Abstract
Helicobacter pylori infects about 50% of the world’s population and inevitably results in the development of gastritis. Of those infected, about 10% develop peptic ulcer disease and roughly 1% develop gastric cancer. Conversely, some take the view that H. pylori infection provides some protection against gastro-esophageal reflux disease and possibly asthma. This review aims to explore the case for and against eradication of the bacterium using a “test and treat” approach amongst the general population.
Introduction
Helicobacter pylori infects about 50% of the world’s population. It is generally agreed that infection by this organism inevitably results in the development of gastritis. Of those infected, about 10% develop peptic ulcer disease, roughly 1% develop gastric cancer and less than 0.1% MALT (mucosa-associated lymphoid tissue) lymphoma [1-3] (Figure 1). These findings indicate that the organism should be eradicated, especially if the treatment regimen is highly effective and safe. This approach, namely "test and treat", has not been met with universal acceptance, and based on the NIH Consensus Conference, only symptomatic patients are to be treated leaving many infected individuals untreated. This is because the pathogenicity of this organism is regarded by some as equivocal and thus the issue as to whether eradication is indicated remains controversial. In this review, we aim to provide both sides of the debate to better inform the practicing physician and patient as to whether eradication would be the best approach.
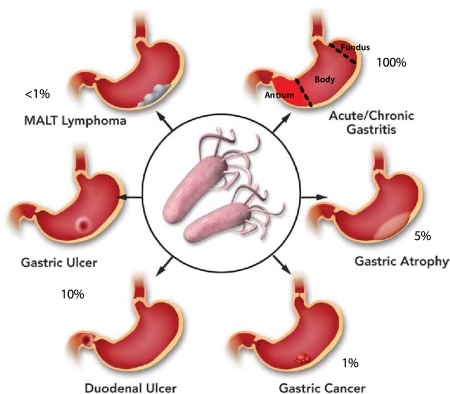
Figure 1. Consequences of H. pylori infection.
An outline of the gastric disorders resulting from infection by H. pylori showing the high incidence of gastritis followed by peptic ulcer diseases (duodenal or gastric ulcer), gastric atrophy gastric cancer and MALT lymphoma. Evidently, eradication of the organism would prevent these effects. Other systemic disorders have also been linked to infection.
The controversy centers around whether to eliminate the organism in all infected individuals or only in symptomatic patients on the grounds that the organism may be a commensal and provide benefits to the asymptomatic infected person. “Treaters” would suggest that eradication of the organism would likely reduce the development of gastric inflammation, development of peptic ulcer disease (especially in those patients who are also taking nonsteroidal anti-inflammatory drugs [NSAIDs]), and the theoretical benefit of reducing the progression to frank gastric malignancy. “Commensalists” suggest that H. pylori, rather than being a pathogen, is in fact a commensal, and eradication worsens gastro-esophageal reflux disease or even induces asthma. This is predicated on relatively few studies with the underlying concept that gastric atrophy generated by active infection, or perhaps ammonia generation by the organism, reduces gastric acidity and hence protects against gastro-esophageal reflux disease [4]. However, while clear that the inhabitants of the lower gut do not cause inflammation and hence can be regarded as commensals, in the case of H. pylori there is inevitable gastritis and a robust immune response, not characteristics of symbionts. The grave consequences of chronic infection argue strongly for a more vigorous physician response, whether in symptomatic or non-symptomatic individuals.
H. pylori and gastro-esophageal reflux disease
A recent review evaluated the results of 11 studies and concluded that H. pylori eradication did not result in a significant difference in the frequency of symptomatic or endoscopically proven erosive gastro-esophageal reflux disease, when compared with persistent infection, regardless of follow-up period, location, or the baseline disease [5]. Another in depth review of the literature agreed with these conclusions, except that in patients with peptic ulcer disease, H. pylori infection doubled the risk of developing gastro-esophageal reflux disease [6].
In contrast to this analysis, an argument has been put forward for the beneficial effects of H. pylori infection [7,8]. In particular, a recent analysis was interpreted to show that infection with H. pylori reduced the onset of Barrett’s esophagus, which is generally regarded as a step on the way to cancer of the esophagus. However, while the correlation between infection and gastritis and intestinal metaplasia was very strong, there was a weak correlation between esophageal intestinal metaplasia and infection [9]. This remains a controversial area [10]. A recent review of the literature concluded that there is no significant difference in the incidence of H. pylori infection between patients with Barrett’s esophagus and controls [11].
