Abstract
Lyme disease in the United States is caused by Borrelia burgdorferi sensu stricto, which is transmitted to mammals by infected ticks. Borrelia spirochetes differentially express immunogenic outer surface proteins (Osp). Our aim was to evaluate antibody responses to Osp antigens to aid the diagnosis of early infection and the management of Lyme disease. We analyzed antibody responses during the first 3 months after the experimental infection of dogs using a novel multiplex assay. Results were compared to those obtained with two commercial assays detecting C6 antigen. Multiplex analysis identified antibodies to OspC and C6 as early as 3 weeks postinfection (p.i.) and those to OspF by 5 weeks p.i. Antibodies to C6 and OspF increased throughout the study, while antibodies to OspC peaked between 7 and 11 weeks p.i. and declined thereafter. A short-term antibody response to OspA was observed in 3/8 experimentally infected dogs on day 21 p.i. Quant C6 enzyme-linked immunosorbent assay (ELISA) results matched multiplex results during the first 7 weeks p.i.; however, antibody levels subsequently declined by up to 29%. Immune responses then were analyzed in sera from 125 client-owned dogs and revealed high agreement between antibodies to OspF and C6 as robust markers for infection. Results from canine patient sera supported that OspC is an early infection marker and antibodies to OspC decline over time. The onset and decline of antibody responses to B. burgdorferi Osp antigens and C6 reflect their differential expression during infection. They provide valuable tools to determine the stage of infection, treatment outcomes, and vaccination status in dogs.
INTRODUCTION
Lyme disease is the most common vector-borne disease in the United States. It is caused by B. burgdorferi sensu stricto bacteria, which is transmitted to mammalian hosts by infected ticks (Ixodes spp.) (9, 32). Clinical signs of Lyme disease in dogs are fever, acute arthritis, arthralgia, lameness, and nephritis in some cases. Central nervous system involvement, heart block, and uveitis are less frequently reported in dogs (2, 10, 12).
The diagnosis of Lyme disease is made on the basis of symptoms, including the animal living in an area where the disease is endemic, ruling out other causes of clinical signs, and a high titer of B. burgdorferi-specific antibodies. In dogs, the latter was initially accomplished by the detection of serum antibodies by a quantitative but rather nonspecific enzyme-linked immunosorbent assay (ELISA) followed by a qualitative but more specific Western blotting (WB) (2, 11, 43). Other, more recent tests are based on the detection of an invariable domain (IR6) of the variable surface antigen VlsE of B. burgdorferi. IR6, also commonly known as C6, is immunodominant in human patients with Lyme disease and also in dogs infected with B. burgdorferi (14–16).
Fluorescent bead-based, multiplex analysis of antibodies to B. burgdorferi is a novel approach of high analytical sensitivity and allows for the simultaneous detection of immune responses to several antigens in dogs and horses (39, 40). Several antigens of B. burgdorferi, including outer surface protein A (OspA), OspC, and OspF, are differentially expressed in ticks (OspA) (23, 24, 30, 36, 44) during transmission to a warm-blooded animal (OspC) (7, 25, 28) or later in the mammalian host (OspF) (1, 18, 21). Although the surface antigen expression patterns of B. burgdorferi have been thoroughly investigated in ticks and during transmission, less information is available about the antigen expression of the spirochetes in the mammalian host. Besides the studies on C6 mentioned above, almost no data exist about the dynamics of antibodies to various B. burgdorferi antigens after the infection of dogs. Considering the ability of B. burgdorferi to regulate its surface antigen expression in adjustment to its current environment, it is likely that the differential expression of these antigens during early or persistent infection results in a variation of the immune response over time. Thus, a more detailed analysis of the dynamics of antibody to different B. burgdorferi antigens in dog serum will likely provide us with greater insights into various stages of infection, could improve our understanding of this persistent pathogen, and is likely to influence prognosis and treatment decisions for Lyme disease.
The aim of this study was to identify markers for early and late infection by comparing antibody responses to different surface antigens of B. burgdorferi in two sample sets. First, sera of experimentally infected dogs were used to compare antibody responses to OspA, OspC, OspF, flagellin B (FlaB), and two C6 peptides during the first 3 months of infection and by using a novel multiplex assay for all six antigens and two commercially available tests based on C6 peptide. Second, we analyzed sera from canine patients by multiplex analysis to further evaluate the infection markers OspC, OspF, and C6.
MATERIALS AND METHODS
Experimental dogs and samples.
A total of 12 clinically healthy purpose-bred beagles were enrolled in this study. Dogs were 9 to 10 weeks of age at the time of tick challenge. Five of them were male and seven female. Dogs were experimentally infected with B. burgdorferi by exposure to 25 (n = 4) or 50 (n = 4) wild-caught ticks (Ixodes scapularis). A control group (n = 4) was not exposed to ticks. Adult Ixodes scapularis ticks were collected in the spring of 2008 in southern Rhode Island from an area in which B. burgdorferi was endemic and were stored in the laboratory (in ventilated tick vials in tick incubators at 12 to 15°C and 95% relative humidity) for a brief period before infestation on dogs. Borrelia infection rates in ticks were determined by dark-field assay to be 52% (20). On day 0, adult ticks were placed in infestation chambers affixed to both sides of each dog. Ticks were allowed to feed to repletion (between days 7 and 14) and were removed on day 14. Starting on the day of tick challenge through to the end of the study, 92 days later, animals were examined daily for clinical signs associated with Borrelia infection, including, but not limited to, lameness, ataxia, joint swelling, enlarged lymph nodes, depression, and fever (>39.5°C) (42). Blood samples were collected from the dogs 13 days before exposure to ticks and on days 21, 35, 49, 63, 77, and 92 and were analyzed for antibodies to Borrelia burgdorferi. The blood was allowed to clot. Three drops of serum were used immediately in the SNAP 4Dx test, and the remainder of the serum was stored at −70°C until being evaluated using the Lyme Quant C6 test and Cornell's multiplex Lyme assay (see below). All tests were performed in a masked manner. Body skin punch biopsy specimens (2 to 4 mm) were collected on days 49 and 92 from each dog to confirm the presence or absence of Borrelia. Two biopsy specimens were taken from body regions close to the tick attachment sites at each sampling day. Half of the biopsy specimens were used for culturing and were placed directly into screw-cap tubes containing 6 ml Barbour-Stoenner-Kelly II (BSK II) medium. The other samples were used for PCR and were frozen immediately and stored at −70°C until analyzed. PCR was performed with primers amplifying a fragment of the housekeeping gene encoding FlaB (FlaB-F, 5′ GACGACGACAAGATGATTATCAATCATAATACATCAGC 3′; and FlaB-R, 5′ GAGGAGAAGCCCGGTTTATCTAAGCAATGACAAAACATA 3′). The dogs were euthanized at the end of the study (day 92). A full necropsy was performed, and samples were collected to investigate Borrelia-associated histopathology and tissue distribution of spirochetes as described previously (35). The following tissues were collected: superficial lymph nodes, skin, the capsules of the left and right carpi, elbows, shoulders, stifles, and tarsi. For each of these samples, the presence or absence of Borrelia-associated pathology was assessed and a score was applied. Histopathological findings in the skin were rated with the following scale: 0, scar (fibrosis/loss of adnexae); 1, no change to minimal change; 2, dermatitis, superficial, mild; and 3, lymphoid proliferation, nodular, perineural. Histopathological findings in synovial membranes were rated with the following scale: 0, no change to minimal change; 1, focal to regional hyperplasia/hypertrophy of synovial lining cells; 2, generalized hyperplasia of synovial lining cells; and 3, generalized hyperplasia plus inflammatory cells plus fibrin deposition; lymph nodes were determined to be either quiescent or reactive.
