Abstract
In Crohn's disease (CD), chronic gut inflammation leads to loss of mucosal barrier integrity. Subsequent leakage of IgG to the gut could produce an increase of IgG coating of intestinal bacteria. We investigated if there is more IgG coating in patients than in volunteers and whether this is dependent on the host IgG response or on the gut bacteria. Fecal and serum samples were obtained from 23 CD patients and 11 healthy volunteers. Both the in vivo IgG-coated fecal bacteria and in vitro IgG coating after serum addition were measured by flow cytometry and related to disease activity. The bacterial composition in feces was determined using fluorescence in situ hybridization. The IgG-binding capacities of Escherichia coli strains isolated from feces of patients and volunteers were assessed. The results showed that the in vivo IgG-coated fraction of fecal bacteria of patients was slightly larger than that of volunteers but significantly larger after incubation with either autologous or heterologous serum. This was dependent on the bacteria and independent of disease activity or the serum used. The presence of more Enterobacteriaceae and fewer faecalibacteria in patient feces was confirmed. E. coli isolates from patients bound more IgG than isolates from volunteers (P < 0.05) after the addition of autologous serum. Together, these results indicate that CD patients have more IgG-binding gut bacteria than healthy volunteers. We showed that the level of IgG coating depends on the bacteria and not on the serum used. Furthermore, CD patients have a strong specific immune response to their own E. coli bacteria.
INTRODUCTION
Ulcerative colitis (UC) and Crohn's disease (CD) are characterized by chronic gut inflammation caused by an abnormal immune response against normal intestinal bacteria (13). Especially in CD, this loss of tolerance for the normal intestinal microbiota becomes apparent (36). Immune reactions are targeted against pathobionts (13), which are commensal bacteria that have the potential to become pathogenic when they are not sufficiently suppressed by beneficial commensal bacteria, the so-called symbionts (25). Pathobionts like adherent Escherichia coli, Salmonella, Campylobacter, Mycobacterium, and Helicobacter may induce strong immune responses and may increase the risk for inflammatory bowel disease (IBD) (5). Protective properties of polysaccharide A-producing Bacteroides fragilis and Faecalibacterium prausnitzii as symbionts in most humans have been reported (13, 24). The current dogma is that there is an imbalance between the pathobionts and the symbionts that may cause chronic inflammation in a genetically susceptible host (28, 36). Normally, there are only limited amounts of bacteria in the mucus layer, but in patients with IBD, an increased number of bacteria are found in the mucus (10, 22, 27, 30). A temporary loss of mucosal barrier function, which results in an influx of commensal bacteria and their antigens into the mucosa, can be due to bacterial or viral enteric infections (4) or hypersensitivity to food antigens (8, 19), and this may precipitate exacerbation of CD in susceptible patients. This inflammation destroys the integrity of the gut mucosa, resulting in an impaired barrier function, which may facilitate an open passage for bacteria into the surrounding tissues and bloodstream. The resulting immune reactions against these bacteria aggravate the illness. Although CD is associated with a Th1 T cell-mediated response, (14), there is also a humoral immune response against the invading bacteria. Therefore, more IgG against gut bacteria will circulate in the bloodstream, and combined with the ulcerated gut mucosa, more IgG may leak into the lumen of the gut. This leads to increased IgG coating of commensal bacteria in the feces of IBD patients (31). It is not known what triggers this IgG coating or if the serum-derived IgG response indicates a history of contact with certain species. van der Waaij et al. (31) indicated that there may be a relationship between the amount of IgG coating and disease activity. Objective measurement of the disease activity is difficult, even for specialized physicians using invasive techniques. Fecal markers are a potentially useful noninvasive measure of gastrointestinal inflammation (16). Calprotectin is one of the most reliable markers for the measurement of inflammation in CD patients (15). Calprotectin can be easily measured in stool samples and can be correlated with the degree of mucosal inflammation (9, 20, 21).
Here, we want to investigate whether the humoral immune response against commensal gut bacteria in CD patients is greater than in healthy individuals. This is done by measuring the spontaneous in vivo IgG-coated fraction of fecal bacteria in patients and healthy controls by flow cytometry analysis with fluorescent anti-human IgG antibodies. In contrast to what was done previously (31), the percentage of in vitro IgG-coated bacteria was analyzed by measuring the IgG coating of bacteria after incubation with the person's own serum (autologous serum) and with serum of another patient or healthy volunteer (heterologous serum) to see if the response is specific for that individual. Calprotectin levels were used to investigate the relationship between IgG coating and disease activity. The microbial fecal composition was determined using a set of 10 different 16S rRNA fluorescence in situ hybridization (FISH) probes for the quantification of 8 different bacterial groups. Different E. coli strains were isolated from the fecal samples of the CD patients and healthy volunteers participating in this study, and the IgG-binding capacities of these E. coli strains was assessed to see whether the response was host specific.
