. Pilmore A. Tiller DJ. Harris DC. Pollack CA. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 40.Murtagh FE. Murphy E. Shepherd KA. Donohoe P. Edmonds PM. End-of-life care in end-stage renal disease: Renal and palliative care. British J Nursing. 2006;15:8–11. doi: 10.12968/bjon.2006.15.1.20301. [DOI] [PubMed] [Google Scholar]
- 41.Chan CH. Noble H. Lo SH. Kwan TH. Lee SL. Sze WK. Palliative care for patients with end-stage renal disease: Experiences from Hong Kong. Int J Palliat Nurs. 2007;13:310–314. doi: 10.12968/ijpn.2007.13.7.24342. [DOI] [PubMed] [Google Scholar]
- 42.Noble H. Rees K. Caring for people who are dying on renal wards: A retrospective study. Edtna-Erca Journal. 2006;32:89–92. doi: 10.1111/j.1755-6686.2006.tb00458.x. [DOI] [PubMed] [Google Scholar]