The animal study was executed according to Pfizer standard operating procedures (SOPs) and legal requirements in the conduct of the study, national animal welfare regulations, and other applicable national regulatory requirements. The study protocol was reviewed and approved by Pfizer's Animal Use Committee (IACUC) prior to the start of the study.
Sera from canine patients.
In addition to sera from experimentally infected dogs, 125 serum samples from client-owned dogs were tested using the multiplex assay to compare the performance of C6 and OspF as markers for chronic infection. The canine patient sera were submitted for serological Lyme testing to the Animal Health Diagnostic Center at Cornell University between July 2008 and January 2009, and antibodies to B. burgdorferi were analyzed by Western blotting using a whole-cell lysate from cultured bacteria (2, 11). Whole-cell lysates from B. burgdorferi contain multiple antigens, including OspA, OspC, OspF, FlaB, and VlsE (39). Information about clinical signs or a disease history was not available for these dogs. The presence (positive) or absence (negative) of serum antibodies to the 39-kDa (VlsE containing the C6 region) and 29-kDa (OspF) proteins on the blots was determined blindly by an observer who was not aware of the multiplex assay results. All serum samples included in this comparison had agreeable results by Western blotting and multiplex analysis, i.e., both tests were either negative or positive for OspF and also for C6-P1, respectively. Patterns with positive C6 and negative OspF results or the opposite were included in the comparison.
B. burgdorferi antigens.
A fluorescent bead-based multiplex assay was used to measure antibodies to B. burgdorferi OspA, OspC, and OspF antigens in canine serum (39). For this study, three additional antigens were added to the existing assay for the simultaneous measurement of antibodies to six antigens of B. burgdorferi. Recombinant FlaB antigen was expressed in Escherichia coli using an expression system previously described for OspA, OspC, and OspF (39). In brief, a 909-bp partial FlaB gene corresponding to the extracellular part of the antigen was amplified from DNA was isolated from B. burgdorferi that originated from infected Ixodes ticks that were collected in a forested area in Westchester County, NY (2). Primers spanning bases 1 to 20 and 909 to 889 of the B. burgdorferi strain B31 FlaB gene (GenBank accession no. NC_001318) were designed. Primers contained BamHI and KpnI restriction sites for the cloning of the FlaB gene into the pQE-30Xa expression vector (Qiagen Inc., Valencia, CA). After induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), the His-tagged protein was expressed in E. coli SG13009 cells (Qiagen Inc., Valencia, CA). The bacteria were lysed, and the His-tagged FlaB protein was purified using a HisTrapFF and an AKTA-fast protein liquid chromatography instrument (both from GE Healthcare, Piscataway, NJ). The protein concentration was determined by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). In addition, two 26-amino-acid C6 peptides from the B31 and 297 strains of B. burgdorferi sensu stricto (15) were used as antigens for the multiplex assay. One C6 peptide sequence (C6-P1) originated from strain B31 (MKKDDQIAAAIALRGMAKDGKFAVKD), and the other C6 peptide (C6-P2) was from strain 297 (MKKNDQIAAAIVLRGMAKDGEFALKD). The two C6 peptides differed in amino acids in positions 4, 12, 21, and 24 (in boldface). The peptides were synthesized, desalted by high-performance liquid chromatography (HPLC), and conjugated to bovine serum albumin (BSA) (GenScript USA, Piscataway, NJ). Before coupling to the multiplex beads, the FlaB protein and the C6 peptides coupled to BSA were run on an SDS gel and tested by Western blotting with positive and negative canine sera as previously described (39). The analysis confirmed the specific detection of the FlaB protein or the C6 peptides by sera that had previously tested positive (see Fig. S1A in the supplemental material). BSA alone was not detected.
Fluorescent bead-based multiplex assay.
Six B. burgdorferi antigens were coupled to fluorescent beads (Luminex, Austin, TX), and the multiplex assay was performed as previously described (39). In brief, OspA was coupled to bead 33, OspC to bead 34, OspF to bead 37, FlaB to bead 36, C6-P1 to bead 35, and C6-P2 to bead 38. For each antigen, 5 × 103 beads were used per microtiter plate well (Multiscreen HTS plates, Millipore, Danvers, MA). Canine serum samples were diluted 1:600 in blocking buffer (phosphate-buffered saline [PBS] with 1% [wt/vol] BSA and 0.05% [wt/vol] sodium acid) and incubated with the beads for 30 min on a shaker at room temperature and in the dark. The plate was washed with PBS-Tween (PBS with 0.1% [wt/vol] BSA, 0.02% [vol/vol] Tween 20, and 0.05% [wt/vol] sodium acid), and 50 μl of biotinylated rabbit anti-dog IgG(H+L) (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:5,000 in blocking buffer was added to each well and incubated for 30 min as described above. After washing, 50 μl of streptavidin-phycoerythrin (Invitrogen, Carlsbad, CA) diluted 1:100 in blocking buffer was added. Plates were incubated for 30 min as described above and washed. The beads were resuspended in 100 μl of blocking buffer, and each plate was placed on the shaker for 15 min to resuspend the beads. The assay was analyzed in a Luminex IS 100 instrument (Luminex, Austin, TX). The data were reported as median fluorescent intensities (MFI). The positive cutoff values for the multiplex assay were established by comparing MFI values and Western blotting results of each antigen by receiver-operating curve (ROC) analyses using 188 canine patient sera as described previously (39) or as shown in Fig. S1B in the supplemental material for FlaB and the C6 peptides. Positive cutoff values were ≥1,000 MFI (OspC and C6-P2) and ≥1,500 MFI (OspA, OspF, C6-P1, and FlaB).
Antibody testing by SNAP 4Dx test and Lyme QuantC6 ELISA.
