Abstract
A peptide cocktail derived from the mycobacterial antigens ESAT-6, CFP-10, and Rv3615c allowed differentiation between Mycobacterium bovis-infected and M. bovis bacillus Calmette-Guérin (BCG)-vaccinated cattle when used as a skin test reagent for a “DIVA” test (i.e., a test capable of differentiating infected and uninfected vaccinated animals). Addition of the antigen Rv3020c improves the diagnostic sensitivity without compromising specificity in the face of BCG or Johne's disease vaccination.
TEXT
Bovine tuberculosis (BTB) caused by the bacterial pathogen Mycobacterium bovis poses a major economic threat to the farming industry, and despite the current “test and slaughter” control policy, the incidence of BTB in Great Britain has been steadily rising over the last 20 years (5). Thus, the British government has acknowledged the urgent need for an effective vaccine. Current promising vaccines are based on heterologous prime-boost approaches that include the live attenuated M. bovis vaccine strain bacillus Calmette-Guérin (BCG) (14, 16–19). However, BCG vaccination sensitizes the animal to bovine tuberculin and compromises the specificity of the tuberculin tests currently used for diagnosis of BTB. Therefore, it is essential that diagnostic tests capable of differentiating infected and uninfected vaccinated animals (“DIVA” tests) are developed in parallel with vaccine initiatives. Although the application of such DIVA tests is largely evaluated in the form of blood-based gamma interferon (IFN-γ) release assays (IGRAs), a skin test format would provide a practical additional option, given the high degree of familiarity with and application of the tuberculin skin test by veterinarians and clinicians.
Recently, we described a DIVA skin test reagent that induced a delayed-type hypersensitivity response in M. bovis-infected cattle but not in BCG-vaccinated animals (20). This reagent consisted of recombinant proteins or a cocktail of synthetic immunogenic peptides. The peptide cocktail contained (i) peptides spanning the entire amino acid sequence of the mycobacterial proteins ESAT-6 and CFP-10 (11 and 10 peptides, respectively); (ii) a single peptide from the mycobacterial protein MPB83 (p195-214) (15), and (iii) three peptides from the mycobacterial protein Rv3615c (p65-84, p73-92, and p84-103) (13). The specificities of ESAT-6, CFP-10, and Rv3615c are due to either their location in the RD-1 region that is deleted from all BCG strains (e.g., ESAT-6 and CFP-10) (4, 6, 7, 10) or because, although not located within the RD-1 deletion, secretion of the protein is dependent upon the Esx-1 secretion system contained within the RD-1 region (e.g., Rv3615c) (11).
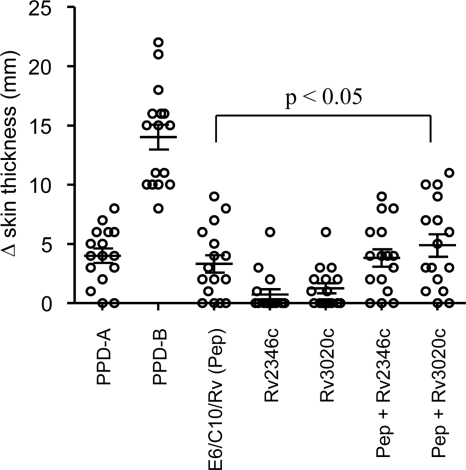
Further development of this DIVA reagent revealed that the MPB83 peptide could be omitted from the cocktail without compromising test sensitivity (data not shown). Next, we investigated whether addition of further immunogenic peptides to the cocktail would enhance its sensitivity in disclosing M. bovis-infected animals. We have previously demonstrated that peptide pools representing the antigens Rv2346c and Rv3020c induced IFN-γ production in whole blood from M. bovis-infected animals but not from BCG-vaccinated cattle (8). Thus, these peptide cocktails were chosen for evaluation in the DIVA skin test in 16 M. bovis-infected field reactors (ages, 12 to 27 months; median, 15 months). Subsequent postmortem analysis revealed that all 16 tuberculosis (TB) reactor animals had visible tuberculous lesions, and all but one were M. bovis culture positive. These animals received intradermal