Abstract
A shortcoming of currently available oral cholera vaccines is their induction of relatively short-term protection against cholera compared to that afforded by wild-type disease. We were interested in whether transcutaneous or subcutaneous boosting using a neoglycoconjugate vaccine made from a synthetic terminal hexasaccharide of the O-specific polysaccharide of Vibrio cholerae O1 (Ogawa) coupled to bovine serum albumin as a carrier (CHO-BSA) could boost lipopolysaccharide (LPS)-specific and vibriocidal antibody responses and result in protective immunity following oral priming immunization with whole-cell cholera vaccine. We found that boosting with CHO-BSA with immunoadjuvantative cholera toxin (CT) or Escherichia coli heat-labile toxin (LT) following oral priming with attenuated V. cholerae O1 vaccine strain O395-NT resulted in significant increases in serum anti-V. cholerae LPS IgG, IgM, and IgA (P < 0.01) responses as well as in anti-Ogawa (P < 0.01) and anti-Inaba (P < 0.05) vibriocidal titers in mice. The LPS-specific IgA responses in stool were induced by transcutaneous (P < 0.01) but not subcutaneous immunization. Immune responses following use of CT or LT as an adjuvant were comparable. In a neonatal mouse challenge assay, immune serum from boosted mice was associated with 79% protective efficacy against death. Our results suggest that transcutaneous and subcutaneous boosting with a neoglycoconjugate following oral cholera vaccination may be an effective strategy to prolong protective immune responses against V. cholerae.
INTRODUCTION
Cholera is an acute secretory diarrhea caused by Vibrio cholerae, a Gram-negative organism. Cholera affects 3 to 5 million individuals each year, killing over 100,000 (47, 49). Epidemic cholera is caused by V. cholerae O1 and O139, and immunity against the disease is serogroup specific. Previous infection with V. cholerae O1 does not provide immunity against O139 and vice versa (2). This is despite the fact that the O1 and O139 serogroup organisms are very highly homologous and produce identical cholera toxins (CTs) (18). V. cholerae O1 serogroup organisms are subdivided into two serotypes, Ogawa and Inaba, which differ by the presence of a methyl group on the terminal saccharide of the O antigen portion of the lipopolysaccharide (LPS) of the Ogawa serotype (45).
Although a number of cholera vaccines exist (35, 38), only two oral killed cholera vaccines are presently available for immunization purposes (38, 46). Oral killed cholera vaccines induce protective immunity, but their use has been hampered by the requirement for two or three priming doses (depending on the age of the recipient and specific vaccine) and their relatively short-term protection (39, 41). Protective immunity against moderate to severe disease following one episode of wild-type cholera lasts for 3 to 10 years (3, 20, 23), but protective immunity following oral cholera vaccination often lasts for 1 to 3 years, with responses in young children being of lower magnitude and shorter duration (39, 41). The mechanism of protective immunity against cholera is not currently known, but the vibriocidal response that largely targets lipopolysaccharides is the best indirect correlate of protection (15, 27).
We have previously shown that transcutaneous and subcutaneous immunization with a synthetic neoglycoconjugate containing a terminal hexasaccharide of the Ogawa O-specific polysaccharide of V. cholerae O1 LPS can induce anti-V. cholerae lipopolysaccharide antibody responses but not vibriocidal responses or protection in a challenge assay (32). We were, therefore, interested in whether transcutaneous or subcutaneous immunization with the neoglycoconjugate might boost anti-V. cholerae immune responses induced by prior intestinal priming with oral cholera vaccine. If successful, such an approach could be used in humans to boost the duration of protection afforded by currently available oral cholera vaccines and possibly to consolidate longer-term immunity following wild-type disease.
MATERIALS AND METHODS
Bacterial strains and media.
To orally vaccinate mice, we used V. cholerae O395-NT, a classical Ogawa V. cholerae O1 vaccine strain deficient in ctxAB (26). We used wild-type Vibrio cholerae O395 in 50% lethal dose (LD50) challenge assays to assess protection.
Carbohydrate conjugates.
We chemically synthesized the upstream, terminal hexasaccharide fragment of the O-specific polysaccharide of V. cholerae O1 Ogawa LPS and conjugated it to bovine serum albumin (BSA) at a molar ratio of 5 (saccharides) to 1 (protein) using squaric acid diethyl ester as a conjugation reagent. The synthesis and characterization of this V. cholerae O1 Ogawa neoglycoconjugate (CHO-BSA) have been previously described (4, 5, 32, 37, 44, 48).
Production of LT.
Escherichia coli heat-labile toxin (LT) was prepared by galactose affinity chromatography as described previously (8, 11, 28). The endotoxin content of the final products was <1 endotoxin unit/mg.
Mice and oral priming immunizations.
We orally immunized 3- to 5-week-old female Swiss Webster germfree mice (Taconic Farms, Germantown, NY) using a previously established mouse cholera oral vaccination model (10, 17, 31). In brief, we removed mice from their germfree shipper and orally immunized animals with 109 CFU of V. cholerae O395-NT grown in Luria-Bertani broth at 37°C overnight with aeration (oral priming, day 0). We then maintained mice in routine (non-germfree) housing conditions, and we orally reimmunized them on days 2, 4, and 6 and then every 14 days until day 100, when mice had developed a mean reciprocal vibriocidal antibody titer of approximately 100.
