Abstract
Porcine reproductive and respiratory syndrome (PRRS) is one of the most devastating and costly diseases to the swine industry worldwide. Overall, the adaptive immune response to PRRS virus (PRRSV) is weak, which results in delayed elimination of virus from the host and inferior vaccine protection. PRRSV has been shown to induce a meager alpha interferon (IFN-α) response, and we hypothesized that elevated IFN-α levels early in infection would shorten the induction time and increase elements of the adaptive immune response. To test this, we measured both antibody and cell-mediated immunity in pigs after the administration of a nonreplicating human adenovirus type 5 vector expressing porcine IFN-α (Ad5–pIFN-α) at the time of PRRSV infection and compared the results to those for pigs infected with PRRSV alone. Viremia was delayed, and there was a decrease in viral load in the sera of pigs administered the Ad5–pIFN-α. Although seroconversion was slightly delayed in pigs receiving Ad5–pIFN-α, probably due to the early reduction in viral replication, little difference in the overall or neutralizing antibody response was seen. However, there was an increase in the number of virus-specific IFN-γ-secreting cells detected in the pigs receiving Ad5–pIFN-α, as well as an altered cytokine profile in the lung at 14 days postinfection, indicating that the presence of IFN-α at the time of infection can alter innate and adaptive immune responses to PRRSV.
INTRODUCTION
Porcine reproductive and respiratory syndrome virus (PRRSV) is a widely disseminated virus of swine that causes interstitial pneumonia and abortions and late-term fetal death in sows (7, 30). PRRSV is a member of the Arteriviridae family (positive-sense single-stranded RNA) and primarily infects cells of the monocyte/macrophage lineage (24). Infection with PRRSV is characterized by prolonged viral persistence, and current vaccines fail to provide disease control, especially against genetically unrelated strains (38). The recent emergence of highly pathogenic strains of PRRSV in Asia highlights the importance of finding methods to control PRRSV disease and spread (15, 35, 36).
In general, both the innate and adaptive immune responses to PRRSV are suppressed. Compared to other viruses that infect the respiratory epithelial cells, such as swine influenza virus or porcine respiratory coronavirus, PRRSV appears to induce only modest levels of alpha interferon (IFN-α) and proinflammatory cytokines (1, 14, 18, 25, 37). Additionally, the host response following PRRSV infection has been characterized as both ineffective and delayed. Although nonneutralizing antibodies are rapidly produced following infection, there is a deficiency in neutralizing antibody production (40). Cell-mediated immune responses, typically measured by increases in PRRSV-specific IFN-γ-producing cells, can take 4 to 8 weeks to develop (4, 16, 22). Several groups have reported increased interleukin-10 (IL-10) production in response to PRRSV infection and suggested that this may be immunosuppressive, since IL-10 has been shown to suppress antigen-presenting cell activities, such as processing and presenting antigen, and IL-1, IL-12, IL-18, tumor necrosis factor alpha (TNF-α), and type I IFN expression (8, 10, 26, 33, 34).
Type I interferons, such as IFN-α and IFN-β, have an important role in the innate and adaptive immune response. They contribute to innate antiviral immunity by promoting production of antiviral mediators such as PKR (double-stranded RNA-dependent protein kinase) and Mx (myxovirus resistant; IFN-inducible GTPase) and elicit NK cell activity for the killing of virus-infected cells (2, 3, 13, 27). IFN-α and -β also play a role in the adaptive immune response by inducing both the maturation of dendritic cells into professional antigen-presenting cells and macrophage development and maturation and, along with IL-6, promoting B cell differentiation into plasma cells (5, 11, 21). Several mechanisms as to how PRRSV inhibits type I IFN production have been proposed, and multiple mechanisms may apply. PRRSV has been shown to inhibit double-stranded RNA activation of interferon regulatory factor 3 (IRF3) via inactivation of IFN-β promoter stimulator 1 (IPS-1), an adaptor molecule in the retinoic acid-inducible gene 1 (RIG-1) pathway (20). Others have proposed that PRRSV interferes with modification of IκB through either nsp2 ovarian tumor domain-mediated inhibition of polyubiquitination or nsp1α-mediated inhibition of phosphorylation, ultimately leading to impairment of NF-κB activity (31, 32).