Three drops of serum from experimentally infected dogs were tested immediately after blood collection and processing using SNAP 4Dx tests as described in the test kit (IDEXX Laboratories, Westbrook, ME). Samples were considered positive if any color developed at the site of the B. burgdorferi antigen spot within 8 min. Frozen samples also were sent to IDEXX Laboratories to perform the Lyme Quant C6 ELISA (C6 ELISA) testing. Sera were considered positive for antibodies to C6 if the ELISA result was ≥30 U/ml.
Data and statistical analysis.
For the C6 and FlaB bead assays within the multiplex assay format, ROCs were generated by 188 blindly measured canine serum samples (39). The Western blotting result (positive/negative) of the corresponding proteins (39 kDa for C6 and 41 kDa for FlaB) served as relative gold standards for the comparison to MFI values obtained by multiplex analysis. The procedure was previously described for the multiplex assay using OspA, OspC, and OspF antigens (39). The ROCs were generated using the MedCalc program, version 11.2.0.0 (F. Schoonjans, Mariakerke, Belgium).
Serum samples from experimentally infected and control dogs were coded on collection, and all antibody testing was performed masked by the different laboratories. Once all of the assays had been run, the samples were decoded for data analysis. Differences in antibody responses between the 25- and 50-tick exposure groups were calculated by two-factor repeated-measures analysis of variance (RM ANOVA). The model was run for each B. burgdorferi antigen in the multiplex analysis and for the C6 Quant ELISA, and a 2-sided P value of 0.05 was considered significant.
For the analysis of antibodies to the infection markers OspC, OspF, and C6 in canine patient sera, we used 125 serum samples that were submitted for Lyme antibody testing to Cornell University. All of these sera were tested by Western blotting and in the multiplex assay and were either positive or negative for the respective antigens in both assays. The spearman's rank correlation between antibodies to C6 and OspF was calculated based on multiplex assay results. In addition, antibodies to OspC, OspF, and C6 antigens were compared by a one-way RM ANOVA with Bonferroni post tests to compare the responses between the three antigens. All analyses were performed with 95% confidence intervals and P < 0.05 for significance. Calculations were performed and the graphs were created by using the GraphPad Prism program, version 5.01.
RESULTS AND DISCUSSION
Infection with B. burgdorferi after exposure of experimental dogs to ticks.
The successful transmission of Borrelia spirochetes from the ticks to the dogs was confirmed using both PCR and culture of skin samples taken close to the tick attachment site. On days 49 and 92 after exposure to ticks, skin biopsy specimens were obtained from the dogs and used to amplify the FlaB gene of B. burgdorferi by PCR (Table 1). Bacterial DNA was detected in all samples from dogs in the 25-ticks/dog group at both time points. For dogs in the 50-tick group, all samples obtained on day 49 were positive and two out of four samples were positive on day 92. All PCRs from control dog samples were negative for B. burgdorferi. The presence of viable spirochetes transmitted by the wild-caught ticks was further confirmed by bacterial cultures from the same skin biopsy specimens (Table 1). B. burgdorferi could not be cultured from any of the tissues of the control group. Spirochetes were present in all noncontaminated cultures from dogs exposed to 25 ticks and in all but one (day 92) culture from dogs in the 50-tick group (Table 1). Bacteria were found in cultured samples from all dogs in the 50-tick group, i.e., in at least one of the skin samples. PCR and bacterial culture confirmed that all dogs exposed to wild-caught ticks were infected with B. burgdorferi. Because infection with B. burgdorferi was confirmed in all dogs that were exposed to ticks, we will refer to day 0 as the day of infection and to the ensuing days as days postinfection (p.i.).
Table 1.
Number of positive results obtained by amplification of the FlaB gene by PCR and bacterial culture of B. burgdorferi using skin biopsy specimens from experimentally infected dogs
| Detection method and day p.i. | No. positive/total no. tested by exptl group |
||
|---|---|---|---|
| Control | 25 ticks/dog | 50 ticks/dog | |
| PCR | |||
| 49 | 0/4 | 4/4 | 4/4 |
| 92 | 0/4 | 4/4 | 2/4 |
| Culture | |||
| 49 | 0/3a | 3/3a | 3/3a |
| 92 | 0/4 | 4/4 | 2/3a |
The fourth culture could not be evaluated due to contamination.
Similar experimental infection models for dogs using wild-caught ticks were previously reported (2, 34). In these models, dogs were also successfully infected by tick exposure as indicated by PCR and long-lasting antibody titer to B. burgdorferi, while the needle injection of the spirochetes resulted in considerably lower antibody titers (2).
Clinical signs and tissue histopathology.
Sporadic fever (body temperature of >39.5°C) was observed in individual dogs of the infected groups between days 9 and 21 (see Fig. S2A in the supplemental material) and on days 83 and 92 in two dogs of the control group. Clinical signs, such as weight loss, ataxia, joint swelling, or depression, were not observed in any of the dogs during the study. Lameness was seen on days 74 to 76 p.i. and again on days 90 to 92 p.i. in one dog that had been exposed to 50 ticks. Enlarged lymph nodes were frequently found from day 15 p.i. on until the end of the study in control and tick-exposed dogs. At the end of the study, histology was performed on skin, lymph nodes, and several synovial membranes, and a score was applied. Lymph node tissues were reactive in all tick-exposed dogs and in two of the four control dogs. By comparing synovial membrane tissues, a slight increase in histopathological changes was found in the infected groups (see Fig. S2B). Increased inflammation in skin regions close to the tick attachment sites was observed in both infected groups but not in the control dogs (see Fig. S2C). Overall, clinical signs within the first 3 months after experimental infection with B. burgdorferi were sporadic, and pathological tissue damage was rather mild in the infected dogs compared to that of the control group. The clinical findings are in agreement with previous studies of dogs reporting lameness as the predominant clinical sign after experimental infection with B. burgdorferi. Lameness was observed 2 to 5 months after tick exposure, and the most severe histopathological changes were found at the time when clinical signs occurred (2, 34).
Multiplex analysis of sera from experimentally infected dogs.
Multiplex analysis for the simultaneous detection of antibodies to OspC, OspF, C6-P1,C6-P2, FlaB, and OspA antigens of B. burgdorferi was performed on serum samples from all experimentally infected dogs on day 13 prior to tick exposure and on days 21, 35, 49, 63, 77, and 92 p.i. (Fig. 1). Antibody values were negative in sera from all dogs in the control group at all time points and in sera from all infected dogs on day 13 prior to tick exposure. Positive values for antibodies to B. burgdorferi were detected by multiplex analysis on the first sampling day after infection (day 21) for selected antigens (see below). Between days 63 and 92 p.i., all infected dogs were positive for antibodies to five antigens, OspC, OspF, C6-P1, C6-P2, and FlaB. The multiplex data were further evaluated for two parameters: (i) the earliest detectable onset of antibody responses to an antigen (positive values) and the time point when all infected dogs in a group were positive for that antigen; and (ii) the dynamics of antibody values to different antigens during the first 3 months after infection.