Boosting immunization of mice.
On day 117, we randomly assigned primed mice in one of several cohorts to receive a boosting vaccination (Table 1). Some cohorts received CHO-BSA (10 μg saccharide/immunization/mouse) transcutaneously (TCI) with or without 25 μg of immunoadjuvantative cholera toxin (CT; List Biological Laboratories, Campbell, CA) or E. coli heat-labile toxin (LT) (9). We immunized additional cohorts with CHO-BSA (10 μg saccharide/immunization/mouse) subcutaneously (SCI) with or without 5 μg of immunoadjuvantative CT. Subcutaneous boosting immunization (SCI) was administered via a 25-gauge needle on the central dorsal surface; transcutaneous boosting immunization (TCI) was administered to the central dorsal surface as previously described (32). Briefly, we clip-shaved the dorsa of mice and then rested the mice for 24 h. We then hydrated the shaved area with sterile water and gently brushed it with emery paper (to remove the outer layers of the stratum corneum) and then rehydrated, blot dried, and applied the vaccine antigens to approximately 1 cm2 of shaved, hydrated skin surface area. Control mice received CT or LT boosting alone or no boosting immunizations following oral priming. Additional control cohorts received no priming oral immunization and received only CHO-BSA with CT or LT transcutaneously or subcutaneously. When booster doses were given, they were administered at 2-week intervals for a total of four immunizations. Transcutaneous immunizations were carried out as previously described (32).
Table 1.
Immunization cohorts
| Cohorta | Oral priming by VCO395NTb | Boosting |
|---|---|---|
| Orally primed and transcutaneously boosted | ||
| Oral+TCI-CHO-BSA/CT | + | CHO-BSA, 10 μg saccharide/mouse; CT, 25 μg/mouse; transcutaneously |
| Oral+TCI-CT | + | CT, 25 μg/mouse; transcutaneously |
| Oral+TCI-CHO-BSA/LT | + | CHO-BSA, 10 μg saccharide/mouse; LT, 25 μg/mouse; transcutaneously |
| Oral+TCI-LT | + | LT, 25 μg/mouse; transcutaneously |
| Orally primed and subcutaneously boosted | ||
| Oral+SCI-CHO-BSA/CT | + | CHO-BSA, 10 μg saccharide/mouse; CT, 5 μg/mouse; subcutaneously |
| Oral+SCI-CT | + | CT, 5 μg/mouse; subcutaneously |
| Orally primed and no boosting | ||
| Oral | + | No boosting |
| No oral priming | ||
| TCI-CHO-BSA/CT | − | CHO-BSA, 10 μg saccharide/mouse; CT, 25 μg/mouse; transcutaneously |
| TCI-CHO-BSA/LT | − | CHO-BSA, 10 μg saccharide/mouse; LT, 25 μg/mouse; transcutaneously |
| TCI-LT | − | LT, 25 μg saccharide/mouse; transcutaneously |
| SCI-CHO-BSA/CT | − | CHO-BSA, 10 μg saccharide/mouse; CT, 5 μg/mouse; subcutaneously |
Oral, oral priming; TCI, transcutaneous boosting immunization; SCI, subcutaneous boosting immunization; CHO, neoglycoconjugate Ogawa hexasaccharide; BSA, bovine serum albumin; CT, cholera toxin; LT, E. coli heat-labile enterotoxin; Oral+TCI-CHO-BSA/CT, orally primed and transcutaneously boosted with CHO-BSA and the CT adjuvant.
VCO395NT, V. cholerae O395-NT vaccine strain.
Immunological sampling.
We collected blood for immunological analyses 2 weeks after each immunization and at the time of sacrifice. We collected stool samples for immunological analysis 2 weeks after the last vaccination. We collected, processed, aliquoted, and stored samples as previously described (10, 17, 36).
Measurement of immune responses.
To detect antibody responses against V. cholerae LPS, we used enzyme-linked immunosorbent assay (ELISA) plates previously coated with LPS from V. cholerae O1 Ogawa strain X25049, as previously described (32). We diluted mouse sera in phosphate-buffered saline (PBS)-0.05% Tween 20-0.1% BSA (PBST/BSA) (for IgA, a 1:50 dilution; for IgG, a 1:1,000 dilution) and used a 1:1,000 dilution of goat anti-mouse IgA–horseradish peroxidase (HRP) or anti-mouse IgG–HRP (Southern Biotech, Birmingham, AL) as a secondary antibody for detection. We developed plates using a 0.55-mg/ml solution of 2,2′-azinobis (3-ethylbenzthiazo-line-6-sulfonic acid) (ABTS) (Sigma), adding 30% H2O2 (Sigma) just before use, and a Vmax microplate reader (Molecular Devices Corp., Sunnyvale, CA), reporting the maximum slope for an optical density change of 0.2 U in milli-optical density units per minute (mOD/min). We calibrated samples using pooled control samples to allow comparisons across plates and reported results as ELISA units as previously described (32). To detect antitoxin responses via ELISA, we used microtiter plates sequentially coated with ganglioside type III (Sigma) (1 μg/ml in 50 mM carbonate buffer, pH 9.6) and cholera toxin (List Biological Laboratories, Campbell, CA) (1 μg/ml per well in PBS). We diluted mouse sera in PBST/BSA (for IgA, 1:50; for IgG, 1:1,000) and used a 1:1,000 dilution of goat anti-mouse IgA–HRP or anti-mouse IgG–HRP (Southern Biotech, Birmingham, AL) as a secondary antibody for detection.