In a previous report, we showed, using a nonreplicating adenovirus type 5 (Ad5) vector to deliver porcine IFN-α (pINF-α) to the pig, that increased levels of IFN-α at the time of challenge delays PRRSV viremia and lessens the severity of disease (6). The goal of this study was to test the effect of elevated IFN-α early in infection on the timing and quality of the adaptive immune response to PRRSV. Because there is little IFN-α produced during PRRSV infection and IFN-α plays a role in the development of the adaptive immune response as well as the innate immune response, we hypothesized that this might be one reason that there is an inadequate adaptive response to the virus. To test this hypothesis, we followed aspects of the adaptive immune response, both antibody- and cell-mediated immunity, in pigs simultaneously administered Ad5–pIFN-α and PRRSV and compared them to the response in pigs exposed only to PRRSV.
MATERIALS AND METHODS
Experimental design and viruses.
A recombinant, replication-defective human adenovirus type 5 was used to deliver porcine IFN-α (Ad5–pIFN-α) as previously described (6). Twenty-eight, 6-week-old pigs were divided into 3 groups of 9 to 10 pigs each and given the following treatments: group 1, Ad5–pIFN-α but no PRRSV; group 2, Ad5–pIFN-α and PRRSV; and group 3, Ad5 not expressing pIFN-α (Ad5-null) and PRRSV. On day 0 of the experiment, pigs were given 5 × 109 focus-forming units (FFU) of the respective Ad5 constructs intramuscularly in the neck, and pigs in groups 2 and 3 were challenged with 104 median cell culture infectious doses (CCID50s) of the SDSU73 strain of PRRSV intranasally, while the pigs in group 1 were mock challenged with medium from noninfected cell cultures. Blood samples were collected on days 0 to 7, 9, 11, 13 or 14, and 16 and then weekly from days 21 through 56. Four to five pigs from each group were euthanized 14 days after challenge with PRRSV, and the other five pigs from each group were euthanized 56 days after challenge with PRRSV.
IFN-α ELISA.
IFN-α levels in the sera were determined by enzyme-linked immunosorbent assay (ELISA) using monoclonal antibody (MAb) F17 and MAb K9 conjugated to horseradish peroxidase (PBL Interferon Source) as previously described (9, 14). Briefly, 96-well Immulon-2 plates were coated with 3 μg F17 MAb. Samples set up in duplicate were added to each well, and plates were incubated and washed prior to the addition of peroxidase-conjugated K9 (1:10,000). Plates were again washed, tetramethylbenzidine substrate solution was added to each well, and the optical density was measured at 450 nm by an ELISA plate reader. Quantified recombinant porcine IFN-α was used as a standard, and IFN-α concentrations were calculated on the basis of a standard curve.
PRRSV isolation and detection.
Viremia was measured by both virus isolation and real-time reverse transcription-PCR (RT-PCR). Virus isolation was performed by adding 100 μl of serum to 1 well of a 24-well plate containing a monolayer of MARC-145 cells in 1 ml of cell culture medium. After 2 h, medium in the well was removed and replaced with fresh medium. Each well was examined for cytopathic effect (CPE) and assessed as positive or negative after 1 week of culture. A real-time RT-PCR assay for PRRSV was used to quantify viral RNA (vRNA) in the serum of pigs as previously described (18). Briefly, viral RNA was isolated from 140 μl of each serum sample using a commercial kit (viral RNA isolation kit; Qiagen), and the viral RNA was eluted into 60 μl. Eight microliters of the eluted RNA sample was then used with a one-step RT-PCR kit (Qiagen) according to the manufacturer's recommendations. Viral RNA was extracted from a stock of SDSU73 virus, and an 8-point standard curve (1:10 dilutions) was generated and used in the same one-step RT-PCR as RNA extracted from each sample. Results are expressed as the amount of vRNA relative to the standard curve.
PRRSV antibody detection.
Seroconversion to PRRSV was determined by an ELISA kit. ELISA sample-to-positive (S/P) ratios were generated on collected serum samples by performing the HerdCheck PRRS ELISA 2XR (IDEXX Laboratories) according to the manufacturer's instructions.
Virus-neutralizing antibody responses were determined using a fluorescent focus neutralization (FFN) assay as previously described (39). Briefly, 2-fold dilutions of heat-inactivated serum samples were incubated with the homologous virus isolate (SDSU73) prior to infection of MARC-145 cells. Endpoints were reported as the highest serum dilution showing a 90% or greater reduction in the number of fluorescent foci.
ELISpot assays.
