Abstract
Patients with primary immunodeficiency (PIDs) depend on the presence of a variety of antibody specificities in intravenous immunoglobulin (IVIG). Using the tick-borne encephalitis virus (TBEV), geographic variability in IVIG antibody content was shown. Care should therefore be exercised when treating PIDs in a given geography, as only locally sourced plasma contains the antibody specificities against the circulating pathogens in the given locality.
TEXT
Intravenous immunoglobulin (IVIG) contains the pooled antibodies (Abs) from at least 3,000 to 60,000 healthy blood and plasma donors (10). One of the main areas of IVIG application is as Ab replacement therapy in patients with primary immunodeficiency (PIDs) (22). These persons critically depend on the presence of a variety of antibody specificities in IVIG, which ensures continued protection against any infectious agent they might encounter. As IVIG is manufactured from the variable resource human plasma, lot-to-lot variation in Ab levels of IVIG products are inevitable and have been reported (18). In addition, the Ab content in IVIG differs depending on the geographic origin of the plasma that was used in manufacture: U.S.-sourced (US-IVIG) or European Union-sourced (EU-IVIG) IVIG contains significantly different Ab levels against hepatitis A virus (8, 21), West Nile virus (WNV) (25), cytomegalovirus (21, 27), and the different echovirus serotypes (24), with some evidence of a difference in Ab content for measles and rubella (21). The reason for this geographic variability in IVIG Ab content is often not clear, yet one of the more obvious variables affecting the quantity and specificity of Abs in IVIG is the endemicity of a pathogen, where a change in the temporal or geographic pattern of virus circulation affects the antibody content of the final IVIG. When a virus or microbial agent is expanding its geographical range, exposure of the formerly naïve population to this novel agent is reflected in the Ab content of IVIG. This was demonstrated, e.g., for US-IVIG after the introduction of WNV into the United States in 1999 (4, 25, 31) and recently also for EU-IVIG, where increasing WNV-neutralizing Ab titers were detected in EU-IVIG lots manufactured after 2009, even though no human WNV infections have yet been reported from the countries in which the plasma was sourced (31).
To further increase the understanding of the geographical difference in IVIG Ab content, we determined the neutralizing Ab titers of IVIG preparations for tick-borne encephalitis virus (TBEV). This member of the Flaviviridae, for which three subtypes (European, Siberian, and Far Eastern) are distinguished, may cause tick-borne encephalitis (TBE), a potentially serious neurological disease with high fever and encephalitis (33), while 70 to 95% of human infections in regions in which the virus is endemic are either subclinical or totally asymptomatic (12). During the last 30 years, TBE morbidity has increased by 400% in Europe (36), and TBEV is currently expanding from its geographical range in Central Europe, Scandinavia, Russia, and Northern Asia (7). However, in the United States, TBEV remains a purely travel-related concern and TBE cases among travelers to Europe, Russia, and China have been reported (5). Disease prevention is possible through vaccination, and four different vaccines are currently available (20). A TBE immunoglobulin for after-exposure treatment is no longer available in Europe, but TBE emergency treatment in Russia includes the use of specific immunoglobulins (28, 29). This therapy can be effective in a curative and prophylactic context (6).
Using a fully validated microneutralization assay, the TBEV neutralization titers of 26 EU-IVIG (KIOVIG; Baxter AG, Vienna, Austria) lots, for which the plasma was mainly sourced in Austria (∼40 to 45%), Germany (∼40 to 45%), and, to a lesser degree, the Czech Republic (∼15%), of 3 Russian TBEV IVIG (Russian-IVIG; Microgen FGUP NPO) lots, manufactured from the plasma of TBEV-vaccinated donors (http://privivka.spb.ru/vaccination/100/), and of 29 US-IVIG (Gammagard Liquid; Baxter Healthcare Corp., Westlake Village, CA) lots were determined essentially as described previously (26). IVIG samples were used undiluted for US-IVIG or initially diluted 1:5 and then serially diluted in 2-fold steps with cell culture medium. TBEV strain Neudörfl (European subtype) (2 × 103 50% tissue culture infectious doses per milliliter) was added, and eight replicates per dilution were subsequently titrated on A549 cells (ATCC CCL-185). The virus-induced cytopathic effect was assessed after 7 days of incubation, and the neutralization titer was determined at least in duplicate. The reciprocal dilutions of a 1:2 dilution series resulting in 50% virus neutralization (NT50; detection limits, ≤0.8 for undiluted and ≤3.8 for 1:5 prediluted IVIG) were reported as means ± standard errors of the means (SEM) (Fig. 1). Graphs and statistical analysis (Student's t test) were done using GraphPad Prism v5.0 software (San Diego, CA).