There have also been multiple studies on the association between gastro-esophageal reflux disease and esophageal adenocarcinoma, and the consensus is that acid reflux predisposes the squamous epithelium of the esophagus to development of columnar metaplasia [10]. However, as discussed above, there is no consensus as to the role of infection in protection against gastro-esophageal reflux disease. Given the mixed results, more studies are needed in this area, but, to date, evidence for the objection that has been made to the eradication of H. pylori, either on the grounds that it ameliorates reflux disease or esophageal intestinal metaplasia, is rather weak. Given the possible explanations for the lower rates of gastro-esophageal reflux disease and Barrett's esophagus seen in H. pylori infections, in some studies other treatments could compensate for this were the infection removed, so this is not an argument against eradication. For example, there are many ways of treating acid reflux, which would obviate the possible consequences of eradication.
There are also very few prospective studies on the association between infection by H. pylori and gastro-esophageal reflux disease or Barrett’s esophagus. A recent community-based study showed that there was indeed an inverse association between H. pylori antibody status and gastro-esophageal reflux disease diagnosis in 611 patients [12], suggesting that a portion of the risk of gastro-esophageal reflux disease may be higher in the absence of infection. A similar set of conclusions was drawn from a study investigating the incidence of new diagnosis of Barrett’s esophagus and gastro-esophageal reflux disease, in patients infected with H. pylori (particularly with patients with CagA+ strains) versus uninfected controls [13]. The percentage of infection was 11.7 in Barrett’s esophagus patients, 9.6 in gastro-esophageal reflux disease patients and 22.7 in healthy control subjects with a total of 929 subjects. There are various possible explanations for this observation. For example, infected patients may have suppressed acid secretion and had gastric atrophy. Alternatively, they may have increased gastric emptying and hence decreased acid reflux or increased appetite due to increased ghrelin (a hormone that regulates appetite) and consequent weight gain. A third possibility is that the ammonia generated by the organism could partially neutralize gastric acid. Whether or not infection is associated with decreased acid reflux, clearly gastro-esophageal reflux disease should be treated so that symptoms disappear. This is certainly less challenging than treating gastric cancer.
There have also been multiple studies on the association between gastro-esophageal reflux disease and esophageal adenocarcinoma, and the consensus clearly is that acid reflux predisposes the squamous epithelium of the esophagus to development of columnar metaplasia [10]. However, as discussed above, there is no consensus as to the role of infection in protection against gastro-esophageal reflux disease.
H. pylori and gastric cancer
It is now well accepted that habitation of the body of the stomach by H. pylori results in a large increase in the incidence of gastric adenocarcinoma [14-16]. Although the mechanism of neoplastic transformation remains an area of active investigation, the induction of neoplasia by infection has resulted in the organism being classified as a type I carcinogen by WHO.
The interaction between H. pylori and the gastric epithelium involves many factors. The major barrier that the organism must overcome in the stomach is the acidity of the gastric surface, which is greater in the fundus than the antrum. The antrum is the most frequent site of infection suggesting that the pH at the surface of antral glands is well tolerated by H. pylori allowing both survival and growth. With proton pump inhibitor administration, the organism is found largely in the fundus, suggesting that the increase of intragastric pH induced by inhibition of the gastric H, K ATPase favors colonization of the fundus rather than the antrum [17].
The first consequence of H. pylori infection is gastritis, which may be the direct result of ammonia secreted by the bacteria or products of the cag pathogenicity island (one of the major pathogenicity determinants in H. pylori), such as the virulence factor cagA (mediated by a type IV secretion system) [18]. Another toxic virulence factor is vacA, which, after proteolysis, is able to insert into the membrane of host cells and after penetration induce vacuolization [19]. Perhaps a major contribution to carcinogenicity is the induction of a local immune response, and in vitro models have shown that H. pylori is able to induce cytokine production [20]. On immune cells themselves, toll-like receptors 2 and 4 that recognize lipopolysaccharide (LPS) mediate the innate immune response to infection [21].
The burden of H. pylori infection for gastric cancer is illustrated in a Japanese study of about 1,500 symptomatic patients. About 3% of those infected by H. pylori had developed gastric cancer, while none of the uninfected group did so. Furthermore, those patients with duodenal ulcer did not show a propensity to develop cancer while those with gastric ulcer or non-ulcer dyspepsia did [22]. This indicates that infection of the body of the stomach results in a more serious consequence than infection of the antrum (the portion of the stomach nearest the duodenum) or duodenum. This is particularly relevant in symptomatic patients undergoing treatment with proton pump inhibitors, in whom the organism has a tendency to leave the antrum and inhabit the fundus. Fundic infection predisposes to intestinal metaplasia and eventually adenocarcinoma, so patients placed on chronic proton pump inhibitor therapy should be checked for active infection and if present, every effort should be made to eradicate the organism.