To assess antigen-specific IFN-γ responses, approximately 8 ml of blood was collected at the time points indicated into BD Vacutainer CPT tubes with sodium citrate, and the peripheral blood mononuclear cell (PBMC) fraction was collected according to the manufacturer's recommendations. PBMCs were washed once with RPMI 1640 (Invitrogen), passed through a 40-μm-pore-size screen filter, washed a second time, and enumerated. An enzyme-linked immunosorbent spot (ELISpot) assay for IFN-γ-secreting cells was performed as previously described with slight modifications (42). Briefly, 96-well membrane plates (MAIPS4510) were prewetted with 35% ethanol, washed, and coated overnight at 4°C with 6 μg/ml anti-pIFN-γ (P2G10; BD Biosciences). The next day, the plate was washed and blocked with complete RPMI (RPMI 1640, 10% fetal bovine serum, 2 mM l-glutamine, 1% antibiotic/antimycotic [Invitrogen], and gentamicin) for 2 h at 37°C. The blocking medium was removed, and 5 × 105 PBMCs were plated per well. Appropriate wells were treated with live homologous PRRSV at a multiplicity of infection of 1 (SDSU73), control MARC medium, or phytohemagglutinin (PHA) added to a final concentration of 10 μg/ml (each treatment in triplicate), and the plates were incubated for 18 h at 37°C in 5% CO2. After 18 h, plates were washed and incubated with anti-IFN-γ detection antibody (0.5 μg/ml; P2C11; BD Biosciences) for 2 h at 37°C. Plates were washed and developed using an ELISpot blue color module (R&D Systems) according to the manufacturer's recommendations. Plates were scanned, and spots were enumerated using a CTL-ImmunoSpot S5 UV analyzer and ImmunoSpot software. The number of PBMC samples analyzed for each treatment group ranged from 4 to 5.
Multiplex cytokine assay.
Approximately 5 ml of bronchoalveolar lavage fluid (BALF) was centrifuged at 300 × g for 10 min at 4°C to pellet cellular debris. The cell-free BALF was tested for levels of IL-1β, IL-8, IL-6, TNF-α, IL-2, IL-4, IL-12p70, IFN-γ, and IL-10 by a SearchLight multiplex ELISA performed according to the manufacturer's recommendations (Aushon Biosystems, Billerica, MA). The average of duplicate wells for each sample was used for statistical analysis.
Statistical analysis.
Viremia, ELISpot assay, and antibody response data were analyzed using a mixed linear model for repeated measures (SAS, version 9.1, for Windows; SAS Institute Inc.). Linear combinations of the least-squares sample estimates were used in a priori contrasts, after testing for either a significant (P < 0.05) treatment effect or an interaction effect between time point and experimental treatment. Comparisons between treatment groups were made at each time point using a 5% level of significance (P < 0.05) to assess statistical differences. Cytokine data were compared using a one-way analysis of variance with a Tukey's posttest at a significance level of P of <0.05.
RESULTS
Serum IFN-α levels.
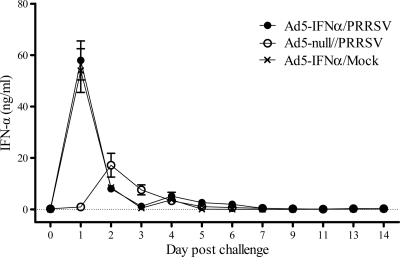
An IFN-α ELISA was run on serum samples to determine the magnitude and duration of IFN-α production in response to administration of the Ad5–pIFN-α and/or PRRSV infection. No IFN-α was detected in the serum of any of the pigs on day 0. Pigs in both groups given the Ad5–pIFN-α had similar levels of IFN-α in their serum, which were high on day 1 but declined rapidly after that (Fig. 1). IFN-α levels in the pigs given Ad5-null and challenged with PRRSV peaked on day 2 but were much lower than peak levels induced by the Ad5–pIFN-α (Fig. 1).
Fig 1.
Serum levels of IFN-α after inoculation with Ad5–IFN-α or Ad5-null and PRRSV or mock challenge with medium from noninfected cell culture. Data are expressed as the mean ± SEM of 9 pigs in each of the Ad5–IFN-α/PRRSV and Ad5–IFN-α/mock groups and 10 pigs in the Ad5-null/PRRSV group through day 9; subsequently, the mean is based on 4 to 5 pigs per group.
Viremia and viral RNA loads.