To assess antigen-specific IgA responses in stool, we first measured total IgA in stool preparations as previously described (36), using rat monoclonal anti-mouse IgA antibody C10-3 (Pharmingen, San Diego, CA) at a dilution of 1:1,000 in 50 mM carbonate buffer (17) and detecting with a 1:1,000 dilution of goat anti-mouse IgA–HRP (Southern Biotech). We quantified total stool IgA using a mouse IgA standard (Kappa TEPC 15; Sigma). We then assessed antigen-specific antitoxin and anti-LPS IgA responses in stool preparations with 100 ng of total stool IgA per well in PBST/BSA by using the above-described ELISA procedures.
Vibriocidal antibody response.
We assessed serum vibriocidal antibody titers against V. cholerae O395-NT with an in vitro microdilution assay as previously described (32). We calculated the vibriocidal titer as the dilution of serum causing 50% reduction of optical density compared with that of control wells containing no serum (32). To assess vibriocidal antibody responses against an Inaba serotype organism, we also calculated vibriocidal titers using V. cholerae O1 Inaba strain Peru-2, an attenuated derivative of C6709, by following the same procedure (6).
Neonatal challenge experiments and LD50s.
To assess protection afforded by antibodies generated by immunization, we used the standard cholera neonatal mouse challenge assay, as previously described (32), with wild-type V. cholerae O395 as the challenge strain. Briefly, we separated 2- to 4-day-old unimmunized CD-1 suckling mice from dams 3 h prior to inoculation and orally challenged pups with 1 LD50 (106 CFU/neonate) (32) of virulent wild-type V. cholerae O395 grown in LB medium, pH 6.5, at 30°C for 18 h (5, 42). We intragastrically administered to pups a 50-μl mixture containing 25 μl of bacteria and 25 μl of pooled serum collected at the time of sacrifice from different immunized mouse cohorts. Following oral challenge, we kept pups separated from dams at 30°C and monitored them every 2 h for 36 h. After 36 h, we euthanized any surviving animals.
Statistical analyses.
We used GraphPad Prism 4.0 for statistical analysis and figure generation. We used the Mann-Whitney test to compare antigen-specific and vibriocidal antibody data between different experimental groups and the Kaplan-Meier and log rank analyses to compare survival curves within the LD50 assay.
RESULTS
Antigen-specific immune responses.
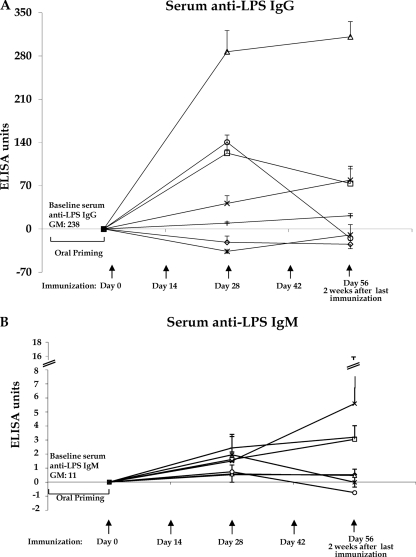
Following oral priming and transcutaneous or subcutaneous boosting immunization with CHO-BSA and immunoadjuvantative CT or LT, we detected significantly boosted LPS IgG-specific antibody responses in sera compared to responses following boosting with CT or LT alone and compared with no boosting across evaluated time points (P < 0.02) (Fig. 1A). We observed the highest anti-LPS IgG responses in animals previously orally primed with V. cholerae O395-NT and then subcutaneously boosted with CHO-BSA with an immunoadjuvant (Fig. 1A). We detected significant anti-LPS IgM responses in mice orally primed and then transcutaneously (P = 0.003) boosted with CHO-BSA with an immunoadjuvant compared to those of nonboosted mice (Fig. 1B). We also detected significant anti-LPS IgM responses in mice receiving transcutaneous (P = 0.007) or subcutaneous (P = 0.006) vaccination with CHO-BSA and an adjuvant without priming. We detected prominent CT IgG-specific antibody responses in all animal cohorts receiving CT or LT, irrelevant of route or priming regimen (P ≤ 0.01 or P ≤ 0.001).
Fig 1.
LPS IgG-specific (A) and IgM-specific (B) antibody responses in sera in different immunized mouse cohorts, assigning postpriming/preboosting antibody levels a value of 0. Mice were primed with V. cholerae O395-NT Ogawa and then randomized to receive booster immunizations at 2-week intervals with immunoadjuvant cholera toxin (CT) with or without Ogawa neoglycoconjugate (CHO-BSA) either transcutaneously (TCI) or subcutaneously (SCI). Control animals received no booster immunization or were never orally primed (see text). Assays were performed by kinetic ELISA, and data were plotted as ELISA units. The geometric mean and standard error of the mean for each immunization cohort are shown. △, oral priming with SCI CHO-BSA/CT boosting (oral priming+SCI-CHO-BSA/CT); □, oral priming+TCI-CHO-BSA/CT boosting; *, oral priming+SCI-CT boosting; ○, oral priming+TCI-CT boosting; ◊, oral priming only, no boosting; ×, no priming+SCI-CHO-BSA/CT; +, no priming+TCI-CHO-BSA/CT. To simplify the figure, cohorts receiving LT as an immunoadjuvant are not included (see text and Fig. 5). GM, geometric mean.