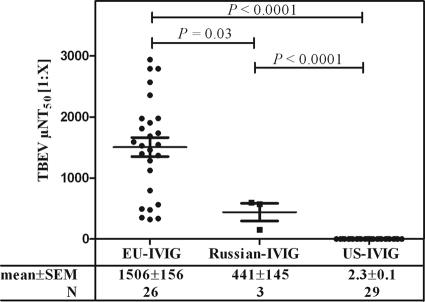
Fig 1.
Determination of TBEV neutralization titers of IVIG manufactured from plasma sourced in Austria, Germany, and the Czech Republic (EU-IVIG), a Russian TBEV IVIG (Russian-IVIG), and IVIG manufactured from plasma sourced in the US (US-IVIG). Titers were determined at least in duplicate using TBEV strain Neudörfl, and the reciprocal dilution resulting in 50% virus neutralization (NT50; detection limits, ≤0.8 for undiluted and ≤3.8 for 1:5 prediluted IVIG) is reported as mean ± SEM.
Whereas high TBEV NT50 values ranging from 321 to 2,940 were obtained for EU-IVIG, significantly less (P = 0.03) TBEV neutralization was seen with the Russian-IVIG (152 to 597 NT50) and even less (P < 0.0001) with US-IVIG (1.6 to 2.8 NT50) (Fig. 1). Our data therefore indicate that in the case of TBEV, the quantity of neutralizing TBEV Abs in IVIG is directly correlated to the endemicity of the pathogen. The high TBEV NT50 titers obtained for EU-IVIG likely reflect the high TBEV immunization rate of 88% in Austria (13), the 16% vaccination coverage in the Czech Republic (7), and possibly asymptomatic infection of the respective plasma donor population, which has been shown to induce neutralizing Abs against TBEV at a level equivalent to that of a completed three-part vaccination scheme (16). In contrast, US-IVIG has no TBEV neutralizing antibody capacity, which reflects the lack of TBEV circulation in this country. The significantly lower TBEV neutralization titer in Russian-IVIG compared to EU-IVIG is surprising, as the product is marketed as a specific “human TBE immunoglobulin” for prophylactic or curative use after suspected exposure to TBEV (28–30) and is manufactured from the plasma of previously TBEV-vaccinated donors (http://privivka.spb.ru/vaccination/100/). Due to the limited availability of Russian-IVIG, only three lots were tested. However, TBEV Ab titers similar to or higher than those present in EU-IVIG would be expected even in this small sample size, as it has been shown that the Russian TBEV vaccines are as immunogenic as the European TBEV vaccines (9, 19). The detection of lower levels of neutralizing TBEV Abs in Russian-IVIG cannot be due to the difference in circulating TBEV subtypes between Europe and Russia (35) or the use of a European subtype TBEV strain for the neutralization experiments reported here, as it has been shown that vaccination with any of the three TBEV subtypes raises Abs that are also cross-neutralizing against the other subtypes (15, 23, 39). As IgG3 has the highest neutralization effect for, e.g., dengue virus (32), cytomegalovirus (11), and rubella and polioviruses (3) and IgG3 degradation during manufacture has been a problem in narrow-spectrum IVIG products (1, 27, 37), the IgG3 content was determined for the three Russian-IVIG lots by enzyme-linked immunosorbent assay (ELISA), as previously described (14). The results (data not shown) showed IgG3 content that was within the physiological range, and IgG3 degradation could therefore be excluded as a contributing factor for the reduced TBEV neutralization capacity of the Russian-IVIG. Currently, no explanation can be offered for the reduced TBEV neutralization titer in Russian-IVIG.