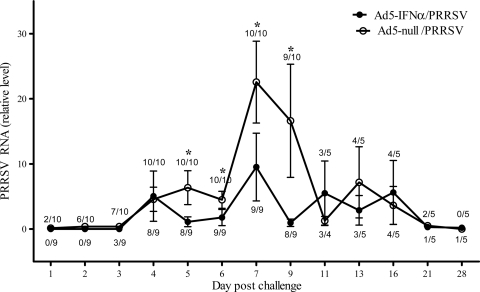
Both virus isolation and quantitative PCR were used to determine the extent of viremia as a measure of the degree of virus replication in the pigs. PRRSV was not isolated from the serum of any mock-infected pig or from any sample from PRRSV-infected pigs taken on day 0. PRRSV was detected by virus isolation in the serum of pigs receiving the Ad5-null earlier than in the serum of pigs receiving the Ad5–pIFN-α, indicating that the presence of IFN-α delayed viremia (Fig. 2). Virus was isolated from the sera of pigs in the Ad5-null/PRRSV group as early as day 1 postinfection, whereas the earliest isolation of virus from the sera of pigs in the group receiving Ad5–pIFN-α was day 3. Differences in virus isolation between the two experimental groups were pronounced at days 2 and 3 postinfection, when the isolation of PRRSV from serum samples was >60% in the Ad5-null/PRRSV group. In addition, the level of PRRSV in the serum detected by quantitative PCR on days 5 through 9 was also significantly lower for pigs that received the Ad5–pIFN-α (Fig. 2), even though the incidence of detecting PRRSV in serum during this time was similar between the 2 groups. At later time points postinfection, there was no significant difference in the amount of vRNA detected between the 2 challenged groups. On day 14 postinfection, PRRSV was isolated from the lung lavage fluid of all PRRSV-challenged pigs, regardless of Ad5–pIFN-α treatment (data not shown).
Fig 2.
Levels of PRRSV vRNA in serum detected by real-time PCR after inoculation with Ad5–IFN-α or Ad5-null and PRRSV or mock challenge with medium from noninfected cell culture. The data are expressed as the mean ± SEM of 9 pigs in the Ad5–IFN-α/PRRSV group and 10 pigs in the Ad5-null/PRRSV group through day 9 postinfection; subsequently, the mean is based on 4 to 5 pigs per group. The values above each data point on the graph refer to the number of positive samples based on virus isolation per total number of samples for the Ad5-null/PRRSV group, and the values below each data point refer to the same data for the Ad5–IFN-α/PRRSV group. No PRRSV vRNA was detected, nor was PRRSV isolated from any of the samples collected from pigs in the Ad5–IFN-α/mock group. *, statistically significant differences at P < 0.05.
Antibody response.
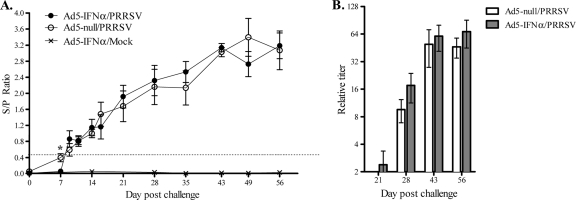
An ELISA was run on serum samples to determine when pigs seroconverted and the total antibody response to PRRSV. On the basis of the ELISA results, pigs given Ad5–pIFN-α and then challenged with PRRSV seroconverted to PRRSV later than pigs challenged with PRRSV given Ad5-null (Fig. 3A). Using the recommended cutoff value of an S/P ratio of 0.400, the pigs in the group given Ad5-null, on average, seroconverted to PRRSV by day 7 postinfection, and the S/P ratio on this day was significantly higher for these pigs than for the pigs in the group given Ad5–pIFN-α, none of which had seroconverted by day 7. After day 7, there was no significant difference in the antibody levels between the groups challenged with PRRSV. None of the pigs in the noninfected group seroconverted to PRRSV. The IDEXX ELISA is useful for measuring seroconversion, but it does not specifically measure neutralizing antibody levels. To better evaluate neutralizing antibody responses, an FFN assay was performed. While there was a trend toward increased neutralizing antibody levels and a more rapid appearance of neutralizing antibody (day 21) in the group given Ad5–pIFN-α, the differences were not statistically significant (Fig. 3b).
Fig 3.
Antibody response to PRRSV after inoculation with Ad5–IFN-α or Ad5-null and PRRSV or mock challenge with medium from noninfected cell culture. Serum antibody response to PRRSV was evaluated by determination of the S/P ratio using IDEXX ELISA (A) or FFN assay (B). (A) Data are expressed as the mean ± SEM of 9 pigs in each of the Ad5–IFN-α/PRRSV and Ad5–IFN-α/mock groups and 10 pigs in the Ad5-null/PRRSV group until 9 days postinfection; subsequently, the mean is based on 4 to 5 pigs per group. (B) Data are expressed as the mean ± SEM for 5 pigs in both the Ad5–IFN-α/PRRSV and Ad5-null/PRRSV groups. FFN titers in the Ad5–IFN-α/mock group were negative at every time point tested. *, statistically significant differences at P < 0.05.