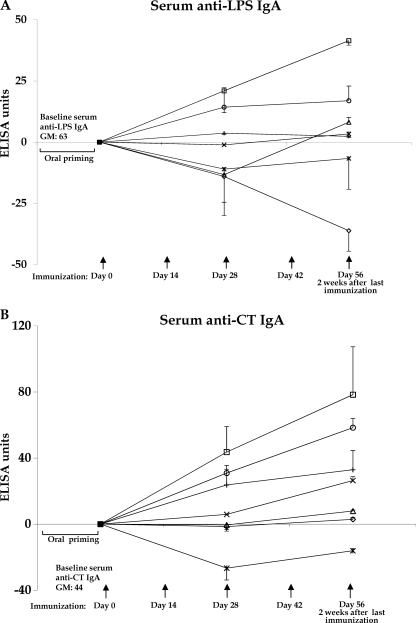
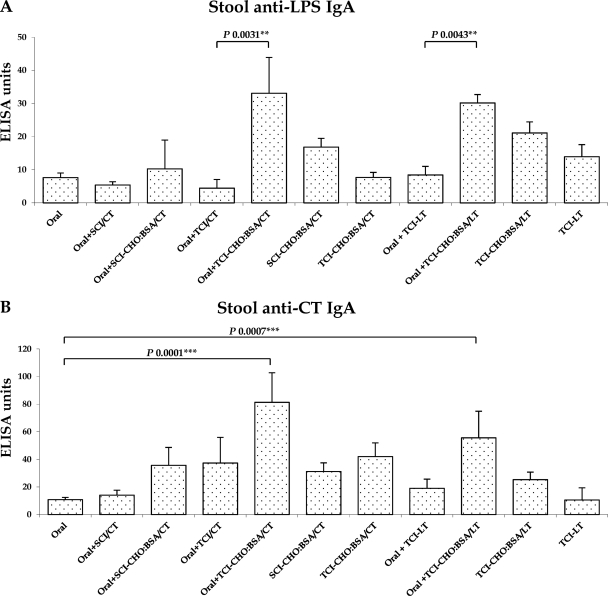
We detected the most prominent anti-LPS IgA (Fig. 2A) and anti-CT IgA (Fig. 2B) responses in animals previously orally primed with V. cholerae O395-NT and then transcutaneously boosted with CHO-BSA with an immunoadjuvant. Similarly, we detected the most prominent anti-LPS IgA (Fig. 3A) and anti-CT IgA (Fig. 3B) responses in stool from animals that were previously orally primed and then transcutaneously boosted with CHO-BSA with an immunoadjuvant.
Fig 2.
LPS IgA-specific (A) and CT IgA-specific (B) responses in sera in different immunized mouse cohorts, assigning postpriming/preboosting antibody levels a value of 0. Mice were primed with V. cholerae O395-NT Ogawa, as described in the text, and then randomized to receive booster immunizations at 2-week intervals with immunoadjuvant cholera toxin (CT) with or without Ogawa neoglycoconjugate (CHO-BSA) either transcutaneously (TCI) or subcutaneously (SCI). Control animals received no booster immunization or were never orally primed (see text). Assays were performed by kinetic ELISA, and data were plotted as ELISA units as described in the text. The geometric mean and standard error of the mean for each immunization cohort are shown. △, oral priming+SCI-CHO-BSA/CT boosting; □, oral priming+TCI-CHO-BSA/CT boosting; *, oral priming+SCI-CT boosting; ○, oral priming+TCI-CT boosting; ◊, oral priming only, no boosting; ×, no priming+SCI-CHO-BSA/CT; +, no priming+TCI-CHO/CT. To simplify the figure, cohorts receiving LT as an immunoadjuvant are not included (see text and Fig. 5). GM, geometric mean.
Fig 3.
Stool LPS IgA-specific (A) and CT IgA-specific (B) antibody responses in stool specimens collected on day 56 from different immunized mouse cohorts. Mice, previously primed with V. cholerae O395-NT Ogawa (Oral), were randomized to receive booster immunizations at 2-week intervals with immunoadjuvant cholera toxin (CT) or E. coli heat-labile toxin (LT) with or without Ogawa neoglycoconjugate (CHO-BSA) either transcutaneously (TCI) or subcutaneously (SCI) (see text). Control animals received no booster immunization or were never orally primed. The geometric mean and standard error of the mean for each immunization cohort are shown.
Vibriocidal responses.