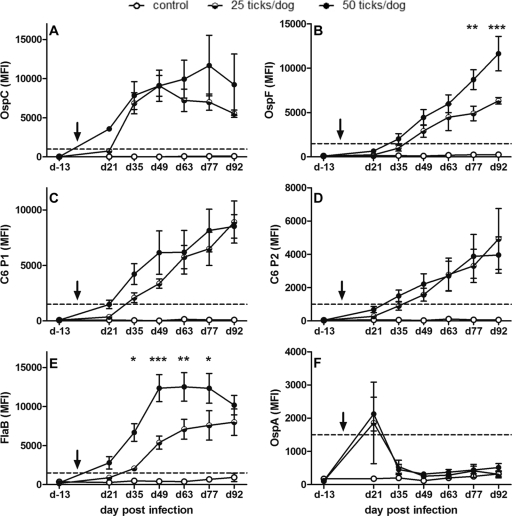
Fig 1.
Multiplex antibody values (MFI) to six surface antigens of B. burgdorferi in serum samples of experimentally infected dogs. Dogs were divided into three groups with four dogs per group: a noninfected control group and two infected groups exposed to 25 or 50 ticks, respectively. Serum samples were obtained on day 13 prior to tick exposure (d-13) and on days 21, 35, 49, 63, 77, and 92 postexposure. All sera were analyzed in a multiplex assay to simultaneously measure antibodies to six surface antigens of B. burgdorferi (A to F). The graphs show the mean values and standard errors for each time point and group of dogs. The dotted lines indicated the positive cutoff values for individual markers. The arrow shows the time of exposure to infected ticks. Asterisks indicate significant differences in antibody values between the 25- and 50-tick exposure groups: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Early markers for infection with B. burgdorferi in dogs.
After exposure to 25 ticks, antibodies to OspC were first detected on day 21 p.i. in one out of four dogs (Table 2). On day 35 (25 ticks), antibodies to OspC and FlaB were detected in all dogs, and those to OspF, C6-P1, and C6-P2 were detected in two or three animals. In the 50-tick group, antibodies to OspC were detected in all dogs by day 21 p.i. At the same time, positive antibody values to C6-P1, C6-P2, and FlaB were observed in one or two of the four dogs while antibodies to OspF were first observed at day 35 p.i. in three out of four dogs. Looking at sera from all experimentally infected dogs (both tick loads), we found that 5/8 dogs had antibodies to OspC in their serum on day 21 p.i. and 8/8 dogs were positive for OspC antibodies on day 35 p.i. The OspF, C6-P1, or C6-P2 infection markers resulted in lower numbers of positive dog samples on day 21 p.i., with 0/8, 2/8, or 1/8, respectively, and on day 35 p.i., with 5/8 or 7/8 positive samples. None of the three dogs that were negative for OspC on day 21 p.i. had positive antibody values for OspF, C6-P1, or C6-P2 (see Tables S1 and S2 in the supplemental material). Based on these patterns obtained by simultaneous antibody detection in samples from experimentally infected dogs, it can be concluded that antibodies to OspC are the first indicators of infection with B. burgdorferi, and antibodies to FlaB and C6 develop afterwards, followed by antibodies to OspF. The finding that antibodies to OspC are a marker of early infection in dogs is in agreement with the role of OspC during spirochete transmission (9). OspC expression is required for the effective migration of the bacteria from the tick intestine to the salivary glands (25). After spirochetes enter the mammalian host, OspC also inhibits bacterial killing by antibody-mediated cytotoxicity (28). As a consequence of OspC expression during and directly after transmission, it is one of the first antigens of B. burgdorferi that is recognized by the mammalian immune system and can subsequently induce antibody responses. In humans, antibodies to OspC are also considered to be markers of early infection (1, 25).
Table 2.
Number of dogs with positive antibody responses to B. burgdorferi antigens in serum samples obtained between days 21 and 49 p.i. and measured by multiplex analysisa
| Infection marker | No. positive/total no. tested by exptl group and day p.i. |
|||||
|---|---|---|---|---|---|---|
| 25 ticks/dog |
50 ticks/dog |
|||||
| 21 | 35 | 49 | 21 | 35 | 49 | |
| OspC | 1/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| OspF | 0/4 | 2/4 | 3/4 | 0/4 | 3/4 | 4/4 |
| C6-P1 | 0/4 | 3/4 | 4/4 | 2/4 | 4/4 | 4/4 |
| C6-P2 | 0/4 | 2/4 | 3/4 | 1/4 | 3/4 | 4/4 |
| FlaB | 0/4 | 4/4 | 4/4 | 2/4 | 4/4 | 4/4 |
| OspA | 1/4 | 0/4 | 0/4 | 2/4 | 0/4 | 0/4 |
On day 13 prior to infection, all antibody values were negative for all antigens. From days 63 to 92 p.i., all antibody values were positive by multiplex analysis for both infected groups and all infection markers, with the exception of OspA, which resulted in positive values on day 21 p.i. only. The time points when antibodies to a particular antigen were first detectable are shown in boldface.
In addition, we observed a small but clear increase in antibody responses to OspA in all experimentally infected dogs on day 21 p.i. compared to results for the preexposure samples (see Table S1 in the supplemental material). Some of the OspA antibody values were in the positive interpretation range for this antigen (Table 2). OspA is expressed in the midgut of infected ticks and becomes downregulated during transmission (9, 23, 24, 30, 36, 44). The immune system thus is not exposed to significant OspA expression after infection with B. burgdorferi. Nevertheless, OspA is highly immunogenic, and antibodies to OspA have been shown to protect mice (6, 29) and dogs from infection (3, 4, 41). OspA is a component of all currently available Lyme vaccines for dogs. In serological diagnostics, antibodies to OspA therefore have been interpreted as vaccination markers in dogs (8, 11, 37, 38). However, our current data show that OspA antibodies also form in response to infection. Compared to the usually high and long-lasting anti-OspA vaccination titers (39), OspA antibody responses in the experimentally infected dogs were low and temporary, as indicated by the small antibody peak on day 21 p.i. (Fig. 1F). This suggested that OspA expression is not completely downregulated by the spirochetes at the time of infection and can induce a weak but detectable antibody response early after infection in some dogs.
Dynamics of antibodies to different antigens of B. burgdorferi after infection.