The geographical variance in IVIG TBEV Ab content reported here should be of particular interest to clinicians treating PIDs, as we demonstrated, using TBEV as an example, that only IVIG produced from locally sourced plasma contains all the Ab specificities against the circulating pathogens in the given locality. This issue has been raised before: Bayry et al. even suggested the fractionation of IVIG from plasma donations collected around the world, to produce IVIG with universal rather than with only regional Ab content (2). This, however, is not currently feasible, as, e.g., in the United States only plasma collected at U.S. donor centers may be used in the manufacture of plasma-derived products destined for the U.S. market (10). The United States remains the single most important source of human plasma for fractionation, and IVIG manufacture in Europe depends on U.S. plasma donations. Care should therefore be exercised when PIDs living in Central Europe are treated with IVIG, as many of the IVIG lots available in Europe are fractionated from U.S. plasma rather than from locally sourced plasma. Details of plasma content in a given IVIG lot can be requested from the respective IVIG manufacturer.
The geographic variance in IVIG Ab content also needs to be taken into consideration for novel treatment approaches, as recently suggested by Seidel et al., who proposed the use of a TBE vaccine as a “neo-antigen” to test B-cell function in patients under regular IgG substitution therapy (34). The results obtained in the current investigation confirm that this proposed approach might be feasible as long as IVIG treatment is done using US-IVIG only, i.e., when no passively transferred TBEV Abs are administered at the same time as an active immunization against TBEV, as this is known to result in remarkably lower levels of actively produced antibodies (17, 38). In PIDs, administration of EU-IVIG, i.e., TBEV Ab high-titer IVIG for substitution therapy prior to TBE vaccination, would thus limit the development of specific antibodies, which in turn would be misconstrued as a reflection of a continued immunodeficient status of the patient. In addition, treatment with US-IVIG should be done only during the winter months in Central Europe, as TBEV has a typical seasonal distribution and occurs only during the warmer months of the year (6) and protective levels of TBEV Abs would therefore not be required during the cold season.
In conclusion, we investigated the TBEV-neutralizing Ab content of EU-IVIG, Russian-IVIG, and US-IVIG and found further evidence for a geographic variability in IVIG Ab content, which for TBEV was directly correlated to the lack of circulation in the United States or the high seroprevalence due to vaccination and possible subclinical infection in Europe and Russia. This finding is of importance to PIDs in Central Europe, who depend on the presence of protective levels of different antibody specificities in IVIG that, in the case of TBEV, can be achieved only through the administration of EU-IVIG.
ACKNOWLEDGMENTS
The contributions of the entire Pathogen Safety team, most notably Bettina York, Claudia Schwarr, Sonja Kurzmann, Cornelia Lassl (cell culture, virus propagation), Florian Kaiser, and Christian Medek (equipment), are herewith gratefully acknowledged. Alfred Weber provided the IgG subclass ELISA data. Baxter's Virology/Preclinical research group is acknowledged for providing the TBEV working stock.
This work was funded by Baxter BioScience. All authors are employees of Baxter Innovations GmbH.
