Abstract
Paracoccidioidomycosis (PCM) is a serious infectious disease that progresses toward death if untreated. Its confirmatory diagnosis is made by the detection of the fungus Paracoccidioides brasiliensis in a direct mycological examination or by histopathology. However, these techniques are of low sensitivity. Serological tests seem to be more promising. The objective of this study was to test Western blot (WB) analysis using sera from patients suspected of PCM to determine whether it represents a safe and sensitive serological technique for a rapid and effective diagnosis for this disease. Sera from 517 patients were analyzed through WB analysis and double-immunodiffusion (DID) techniques using a crude exoantigen of P. brasiliensis 339. DID gave positive reactions for 140 sera (27%) and WB for 250 sera (48.4%). All sera that had a positive reaction by DID also had a positive result with a 43-kDa glycoprotein by WB analysis. Among the 377 samples that were negative by DID, 29.1% were reactive in WB analysis. For the cutoff dilution used (1:400), a positive reaction was not observed with any of the 102 sera from patients with other diseases in regions where such diseases are endemic and 30 healthy individuals tested as negative controls. These results prove WB analysis to be a sensitive technique and suggest its inclusion among routine laboratory assays as a safe method for PCM diagnosis.
INTRODUCTION
Paracoccidioidomycosis (PCM) is a systemic mycosis that is endemic in several Latin American countries. It occurs in individuals during the most productive period of their lives, rendering them unable to work. PCM is a serious public health problem, since it involves high social and economic costs (23). In Brazil, among chronic diseases of infectious and parasitic origin, PCM is the eighth leading cause of death and corresponds to a higher mortality rate among systemic mycoses (5). Its yearly mortality rate (3,181 cases) is considered high and is equivalent to 1.45 cases per million (5, 19). However, it is believed that these data are still underestimated (17) because they were based on death certificates, which are not a reliable source for statistics on mortality (4).
The diagnosis of PCM is confirmed by methods testing for the presence of P. brasiliensis fungus in the tissues, but the utility of these methods is quite limited due to poor access to samples of deep organs and to the fact that identification of the fungus is often not feasible, mainly because of previous treatments. Serological techniques would be useful, but those used routinely do not offer high sensitivity or high specificity.
Several serological tests for detection of anti-P. brasiliensis antibodies, such as complement fixation (14), double immunodiffusion (DID) (6), counterimmunoelectrophoresis (8), the enzyme-linked immunosorbent assay (ELISA) (11), and dot blot analysis (11, 21), can be used in the diagnosis of PCM. However, none of these has the characteristics of high sensitivity and specificity needed for an accurate diagnosis (1, 8, 10). DID is the most widely used serological test and is considered a reference for diagnosis, treatment, and monitoring of PCM (6). It is a simple and inexpensive technique, but several studies have shown false-negative results (8, 10). Counterimmunoelectrophoresis shows sensitivity similar to that of DID (8) and does not increase the specificity. ELISA has been used to detect antibodies in diagnosis of almost all systemic mycoses. In the case of PCM, however, it has not contributed adequately due to cross-reactivity (12) with sera from patients with tuberculosis (15, 16, 23), Hansen's disease and squamous cell carcinoma (which can be confused with PCM in its stomatological manifestations), syphilis, Wegener's granulomatosis, leishmaniasis, and sarcoidosis and especially with sera from patients with histoplasmosis (23), candidiasis, and Jorge Lobo's disease (1). In addition, high levels have been found in sera of apparently healthy individuals living in areas where PCM is endemic (1, 10). Attempts to reduce the high levels of cross-reactivity and increase the specificity of the technique have been made (1); however, it was not possible to significantly increase the test performance. Thus, so far none of the procedures used was sufficient to eliminate cross-reactivity of ELISA for PCM. On the other hand, Western blot (WB) analysis offers an advantage over ELISA, since prior protein electrophoresis ensures the presence of markers such as gp43 and, according to some studies, the sensitivity is high (10, 20, 22), but results have been obtained with a small number of patients, and the technique needs to be tested using a larger sample population. This study aimed at evaluating WB analysis using sera of patients suspected of PCM to determine whether it represents a specific and sensitive serological technique for a rapid and effective diagnosis of this disease.