Antigen-specific IFN-γ response.
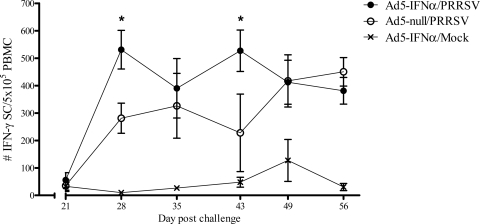
To better understand the effects of Ad5–pIFN-α administration on the cellular immune response to PRRSV, an IFN-γ ELISpot assay was used to enumerate the number of antigen-specific IFN-γ-secreting cells (ISCs) circulating in the peripheral blood. It was not until 28 days following challenge that PRRSV-specific ISCs were detected, regardless of the administration of Ad5–pIFN-α. However, on day 28 there were significantly more ISCs in pigs that received the Ad5–pIFN-α. Overall, higher numbers of ISCs were detected in the peripheral blood cells of pigs given Ad5–pIFN-α from days 28 through 43, and these numbers were significantly higher on days 28 and 43 (Fig. 4).
Fig 4.
Number of PRRSV-specific IFN-γ-secreting cells (SC) after inoculation with Ad5–IFN-α or Ad5-null and PRRSV or mock challenge with noninfected cell culture and medium. Data are expressed as the mean ± SEM for 5 pigs in all the groups. *, statistically significant differences at P < 0.05.
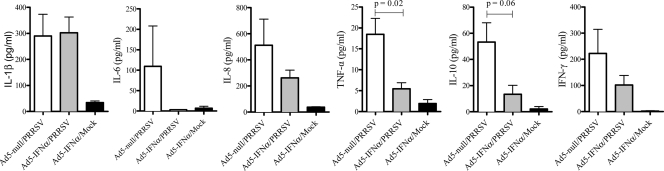
Cytokines in BALF.
A subset of pigs in each group was euthanized on day 14 postinfection to evaluate local host responses in the lung. In general, a trend toward increased levels of IL-1β, IL-8, TNF-α, IL-10, and IFN-γ was detected in the BALF of PRRSV-infected pigs (regardless of Ad5–pIFN-α administration) than that of noninfected pigs (Fig. 5). However, PRRSV-infected pigs that were given Ad5–pIFN-α had lower levels of IL-6, IL-8, TNF-α, IL-10, and IFN-γ than PRRSV-infected pigs given Ad5-null, though the difference was statistically significant only for TNF-α. The remaining cytokine analyzed, IL-1β, was expressed at very similar levels in both groups of PRRSV-infected pigs (Fig. 5). There were no significant differences in the levels of IL-12p70, IL-2, or IL-4 among the 3 groups (data not shown).
Fig 5.
Cytokine levels in BALF 14 days after inoculation with Ad5–IFN-α or Ad5-null and PRRSV or mock challenge with medium from noninfected cell culture. Data are expressed as the mean ± SEM of 4 pigs in the Ad5–IFN-α/PRRSV and Ad5–IFN-α/mock groups and 5 pigs in the Ad5-null/PRRSV group.
DISCUSSION
The pleiotropic cytokine IFN-α is involved in many aspects of the host immune response at both the innate and the adaptive levels. It can exert potent antiviral effects by initiating the production of various antiviral mediators that hinder viral replication and packaging. IFN-α can also act on antigen-presenting cells to enhance their ability to activate lymphocytes through production of cytokines and expression of antigen-presentation molecules. In addition, IFN-α can act directly on T cells to drive maturation from a naïve T cell to an effector cell (12). IFN-α is typically produced in response to a viral infection, but it is well accepted that PRRSV does not induce a strong IFN-α response, which may secondarily affect development of the adaptive immune response. It has been hypothesized that administration of IFN-α, as a recombinant protein or through in vivo expression of the cloned gene, may enhance the host response to PRRSV, aiding in clearance and increased adaptive immunity.
A previous report by our group showed that prophylactic administration of Ad5–pIFN-α to pigs resulted in increased levels of circulating IFN-α for approximately 2 days and this was able to attenuate PRRSV disease severity when given 1 day prior to PRRSV exposure (6). However, effects on development of the antigen-specific immune response were not considered in that first study. The study reported here was extended to 56 days postinfection in order to monitor PRRSV-specific responses. The timing of Ad5–pIFN-α administration was also altered to assess the effectiveness of the construct as a potential adjuvant or metaphylactic when given coincident with potential exposure to PRRSV, for example, when pigs are moved to a new facility and/or mixed with pigs potentially infected with PRRSV.