H. pylori and other diseases
A variety of ailments have been associated with infection, both positively and negatively affected by eradication (see [23] for a recent review). Prominent among those that are worsened by infection are idiopathic thrombocytic purpura, where the platelet count returns to normal after eradication, and iron deficiency anemia where eradication improves the efficacy of iron administration.
Various allergic diseases, such as asthma and various allergies, show a negative association epidemiologically with H. pylori eradication [24]. It may be that activation of Th1 cells by H. pylori neutrophil-activating protein, and reduced activation of Th2, could account for the beneficial effect on allergic responses [25].
Strategies for eradication of H. pylori
Perhaps if eradication of the infection was straightforward, a test and treat strategy would be more accepted. For an infection, where routine treatment is adopted without testing for susceptibility, a rate of > 90% is usually required for satisfactory outcome for treatment of infections, but routine, uncritical treatment often prevents recognition that this treatment is not meeting the requirement for success. In the case of eradication therapy, there was early recognition that more than one drug was required and the first generally used regimen was a combination of bismuth, tetracycline and metronidazole, quickly followed by the addition of a proton pump inhibitor such as omeprazole. This treatment required 3 or 4 doses per day and was not widely accepted. It was later replaced by the now classical triple therapy requiring twice daily dosing of a proton pump inhibitor, clarithromycin and amoxicillin or metronidazole. However, treatment success with this therapy has fallen below 80%, largely due to clarithromycin or metronidazole resistance [26]. Another issue is compliance in symptomatic patients where the proton pump inhibitor ablates the symptoms and the patient stops taking the medications. Currently, the state of eradication therapy is in flux with no one regimen offering the combination of simplicity and success.
What is now universal in therapy is the accompaniment of antibiotics by an inhibitor of acid secretion, now usually a proton pump inhibitor. This raises the question of the reason for the requirement for acid secretory inhibition. H. pylori is a slow-growing organism, even at neutral pH, with a division time of 4-6 hours in contrast to 20 minutes for E. coli. Its growth slows at acidic pH and stops at pH 3.0. Although there has been controversy as to the pH of its habitat in the stomach, transcriptomal analysis provides evidence that its habitat is indeed acidic, probably at about a median pH of 3.5. For antibiotics such as tetracycline, clarithromycin and amoxicillin, growth is essential for their efficacy. So it is likely that the elevation of pH by proton pump inhibitors accounts for their synergism with these antibiotics. However, the median intragastric pH achieved by proton pump inhibitors is ~ 4.0, still low enough for many of the organisms to remain quiescent. More effective inhibition of acid secretion would likely improve eradication with a bactericidal drug such as amoxicillin, to which current resistance is < 1%. This would result in dual therapy with BID (twice daily) administration of the inhibitor of acid secretion and amoxicillin. In Japan, where the incidence of slow proton pump inhibitor metabolizers is quite high, eradication of H. pylori was achieved with the proton pump inhibitor omeprazole and amoxicillin alone rather than triple therapy [27]. Given this result, it seems unfortunate that the pharmaceutical industry appears to have abandoned H. pylori as a desirable therapeutic target.
Another point to be made is that if eradication is undertaken, there must be quantitative assessment of successful therapy, with either the stool antigen test or urea breath test, and this should be a requirement for eradication treatment.
Conclusions
The controversy over whether H. pylori should be eradicated in all infected individuals or just in symptomatic patients, reflects the risk to benefit ratio. There is no controversy as to the sequelae of infection: peptic ulcer disease, gastric cancer and MALT lymphoma. In contrast, there is disagreement as to effects of eradication on gastro-esophageal reflux disease or Barrett’s esophagus. In light of this, the weight of evidence argues for eradication as preventive medicine and, importantly, if gastro-esophageal reflux disease symptoms are present these should be treated vigorously.
The therapeutic choices available currently, however, highlight the problem that eradication may not be easy. Current therapy is complicated and less than optimal. In principal, if better acid suppressing agents become available, then it seems likely that eradication could be improved and become simpler.