injections (0.1-ml volume) in the neck with the following antigens: avian tuberculin (purified protein derivative A [PPD-A]; 2,500 IU [Prionics, Lelystad, Netherlands]), bovine tuberculin (PPD-B; 3,000 IU [Prionics, Lelystad, Netherlands]), Rv2346c peptide cocktail, Rv3020c peptide cocktail, and ESAT-6/CFP-10/Rv3615c peptide cocktail with or without either Rv2346c or Rv3020c peptide cocktails. In all cases, the peptide cocktails were tested at a concentration of 10 μg/peptide/injection, respectively. Skin induration at the injection sites was measured using calipers by the same operator prior to and 72 h after the skin test, and the results are expressed as the difference in skin thickness (mm) between the two readings. As shown in Fig. 1, all 16 M. bovis-infected cattle demonstrated PPD-B skin test reactions. All 16 tested positive to the single intradermal tuberculin (SIT) test and to the single intradermal cervical comparative tuberculin (SICCT) test at the “severe” level of interpretation (Table 1). When the standard interpretation of the SICCT test was applied, 87.5% (14/16) tested positive (Table 1). The ESAT-6/CFP-10/Rv3615c peptide cocktail (Pep) induced detectable responses in 12/16 animals, demonstrating again its potential as a BTB diagnostic skin test reagent (Fig. 1 and Table 1). The Rv2346c and Rv3020c peptide cocktails on their own elicited skin test responses in only 4/16 and 8/16 animals, respectively (Fig. 1). When used in combination with ESAT-6/CFP-10/Rv3615c (Pep), both Rv2346c and Rv3020c enhanced the numbers of animals mounting a skin test response to 13/16 and 14/16, respectively (Fig. 1). However, only addition of Rv3020c peptides to the ESAT-6/CFP-10/Rv3615c cocktail induced a significant increase in skin thickening compared to ESAT-6/CFP-10/Rv3615c alone (P < 0.05; repeated-measures analysis of variance [ANOVA], Tukey's posttest analysis). It should be noted that responses to the ESAT-6/CFP-10/Rv3615c/Rv3020c peptide cocktail were lower than those to PPD-B, highlighting that there is still room for further enhancement of skin test DIVA reagents.
Fig 1.
Skin test responses to defined antigens in M. bovis-infected cattle. Results are expressed as the difference in skin thickness (mm) between the pre- and postskin test readings. Pep, peptide. Each open circle represents an individual animal, while the horizontal line provides the mean ± standard error of the mean (SEM). P < 0.05, repeated-measures ANOVA and Tukey's posttest analysis.
Table 1.
Performance of the skin test reagents
| Group | No. of animals | % positive (95% CI)g |
||||
|---|---|---|---|---|---|---|
| SICCT |
SITe | E/C/RVf | E/C/Rv + Rv3020cf | |||
| Standardc | Severed | |||||
| M. bovis infecteda | 16 | 87.5 (61.7–98.5) | 100 (79.4–100) | 100 (79.4–100) | 75.0 (47.7–92.7) | 87.5 (61.7–98.5) |
| Noninfected and vaccinatedb | ||||||
| Naïve controls | 7 | 0 (0–41.0) | 0 (0–41.0) | 0 (0–41.0) | 0 (0–41.0) | 0 (0–41.0) |
| BCG alone | 7 | 14.3 (0.4–57.9) | 57.1 (18.4–90.1) | 71.4 (29.1–96.3) | 0 (0–41.0) | 0 (0–41.0) |
| BCG followed by Gudair | 7 | 0 (0–41.0) | 0 (0–41.0) | 100 (59.0–100) | 0 (0–41.0) | 0 (0–41.0) |
| Gudair followed by BCG | 7 | 0 (0–41.0) | 0 (0–41.0) | 85.7 (42.1–99.6) | 0 (0–41.0) | 0 (0–41.0) |
| BCG + Gudair at same time | 6 | 0 (0–45.9) | 0 (0–41.0) | 50.0 (11.8–88.2) | 0 (0–45.9) | 0 (0–45.9) |
| Gudair alone | 7 | 0 (0–41.0) | 0 (0–41.0) | 57.1 (18.4–90.1) | 14.3 (0.4–57.9) | 14.3 (0.4–57.9) |
| Total noninfected | 41 | 2.4 (0.1–12.9) | 9.8 (2.7–23.2) | 61.0 (44.5–75.8) | 2.4 (0.1–12.9) | 2.4 (0.1–12.9) |
Values in this row represent test sensitivities.