MATERIALS AND METHODS
Patients and volunteers.
Fresh fecal and blood samples from patients with active and quiescent CD (9 males and 14 females; mean [range] age, 38 [19 to 69] years) were obtained. The exclusion criteria were use of antibiotics 6 weeks prior to providing blood and fecal samples and/or an enterostomy. Table 1 shows the characteristics of the patients and volunteers. Determination of whether patients were in remission or in an active state of CD was based on a physician's global assessment and fecal calprotectin levels (see below). As controls, fresh fecal and blood samples from healthy volunteers (7 males and 4 females; mean [range] age, 42 [22 to 61] years) were obtained. The exclusion criterion was use of antibiotics 6 weeks prior to providing the samples. The study was approved by the local medical ethical committee (number 2005.141).
Table 1.
Characteristics of patients with CD and healthy volunteers
| Characteristic | Value |
|
|---|---|---|
| CD patients | Volunteers | |
| n | 23 | 11 |
| Mean age (range) (yr) | 38 (19–69) | 42 (22–61) |
| Sex ratio (male/female) | 9/14 | 7/4 |
| No. active/in remission | 9/14 | |
| Medical therapy (no. receiving) | ||
| Corticosteroids | 4 | |
| Immunosuppressive drugs (thiopurine/methotrexate) | 12 | |
| Anti-tumor necrosis factor alpha | 3 | |
| Mesalazines | 7 | |
| Location of the disease (no.) | ||
| Ileal | 9 | |
| Colonic | 3 | |
| Ileocolonic | 11 | |
Sample preparation.
Blood samples were taken in a serum tube with serum separation gel. After centrifugation (2 min; 3,000 rpm), the serum was divided into aliquots and stored at −20°C until it was used.
Fresh stool samples were collected in a screw-cap container (50 ml), cooled (4°C), transported to the laboratory, and processed within 8 h. Samples for flow cytometry analysis were prepared as follows: 0.5 g feces was homogenized in 24.5 ml of filtered (Millipore, Billerica, MA; 0.22 μm) phosphate-buffered saline (PBS) ([per liter] 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4) on a vortex mixer and centrifuged at low speed (700 × g) for 5 min to separate larger fecal particles from bacteria. The supernatant was stored in 1-ml aliquots at −20°C until it was used.
Flow cytometry.
The supernatant of the stool samples described above, containing the bacteria, was centrifuged at 13,000 × g for 5 min. The bacterial pellet was washed in 1 ml of PBS and centrifuged at 13,000 × g for 5 min. The pellet was suspended either in 100 μl PBS or in 100 μl autologous or heterologous serum (1:100 in PBS) and incubated for 30 min on ice. The suspensions were washed with 1 ml PBS and centrifuged at 13,000 × g for 5 min. The bacterial pellet was suspended in 100 μl γ-chain-specific anti-human IgG-fluorescein isothiocyanate (FITC) conjugate (Sigma-Aldrich, Saint Louis, MO) that was diluted 1:100 in PBS. After a 30-min incubation time, the suspensions were washed twice in 1 ml of PBS and centrifuged at 13,000 × g for 5 min. Finally, the bacterial pellet was suspended in 500 μl PBS, mixed with 20 μl propidium iodine (PI) (1 μg/ml; Sigma-Aldrich, Saint Louis, MO), stored on ice in the dark, and analyzed within 1 h. Flow cytometry analysis of the fecal bacteria was performed with a flow cytometry Calibur (Becton Dickinson). Measurements were performed on 40,000 PI-positive events at a flow rate of 2,000 to 3,000 events/s. Analysis was done with FlowJo (Tree Star Inc., Ashland, OR) to determine the numbers of IgG-coated bacteria and bacteria that could be coated by serum IgG.
FISH.
The fecal samples were diluted and fixed for FISH analysis as described previously (6). Briefly, 2.5 g of feces was diluted 10-fold in filtered phosphate-buffered saline (PBS), and after homogenizing, the mixture was centrifuged at 700 × g. One milliliter of the supernatant was mixed with 3 ml freshly prepared 4% paraformaldehyde in PBS and incubated overnight at 4°C to fix the bacteria. These 40-fold-diluted fecal samples were stored at −80°C until use. FISH analysis and 4′,6-diamidino-2-phenylindole (DAPI) staining were performed as described previously (6) on different fecal dilutions in PBS ranging from 40- to 1,600-fold, depending on the relative amount of targeted bacteria. The analysis was performed on all 34 samples by DAPI staining and with a set of probes for the most predominant groups of fecal bacteria described (see Table 3).