Acknowledgments
Supported in part by USVA and NIH grant #’s 5R01 DK53642 and 5R01 DK58333
Abbreviations
- MALT
mucosa-associated lymphoid tissue
- NSAIDs
nonsteroidal anti-inflammatory drugs
- LPS
lipopolysaccharide
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/m/4/7
References
- 1.Kuipers EJ. Review article: exploring the link between Helicobacter pylori and gastric cancer. Aliment Pharmacol Ther. 1999;13(Suppl 1):3–11. doi: 10.1046/j.1365-2036.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 2.Parsonnet J. The incidence of Helicobacter pylori infection. Aliment Pharmacol Ther. 1995;9(Suppl 2):45–51. [PubMed] [Google Scholar]
- 3.Parsonnet J, Isaacson PG. Bacterial infection and MALT lymphoma. N Engl J Med. 2004;350:213–5. doi: 10.1056/NEJMp038200. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 4.Sonnenberg A. Effects of environment and lifestyle on gastroesophageal reflux disease. Dig Dis. 2011;29:229–34. doi: 10.1159/000323927. [DOI] [PubMed] [Google Scholar]
- 5.Qian B, Ma S, Shang L, Qian J, Zhang G. Effects of Helicobacter pylori eradication on gastroesophageal reflux disease. Helicobacter. 2011;16:255–65. doi: 10.1111/j.1523-5378.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 6.Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. Am J Gastroenterol. 2010;105:1007–13. doi: 10.1038/ajg.2009.734. quiz 1006, 1014. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 7.Blaser M. Antibiotic overuse: Stop the killing of beneficial bacteria. Nature. 2011;476:393–4. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 8.Blaser MJ. Helicobacter pylori and esophageal disease: wake-up call? Gastroenterology. 2010;139:1819–22. doi: 10.1053/j.gastro.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139:1894–1901.e2. doi: 10.1053/j.gastro.2010.08.018. quiz e12. [DOI] [PubMed] [Google Scholar]; F1000 Factor 10Evaluated by Jay Solnick and Michael Hornsby 21 Jan 2011
- 10.Appelman HD, Umar A, Orlando RC, Sontag SJ, Nandurkar S, El-Zimaity H, Lanas A, Parise P, Lambert R, Shields HM. Barrett's esophagus: natural history. Ann N Y Acad Sci. 2011;1232:292–308. doi: 10.1111/j.1749-6632.2011.06057.x. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 11.Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett's esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492–500. doi: 10.1038/ajg.2008.37. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 12.Corley DA, Kubo A, Levin TR, Block G, Habel L, Rumore G, Quesenberry C, Buffler P, Parsonnet J. Helicobacter pylori and gastroesophageal reflux disease: a case-control study. Helicobacter. 2008;13:352–60. doi: 10.1111/j.1523-5378.2008.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, Leighton P, Rumore G, Quesenberry C, Buffler P, Parsonnet J. Helicobacter pylori infection and the risk of Barrett's oesophagus: a community-based study. Gut. 2008;57:727–33. doi: 10.1136/gut.2007.132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 15.Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–48. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 16.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 17.Stolte M, Meining A. Veränderungen der Helicobacter-pylori-Gastritis durch säuresekretionshemmende Therapie. Z Gastroenterol. 1999;37:1029–36. [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 18.Figura N. Helicobacter pylori factors involved in the development of gastroduodenal mucosal damage and ulceration. J Clin Gastroenterol. 1997;25(Suppl 1):S149–63. doi: 10.1097/00004836-199700001-00025. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 19.Isomoto H, Moss J, Hirayama T. Pleiotropic actions of Helicobacter pylori vacuolating cytotoxin, VacA. Tohoku J Exp. Med 2010;220:3–14. doi: 10.1620/tjem.220.3. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 20.Shimoyama T, Crabtree JE. Mucosal chemokines in Helicobacter pylori infection. J Physiol Pharmacol. 1997;48:315–23. [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 21.Pece S, Giuliani G, Di Leo A, Fumarola D, Antonaci S, Jirillo E. Role of lipopolysaccharide and related cytokines in Helicobacter pylori infection. Recenti Prog Med. 1997;88:237–41. [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 22.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 23.Malfertheiner P, Selgrad M. Helicobacter pylori infection and current clinical areas of contention. Curr Opin Gastroenterol. 2010;26:618–23. doi: 10.1097/MOG.0b013e32833efede. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 24.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–73. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codolo G, Mazzi P, Amedei A, Del Prete G, Berton G, D'Elios MM, Bernard M de. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cell Microbiol. 2008;10:2355–63. doi: 10.1111/j.1462-5822.2008.01217.x. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 26.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79–88. doi: 10.1038/nrgastro.2010.210. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012
- 27.Furuta T, Sugimoto M, Shirai N, Ishizaki T. CYP2C19 pharmacogenomics associated with therapy of Helicobacter pylori infection and gastro-esophageal reflux diseases with a proton pump inhibitor. Pharmacogenomics. 2007;8:1199–210. doi: 10.2217/14622416.8.9.1199. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6Evaluated by George Sachs 15 Mar 2012