Primary vaccination at 6 weeks of age, second vaccination (where given) at 12 weeks of age, and skin test at approximately 23 weeks of age. Values in these rows represent the percentage of false-positive test results.
Response considered positive if Δ skin thickness for PPD-B − PPD-A is >4 mm (standard OIE and United Kingdom test interpretation).
Response considered positive if Δ skin thickness for PPD-B − PPD-A is >2 mm (severe United Kingdom test interpretation).
Response considered positive if Δ skin thickness for PPD-B is ≥4 mm.
Response considered positive if Δ skin thickness is ≥1 mm. E/C/Rv denotes ESAT-6/CFP-10/Rv3615c peptide cocktail.
95% CI, 95% confidence interval.
Having shown that ESAT-6/CFP-10/Rv3615c/Rv3020c was the best peptide cocktail for disclosing M. bovis-infected animals, we next wished to confirm that the specificity of this reagent was not compromised by the addition of Rv3020c peptides. To this end, we tested this reagent in naïve calves and in calves that had been vaccinated subcutaneously with a single dose of 1 × 106 CFU of BCG Danish (Staten Serum Institute, Sweden). Furthermore, given that Mycobacterium avium subsp. paratuberculosis infection (1–3) and immunization with M. avium subsp. paratuberculosis vaccines (9) are confounding factors in the diagnosis of M. bovis infection, we also compared the skin test specificity of the ESAT-6/CFP-10/Rv3615c/Rv3020c reagent with that of PPD-based tests in animals that had been vaccinated subcutaneously with 2 ml of the M. avium subsp. paratuberculosis Gudair vaccine (CZ Veterinaria, Spain), either alone or in combination with BCG vaccination. The calves used in this study were aged between 159 and 194 days (median age of 162 days) at the time of skin testing (see Table 1 footnotes for timings of the vaccinations and skin testing). The criteria for a positive skin test with the different antigens were as follows: (i) SICCT responses were considered positive if the change in (Δ) skin thickness for PPD-B – PPD-A is >4 mm (standard World Organisation for Animal Health [OIE] and United Kingdom test interpretation) or >2 mm (severe United Kingdom test interpretation); (ii) SIT responses were considered positive if Δ skin thickness for PPD-B is ≥4 mm; and (iii) peptide cocktail responses were considered positive if Δ skin thickness is ≥1 mm. The SICCT was only compromised in a proportion of calves that been vaccinated with BCG only, none of which responded to the DIVA cocktail (Table 1). Across all 41 noninfected/vaccinated animals tested, only 1 (2.4%) mounted a detectable (1-mm) skin test response to ESAT-6/CFP-10/Rv3615c/Rv3020c, while 25 (61%) were SIT positive to bovine tuberculin. Thus, these results demonstrate that in contrast to the SIT skin test, the ESAT-6/CFP-10/Rv3615c/Rv3020c skin test reagent demonstrated a high level of specificity when used in animals vaccinated with combinations of BCG and/or Mycobacterium avium subsp. paratuberculosis vaccines.
The Bovigam IFN-γ test (Prionics, AG, Switzerland) is an alternative blood-based test for diagnosis of BTB (21). Briefly, cattle whole blood is cultured for 24 h with and without test antigens (including PPD-B, PPD-A, and peptide cocktails), following which IFN-γ levels in the culture supernatants are measured via enzyme-linked immunosorbent assay (ELISA). However, the specificity of this test is compromised in young cattle (<6 months of age), due in part to the induction of nonspecific IFN-γ production from natural killer cells (12). Given that the 41 noninfected animals studied herein were less than 6 months old, the use of the Bovigam IFN-γ test is counterindicative as a diagnostic test for these animals. Confirming this observation, when our study animals were tested with the peptide cocktails using the Bovigam IFN-γ test, 6 and 12 of the 41 non-M. bovis-infected animals mounted IFN-γ responses to ESAT-6/CFP-10/Rv3615c and ESAT-6/CFP-10/Rv3615c/Rv3020c, respectively (data not shown). (ELISA responses were considered positive if the optical density at 450 nm [OD450] for peptide cocktail-stimulated cultures − nil antigen-stimulated cultures was >0.1.) Responses to these peptide cocktails were also observed in naïve cattle, further confirming the confounding effect of young age on this test (data not shown). Importantly, this age effect had no negative impact on the performance of the DIVA peptide cocktails when used as a skin test reagent, highlighting the potential specificity benefits of this test format for use in young cattle.