Table 3.
Numbers and percentages of fecal microorganisms from 11 volunteers and 23 CD patients as determined by DAPI staining (total cells) and FISH with group-specific probes
| Population | Stain or probe (reference) | Mean no. of cells/g fecesa |
% of total cells (DAPI) |
||
|---|---|---|---|---|---|
| Volunteers | Patients | Volunteers | Patients | ||
| Total cells | DAPI | 6.0 × 1010 | 3.0 × 1010 | ||
| Total bacteria | Eub338 (1) | 4.2 × 1010 | 2.0 × 1010 | 70.4 | 62.2 |
| Enterobacteriaceae | E.coli153 (17) | 1.3 × 107b | 2.0 × 108b | 0.03b | 0.97b |
| Faecalibacterium | Fprau645 (26) | 2.3 × 109b | 4.1 × 109b | 2.89 | 1.51 |
| Bacteroides | Bac303 (12) | 2.4 × 109 | 6.7 × 109 | 3.33 | 31.2 |
| Ruminococci | Rfla729/Rbro730 (6) | 2.2 × 108 | 3.6 × 108 | 0.48b | 1.26b |
| Enterococcus faecalis | Enfl3 (32) | 3.8 × 106 | 4.0 × 106 | 0.01 | 0.02 |
| Clostridium sp. strain XIVa | Erec482 (3) | 6.8 × 109 | 3.4 × 109 | 13.1 | 12.0 |
| Streptococci/lactococci | Strc493 (3) | 2.1 × 108 | 1.5 × 108 | 0.35 | 0.55 |
| Atopobia | Ato291 (7) | 8.8 × 108 | 6.8 × 108 | 1.79 | 2.34 |
Median numbers from fecal samples were calculated per gram (wet weight) feces, assuming that the numbers below the detection limit were zero.
Significant differences (P < 0.005) between patients and volunteers as tested by the Mann-Whitney U test.
Isolation, IgG coating, and flow cytometry of E. coli strains.
Fecal samples from patients and volunteers diluted in PBS were plated on McConkey plates. This resulted in 25 different E. coli isolates. In addition, two laboratory strains were used, namely, ATCC 25422 and O157:H7. These laboratory strains were treated as nonpatient strains. Identification of the strains was done with the API-20E test kit (bioMérieux Inc., Hazelwood, MO) following the manufacturer's protocol. All isolates were tested for IgG binding after addition of their own serum (autologous serum) or the sera of patients or volunteers (heterologous serum). Reference strains were tested with patient P1 serum.
For IgG coating, a colony was picked from a pure culture on McConkey agar and grown overnight in brain heart infusion (BHI) (Mediaproducts BV, Groningen, Netherlands) broth at 37°C. One milliliter of the broth was centrifuged for 5 min at 8,000 × g. Then, the pellet was resuspended in 1 ml of PBS. This was centrifuged for 4.5 min at 8,000 × g and resuspended in 985 μl of PBS and 15 μl of blood serum. The sample was then incubated for 30 min and subsequently centrifuged for 5 min at 8,000 × g. The pellet was resuspended in 500 μl of PBS plus 1% bovine serum albumin (BSA) and 100× diluted goat anti-human IgG-FITC conjugate (Sigma-Aldrich, Saint Louis, MO). The suspension was incubated for 30 min and centrifuged for 5 min at 8,000 × g. The pellet was resuspended in 500 μl of PBS, mixed with 15 μl PI (1 μg/ml), and analyzed by flow cytometry on an Accuri C6 cytometer (Accuri Europe Ltd., St. Ives, United Kingdom). Measurements were performed, counting 30,000 PI-positive events at a flow rate of 2,500 to 3,500 events/s, scoring the mean FITC fluorescence of cells above the background threshold times the fraction of cells that were positive. The background was determined as the signal of bacteria without the addition of the anti-human IgG-FITC conjugate.
Calprotectin.
Calprotectin was measured in stool samples from patients and volunteers. Calprotectin was quantified using the Calprest ELISA kit (NovaTec Immundiagnostica, Germany) according to the manufacturer's instructions. The cutoff level for CD is 60 mg/kg. The optical density (OD) of the samples was measured at 405 nm with a microtiter plate reader and then plotted as the log value.