Footnotes
Published ahead of print 28 February 2012
REFERENCES
- 1.Audet S, et al. 2006. Measles-virus-neutralizing antibodies in intravenous immunoglobulins. J. Infect. Dis. 194:781–789 [DOI] [PubMed] [Google Scholar]
- 2.Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. 2003. Intravenous immunoglobulin for infectious diseases: tailor-made or universal? J. Infect. Dis. 188:1610–1611 [DOI] [PubMed] [Google Scholar]
- 3.Beck OE. 1981. Distribution of virus antibody activity among human IgG subclasses. Clin. Exp. Immunol. 43:626–632 [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Nathan D, et al. 2009. Using high titer West Nile intravenous immunoglobulin from selected Israeli donors for treatment of West Nile virus infection. BMC Infect. Dis. 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention 2010. Tick-borne encephalitis among U.S. travelers to Europe and Asia—2000–2009. MMWR Morb. Mortal. Wkly. Rep. 59:335–338 [PubMed] [Google Scholar]
- 6.Charrel RN, et al. 2004. Tick-borne virus diseases of human interest in Europe. Clin. Microbiol. Infect. 10:1040–1055 [DOI] [PubMed] [Google Scholar]
- 7.Donoso MO, Escadafal C, Niedrig M, Pfeffer M, on behalf of the Working Group For Tick-Borne Encephalitis Virus. 2011. Tick-borne encephalitis in Europe, 2007 to 2009. Euro Surveill. 16(39):pii=19976 [DOI] [PubMed] [Google Scholar]
- 8.Farcet MR, Planitzer CB, Stein O, Modrof J, Kreil TR. 2010. Hepatitis A virus antibodies in immunoglobulin preparations. J. Allergy Clin. Immunol. 125:198–202 [DOI] [PubMed] [Google Scholar]
- 9.Fritz R, et al. 2012. Quantitative comparison of the cross-protection induced by tick-borne encephalitis virus vaccines based on European and Far Eastern virus subtypes. Vaccine 30(6):1165–1169 [DOI] [PubMed] [Google Scholar]
- 10.GAO. Blood plasma safety—plasma product risks are low if good manufacturing practices are followed—report to the Chairman, Subcommittee on Human Resources, Committee on Government Reform and Oversight, House of Representatives. 1998. http://www.gao.gov/assets/230/226266.pdf.
- 11.Gilljam G, Wahren B. 1989. Properties of IgG subclasses to human cytomegalovirus. J. Virol. Methods 25:139–151 [DOI] [PubMed] [Google Scholar]
- 12.Gritsun TS, Lashkevich VA, Gould EA. 2003. Tick-borne encephalitis. Antiviral Res. 57:129–146 [DOI] [PubMed] [Google Scholar]
- 13.Heinz FX, Holzmann H, Essl A, Kundi M. 2007. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 25:7559–7567 [DOI] [PubMed] [Google Scholar]
- 14.Hofmeister Y, et al. 2011. Human IgG subclasses: in vitro neutralization of and in vivo protection against West Nile virus. J. Virol. 85:1896–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzmann H, et al. 1992. Molecular epidemiology of tick-borne encephalitis virus: cross-protection between European and Far Eastern subtypes. Vaccine 10:345–349 [DOI] [PubMed] [Google Scholar]
- 16.Klockmann U, et al. 1991. Humoral immunity against tick-borne encephalitis virus following manifest disease and active immunization. Vaccine 9:42–46 [DOI] [PubMed] [Google Scholar]
- 17.Kreil TR, Burger I, Attakpah E, Olas K, Eibl MM. 1998. Passive immunization reduces immunity that results from simultaneous active immunization against tick-borne encephalitis virus in a mouse model. Vaccine 16:955–959 [DOI] [PubMed] [Google Scholar]
- 18.Lejtenyi D, Mazer B. 2008. Consistency of protective antibody levels across lots of intravenous immunoglobulin preparations. J. Allergy Clin. Immunol. 121:254–255 [DOI] [PubMed] [Google Scholar]
- 19.Leonova GN, Pavlenko EV. 2009. Characterization of neutralizing antibodies to Far Eastern of tick-borne encephalitis virus subtype and the antibody avidity for four tick-borne encephalitis vaccines in human. Vaccine 27:2899–2904 [DOI] [PubMed] [Google Scholar]