MATERIALS AND METHODS
Samples.
We analyzed sera from 517 patients, 394 of whom were suspected of having PCM (301 patients in the University Hospital of Cascavel-PR, Brazil, and 93 patients in the Central Laboratory of Parana State [LACEN], Curitiba-PR, Brazil). Another 123 patients from Maringá-PR, Brazil, were selected from a research project that includes patients with PCM and concomitant pulmonary tuberculosis. In order to eliminate cross-reactivity, 102 serum samples positive for other regional diseases (leishmaniasis [30], Chagas' disease [30], tuberculosis [37], and histoplasmosis [5]) were tested. Furthermore, sera from 30 healthy individuals were included in the study.
Antigen.
A crude soluble exoantigen (rich in a 43-kDa component) (Fig. 1) was used that was obtained from a 7-day culture filtrate of strain P. brasiliensis 339 in YPD (yeast extract-peptone-dextrose) medium provided by the Centre for Immunobiological Production and Research (CPPI) organ of the Health Secretariat of the state of Paraná (18).
Fig 1.
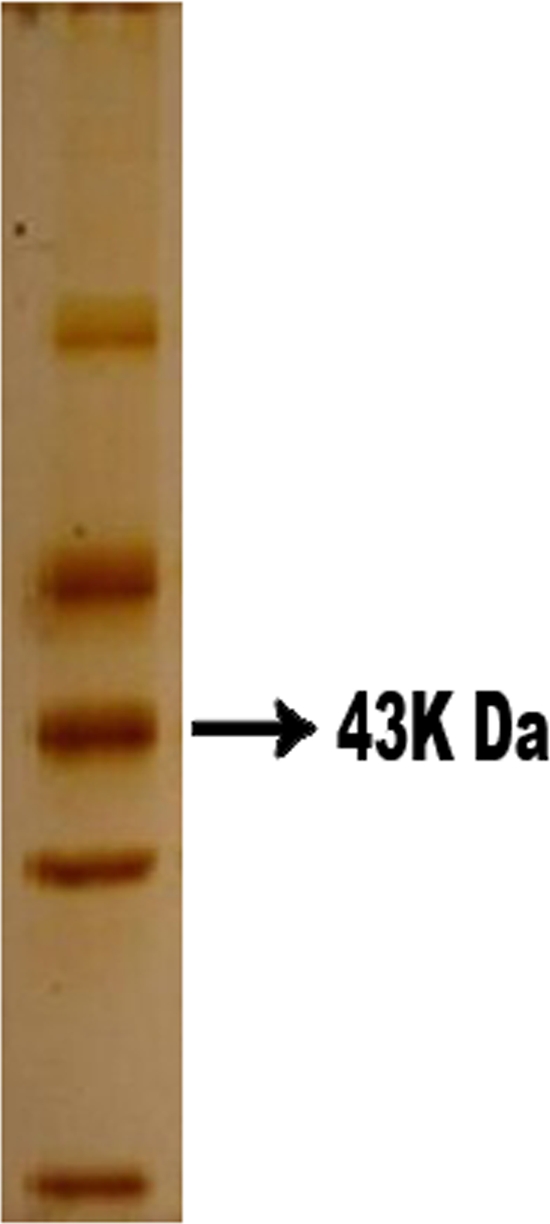
SDS-PAGE was followed by silver staining. The figure shows the exoantigen protein profile of P. brasiliensis used in DID and WB analysis; note that the 43-kDa component was expressed in large quantities.
Double immunodiffusion.