Similar to the results reported in the prior study, in the current study there was an initial delay in the detection of vRNA in the sera of pigs given the Ad5–pIFN-α construct, concurrent with the time at which serum IFN-α levels were highest (6). Whereas by day 3 postinfection most of the pigs in the previous experiment were viremic whether or not they received IFN-α, the majority of pigs receiving IFN-α in this experiment did not become viremic until day 4. This is likely due to the timing of Ad5–pIFN-α administration. Specifically, Ad5–pIFN-α was given 1 day prior to infection in the previous experiment, as opposed to the same day as PRRSV challenge in this experiment. Unlike the previous experiment, however, exposure to IFN-α did result in a significant decrease in the amount of PRRS vRNA between days 5 and 9 postinfection, although it did not affect the incidence of viremia during the same time period. Thus, while administration of the Ad5–pIFN-α construct did not prevent the occurrence of viremia, it did significantly decrease the amount of vRNA circulating in the blood. Our data demonstrate that a single dose of Ad5–pIFN-α, when administered on the same day as PRRSV challenge, can control early viral replication and viremia and lower peak virus levels found in the serum.
In a prior report, we demonstrated that the onset of viremia in pigs given a low dose of PRRSV is delayed compared to the onset of viremia in pigs receiving a higher dose, although the amount of vRNA subsequently detected in the sera is unaffected (19). This mirrors the delay in viremia observed in our previous experiment giving Ad5–pIFN-α 1 day prior to challenge with PRRSV (6). In the current experiment, not only was there a delay in the onset of viremia but also there were subsequently decreased viral loads in the peripheral blood as well. Thus, it is unlikely that Ad5–pIFN-α alone controlled viral loads in the current experiment because we would have expected vRNA levels to be the same between groups once IFN-α levels returned to normal in the Ad5–pIFN-α group, which was by day 3. Because vRNA levels remained relatively low in the Ad5–pIFN-α group, we speculate that additional control/activation mechanisms were initiated with Ad5–pIFN-α administration. Further work is warranted to understand the innate antiviral response and to define mechanisms of controlling viral load at early time points following Ad5–pIFN-α administration.
By evaluating the adaptive immune responses, our data show that administration of Ad5–pIFN-α on the day of PRRSV challenge can significantly alter the ensuing T cell response but has a less pronounced effect on antibody responses. Seroconversion, as measured by IDEXX ELISA, was delayed in pigs given the Ad5–pIFN-α construct compared to pigs given the Ad5-null construct, presumably due to the delay in viral replication and/or antigen load. However, this was detected only on day 7 postinfection, and there was no significant difference between the groups by day 9 postinfection. Neutralizing antibody titers also did not differ significantly between the two groups, though a trend toward increased neutralization titers on days 21 and 28 in the Ad5–pIFN-α group was appreciated. There was, however, an increase in the number of antigen-specific ISCs in the periphery of pigs given the Ad5–pIFN-α construct. These results are similar to those reported by Meier et al., who coadministered IFN-α with a modified live PRRSV vaccine and found an increase in PRRSV antigen-specific ISCs but no effect on the production of antivirus antibodies (23). IFN-α is known to have effects on antigen-presenting cells but has also been shown to act directly on naïve T cells to drive differentiation into effector T cells (reviewed in reference 12). The contribution of ISCs to PRRSV clearance has not been clearly defined, and it is difficult to assess their contribution in the current study, as viral clearance or control in the sera was apparent on day 21 postinfection but PRRSV ISCs were not detectable in the peripheral blood until day 28 postinfection. Neutralizing antibody titers were detectable in a few pigs in the Ad5–pIFN-α group on day 21, although viral loads were decreasing by this time point. There are several reports suggesting that neutralizing antibodies do not correlate with decreased viremia (29, 41), while some papers suggest that titers must be extremely high to provide protection (17, 28). Since we did not attempt rechallenge, we do not know whether the changes in the immune response to PRRSV seen in the presence of IFN-α would have been more efficacious in preventing clinical signs or inducing viral clearance in pigs reexposed to homologous or heterologous PRRSV. Further studies would be needed to explore this possibility.
The presence of IFN-α at PRRSV challenge altered the cytokine profile observed in the lung lavage fluid at day 14 postinfection. Although IL-1β levels were unchanged, there was a trend toward lower levels of IL-10 and IFN-γ, as well as the proinflammatory cytokines IL-6, IL-8, and TNF-α. Whether the delay in viral replication, lowered viral titers, and/or activation mechanisms initiated by increased levels of IFN-α influenced these differences is unknown, and these results do reflect a snapshot in time. However, PRRSV induction of IL-10 has been postulated to be at least partially responsible for some of its immunosuppressive effects (8, 10, 33, 34). Thus, it is tempting to speculate that the decrease in IL-10 may be associated with the increase in PRRSV-specific ISCs detected in pigs administered the Ad5–pIFN-α.