During the first 3 months of infection with B. burgdorferi, the dynamics of antibodies to OspC differed from those to the other antigens. Antibodies to C6-P1, C6-P2, OspF, and FlaB (25 ticks) increased constantly (Fig. 1B to E), indicating the presence of B. burgdorferi in the host and suggesting a continuous immune stimulus provided by these antigens. Antibodies to OspF, C6, and FlaB all provided robust infection markers by 2 months after infection. Their kinetics also suggested a titer increase beyond 3 months of infection which eventually may develop into a constant titer in chronically infected dogs, as previously described in long-term infection studies for antibodies to whole bacterial lysate antigen (2, 34) or to C6 (16, 26). In contrast, antibodies to OspC peaked on day 49 (25 ticks) or 77 (50 ticks) and decreased afterwards (Fig. 1A). This is in accordance with the differential expression pattern of the OspC antigen during transmission and its downregulation after infecting mammalian hosts. The missing antigenic stimulus likely leads to the decline of antibodies to OspC a few weeks after infection. Our findings also suggested that OspC antibodies further decline after day 92 p.i. and may become undetectable in late infection stages. The only other decline of antibodies was observed for FlaB between days 77 and 92 in the 50-tick exposure groups. However, this was not consistent between the two exposure groups (Fig. 1E).
Exposure to 50 ticks resulted in slightly earlier detection of antibodies to most antigens compared to that for the 25-tick exposure groups (Table 2). We also analyzed if significant differences in the magnitude of antibody responses were observed for individual antigens after exposure to 25 or 50 infected ticks. Compared to the 25-tick group, a significantly increased antibody production after exposure to 50 ticks was observed only for OspF on days 77 and 92 p.i. and FlaB on days 35 to 77 postinfection (Fig. 1B and E). Overall, this suggests that the influence of infection dose on the magnitude of antibody responses to B. burgdorferi is small, and that antibody values instead are influenced by the individual animal's immune response (see Tables S1 and S2 in the supplemental material). Quantitative antibody values, although valuable indicators for infection, thus are unlikely to give a direct correlation to spirochete loads. Nevertheless, this requires further confirmation, because the number of spirochetes per tick was unknown in this study and possible variations in this parameter may have influenced the antibody responses of these dogs.
After experimental infection, FlaB appeared to be a robust marker of infection inducing strong antibody responses (Fig. 1E). Although FlaB may be a valuable infection marker under experimental conditions, dogs are usually exposed to various other bacteria expressing flagellin. In canine patient sera, cross-reactivities with other flagellins were described previously (17, 31). Antibodies to FlaB thus should not be considered specific markers for infection with B. burgdorferi in canine patients.
In summary, the multiplex analysis of sera from experimental infection of dogs confirmed that (i) antibodies to OspC are useful markers for early infection with B. burgdorferi, (ii) antibodies to C6 or OspF antigens are developed at similar times after infection, with antibodies to C6 slightly preceding those to OspF in some dogs, and (iii) that antibodies to OspF and C6 are maintained at similar levels during the first 3 months of infection.
Analysis of Lyme QuantC6 ELISA titers in experimentally infected dogs.
Serum samples were also analyzed by SNAP 4Dx tests and QuantC6 ELISA. The SNAP test was negative for all control animals during the course of the study. In infected dogs, the test was negative on day 13 prior to infection and day 21 p.i. in all animals, positive in two out of four dogs per group at day 35 p.i., and positive in all infected dogs from day 49 on (Table 3). Differences between exposures to 25 or 50 ticks were not observed by using the SNAP test. On day 13 prior to infection, the C6 ELISA identified one control dog as being positive for antibodies to C6 (34 U/ml). This sample was negative in all other tests performed in this study. All other samples from control dogs and from infected dogs at day 13 before exposure were negative using the C6 ELISA (Table 3). In tick-exposed groups, antibodies were identified at day 21 p.i. in two out of four (25 ticks) or all dogs (50 ticks). The antibodies to C6 increased until day 49 p.i. and decreased afterwards in both infected groups (Fig. 2). The decrease in antibody means between days 49 and 92 as identified by the C6 ELISA was 81 U/ml and 64 U/ml for 25- and 50-tick exposure groups, respectively. The assay did not result in significant differences in antibodies to C6 between the 25- and 50-tick exposure groups. Individual dog antibody results for the C6 ELISA are shown in Table S3 in the supplemental material. Antibodies to C6 identified by ELISA or SNAP tests are valuable markers to identify infection with B. burgdorferi in dogs (5, 13, 16, 26, 33). Here, the C6 ELISA identified antibodies to infection as early as 21 days p.i., which was similar to antibodies to OspC in the multiplex analysis using the same samples. The SNAP test detected infection at day 35 or 49 p.i. depending on the dog, indicating the improved analytical sensitivity of quantitative tests compared to the qualitative SNAP 4Dx test.
Table 3.
Detection of antibodies to C6 by SNAP4Dx test and Lyme Quant C6 ELISA in sera from experimentally infected dogs
| Assay and group | No. positive/total no. tested by day p.i.a |
||||||
|---|---|---|---|---|---|---|---|
| −13 | 21 | 35 | 49 | 63 | 77 | 92 | |
| SNAP4Dx | |||||||
| Control | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 25 ticks/dog | 0/4 | 0/4 | 2/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| 50 ticks/dog | 0/4 | 0/4 | 2/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| Lyme Quant C6 ELISA | |||||||
| Control | 1/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 25 ticks/dog | 0/4 | 2/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| 50 ticks/dog | 0/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
The time points when antibodies to C6 were first detectable by each of the assays are shown in boldface.
Fig 2.
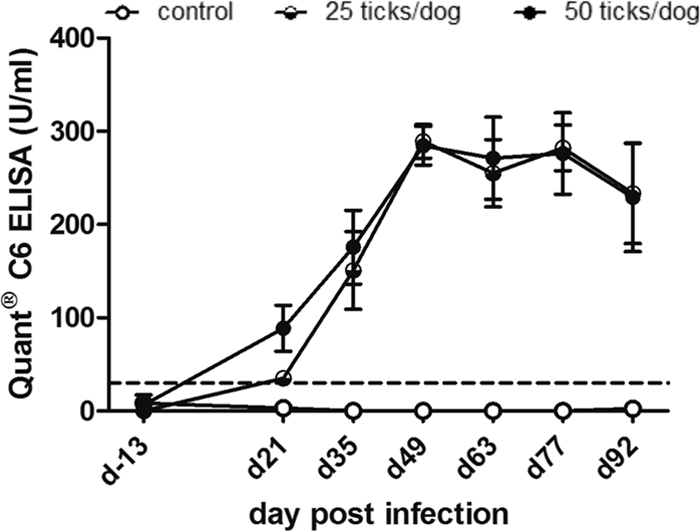
Antibody titers in sera from experimentally infected dogs identified by Lyme Quant C6 ELISA. Serum samples were obtained on day 13 prior to infection (d-13) and on days 21, 35, 49, 63, 77, and 92 p.i. The graph shows mean values and standard errors for C6 antibody values of the control group and two experimentally infected groups by exposure to 25 or 50 ticks per dog. The dotted line indicates the positive cutoff value of the C6 ELISA (>30 U/ml).