Glass slides (75 by 25 mm) were covered with 3.0 ml of a 1% agarose gel in a 0.85% physiological saline solution. We placed 10 μl of crude exoantigen of P. brasiliensis in the central well and 10 μl of patient sera (undiluted and diluted up to 1:128) in radially arranged holes. After they were allowed to rest in a humid chamber at room temperature for 24 h and were washed with 5% sodium citrate for 1 h and with 0.85% saline for 24 h, the slides were dried and stained for 7 min with Coomassie brilliant blue R-250 (0.15%) in a mixture of water-acetic acid-ethanol (4:2:4). The reading was done by observing the precipitation lines between antigen and antibodies in the serum of patients, as long as the lines formed between the antigen and positive-control sera were clearly identifiable.
Western blot analysis.
For the electrophoretic separation of the antigen, a denaturing polyacrylamide gel was prepared at 12% (sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]). A 200-μl aliquot of the exoantigen reconstituted as recommended by the manufacturer was diluted in 800 μl of Tris-HCl buffer (0.125 M; pH 6.8) containing 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, and 0.004% bromophenol blue. The mixture was boiled for 5 min before being applied to the gel.
The running buffer consisted of Tris (25 mM), glycine (190 mM), and 0.1% SDS, and the electrophoresis conditions were maintained with a current of 30 mA for about 3 h (Mini-Protean Tetra cell; Bio-Rad).
The antigens that separated in electrophoresis were transferred to a 0.20-μm-pore-size nitrocellulose membrane (Sigma Chemical Co., St. Louis, MO) in a Trans-Blot SD machine (semidry transfer cell; Bio-Rad) using a transfer buffer consisting of Tris (25 mM), glycine (192 mM), and 20% methanol (pH 8.3). The transfer process was maintained at 16 V for 1 h. The membrane was cut into strips of about 3 cm, which were blocked with 20% PBS–Tween (PBS-T) containing 5% skim milk for 2 h with stirring and then washed 3 times with PBS-T for 5 min each time.
Serum samples were diluted (1:50, 1:100, 1:200, and 1:400) in PBS-T buffer containing 1% skim milk and incubated for 3 h with stirring at 35°C and were again washed 3 times with PBS-T for 5 min each time. The conjugate (anti-human IgG protein plus peroxidase; Sigma catalog no. A8667) was then added, and the reaction mixture was diluted 1:1,000 in PBS-T containing 1% skim milk and incubated for 1 h. The membrane strips were washed 3 times with PBS-T for 5 min. The reaction was revealed with Tris-HCl (0.1 M; pH 7.4)–diaminobenzidine (0.2 mg)–30% H2O2 (0.4 μl).
RESULTS
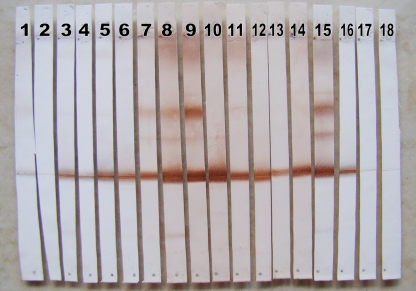
Of the 517 sera referred for investigation of PCM, 250 (48.4%) were reactive in WB analysis (Fig. 2). Of these, DID was positive for 140 samples (27%), and those results were confirmed by WB analysis. In addition, WB analysis performed using a glycoprotein of 43 kDa gave positive results for 110 (21.4%) sera, as shown in Table 1.
Fig 2.
Illustration of the results found by WB analysis in the present study, showing positive results for paracoccidioidomycosis with sera diluted 1:400 (lanes 3 to 16), negative results for sera from patients suspected of having PCM (lanes 17 and 18), a positive control (lane 1), and a negative control (lane 2) from a healthy person.
Table 1.