- 20.Mansfield KL, et al. 2009. Tick-borne encephalitis virus—a review of an emerging zoonosis. J. Gen. Virol. 90:1781–1794 [DOI] [PubMed] [Google Scholar]
- 21.Matejtschuk P, Chidwick K, Prince A, More JE, Goldblatt D. 2002. A direct comparison of the antigen-specific antibody profiles of intravenous immunoglobulins derived from US and UK donor plasma. Vox Sang. 83:17–22 [DOI] [PubMed] [Google Scholar]
- 22.Orange JS, et al. 2006. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J. Allergy Clin. Immunol. 117:S525–S553 [DOI] [PubMed] [Google Scholar]
- 23.Orlinger KK, et al. 2011. A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J. Infect. Dis. 203:1556–1564 [DOI] [PubMed] [Google Scholar]
- 24.Planitzer CB, Farcet MR, Schiff RI, Ochs HD, Kreil TR. 2011. Neutralization of different echovirus serotypes by individual lots of intravenous immunoglobulin. J. Med. Virol. 83:305–310 [DOI] [PubMed] [Google Scholar]
- 25.Planitzer CB, Modrof J, Kreil TR. 2007. West Nile virus neutralization by US plasma-derived immunoglobulin products. J. Infect. Dis. 196:435–440 [DOI] [PubMed] [Google Scholar]
- 26.Planitzer CB, Modrof J, Yu MY, Kreil TR. 2009. West Nile virus infection in plasma of blood and plasma donors, United States. Emerg. Infect. Dis. 15:1668–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planitzer CB, Saemann MD, Gajek H, Farcet MR, Kreil TR. 2011. Cytomegalovirus neutralization by hyperimmune and standard intravenous immunoglobulin preparations. Transplantation 92:267–270. [DOI] [PubMed] [Google Scholar]
- 28.ProMED-mail. 31 July 2009, posting date. Tick-borne encephalitis—Russia (06): Chelyabinsk. Archive number 20090731.2691. http://www.promedmail.org/direct.php?id=20090731.2691.
- 29.ProMED-mail. 24 May 2009, posting date. Tick-borne encephalitis—Russia (03): Yaroslavl. Archive number 20090524.1944. http://www.promedmail.org/direct.php?id=20090524.1944.
- 30.ProMED-mail. 21 July 2007, posting date. Tick-borne encephalitis—Russia: Irkutsk, Arkhangelsk, Kemerovo. Archive number 20070721.2333. http://www.promedmail.org/direct.php?id=20070721.2333.
- 31.Rabel P, et al. 2011. Increasing West Nile virus antibody titres in central European plasma donors from 2006 to 2010. Euro Surveill. 16(10):pii=19812 [DOI] [PubMed] [Google Scholar]
- 32.Rodrigo WW, et al. 2009. Dengue virus neutralization is modulated by IgG antibody subclass and Fcγ receptor subtype. Virology 394:175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruzek D, Dobler G, Mantke OD. 2010. Tick-borne encephalitis: pathogenesis and clinical implications. Travel Med. Infect. Dis. 8:223–232 [DOI] [PubMed] [Google Scholar]
- 34.Seidel MG, Grohmann E, Sadeghi K, Heitger A, Forster-Waldl E. 2010. Vaccination against tick-borne encephalitis virus tests specific IgG production ability in patients under immunoglobulin substitution therapy. Vaccine 28:6621–6626 [DOI] [PubMed] [Google Scholar]
- 35.Suess J. 2011. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia—an overview. Ticks Tick Borne Dis. 2:2–15 [DOI] [PubMed] [Google Scholar]
- 36.Suess J. 2008. Tick-borne encephalitis in Europe and beyond–the epidemiological situation as of 2007. Euro Surveill. 13(26):pii=18916 [PubMed] [Google Scholar]
- 37.Teschner W, Poelsler G, Butterweck HA, Mais-Paul U, Kreil TR. 2010. Manufacturing of IVIG, p 13–42. In Lazarus AH, Semple JW. (ed), Immunoglobulin therapy. AABB Press, Bethesda, MD. [Google Scholar]
- 38.von Hedenstrom M, Heberle U, Theobald K. 1995. Vaccination against tick-borne encephalitis (TBE): influence of simultaneous application of TBE immunoglobulin on seroconversion and rate of adverse events. Vaccine 13:759–762 [DOI] [PubMed] [Google Scholar]
- 39.Wiedermann U. 2010. Tick borne encephalitis TBE—vaccination in non-endemic countries. Travel Med. Infect. Dis. 8:251–256 [DOI] [PubMed] [Google Scholar]