The Quant C6 ELISA is frequently used to make treatment decisions in dogs and to monitor treatment success by the quantification of antibodies (http://www.idexx.com/pubwebresources/pdf/en_us/smallanimal/reference-laboratories/lyme-quant-c6-white-paper.pdf). In an experimental study, a decrease of C6 antibodies to baseline values was reported at 6 months after antibiotic treatment with ceftriaxone (26). The antibiotic treatment of C6 ELISA-positive client-owned dogs showed a drop in antibody values of 68 to 83% at 12 months after treatment (13). In the same study, a decline in C6 antibody units of up to 31% was observed in C6 antibody-positive, untreated control dogs. Here, we observed a decline of antibody values of 23 to 29% in the two infected groups between days 49 and 92 p.i. in the absence of any treatment. This finding is somewhat unexpected, considering the persistent nature of infection with B. burgdorferi and the previously reported constant increase of antibodies during the first 3 months p.i. using whole-cell lysate ELISA (2, 34). It also was not confirmed by using two C6 peptides in multiplex analysis. In the same serum samples, both C6 multiplex values increased constantly until day 92 p.i. The drop in the C6 ELISA values from day 63 on may reflect the limitations of this ELISA compared to multiplex technology. Multiplex assays have a much wider dynamic range. Samples with antibody values between 0.001 and 1,000 ng/ml will fall into the linear range of the assay and allow the accurate quantification of the samples in a wide range of antibody concentrations (22, 27, 39, 40). ELISAs have a much smaller linear quantification range (about 0.1 to 500 ng/ml). Thus, samples will more frequently fall into the lower and upper plateau ranges, i.e., quantification is not accurate. Thus, one explanation for the decreasing values observed in Fig. 2 is that the samples have challenged the upper plateau of the C6 ELISA, resulting in inaccurate antibody values. However, the technical details about the C6 ELISA are unknown, and it is difficult to speculate about the reasons for its performance.
Comparison of antibodies to OspC, OspF, and C6 in canine patient serum.
Previous long-term experimental infection studies confirmed that antibody titers to B. burgdorferi whole-cell lysate (2, 34) or to C6 (16, 26) are maintained beyond 18 months p.i. The antibody kinetics obtained here for antibodies to OspF and C6 during experimental infection suggested that antibodies to both antigens are maintained and increase further beyond the 3-month interval of the study. Our data also suggested that antibodies to OspC decrease after 7 to 11 weeks of infection and eventually become undetectable in later infection stages. In a previous report using an ELISA, antibodies to OspF and C6 (39 kDa) were found to be the most important indicators of natural exposure to B. burgdorferi in dogs, while antibodies to OspC were identified in less than 10% of the clinical samples, leading to the conclusion that OspC is a less useful marker for Lyme disease in dogs (18, 19). The dogs investigated in these two reports lived in an area in which the disease is endemic and had a history of clinical signs of Lyme disease and positive antibody titers by ELISA. Thus, the majority of these samples likely represented chronic cases.
To compare antibodies to OspF and C6 as long-lasting and robust markers for chronic infection with B. burgdorferi, and to confirm that antibodies to OspC can become undetectable in late infection stages, we analyzed sera from 125 canine patients. For these samples, the Spearman's rank correlations for antibodies to OspF and C6 measured by multiplex analysis was 0.85 (P < 0.0001). Identical (either negative or positive) multiplex results for antibodies to OspF and C6 were found in 115 of these samples, confirming the high level of agreement between antibodies to OspF and C6 as markers for infection with B. burgdorferi. Out of these 115 samples, 51 sera were negative for antibodies to C6 and OspF, and 64 sera were positive for both markers. All but 1 out of 51 negative samples also were negative for antibodies to OspC, confirming that most of these dogs were not infected with B. burgdorferi. The single OspC-positive sample likely represented a dog 2 to 3 weeks after infection, before antibodies to C6 and OspF developed. The 64 sera with antibodies to OspF and C6 clearly identified dogs that were previously infected with B. burgdorferi. Disagreement between OspF and C6 results was found for nine sera that were positive for antibodies to C6 and negative for those to OspF and one serum that showed the opposite result. Out of the nine C6+/OspF− samples, six had positive antibody values to OspC (Fig. 3A, bars 1 to 6), suggesting an early infection stage of about 3 to 7 weeks p.i. The remaining four samples (three C6+/OspF− and one C6−/OspF+) overall had very low positive antibody values for C6 and OspF, respectively, and were negative for OspC. For these samples, infection with B. burgdorferi remained questionable because of overall inconclusive antibody results and only one low positive value that also could represent the high background value of the respective serum.
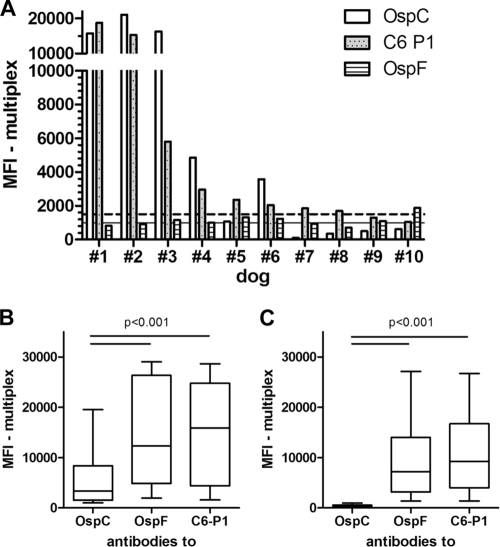
Fig 3.
Comparison of antibodies to OspC, OspF, and C6 obtained from canine patient sera. Antibodies were determined by multiplex analysis. Canine patient sera were submitted to the Animal Health Diagnostic Center at Cornell University between July 2008 and January 2009. (A) OspC antibody values of nine dogs (#1 to 9) with a C6+/OspF− and one dog (#10) with C6−/OspF+ detection pattern in serum. The antibodies to OspC, OspF, and C6 P1 were analyzed in the multiplex assay. These 10 samples, with disagreement on the Lyme antibody status interpretation based on C6 and OspF, were obtained from a total of 125 canine patient sera. For the remaining 115 samples, the multiplex assay interpretation based on OspF and C6 was in agreement. The C6 values decrease from dog 1 to 10. The horizontal lines show the positive OspC (intact line) or C6 and OspF (dotted line) cutoff values. (B) Sera from 21 dogs with antibodies to all three infection markers. (C) Sera from 43 dogs with antibodies to OspF and C6 that were negative for antibodies to OspC. The horizontal lines in plots B and C indicate significant differences between the antigens. The P value applies to both lines in each plot.