Distribution of results for 517 sera tested by two serological techniques, Western blot analysis (WB) and double immunodiffusion (DID)
| WB result | No. of sera with indicated DID result |
Total no. of sera | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 140 | 110 | 250 |
| Negative | 0 | 267 | 267 |
| Total | 140 | 377 | 517 |
We obtained reliable information on the clinical and microbiological characterization of PCM for 422 of 517 patients whose sera were evaluated. Thus, only the results of this group were employed for studies of sensitivity and specificity (Table 2).
Table 2.
Performance of two serological techniques, Western blot analysis (WB) and double immunodiffusion (DID), using 422 sera from patients under investigation for paracoccidioidomycosis
| Assay (dilution) | % sensitivity | % specificity |
|---|---|---|
| DID | 80 | 93.6 |
| WB (1:50) | 95.5 | 54.5 |
| WB (1:400) | 91.8 | 63.5 |
Both DID and WB analysis gave negative reactions with sera from healthy subjects, and with the sera from patients suspected of PCM, DID showed 80% sensitivity and a specificity of 93.6%. Regarding WB analysis, there were no differences in the expression of the 43-kDa component that were attributable to serum dilutions. However, the 1:400 dilution minimized cross-reactivity. With the 1:50 dilution, a positive reaction was obtained for WB analysis of 149 sera that had been negative by DID. At this dilution, sera from patients with tuberculosis, Chagas' disease, or histoplasmosis did not react with the P. brasiliensis exoantigen. However, the 43-kDa component was detected in 10 (30%) of the sera from patients with leishmaniasis. With sera diluted 1:400, on the other hand, the reaction was negative for 100% of patients with leishmaniasis; however, this procedure resulted in decreased sensitivity of the test (Table 2). Thus, among the sera that gave negative test results by DID, 110 were positive by WB analysis (29.1%). Therefore, serology by WB analysis with a dilution of 1:400 gave a sensitivity of 91.8% and was useful for discriminating cross-reactions with other infectious diseases that are frequent in the regions sampled.
DISCUSSION
WB analysis was revealed to be an important tool for the diagnosis of PCM; it was able to provide data representing high sensitivity and reasonable specificity. WB analysis was assessed here with a high number (517) of sera from patients in regions where PCM is endemic. The technique made it possible to detect positive sera that gave negative test results by DID, without false positives or cross-reactions with other diseases.
Until this study, there was no report of any study comparing DID and WB analysis that used a high number of sera from patients suspected of having PCM. The superiority of WB analysis is shown by the fact that the ratio of the number of sera that gave positive test results by that technique but gave negative test results by DID to the total number of sera that gave negative test results by DID was 110/377 (Table 1), representing almost 30%. With regard to cross-reactions, considering that the 43-kDa component is shared by other microorganisms such as Histoplasma capsulatum, Leishmania spp., Mycobacterium tuberculosis, and Trypanosoma cruzi, WB analysis was also efficient.
Our study showed that the cutoff of 1:400 allowed us to consider that a positive reaction indicated the presence of PCM, contributing significantly to the clinical screening of patients.
WB analysis has been used in studies on PCM by several authors (2, 10, 20, 22), but the focus of most studies has been clinical forms and treatment monitoring of PCM (9, 13). There was a previous study similar to ours (9) in which the authors correlated the findings of the same two serological tests, DID and WB analysis, with the clinical diagnosis and follow-up of PCM. However, that study analyzed only confirmed patients, whereas in our study, patients were not screened with respect to PCM diagnosis, and yet their results were in accord with ours. Recently, Silva et al. (20) compared DID and WB analysis using 23 sera and found that 95.4% of sera negative by DID gave a positive reaction to gp43 from an antigen obtained from a P. brasiliensis culture filtrate by WB analysis. Previously, we found similar results (22). These results are consistent with the findings of Del Negro et al. (7); the authors of that study stated that sera from patients with PCM can be negative by DID while reagents for WB analysis can reveal the presence of gp43 in the same sera. Despite being the gold standard test and having low cost, DID requires much higher levels of antibody serum than WB analysis in order to diagnose PCM. In fact, several studies have shown false-negative DID results (7, 8).