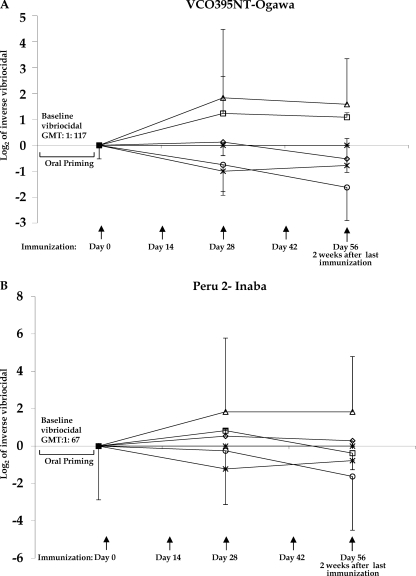
Anti-Ogawa vibriocidal responses increased following oral priming and transcutaneous or subcutaneous boosting with CHO-BSA and an immunoadjuvant compared to those of boosting with only an adjuvant (P = 0.01) (Fig. 4A). Although there was no significant boosting of anti-Inaba vibriocidal responses following transcutaneous immunization with CHO-BSA and an adjuvant, the anti-Inaba vibriocidal titer remained steady in mice subcutaneously immunized with CHO-BSA and an adjuvant following oral priming while it declined in other cohorts (P = 0.05) (Fig. 4B).
Fig 4.
Changes from the baseline vibriocidal level, with the log2 of the reciprocal vibriocidal antibody titer in each vaccine cohort plotted. Geometric means and standard errors of the mean are plotted, assigning postpriming/preboosting vibriocidal levels a value of 0. Mice were primed with V. cholerae O395-NT Ogawa, as described in the text, and then randomized to receive booster immunizations at 2-week intervals with immunoadjuvant cholera toxin (CT) with or without Ogawa neoglycoconjugate (CHO-BSA) either transcutaneously (TCI) or subcutaneously (SCI). Control animals received no booster immunization or were never orally primed (see text). Vibriocidal responses were measured using V. cholerae O1 Ogawa O395-NT (A) or V. cholerae O1 Inaba Peru-2 (a derivative of wild-type C6709) (B). △, oral priming+SCI-CHO-BSA/CT boosting; □, oral priming+TCI-CHO-BSA/CT boosting; *, oral priming+SCI-CT boosting; ○, oral priming+TCI-CT boosting; ◊, oral priming only, no boosting; ×, no priming+SCI-CHO-BSA/CT; +, no priming+TCI-CHO-BSA/CT. To simplify the figure, cohorts receiving LT as an immunoadjuvant are not included (see text and Fig. 5). Please note that no vibriocidal responses were detected in animals that received SCI-CHO-BSA/CT or TCI-CHO-BSA/CT without priming. Those values overlap on the x axis. GMT, geometric mean titer.
Comparison of CT and LT adjuvants.
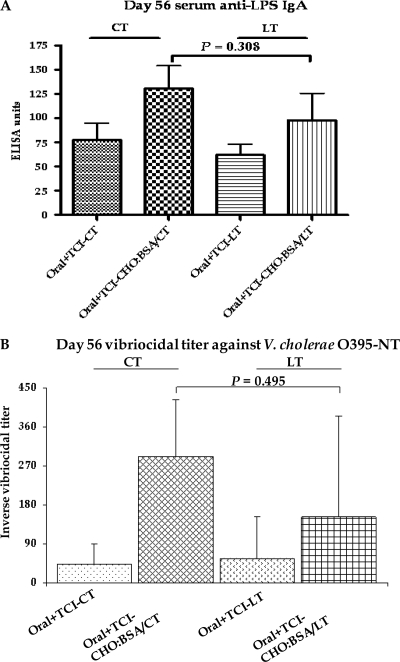
We detected no significant differences when comparing antigen-specific and vibriocidal immune responses following use of CT or LT as the immunoadjuvant (Fig. 3 and 5 and data not shown).
Fig 5.
Comparison of immunoadjuvants used in different mouse cohorts. Mouse, orally primed with V. cholerae O395-NT Ogawa, were randomized to receive booster immunizations transcutaneously with either cholera toxin (CT) or E. coli heat-labile toxin (LT) and with or without neoglycoconjugate (CHO-BSA) (see text). Geometric means and standard errors of the mean of serum anti-LPS IgA (A) and the reciprocal vibriocidal titer against the Ogawa serotype (B) at day 56 (2 weeks after the last immunization) are shown.
Protection assay.
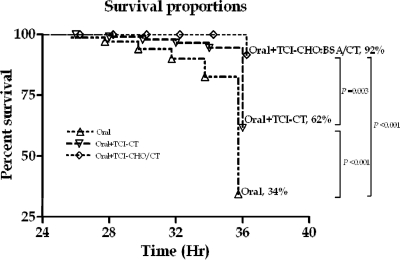
We found significant protection against lethal challenge with wild-type V. cholerae O1 Ogawa O395 organisms (1 LD50, 106 CFU) in mice administered with serum collected from animals orally primed with O395-NT and then transcutaneously boosted with CHO-BSA and CT (92% survival; protective efficacy, 86%) compared to that in mice administered with serum collected from animals that were orally primed but not boosted (34% survival; P < 0.001) and that in mice administered with serum collected from animals orally primed with O395-NT and then boosted with the CT adjuvant alone (62% survival; P = 0.003; protective efficacy, 79%) (Fig. 6). We did not assess protection using sera from subcutaneously boosted mice.
Fig 6.