Statistics.
Differences in the percentages of bacterial IgG coating and differences in the calprotectin levels were evaluated with the nonparametric Kruskal-Wallis test (P < 0.05). Differences in microbiota composition were tested for significance using the Mann-Whitney U test, with a Bonferroni correction for the number of tests performed. Relationships between different parameters were tested with the nonparametric Spearman's rho test. The P value for significance was set at 0.05. For the analysis of IgG binding to E. coli, the Mann-Whitney U test (P < 0.05) or the Wilcoxon signed-rank test (P < 0.05) was used depending on the comparison, as indicated in the text.
RESULTS
CD patients show high autologous IgG coating against their own bacteria.
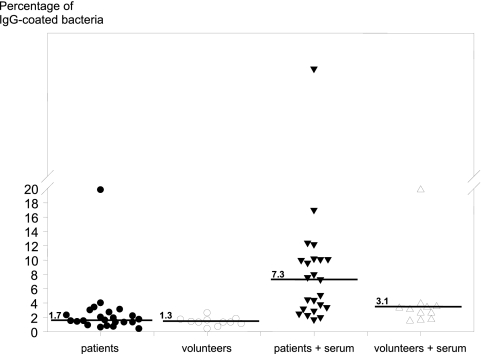
To see if there is an immune response against a person's own gut bacteria, a first series of flow cytometry analyses was performed on the fecal bacteria from CD patients and the volunteers with or without incubation with autologous serum. Table 2 shows the percentages of IgG-coated microbial cells derived from the patients and volunteers and the calprotectin levels. Figure 1 shows the percentages of IgG-coated fecal bacteria in the 4 incubation groups. The median of the percentage of in vivo IgG coating of bacteria was 1.2 times higher in patients than in volunteers, although a nonparametric Kruskal-Wallis test showed only a trend (P < 0.089), due to the large range (0.7 to 19.8 in patients versus 0.4 to 2.6 in volunteers). In contrast to the patients, almost all volunteers showed a low percentage of in vivo IgG-coated fecal bacteria, except volunteer 10, who had a relatively high percentage of IgG coating. When autologous serum was added to feces, the level of IgG-coated bacteria increased 4.3-fold in patients (range, 1.7- to 43.5-fold) and 2.4-fold in volunteers (range, 1.5- to 19.8-fold). This increase, compared to in vivo coating, was significant in both patients (P < 0.001) and volunteers (P < 0.001). Comparing the two groups after autologous-serum incubations showed that the patient group had significantly higher coating (P < 0.019), 2.4 times higher than the volunteer group. Spearman's rho test showed a significant correlation of 0.7 (P < 0.001) between the in vivo coating and the coating after autologous-serum incubations.
Table 2.
Global physician's assessment of disease status, disease location, and calprotectin levels of patients and volunteers and the percentage of IgG-coated bacteria with or without incubation with autologous serum
| Identifiera | Global physician's assessment (in remission/active) | Disease location | Calprotectin amt (mg/kg) | % IgG-coated bacteria |
|
|---|---|---|---|---|---|
| In vivo coating | In vivo + serum | ||||
| P1 | Active | Ileal/colonic | 2,357 | 3.1 | 10.1 |
| P2 | Active | Ileal/colonic | 2,140 | 19.8 | 17.0 |
| P3 | Active | Ileal/colonic | 1,604 | 3.4 | 3.8 |
| P4 | Active | Ileal | 1,091 | 0.7 | 1.7 |
| P5 | Active | Ileal/colonic | 841 | 2.4 | 4.5 |
| P6 | Active | Ileal | 273 | 2.0 | 4.4 |
| P7 | Active | Ileal | 227 | 2.2 | 7.6 |
| P8 | Active | Ileal | 215 | 1.5 | 12.2 |
| P9 | Active | Ileal/colonic | 89 | 0.9 | 2.3 |
| P10 | Remission | Ileal/colonic | 465 | 1.9 | 5.1 |
| P11 | Remission | Ileal | 323 | 2.3 | 12.4 |
| P12 | Remission | Colonic | 322 | 2.7 | 9.6 |
| P13 | Remission | Ileal | 249 | 1.6 | 8.0 |
| P14 | Remission | Colonic | 246 | 1.4 | 7.3 |
| P15 | Remission | Ileal | 229 | 1.5 | 3.2 |
| P16 | Remission | Colonic | 229 | 1.3 | 3.4 |
| P17 | Remission | Ileal | 197 | 0.8 | 2.9 |
| P18 | Remission | Ileal/colonic | 133 | 1.3 | 10.2 |
| P19 | Remission | Ileal | 124 | 0.6 | 2.0 |
| P20 | Remission | Ileal/colonic | 106 | 1.7 | 2.5 |
| P21 | Remission | Ileal/colonic | 97 | 4.0 | 43.5 |
| P22 | Remission | Ileal/colonic | 72 | 1.3 | 10.0 |
| P23 | Remission | Ileal/colonic | <20 | 3.0 | 10.1 |
| Median (23 patients) | 229 | 1.7 | 7.3 | ||
| V1 | NAb | NA | 31 | 1.6 | 3.4 |
| V2 | NA | NA | 24 | 0.7 | 1.6 |