Western blotting has been considered a costly, complex reaction, and the equipment needed has been difficult for clinical laboratories to access (21). However, the equipment is now available, may be purchased at an affordable price, and is easy to handle, enabling the realization of a large number of tests, thus providing quick and reliable results. It has been noted that some authors have evaluated ELISA as a serologic test for diagnosis of PCM (1, 11); however, this technique does not reveal what component of the antigen was recognized by patient's antibodies, and this is a problem that also occurs with dot blot analysis, which is a disadvantage compared to WB analysis. In our opinion, the results we obtained with WB analysis ensure, with the available resources, the achievement of results superior to those of the previous techniques because of reduced cross-reactions. In practice, WB analysis could be used in parallel with DID, complementing that technique and providing improved results for the serological diagnosis of PCM, especially for sera that give negative DID results. Regarding the serum dilution, for safety reasons we recommend the cutoff of 1:400, though it offers a slightly lower sensitivity. A 1:50 dilution could also be used with caution; as shown in Table 2, the sensitivity would be 95.5%, well above that of DID, but there is a risk of cross-reactivity with leishmaniasis. This concern is important, especially in regions of endemicity with an overlap of these two important infectious diseases.
There is an limitation, in relation to the antigen used in WB analysis, which must be overcome: P. brasiliensis antigens are not commercially available and are usually obtained via in-house production centers for research, and the variability of the results according to the antigen used is recognized (8, 20). In this study, we employed a P. brasiliensis antigen produced by the CPPI and distributed to the public laboratories of the state of Paraná, Brazil, which is the same antigen as has been used to perform DID for more than 10 years (18). It is a soluble antigen obtained from a culture filtrate whose analysis by SDS-PAGE shows expression of the important 43-kDa component (Fig. 1).
Finally, this was the first prospective study performed with a great number of sera; our results confirm that WB analysis is more sensitive than DID, suggesting that it could be considered a technique aiding the diagnosis of PCM, delivering safe, reliable, and fast results and confirming the importance of WB analysis in the serological diagnosis of PCM.
ACKNOWLEDGMENTS
This work was supported by CAPES, CNPq, and the Araucaria Foundation.
Footnotes
Published ahead of print 1 February 2012
REFERENCES
- 1. Albuquerque CF, Silva SHMD, Camargo ZP. 2005. Improvement of the specificity of an enzyme-linked immunosorbent assay for diagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 43: 1944–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blotta MH, Camargo ZP. 1993. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: relationship with clinical forms of paracoccidioidomycosis. J. Clin. Microbiol. 31: 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blotta M, et al. 1999. Endemic regions of paracoccidioidomycosis in Brazil: a clinical and epidemiologic study of 584 cases in the southeast region. Am. J. Trop. Med. Hyg. 61: 390–394 [DOI] [PubMed] [Google Scholar]
- 4. Conselho Federal de Medicina 2005. Regulamenta a responsabilidade médica no fornecimento da Declaração de Óbito, p 121 Diário Oficial da União 2005; 5 dez: seção I. Resolução CFM no. 1779/2005 Conselho Federal de Medicina, Brasilia, Brazil: http://www.portalmedico.org.br/resolucoes/cfm/2005/1779_2005.htm [Google Scholar]