Overall, we have demonstrated that the administration of pIFN-α at the time of challenge can reduce the severity of PRRSV disease and decrease vRNA loads. However, until dependable correlates of protection for PRRSV are identified, it will continue to be difficult to assess the impact of different therapeutics and/or adjuvants on specific immune parameters. At this time, without rechallenge, we are unable to say for sure whether the increased antigen-specific IFN-γ-secreting cells would actually contribute to protection. While it is necessary to continue to identify factors that control viremia, such as IFN-α, it is also necessary to understand how PRRSV subverts the host immune response to its benefit and identify the mechanisms that the host uses to clear the virus.
ACKNOWLEDGMENTS
We thank Lea Ann Hobbs, Kim Driftmier, Gwen Nordholm, Mike Mullins, Sarah Shore, Sarah Anderson, and Craig Welbon for excellent laboratory technical assistance and Dalene Whitney, Jason Huegel, Brian Pottebaum, and Jason Crabtree for their excellent animal care.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Footnotes
Published ahead of print 1 February 2012
REFERENCES
- 1. Albina E, Carrat C, Charley B. 1998. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 18: 485–490 [DOI] [PubMed] [Google Scholar]
- 2. Asano A, Ko JH, Morozumi T, Hamashima N, Watanabe T. 2002. Polymorphisms and the antiviral property of porcine Mx1 protein. J. Vet. Med. Sci. 64: 1085–1089 [DOI] [PubMed] [Google Scholar]
- 3. Asano A, Kon Y, Agui T. 2004. The mRNA regulation of porcine double-stranded RNA-activated protein kinase gene. J. Vet. Med. Sci. 66: 1523–1528 [DOI] [PubMed] [Google Scholar]
- 4. Bautista EM, Molitor TW. 1997. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 10: 83–94 [DOI] [PubMed] [Google Scholar]
- 5. Belardelli F, Gresser I. 1996. The neglected role of type I interferon in the T-cell response: implications for its clinical use. Immunol. Today 17: 369–372 [DOI] [PubMed] [Google Scholar]
- 6. Brockmeier SL, et al. 2009. Adenovirus-mediated expression of interferon-alpha delays viral replication and reduces disease signs in swine challenged with porcine reproductive and respiratory syndrome virus. Viral Immunol. 22: 173–180 [DOI] [PubMed] [Google Scholar]
- 7. Christianson WT, Kim HS, Yoon IJ, Joo HS. 1992. Transplacental infection of porcine fetuses following experimental challenge inoculation with encephalomyocarditis virus. Am. J. Vet. Res. 53: 44–47 [PubMed] [Google Scholar]
- 8. Chung HK, Chae C. 2003. Expression of interleukin-10 and interleukin-12 in piglets experimentally infected with porcine reproductive and respiratory syndrome virus (PRRSV). J. Comp. Pathol. 129: 205–212 [DOI] [PubMed] [Google Scholar]
- 9. Diaz de Arce H, et al. 1992. A sensitive immunoassay for porcine interferon-alpha. Vet. Immunol. Immunopathol. 30: 319–327 [DOI] [PubMed] [Google Scholar]
- 10. Flores-Mendoza L, Silva-Campa E, Resendiz M, Osorio FA, Hernandez J. 2008. Porcine reproductive and respiratory syndrome virus infects mature porcine dendritic cells and up-regulates interleukin-10 production. Clin. Vaccine Immunol. 15: 720–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallucci S, Lolkema M, Matzinger P. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5: 1249–1255 [DOI] [PubMed] [Google Scholar]
- 12. Huber JP, Farrar JD. 2011. Regulation of effector and memory T-cell functions by type I interferon. Immunology 132: 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katze MG, He Y, Gale M., Jr 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2: 675–687 [DOI] [PubMed] [Google Scholar]
- 14. Lee SM, Schommer SK, Kleiboeker SB. 2004. Porcine reproductive and respiratory syndrome virus field isolates differ in in vitro interferon phenotypes. Vet. Immunol. Immunopathol. 102: 217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, et al. 2007. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the mid-eastern region of China. Vet. J. 174: 577–584 [DOI] [PubMed] [Google Scholar]
- 16. Lopez Fuertes L, et al. 1999. Analysis of cellular immune response in pigs recovered from porcine respiratory and reproductive syndrome infection. Virus Res. 64: 33–42 [DOI] [PubMed] [Google Scholar]
- 17. Lopez OJ, Osorio FA. 2004. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 102: 155–163 [DOI] [PubMed] [Google Scholar]