In summary, the results presented herein highlight the continued advancement of a practical and sensitive skin-test-based DIVA diagnostic test that can be developed in parallel with current vaccine initiatives for both M. bovis and M. avium subsp. paratuberculosis infections.
ACKNOWLEDGMENTS
We are grateful for the support of the AHVLA Animal Support Unit for animal husbandry provision and to AHVLA officers for assisting with the recruitment of the naturally infected cattle.
This work was funded by the Department for Environment, Food and Rural Affairs (DEFRA), United Kingdom.
Footnotes
Published ahead of print 1 February 2012
REFERENCES
- 1.Alvarez J, et al. 2009. Effect of paratuberculosis on the diagnosis of bovine tuberculosis in a cattle herd with a mixed infection using interferon-gamma detection assay. Vet. Microbiol. 135: 389– 393 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez J, et al. 2008. Interference of paratuberculosis with the diagnosis of tuberculosis in a goat flock with a natural mixed infection. Vet. Microbiol. 128: 72– 80 [DOI] [PubMed] [Google Scholar]
- 3.Aranaz A, et al. 2006. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M. avium subsp. paratuberculosis. Vet. Res. 37: 593– 606 [DOI] [PubMed] [Google Scholar]
- 4.Behr MA, et al. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284: 1520– 1523 [DOI] [PubMed] [Google Scholar]
- 5. DEFRA 2010. The UK Chief Veterinary Officer's 2009 report on animal health and welfare. Department for Environment, Food and Rural Affairs, London, United Kingdom: http://www.defra.gov.uk/corporate/about/who/cvo/documents/2009report.pdf [Google Scholar]
- 6.Garnier T, et al. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. U. S. A. 100: 7877– 7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon SV, et al. 1999. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32: 643– 655 [DOI] [PubMed] [Google Scholar]
- 8.Jones GJ, Hewinson RG, Vordermeier HM. 2010. Screening of predicted secreted antigens from Mycobacterium bovis identifies potential novel differential diagnostic reagents. Clin. Vaccine Immunol. 17: 1344– 1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler H, et al. 2001. Immune reactions in cattle after immunization with a Mycobacterium paratuberculosis vaccine and implications for the diagnosis of M. paratuberculosis and M. bovis infections. J. Vet. Med. B Infect. Dis. Vet. Public Health 48: 185– 195 [DOI] [PubMed] [Google Scholar]
- 10.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178: 1274– 1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millington KA, et al. 2011. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 108: 5730– 5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen I, et al. 2005. Bovine NK cells can produce gamma interferon in response to the secreted mycobacterial proteins ESAT-6 and MPP14 but not in response to MPB70. Infect. Immun. 73: 5628– 5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidders B, et al. 2008. Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect. Immun. 76: 3932– 3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skinner MA, et al. 2005. The order of prime-boost vaccination of neonatal calves with Mycobacterium bovis BCG and a DNA vaccine encoding mycobacterial proteins Hsp65, Hsp70, and Apa is not critical for enhancing protection against bovine tuberculosis. Infect. Immun. 73: 4441– 4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vordermeier HM, et al. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab Immunol. 6: 675– 682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vordermeier HM, Huygen K, Singh M, Hewinson RG, Xing Z. 2006. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect. Immun. 74: 1416– 1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vordermeier HM, et al. 2004. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 112: 461– 470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vordermeier HM, et al. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77: 3364– 3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedlock DN, et al. 2005. Vaccination of cattle with a CpG oligodeoxynucleotide-formulated mycobacterial protein vaccine and Mycobacterium bovis BCG induces levels of protection against bovine tuberculosis superior to those induced by vaccination with BCG alone. Infect. Immun. 73: 3540– 3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelan AO, et al. 2010. Development of a skin test for bovine tuberculosis for differentiating infected from vaccinated animals. J. Clin. Microbiol. 48: 3176– 3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood PR, et al. 1991. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68: 286– 290 [DOI] [PubMed] [Google Scholar]