- 5. Coutinho ZF, et al. 2002. Paracoccidioidomycosis mortality in Brazil (1980–1995). Cad. Saúde Pública 18: 1441–1454 [DOI] [PubMed] [Google Scholar]
- 6. de Camargo ZP. 2008. Serology of paracoccidioidomycosis. Mycopathologia 165: 289–302 [DOI] [PubMed] [Google Scholar]
- 7. Del Negro GM, et al. 1995. Lack of activity of paracoccidioidomycosis sera in the double immunodiffusion test with the gp43 antigen: report of two cases. J. Med. Vet. Mycol. 33: 113–116 [DOI] [PubMed] [Google Scholar]
- 8. Del Negro GMB, et al. 1991. The sensitivity, specificity and efficiency values of some serological tests used in the diagnosis of paracoccidioidomycosis. Rev. Inst. Med. Trop. São Paulo 33: 277–280 [DOI] [PubMed] [Google Scholar]
- 9. Do Valle AC, et al. 2001. Interpretation and clinical correlation of serological tests in paracoccidioidomycosis. Med. Mycol. 39: 373–377 [DOI] [PubMed] [Google Scholar]
- 10. Elias Costa MR, Da Silva Lacaz C, Kawasaki M, De Camargo ZP. 2000. Conventional versus molecular diagnostic tests. Med. Mycol. 38(Suppl. 1): 139–145 [PubMed] [Google Scholar]
- 11. Martins R, Marquez S, Alves M, Fecchio D, Franco M. 1997. Serological follow-up of patients with paracoccidioidomycosis treated with itraconazole using dot-blot, ELISA and Western-blot. Rev. Inst. Med. Trop. São Paulo 39: 261–269 [DOI] [PubMed] [Google Scholar]
- 12. Mendes-Giannini MJS, Camargo ME, Lacaz CS, Ferreira AW. 1984. Immunoenzymatic absorption test for serodiagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 20: 103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mendes-Giannini MJ, Bueno JP, Shikanai-Yasuda MA, Ferreira AW, Masuda A. 1989. Detection of the 43,000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J. Clin. Microbiol. 27: 2842–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moses A. 1916. Fixação de complemento na blastomicose. Mem. Inst. Oswaldo Cruz 8: 68–70 [Google Scholar]
- 15. Paniago AMM, et al. 2003. Paracoccidioidomicose: estudo clínico e epidemiológico de 422 casos observados no Estado de Mato Grosso do Sul. Rev. Soc. Bras. Med. Trop. 36: 455–459 [DOI] [PubMed] [Google Scholar]
- 16. Quagliato Júnior R, et al. 2007. Association between paracoccidioidomycosis and tuberculosis: reality and misdiagnosis. J. Bras. Pneumol. 33: 295–300 [DOI] [PubMed] [Google Scholar]
- 17. Santo AH. 2008. Tendência da mortalidade relacionada à paracoccidioidomicose, Estado de São Paulo, Brasil, 1985 a 2005: estudo usando causas múltiplas de morte. Rev. Panam. Salud Publica 23: 313–324 [DOI] [PubMed] [Google Scholar]
- 18. Secretaria de Estado da Saúde do Paraná 2002. Protocolo de paracoccidioidomicose. Governo do Paraná, Curitiba-PR, Brazil [Google Scholar]
- 19. Shikanai-Yasuda MA, Telles Filho FDQ, Mendes RP, Colombo AL, Moretti ML. 2006. Consenso em paracoccidioidomicose. Rev. Soc. Bras. Med. Trop. 39: 297–310 [DOI] [PubMed] [Google Scholar]
- 20. Silva DF, et al. 2008. Use of immunoblotting assay improves the sensitivity of paracoccidioidomycosis diagnosis. J. Venom. Anim. Toxins Incl. Trop. Dis. 14: 313–321 [Google Scholar]
- 21. Taborda CP, Camargo ZP. 1994. Diagnosis of paracoccidioidomycosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J. Clin. Microbiol. 32: 554–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahachi G, Guilhermetti E, Silva JA, Svidzinski TIE. 1999. Importância do western blot no diagnóstico seguro da Paracoccidioidomicose, abstr. B 87. Abstr. 7th Int. Meet. Paracoccidoidomycosis, Campos do Jordão, Brazil [Google Scholar]
- 23. Wanke B, Aidê MA. 2009. Chapter 6—paracoccidioidomycosis. J. Bras. Pneumol. 35: 1245–1249 [DOI] [PubMed] [Google Scholar]