Survival rates of neonatal CD-1 mice following oral challenge with wild-type V. cholerae O1 Ogawa O395. Two- to four-day-old unimmunized pups were intragastrically administered a 50-μl mixture containing 25 μl of V. cholerae O395 (106 CFU) and 25 μl of pooled sera recovered from vaccinated mouse cohorts (cohorts were orally primed with V. cholerae O395-NT with no booster immunization [Oral] [n = 24], orally primed with V. cholerae O395-NT followed by transcutaneous boosting with CT alone [Oral+TCI-CT] [n = 23], or orally primed with V. cholerae O395-NT followed by transcutaneous boosting with neoglycoconjugate [CHO-BSA] and CT [Oral+TCI-CHO-BSA/CT] [n = 24]). Challenged pups were kept at 30°C and monitored for death every 2 h from 24 to 36 h and then sacrificed. Survival curves were compared by a log rank test.
DISCUSSION
In this study, we have demonstrated that boosting with a neoglycoconjugate cholera vaccine and an adjuvant can induce vibriocidal, V. cholerae LPS-specific, and anti-CT antibody responses in serum and stool and can induce protective immunity in a V. cholerae challenge model. Current oral cholera vaccines are associated with both lower-level and shorter-term protection than that afforded by wild-type cholera. In population-based analyses and volunteer challenge studies, wild-type cholera induces 60 to 100% protection that lasts for 3 to 10 years (3, 20, 23), although some studies suggest a shorter duration (19). In comparison, current oral cholera vaccines provide 60 to 80% protection against cholera for 24 to 36 months when considered for all recipients and 35 to 60% protection for 6 to 24 months in younger children (39, 41). Therefore, the ability to boost the level and duration of protective immunity following oral cholera vaccination would be significant.
Boosting against cholera using repetitive oral immunization may be complicated by issues of partial mucosal immunity or chronic enteropathies inhibiting vaccine “take” (22, 24, 30), concomitant intestinal (7) and parasitic infections (16), and micronutrient deficiency (1). The intestinal and dermal immune systems are, however, in communication, and skin can be used as an alternate route for delivering vaccines to elicit mucosal responses in the gut (13, 14, 43). Antigens applied transcutaneously are processed by dermal dendritic cells that then migrate to regional lymph nodes and, through a poorly defined mechanism, this results in both systemic and mucosal immune responses (14). We therefore examined the ability of boosting immunizations, especially via the transcutaneous route, to affect previously primed mucosal responses. In this study, we used a previously established model of oral live attenuated cholera vaccination for intestinal priming in mice (34).
We used a neoglycoconjugate for boosting immunizations, since we had previously shown that transcutaneous and subcutaneous immunization in unprimed animals with this vaccine did not induce vibriocidal or anti-LPS IgM and IgA responses and did not result in protection in a cholera challenge assay despite the induction of anti-LPS IgG responses in serum (32). We were, therefore, particularly interested in whether boosting immunization with this vaccine could enhance preexisting vibriocidal and other anti-V. cholerae LPS immune responses and induce protection following oral priming.
Both subcutaneous and transcutaneous boosting resulted in increased vibriocidal responses as well as IgG and IgM anti-LPS and anti-CT responses in orally primed mice. IgA responses were most prominent following transcutaneous boosting, both in serum and stool samples, supporting the link between the dermal and mucosal immune systems and suggesting that mucosal priming-transcutaneous boosting immunization strategies may be particularly attractive approaches to protect against mucosal pathogens. We did not detect any vibriocidal or anti-LPS IgM or IgA responses in unprimed mice.
The vibriocidal response is currently one of the best predictors of protective immunity against cholera, although it presumably is a surrogate marker for as-yet poorly defined mucosal immune responses (15). The vibriocidal response is largely composed of IgM antibodies and largely targets V. cholerae LPS (15, 27). Anti-LPS IgA antibodies also correlate with protection from cholera (15). Humans who receive currently available oral killed cholera vaccines are immunized with both Ogawa and Inaba killed V. cholerae organisms. Anti-Ogawa and anti-Inaba vibriocidal antibody responses are cross-reactive, although they are higher for the homologous infecting or immunizing serotype. In this study, we showed that boosting with the neoglycoconjugate following oral priming does increase vibriocidal responses as well as anti-LPS IgM and IgA responses. We also confirmed that vibriocidal antibody boosting was higher for the serotype homologous to the vaccine (Ogawa) but that significant anti-Inaba responses were also observed. Supporting the association of vibriocidal and anti-LPS responses with protection against cholera, we also found that boosting that induced these anti-LPS responses in mice resulted in protection in the cholera challenge assay.
We and others have shown that successful transcutaneous immunization often requires the coapplication of an immunoadjuvant, with the best described ones being ADP-ribosylating proteins, such as cholera toxin (CT) or heat-labile toxin of E. coli (LT) or derivatives of LT (12, 14, 32, 40). Using CT, LT, or their derivatives as adjuvants has the added attraction that antitoxin immunity may supplement anti-V. cholerae immunity and also provide limited protection against enterotoxigenic E. coli (ETEC) (14). Immune responses against CT and LT are cross-reactive (21), and nontoxic derivatives of LT have been developed that maintain immunoadjuvanticity (14, 25, 28, 29, 33). In this study, we found that immune responses following use of CT or LT as an adjuvant were equivalent, advancing the possibility of using nontoxic but immunoadjuvantative derivatives of LT in this approach.