Further analysis of the 64 canine patient sera that were positive for antibodies to OspF and C6 identified 23 samples that also were positive for antibodies to OspC (Fig. 3B), while 41 sera were negative for OspC antibodies (Fig. 3C). In the 23 samples that were positive for all three antigens, OspC antibody values were significantly decreased compared to values obtained for antibodies to OspF and C6 (P < 0.001). In agreement with the antibody pattern that developed in experimentally infected dogs during the first 3 months after infection, sera from canine patients that were positive for antibodies to OspC, OspF, and C6 likely represented dogs that were infected several weeks to a few months ago. Alternatively, samples with antibodies to these three antigens also could originate from chronically infected dogs that were recently reinfected with B. burgdorferi, resulting in a recent response to OspC on top of previously existing antibodies to OspF and C6.
The results also confirmed that antibodies to OspC are not detectable in many canine patient sera with robust OspF and C6 titers and suggested that these patterns are derived from long-term, chronic infection with B. burgdorferi. Thus, the simultaneous appearance of antibodies to OspC, OspF, and C6 is likely characteristic for an intermediate infection stage, and sera with antibodies to OspF and C6 only suggest a late infection stage because antibodies to OspC disappear from the circulation over time. The latter observation is in agreement with the downregulation of OspC on the spirochete surface in the mammalian host (7, 25, 36). The short availability of OspC directly after transmission for the induction of host immunity offers an explanation for the early increase of antibodies to OspC and also for the decline of OspC antibodies over time because of the missing antigenic stimulus. Figure 4 summarizes our results from the experimental infection study and the analysis of canine patient sera with unknown infection history. The projected data for OspF and C6 antibodies after 3 months of infection closely resemble antibody titers to B. burgdorferi that were described in previous long-term infection studies after the experimental infection of untreated dogs (2, 16, 26, 34).
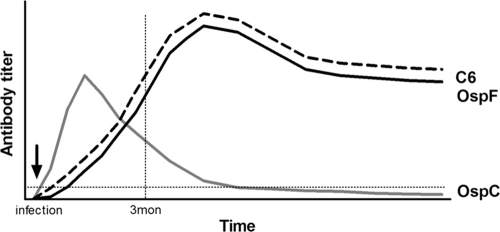
Fig 4.
Summary of experimentally confirmed and projected values based on canine patient sera for antibodies to OspC, OspF, and C6 antigens of B. burgdorferi during early and late infection. The data were obtained by multiplex analysis. The lines for the first 3 months after infection are based on the multiplex results from experimentally infected dogs. After 3 months the lines are projected from the data obtained from patient serum. The horizontal dotted line shows the cutoff value for the multiplex assay. The vertical dotted line indicates 3 months after infection.
In conclusion, our data, obtained from experimentally infected dogs and canine patient sera, confirmed that antibodies to OspC are a sensitive marker for early infection with B. burgdorferi in dogs and showed that antibodies to OspF and C6 are highly comparable as robust markers for infection with B. burgdorferi and the diagnosis of Lyme disease in clinically affected canine patients from areas in which it is endemic. The canine Lyme multiplex assay can distinguish between these antibodies and has a broad quantification range, providing the increased accuracy of antibody detection during infection. The assay allows the simultaneous analysis of antibodies to multiple infection markers that are indicative of very early (a few weeks; OspC+ only), intermediate (2 to 5 months; OspC+/OspF+/C6+), or late infection (>5 months; OspC−/OspF+/C6+). These advantages of the canine Lyme multiplex assay allow an interpretation of the stage of infection that cannot be provided by other assays and is beneficial for the diagnosis, prognosis, and treatment of Lyme disease in dogs.
Supplementary Material
ACKNOWLEDGMENTS
The multiplex assay development and the analysis of serum samples were funded by the Method Development Funds of the Animal Health Diagnostic Center at Cornell University. The experimental infection study, including the SNAP 4Dx and Lyme Quant C6 ELISA, was funded by Pfizer Animal Health.
We acknowledge the expert contributions to this study of Nicole Honsberger, John Johnson, and the animal research services team at Pfizer Animal Health. We also thank T. N. Mather (University of Rhode Island) for collecting the ticks and for determining tick infection levels.
H.F., A.R., H.N.E., C.E., and R.M. declare that they have no competing financial interests in the context of this article. B.W. has submitted a patent application entitled “Methods for diagnosing Lyme disease” that uses technology described in the manuscript. This study has been performed as a collaborative research project between P.M. at Pfizer Animal Health and B.W. at Cornell University. There were no fees or financial support exchanged between the collaborators. D.G.-T. and P.M. work for Pfizer Animal Health, which does not have a diagnostic division.
B. Wagner developed the multiplex evaluation approach, performed some of the data analysis, interpreted the data, and drafted the manuscript. H. Freer and A. Rollins performed the multiplex analysis. H. N. Erb was involved in the statistical analysis and revising the manuscript. C. Earnhardt performed the B. burgdorferi DNA extraction and the FlaB PCR. R. Marconi provided scientific input on DNA isolation and PCR. P. Meeus had oversight of the experimental infection study design and contributed to critically revising the manuscript.