- 18. Loving CL, Brockmeier SL, Sacco RE. 2007. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology 120: 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loving CL, Brockmeier SL, Vincent AL, Lager KM, Sacco RE. 2008. Differences in clinical disease and immune response of pigs challenged with a high-dose versus low-dose inoculum of porcine reproductive and respiratory syndrome virus. Viral Immunol. 21: 315–325 [DOI] [PubMed] [Google Scholar]
- 20. Luo R, et al. 2008. Porcine reproductive and respiratory syndrome virus (PRRSV) suppresses interferon-beta production by interfering with the RIG-I signaling pathway. Mol. Immunol. 45: 2839–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marrack P, Kappler J, Mitchell T. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meier WA, et al. 2003. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 309: 18–31 [DOI] [PubMed] [Google Scholar]
- 23. Meier WA, et al. 2004. Cytokines and synthetic double-stranded RNA augment the T helper 1 immune response of swine to porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 102: 299–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meulenberg JJ, et al. 1993. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology 192: 62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller LC, Laegreid WW, Bono JL, Chitko-McKown CG, Fox JM. 2004. Interferon type I response in porcine reproductive and respiratory syndrome virus-infected MARC-145 cells. Arch. Virol. 149: 2453–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19: 683–765 [DOI] [PubMed] [Google Scholar]
- 27. Muller M, Winnacker EL, Brem G. 1992. Molecular cloning of porcine Mx cDNAs: new members of a family of interferon-inducible proteins with homology to GTP-binding proteins. J. Interferon Res. 12: 119–129 [DOI] [PubMed] [Google Scholar]
- 28. Osorio FA, et al. 2002. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology 302: 9–20 [DOI] [PubMed] [Google Scholar]
- 29. Plagemann PG. 2006. Neutralizing antibody formation in swine infected with seven strains of porcine reproductive and respiratory syndrome virus as measured by indirect ELISA with peptides containing the GP5 neutralization epitope. Viral Immunol. 19: 285–293 [DOI] [PubMed] [Google Scholar]
- 30. Rossow KD, et al. 1994. Experimental porcine reproductive and respiratory syndrome virus infection in one-, four-, and 10-week-old pigs. J. Vet. Diagn. Invest. 6: 3–12 [DOI] [PubMed] [Google Scholar]
- 31. Song C, Krell P, Yoo D. 2010. Nonstructural protein 1alpha subunit-based inhibition of NF-kappaB activation and suppression of interferon-beta production by porcine reproductive and respiratory syndrome virus. Virology 407: 268–280 [DOI] [PubMed] [Google Scholar]
- 32. Sun Z, Chen Z, Lawson SR, Fang Y. 2010. The cysteine protease domain of porcine reproductive and respiratory syndrome virus nonstructural protein 2 possesses deubiquitinating and interferon antagonism functions. J. Virol. 84: 7832–7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suradhat S, Thanawongnuwech R. 2003. Upregulation of interleukin-10 gene expression in the leukocytes of pigs infected with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 84: 2755–2760 [DOI] [PubMed] [Google Scholar]
- 34. Suradhat S, Thanawongnuwech R, Poovorawan Y. 2003. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 84: 453–459 [DOI] [PubMed] [Google Scholar]
- 35. Tian K, et al. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2: e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tong GZ, et al. 2007. Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg. Infect. Dis. 13: 1434–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Reeth K, Labarque G, Nauwynck H, Pensaert M. 1999. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res. Vet. Sci. 67: 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wills RW, et al. 1997. Porcine reproductive and respiratory syndrome virus: a persistent infection. Vet. Microbiol. 55: 231–240 [DOI] [PubMed] [Google Scholar]
- 39. Wu WH, et al. 2001. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology 287: 183–191 [DOI] [PubMed] [Google Scholar]
- 40. Yoon KJ, et al. 1995. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Invest. 7: 305–312 [DOI] [PubMed] [Google Scholar]
- 41. Zuckermann FA, et al. 2007. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet. Microbiol. 123: 69–85 [DOI] [PubMed] [Google Scholar]
- 42. Zuckermann FA, et al. 1998. Interleukin-12 enhances the virus-specific interferon gamma response of pigs to an inactivated pseudorabies virus vaccine. Vet. Immunol. Immunopathol. 63: 57–67 [DOI] [PubMed] [Google Scholar]