Our work builds upon our previous evaluation of the ability of transcutaneous and subcutaneous immunization with a cholera neoglycoconjugate to induce anti-LPS responses (32) and previous work demonstrating that the Ogawa neoglycoconjugate was protectively immunogenic in mice following intraperitoneal immunization (5). Future work could evaluate neoglycoconjugates that contain both Ogawa and Inaba O1 moieties, O139-associated polysaccharides, conjugates using a protein other than BSA, conjugates using other saccharide components of V. cholerae, and the ability of cholera conjugate vaccines to induce memory responses. Importantly, our results suggest that transcutaneous boosting might be a way to prolong protective immunity beyond that afforded by current cholera vaccines or V. cholerae infection. Such a vaccination strategy could improve the usefulness of cholera vaccines, especially in areas of the world where cholera is endemic and the need for protective immunity is ongoing.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health-NIDDK, the International Centre for Diarrheal Diseases Research, Bangladesh (icddr,b) (F.Q., A.A.T.), grants from the National Institutes of Health, including the National Institute of Allergy & Infectious Diseases (U01 AI077883 [E.T.R.], U01 AI058935 [S.B.C., E.T.R.], and R03 AI063079 [F.Q.]), the Fogarty International Center Training Grant in Vaccine Development and Public Health (D43 TW005572 [A.A.T., M.A., A.S., M.S.B., F.Q., E.T.R.]), an American Recovery and Reinvestment Act (ARRA) Postdoctoral Fellowship in Global Infectious Diseases (TW005572 [D.T.L., R.C.L.]), and Career Development Awards (K01 TW07409 [J.B.H.], TW07144 [R.C.L.], and K08 AI089721 [R.C.C.]), a Physician-Scientist Early Career Award from the Howard Hughes Medical Institute (R.C.L.), and a Harvard Initiative for Global Health Postdoctoral Fellowship in Global Infectious Diseases (D.T.L.).
Peru-2 was graciously supplied by John Mekalanos, Harvard Medical School.
Footnotes
Published ahead of print 22 February 2012
REFERENCES
- 1.Ahmed T, Svennerholm AM, Al Tarique A, Sultana GN, Qadri F. 2009. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 27:1433–1439 [DOI] [PubMed] [Google Scholar]
- 2.Albert MJ, Alam K, Rahman AS, Huda S, Sack RB. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J. Infect. Dis. 169:709–710 [DOI] [PubMed] [Google Scholar]
- 3.Ali M, Emch M, Park JK, Yunus M, Clemens J. 2011. Natural cholera infection-derived immunity in an endemic setting. J. Infect. Dis. 204:912–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chernyak A, Karavanov A, Ogawa Y, Kovac P. 2001. Conjugating oligosaccharides to proteins by squaric acid diester chemistry: rapid monitoring of the progress of conjugation, and recovery of the unused ligand. Carbohydr. Res. 330:479–486 [DOI] [PubMed] [Google Scholar]
- 5.Chernyak A, et al. 2002. Induction of protective immunity by synthetic Vibrio cholerae hexasaccharide derived from V. cholerae O1 Ogawa lipopolysaccharide bound to a protein carrier. J. Infect. Dis. 185:950–962 [DOI] [PubMed] [Google Scholar]
- 6.Chiang SL, Mekalanos JJ. 2000. Construction of a Vibrio cholerae vaccine candidate using transposon delivery and FLP recombinase-mediated excision. Infect. Immun. 68:6391–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury F, et al. 2010. Concomitant enterotoxigenic Escherichia coli infection induces increased immune responses to Vibrio cholerae O1 antigens in patients with cholera in Bangladesh. Infect. Immun. 78:2117–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements JD, Finkelstein RA. 1979. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect. Immun. 24:760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements JD, Hartzog NM, Lyon FL. 1988. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 6:269–277 [DOI] [PubMed] [Google Scholar]
- 10.Crean TI, John M, Calderwood SB, Ryan ET. 2000. Optimizing the germfree mouse model for in vivo evaluation of oral Vibrio cholerae vaccine and vector strains. Infect. Immun. 68:977–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson BL, Clements JD. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghose C, et al. 2007. Transcutaneous immunization with Clostridium difficile toxoid A induces systemic and mucosal immune responses and toxin A-neutralizing antibodies in mice. Infect. Immun. 75:2826–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glenn GM, Scharton-Kersten T, Alving CR. 1999. Advances in vaccine delivery: transcutaneous immunisation. Expert Opin. Investig. Drugs 8:797–805 [DOI] [PubMed] [Google Scholar]
- 14.Glenn GM, Scharton-Kersten T, Vassell R, Matyas GR, Alving CR. 1999. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect. Immun. 67:1100–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris JB, et al. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris JB, et al. 2009. Immunologic responses to Vibrio cholerae in patients co-infected with intestinal parasites in Bangladesh. PLoS Negl. Trop. Dis. 3:e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John M, Bridges EA, Miller AO, Calderwood SB, Ryan ET. 2002. Comparison of mucosal and systemic humoral immune responses after transcutaneous and oral immunization strategies. Vaccine 20:2720–2726 [DOI] [PubMed] [Google Scholar]