| V3 | NA | NA | <20 | 0.4 | 1.7 |
| V4 | NA | NA | <20 | 1.8 | 3.3 |
| V5 | NA | NA | <20 | 1.4 | 3.9 |
| V6 | NA | NA | <20 | 1.7 | 3.1 |
| V7 | NA | NA | <20 | 1.3 | 3.5 |
| V8 | NA | NA | <20 | 1.3 | 2.6 |
| V9 | NA | NA | <20 | 1.1 | 2.6 |
| V10 | NA | NA | <20 | 2.6 | 19.8 |
| V11 | NA | NA | <20 | 1.3 | 1.5 |
| Median (11 volunteers) | <20 | 1.3 | 3.1 | ||
P, patient; V, volunteer.
NA, not assessed.
Fig 1.
Flow cytometry analysis of fecal samples from CD patients and healthy volunteers. Each symbol represents a patient or volunteer. The percentages of IgG-coated fecal bacteria for the four different groups (solid circles, in vivo IgG coating patients; open circles, in vivo IgG coating volunteers; solid triangles, patient samples after autologous-serum incubation; open triangles, volunteer samples after autologous-serum incubation). The lines represent the medians of the groups.
The calprotectin levels (Table 2) of the patient group were significantly higher (P < 0.000) than those of the volunteers, using the nonparametric Kruskal-Wallis test. The patient group can be divided into active CD and remission CD, based on a physician's global assessment. Comparing active CD to remission showed that the calprotectin levels were significantly higher in the active-CD group (P = 0.032). The calprotectin levels of both the active and remission groups were also significantly higher than those of the volunteers (both P < 0.001). There was no correlation between calprotectin levels and in vivo IgG coating or with coating after autologous-serum incubation in the patient group.
In contrast, patients in remission with no signs of disease showed higher in vivo coating (P = 0.035) than volunteers. The total amount of IgG in the serum samples was not different between the patients and volunteers and was not correlated with the in vivo coating or coating after serum incubation (data not shown).
The composition of the fecal flora is important for IgG coating.
In addition to the responses against autologous gut bacteria, we investigated whether the bacterial composition of the patients' and volunteers' microbiota or the composition of the serum immunoglobulins was responsible for the differences in IgG coating of autologous gut bacteria. Fecal bacteria from patients P1, P8, and P11, with high IgG coating after incubation with autologous serum, so-called autologous-IgG coating, also showed high IgG coating after incubation with heterologous serum. There was no difference in coating by the serum from volunteer V3 with low autologous-IgG coating (on average, 1.02-fold increase) or with that from V10 with high autologous coating (on average, 0.95-fold decrease). Patients P4, P9, and P19 from the group with low autologous IgG coating were also tested with heterologous sera of V3 and V10. P4 and P19 also showed low heterologous IgG coating (on average, 1.6-fold increase with V3 and 1.1-fold increase with V10). The exception was P9, who had more IgG-coated bacteria with heterologous serum (an increase of 3.1-fold for V3 and 2.7-fold for V10) than with autologous serum.
Fecal bacteria from volunteers were also incubated with heterologous sera from 6 patients and subsequently analyzed by flow cytometry. Fecal samples V3 and V11, with low autologous-IgG coating, and V5 and V10, with high(er) IgG coating, were incubated with sera of patients P2, P8, and P21, with high autologous-IgG coating, and P9, P19, and P20, with low autologous-IgG coating. The heterologous coating of bacteria with low autologous coating increased slightly, on average 1.3-fold for V11 and 2.5-fold for V3. For V5, the coating decreased 1.8-fold, and for V10, almost the same level of high IgG coating was observed (1.1 times lower). All these slight changes were similar for patient sera with high and low autologous coating. These experiments indicate that the bacterial compositions of both the CD patients and the volunteers were more important for IgG coating than the composition of the serum immunoglobulin.