Footnotes
Published ahead of print 15 February 2012
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Akin E, McHugh GL, Flavell RA, Fikrig E, Steere AC. 1999. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67: 173– 181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel MJG, et al. 1993. Experimental Lyme disease in dogs produces arthritis and persistent infection. J. Infect. Dis. 167: 651– 664 [DOI] [PubMed] [Google Scholar]
- 3.Chang YF, et al. 1995. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect. Immun. 63: 3543– 3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conlon JA, Mather TN, Tanner P, Gallo G, Jacobson RH. 2000. Efficacy of a nonadjuvanted, outer surface protein A, recombinant vaccine in dogs after challenge by ticks naturally infected with Borrelia burgdorferi. Vet. Ther. 1: 96– 107 [PubMed] [Google Scholar]
- 5.Duncan AW, Correa MT, Levine JF, Breitschwerdt EB. 2005. The dog as a sentinel for human infection: prevalence of B. burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonot. Dis. 5: 101– 109 [DOI] [PubMed] [Google Scholar]
- 6.Fikrig E, Barthold SW, Kantor FS, Flavell RA. 1990. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250: 553– 556 [DOI] [PubMed] [Google Scholar]
- 7.Grimm D, et al. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101: 3142– 3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerra MA, Walker ED, Kitron U. 2000. Quantitative approach for the serodiagnosis of canine Lyme disease by the immunoblot procedure. J. Clin. Microbiol. 38: 2628– 2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovius JWR, van Dam AP, Fikrig E. 2007. Tick-host-pathogen interaction in Lyme borreliosis. Trends Parasitol. 23: 434– 438 [DOI] [PubMed] [Google Scholar]
- 10.Hutton TA, et al. 2008. Search for Borrelia burgdorferi in kidneys of dogs with suspected “Lyme nephritis.” J. Vet. Intern. Med. 22: 860– 865 [DOI] [PubMed] [Google Scholar]
- 11.Jacobson RH, Chang YF, Shin SJ. 1996. Lyme disease: laboratory diagnosis of infected and vaccinated symptomatic dogs. Semin. Vet. Med. Surg. (Small Anim.) 11: 172– 182 [DOI] [PubMed] [Google Scholar]
- 12.Levy SA, Magnarelli LA. 1992. Relationship between development of antibodies to Borrelia burgdorferi in dogs and the subsequent development of limb/joint borreliosis. J. Am. Vet. Med. Assoc. 200: 344– 347 [PubMed] [Google Scholar]
- 13.Levy SA, et al. 2008. Quantitative measurement of C6 antibody following antibiotic treatment of Borrelia burgdorferi antibody-positive nonclinical dogs. Clin. Vaccine Immunol. 15: 115– 119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang FT, et al. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163: 5566– 5573 [PubMed] [Google Scholar]
- 15.Liang FT, Philipp MT. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect. Immun. 67: 6702– 6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang FT, Jacobson RH, Straubinger RK, Grooters A, Phillipp MT. 2000. Characterization of a Borrelia burgdorferi VlsE invariable region useful in canine Lyme disease serodiagnosis by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38: 4160– 4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenmayer J, Weber M, Bryant J, Marquez E, Onderdonk A. 1990. Comparison of indirect immunofluorescent-antibody assay, enzyme-linked immunosorbent assay, and Western immunoblot for the diagnosis of Lyme disease in dogs. J. Clin. Microbiol. 28: 92– 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnarelli LA, Flavell RA, Padula SJ, Anderson JF, Fikrig E. 1997. Serologic diagnosis of canine and equine borreliosis: use of recombinant antigens in enzyme-linked immunosorbent assays. J. Clin. Microbiol. 35: 169– 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnarelli LA, et al. 2001. Reactivity of dog sera to whole-cell or recombinant antigens of Borrelia burgdorferi by ELISA and immunoblot analysis. J. Med. Microbiol. 50: 889– 895 [DOI] [PubMed] [Google Scholar]
- 20.Mather TN, Telford SR, III, Moore SI, Spielman A. 1990. Borrelia burgdorferi and Babesia microti: efficiency of transmission from reservoirs to vector ticks (Ixodes dammini). Exp. Parasitol. 70: 55– 61 [DOI] [PubMed] [Google Scholar]
- 21.McDowell JV, Sung SY, Price G, Marconi RT. 2001. Demonstration of the genetic stability and temporal expression of select members of the Lyme disease spirochete OspF protein family during infection in mice. Infect. Immun. 69: 4831– 4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan E, et al. 2004. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin. Immunol. 110: 252– 266 [DOI] [PubMed] [Google Scholar]
- 23.Pal U, et al. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106: 561– 569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal U, Fikrig E. 2003. Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microb. Infect. 5: 659– 666 [DOI] [PubMed] [Google Scholar]
- 25.Pal U, et al. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 113: 220– 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philipp MT, et al. 2001. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J. Infect. Dis. 184: 870– 878 [DOI] [PubMed] [Google Scholar]
- 27.Prabhakar U, Eirikis E, Miller BE, Davis HM. 2005. Multiplexed cytokine sandwich immunoassays: clinical applications. Methods Mol. Med. 114: 223– 232 [DOI] [PubMed] [Google Scholar]
- 28.Ramamoorthi N, et al. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436: 573– 577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaible UE, et al. 1990. Monoclonal antibodies specific for outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (SCID) mice. Proc. Natl. Acad. Sci. U. S. A. 87: 3768– 3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. 1995. Induction of outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. U. S. A. 92: 2909– 2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin SJ, et al. 1993. Cross-reactivity between B. burgdorferi and other spirochetes affects specificity of serotests for detection of antibodies to the Lyme disease agent in dogs. Vet. Microbiol. 36: 161– 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steere AC. 2001. Lyme disease. N. Engl. J. Med. 345: 115– 125 [DOI] [PubMed] [Google Scholar]
- 33.Stone EG, Lacombe EH, Rand PW. 2005. Antibody testing and Lyme disease risk. Emerging Infect. Dis. 11: 722– 724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straubinger RK, Straubinger AF, Summers BA, Jacobson RH. 2000. Status of Borrelia burgdorferi infection after antibiotic treatment and the effects of corticosteroids: an experimental study. J. Infect. Dis. 181: 1069– 1081 [DOI] [PubMed] [Google Scholar]
- 35.Summers BA, et al. 2005. Histopathological studies of experimental Lyme disease in the dog. J. Comp. Pathol. 133: 1– 13 [DOI] [PubMed] [Google Scholar]
- 36.Templeton TJ. 2004. Borrelia outer membrane surface proteins and transmission through the tick. J. Exp. Med. 199: 603– 606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Töpfer KH, Straubinger RK. 2007. Characterization of the humoral immune response in dogs after vaccination against Lyme borreliosis agent. A study with five commercial vaccines using two different vaccination schedules. Vaccine 25: 314– 326 [DOI] [PubMed] [Google Scholar]
- 38.Wagner B, Freer HH. 2009. Development of a bead-based multiplex assay for simultaneous quantification of cytokines in horses. Vet. Immunol. Immunopathol. 127: 242– 248 [DOI] [PubMed] [Google Scholar]
- 39.Wagner B, Freer H, Rollins A, Erb HN. 2011. A fluorescent bead-based multiplex assay for the simultaneous detection of antibodies to B. burgdorferi outer surface proteins in canine serum. Vet. Immunol. Immunopathol. 140: 190– 198 [DOI] [PubMed] [Google Scholar]
- 40.Wagner B, et al. 2011. Development of a multiplex assay for detection of antibodies to Borrelia burgdorferi in horses and its validation using Bayesian and conventional statistical methods. Vet. Immunol. Immunopathol. 144: 374– 381 [DOI] [PubMed] [Google Scholar]
- 41.Wieneke CA, et al. 2000. Evaluation of whole-cell and OspC enzyme-linked immunosorbent assays for discrimination of early lyme borreliosis from OspA vaccination. J. Clin. Microbiol. 38: 313– 317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wikle RE, Fretwell B, Jarecki M, Jarecki-Black JC. 2006. Canine Lyme disease: one-year duration of immunity elicited with a canine OspA monovalent Lyme vaccine. Intern. J. Appl. Res. Vet. Med. 4: 23– 28 [Google Scholar]
- 43.Wittenbrink MM, Failing K, Krauss H. 1996. Enzyme-linked immunosorbent assay and immunoblot analysis for detection of antibodies to Borrelia burgdorferi in dogs. The impact of serum absorption with homologous and heterologous bacteria. Vet. Microbiol. 48: 257– 268 [DOI] [PubMed] [Google Scholar]
- 44.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199: 641– 648 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.