- 18.Johnson JA, et al. 1994. Vibrio cholerae O139 synonym Bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect. Immun. 62:2108–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King AA, Ionides EL, Pascual M, Bouma MJ. 2008. Inapparent infections and cholera dynamics. Nature 454:877–880 [DOI] [PubMed] [Google Scholar]
- 20.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696–700 [DOI] [PubMed] [Google Scholar]
- 21.Krueger KM, Barbieri JT. 1995. The family of bacterial ADP-ribosylating exotoxins. Clin. Microbiol. Rev. 8:34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagos R, et al. 1999. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 180:1709–1712 [DOI] [PubMed] [Google Scholar]
- 23.Levine MM, et al. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818–820 [DOI] [PubMed] [Google Scholar]
- 24.Losonsky GA, et al. 1996. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect. Immun. 64:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCluskie MJ, Weeratna RD, Clements JD, Davis HL. 2001. Mucosal immunization of mice using CpG DNA and/or mutants of the heat-labile enterotoxin of Escherichia coli as adjuvants. Vaccine 19:3759–3768 [DOI] [PubMed] [Google Scholar]
- 26.Mekalanos JJ, et al. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551–557 [DOI] [PubMed] [Google Scholar]
- 27.Neoh SH, Rowley D. 1970. The antigens of Vibrio cholerae involved in the vibriocidal action of antibody and complement. J. Infect. Dis. 121:505–513 [DOI] [PubMed] [Google Scholar]
- 28.Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 18:546–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizza M, et al. 2001. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19:2534–2541 [DOI] [PubMed] [Google Scholar]
- 30.Richie EE, et al. 2000. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 18:2399–2410 [DOI] [PubMed] [Google Scholar]
- 31.Rollenhagen JE, et al. 2006. Transcutaneous immunization with toxin-coregulated pilin A induces protective immunity against Vibrio cholerae O1 El Tor challenge in mice. Infect. Immun. 74:5834–5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rollenhagen JE, et al. 2009. Transcutaneous immunization with a synthetic hexasaccharide-protein conjugate induces anti-Vibrio cholerae lipopolysaccharide responses in mice. Vaccine 27:4917–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan EJ, et al. 1999. Mutants of Escherichia coli heat-labile toxin act as effective mucosal adjuvants for nasal delivery of an acellular pertussis vaccine: differential effects of the nontoxic AB complex and enzyme activity on Th1 and Th2 cells. Infect. Immun. 67:6270–6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan ET, et al. 1997. Oral immunization with attenuated vaccine strains of Vibrio cholerae expressing a dodecapeptide repeat of the serine-rich Entamoeba histolytica protein fused to the cholera toxin B subunit induces systemic and mucosal antiamebic and anti-V. cholerae antibody responses in mice. Infect. Immun. 65:3118–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan ET, Calderwood SB, Qadri F. 2006. Live attenuated oral cholera vaccines. Expert Rev. Vaccines 5:483–494 [DOI] [PubMed] [Google Scholar]
- 36.Ryan ET, et al. 1999. In vivo expression and immunoadjuvancy of a mutant of heat-labile enterotoxin of Escherichia coli in vaccine and vector strains of Vibrio cholerae. Infect. Immun. 67:1694–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saksena R, Ma X, Wade TK, Kovac P, Wade WF. 2005. Effect of saccharide length on the immunogenicity of neoglycoconjugates from synthetic fragments of the O-SP of Vibrio cholerae O1, serotype Ogawa. Carbohydr. Res. 340:2256–2269 [DOI] [PubMed] [Google Scholar]
- 38.Shin S, Desai SN, Sah BK, Clemens JD. 2011. Oral vaccines against cholera. Clin. Infect. Dis. 52:1343–1349 [DOI] [PubMed] [Google Scholar]
- 39.Sinclair D, Abba K, Zaman K, Qadri F, Graves PM. 2011. Oral vaccines for preventing cholera. Cochrane Database Syst. Rev. 3:CD008603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snider DP. 1995. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit. Rev. Immunol. 15:317–348 [DOI] [PubMed] [Google Scholar]
- 41.Sur D, et al. 2011. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl. Trop. Dis. 5:e1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U. S. A. 84:2833–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Udey MC. 1997. Cadherins and Langerhans cell immunobiology. Clin. Exp. Immunol. 107(Suppl 1):6–8 [PubMed] [Google Scholar]
- 44.Wade TK, Saksena R, Shiloach J, Kovac P, Wade WF. 2006. Immunogenicity of synthetic saccharide fragments of Vibrio cholerae O1 (Ogawa and Inaba) bound to Exotoxin A. FEMS Immunol. Med. Microbiol. 48:237–251 [DOI] [PubMed] [Google Scholar]
- 45.Wang J, et al. 1998. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. J. Biol. Chem. 273:2777–2783 [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization 2010. Cholera vaccines: WHO position paper. Wkly. Epidemiol. Rec. 85:117–128 [PubMed] [Google Scholar]
- 47.World Health Organization 2009. Cholera: global surveillance summary, 2008. Wkly. Epidemiol. Rec. 84:309–324 [PubMed] [Google Scholar]
- 48.Zhang J, Kovac P. 1999. Studies on vaccines against cholera. Synthesis of neoglycoconjugates from the hexasaccharide determinant of Vibrio cholerae O:1, serotype Ogawa, by single-point attachment or by attachment of the hapten in the form of clusters. Carbohydr. Res. 321:157–167 [DOI] [PubMed] [Google Scholar]
- 49.Zuckerman JN, Rombo L, Fisch A. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7:521–530 [DOI] [PubMed] [Google Scholar]