Composition of the microbiota.
The bacterial compositions of the feces of the 23 CD patients and 11 healthy volunteers were analyzed using FISH. After visual counting and extrapolation to the number of bacteria per gram feces, the median composition was calculated (Table 3). In addition, the median percentage of total bacteria counted by DAPI staining was calculated. The median numbers of Enterobacteriaceae and ruminococci were higher in patients with CD (both P < 0.001), and the number of F. prausnitzii bacteria was lower (P = 0.001). After correction for multiple testing, no significant differences were detected for all the other bacterial groups.
IgG binding of E. coli isolates.
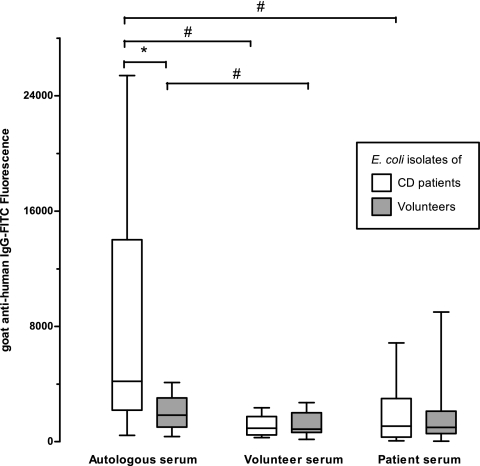
To investigate whether the higher numbers of Enterobacteriaceae in the patient samples also lead to more in vitro IgG coating with patient sera, we tried to culture them from feces that was stored for over a year at −20°C. Out of all 23 patient samples, 14 E. coli isolates were obtained from 8 samples. From the remaining 15 samples, E. coli could no longer be retrieved. In addition, 11 E. coli strains isolated from 7 volunteer samples and two laboratory strains, O157:H7 and ATCC 25422, were analyzed. First, the isolates were identified using the API-20E test kit (bioMérieux Inc., Hazelwood, MO) (data not shown). After the addition of autologous blood serum and incubation with anti-human IgG antibodies, the mean fluorescence above the threshold per cell times the fraction of positive bacteria that bind IgG on their cell surfaces was determined by flow cytometry. A significant difference in fluorescence, and thus the amount of coating per E. coli cell, was seen between CD patient isolates and those from healthy volunteers (Fig. 2). This difference was significant (P = 0.029) despite the small group of isolates and the observation that there were two patient isolates that bound almost no IgG, less than the two reference strains incubated with patient serum (data not shown). To test coating with heterologous sera, the sera were chosen from volunteer 8 and from a CD patient who was not in the study group. No E. coli isolates were obtained from the feces corresponding to these sera. When these heterologous sera were added to the E. coli cells, no differences were found between CD patient and volunteer isolates (Fig. 2). In order to test the differences between all strains treated with either autologous, volunteer, or patient serum, a Wilcoxon signed-rank test was used. All strains treated with autologous serum had 2.5 times greater amounts of IgG binding than with heterologous volunteer serum (P < 0.001) or patient serum (P = 0.015). The same was true when, for only the patient strains, autologous-serum incubations were compared with volunteer serum (P = 0.002) or patient serum (P = 0.013) incubation. In addition, volunteer isolates also bound more IgG from autologous serum than from volunteer serum (P = 0.041), but no difference was detected between volunteer isolates incubated with autologous and patient serum (Fig. 2).
Fig 2.
Box plot showing flow cytometry analysis of the serum IgG-binding capacities of 14 E. coli isolates from CD patients and 11 isolates from healthy volunteers. The E. coli cells were incubated with autologous serum or heterologous serum from a volunteer or a CD patient, as indicated. The medians (the bars in the boxes) of fluorescence per strain are shown. The boxes extend from the 25th to the 75th percentile, and the whiskers indicate the minimum and maximum. The brackets indicate significant differences (P < 0.05; *, Mann-Whitney U test; #, Wilcoxon signed-rank test).
No correlation could be found between API-20E types and the IgG-binding properties of the E. coli strains (data not shown) or between the calprotectin levels in the fecal samples and the amount of IgG coating. In summary, the E. coli strains in the feces of CD patients bound more IgG from autologous serum than the strains from healthy volunteers. This indicates that there is a specific immune reaction to fecal E. coli strains that is stronger in patients with CD than in healthy volunteers.
DISCUSSION
The humoral immune responses against autologous gut bacteria were studied in CD patients and healthy volunteers, and E. coli isolates were screened for the variation in the humoral immune response they provoke. IgG-coated bacteria were found in feces of both patients and volunteers, but this coating was significantly greater in patients, indicating a stronger humoral immune response against intestinal bacteria in CD patients. Furthermore, serum incubations with autologous serum showed binding of blood IgG to the fecal bacteria in both patients and volunteers. Therefore, both patients and volunteers have serum IgG that is able to bind to bacterial antigens. These IgG levels are probably caused by earlier exposure to these antigens, perhaps during a period when the barrier function was impaired.
In eight patients, the percentage of IgG-coated fecal bacteria increased to more than 10% after incubation with autologous serum. Volunteers also showed increased IgG coating after autologous-serum incubation, but only to a range of 1.5 to 3.9%, with one exception, i.e., to 19.8%. This volunteer had high IgG coating of his fecal bacteria after autologous-serum incubation, which may be explained by a current immune reaction, perhaps due to inflammation. Cross-incubations with heterologous sera and fecal bacteria from a selection of patients and volunteers with high and low in vivo IgG coating showed that there was no large difference in IgG coating of fecal bacteria compared to their own serum. This was the case for the selected patient feces with volunteer's serum or vice versa. These data indicate that the bacterial composition of the feces is more important for IgG coating than the composition of the serum. It also indicates that patients have more bacteria that can be coated with serum IgG than healthy volunteers and that this is independent of the type of serum IgG. This finding suggests that the composition of the bacterial microbiota of CD patients is different from that of healthy volunteers, which was also found by others (18, 25, 35).
The low levels of autologous-IgG coating of the fecal bacteria in healthy volunteers fits the idea of some kind of tolerance (or unresponsiveness) of the mucosal immune system for indigenous fecal bacteria. The higher levels of IgG coating in vivo or after autologous-serum incubation in CD patients indicate a breakdown of mucosal tolerance for the intestinal bacteria, perhaps caused by a leaky bowel (34).
Calprotectin levels were significantly higher in active-CD patients than in patients in remission, and both were significantly higher than those of healthy volunteers. This confirms that calprotectin is a useful serum marker for the activity of CD (23, 29), and it shows that patients in remission still have low-grade inflammation, since the calprotectin levels remain above background levels. The amount of IgG coating, however, is not correlated with calprotectin levels, which could indicate that patients in remission still have a gut with an impaired barrier function. To compare the calprotectin levels and the IgG coating as indicators of CD disease activity, a longitudinal study needs to be performed.
The differences in microbial composition can be important for the treatment of CD patients, since microbiota-modulating therapy with, for instance, pro- or prebiotics may normalize this in future and thereby reduce flare ups of CD. FISH analysis confirmed the reduced number of F. prausnitzii bacteria in the feces that were found earlier (25, 35). Low numbers of F. prausnitzii bacteria are counterbalanced by other bacteria, like E. coli, and more specifically adhesive-invasive E. coli (2). These bacteria can adhere to the mucosal layer, where they form biofilms, and they can even penetrate into the epithelial cells of the gut. When they have invaded the body, they can cause symptoms like diarrhea.
The last experiment of the study was focused on E. coli, since the role of E. coli in CD was discussed previously, and it has been shown that in genetically susceptible patients the clearance of E. coli is impaired (11, 33, 35). Here, CD patient isolates showed significantly more IgG binding with autologous serum than the isolates obtained from healthy volunteers. This shows that CD patients indeed have E. coli strains in their feces that are more immunogenic. It is not known what the exact difference is between the E. coli strains found in the CD patients and those from healthy volunteers. Their immune reactions are very strong, most likely against a strain-specific antigen. Whether these high-IgG-binding strains are more virulent needs to be investigated. It would also be intriguing to determine if these strains would cause a loss of barrier integrity by infection or even an invasion after a barrier integrity incident. Animal infection models using our isolates may resolve this. After invasion, the tolerance for E. coli may be lost and a specific reaction against the person's own E. coli bacteria can be generated. This reaction could become chronic.
In conclusion, we showed that CD patients have bacteria in their guts that are more reactive to IgG than those of volunteers, which may be the result of a period of impaired barrier function of the gut. Furthermore, the bacteria from CD patients are more prone to be coated with serum IgG than those from volunteers, regardless of the origin of the serum. This study confirms that there are differences in the numbers of E. coli and F. prausnitzii bacteria present in the feces of CD patients and those in volunteers. Finally, we showed that E. coli strains from CD patients specifically bind autologous IgG, which indicates a strong specific immune reaction against patients' own E. coli strains. In summary, our results show that CD patients have different and more immunogenic gut bacteria than healthy volunteers.
ACKNOWLEDGMENTS
The assistance of Renske Vonk and Danai Dimitropoulou during parts of this study is very much appreciated. We thank Gjalt W. Welling for carefully reading the manuscript.
Footnotes
Published ahead of print 15 February 2012
REFERENCES
- 1.Amann RI, et al. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnich N, Darfeuille-Michaud A. 2007. Adherent-invasive Escherichia coli and Crohn's disease. Curr. Opin. Gastroenterol. 23:16–20 doi: 10.1097/MOG.0b013e3280105a38. [DOI] [PubMed] [Google Scholar]
- 3.Franks AH, et al. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhard RL, et al. 1982. Acute viral enteritis and exacerbations of inflammatory bowel disease. Gastroenterology 83:1207–1209 [PubMed] [Google Scholar]
- 5.Gradel KO, et al. 2009. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 137:495–501 [DOI] [PubMed] [Google Scholar]
- 6.Harmsen HJM, Raangs GC, He T, Degener JE, Welling GW. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmsen HJM, et al. 2000. Development of 16S rRNA-based probes for the Coriobacterium group and the Atopobium cluster and their application for enumeration of Coriobacteriaceae in human feces from volunteers of different age groups. Appl. Environ. Microbiol. 66:4523–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter JO. 1998. Nutritional factors in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 10:235–237 [DOI] [PubMed] [Google Scholar]
- 9.Johne B, Kronborg O, Ton HI, Kristinsson J, Fuglerud P. 2001. A new fecal calprotectin test for colorectal neoplasia. Clinical results and comparison with previous method. Scand. J. Gastroenterol. 36:291–296 [PubMed] [Google Scholar]
- 10.Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. 2002. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand. J. Gastroenterol. 37:1034–1041 [DOI] [PubMed] [Google Scholar]
- 11.Lapaquette P, Glasser A, Huett A, Xavier RJ, Darfeuille-Michaud A. 2010. Crohn's disease-associated adherent-invasive E. coli are selectively favoured by impaired autophagy to replicate intracellularly. Cell. Microbiol. 12:99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer KH. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097–1106 [DOI] [PubMed] [Google Scholar]
- 13.Mazmanian SK, Round JL, Kasper DL. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453:620–625 [DOI] [PubMed] [Google Scholar]
- 14.Monteleone G, et al. 2006. Bacteria and mucosal immunity. Dig. Liver Dis. 38(Suppl. 2):S256–S260 [DOI] [PubMed] [Google Scholar]
- 15.Poullis A, Foster R, Mendall MA, Fagerhol MK. 2003. Emerging role of calprotectin in gastroenterology. J. Gastroenterol. Hepatol. 18:756–762 [DOI] [PubMed] [Google Scholar]
- 16.Poullis A, Foster R, Northfield TC, Mendall MA. 2002. Review article: faecal markers in the assessment of activity in inflammatory bowel disease. Aliment. Pharmacol. Ther. 16:675–681 [DOI] [PubMed] [Google Scholar]
- 17.Poulsen LK, Licht TR, Rang C, Krogfelt KA, Molin S. 1995. Physiological state of Escherichia coli BJ4 growing in the large intestines of streptomycin-treated mice. J. Bacteriol. 177:5840–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin J, et al. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riordan AM, et al. 1993. Treatment of active Crohn's disease by exclusion diet: East Anglian multicentre controlled trial. Lancet 342:1131–1134 [DOI] [PubMed] [Google Scholar]
- 20.Roseth AG, Aadland E, Jahnsen J, Raknerud N. 1997. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion 58:176–180 [DOI] [PubMed] [Google Scholar]
- 21.Roseth AG, Fagerhol MK, Aadland E, Schjonsby H. 1992. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand. J. Gastroenterol. 27:793–798 [DOI] [PubMed] [Google Scholar]
- 22.Schultsz C, Van Den Berg FM, Ten Kate FW, Tytgat GN, Dankert J. 1999. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology 117:1089–1097 [DOI] [PubMed] [Google Scholar]
- 23.Sipponen T, et al. 2008. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm. Bowel Dis. 14:1392–1398 [DOI] [PubMed] [Google Scholar]
- 24.Sokol H, et al. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U. S. A. 105:16731–16736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokol H, et al. 2009. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 15:1183–1189 [DOI] [PubMed] [Google Scholar]
- 26.Suau A, et al. 2001. Fusobacterium prausnitzii and related species represent a dominant group within the human fecal flora. Syst. Appl. Microbiol. 24:139–145 [DOI] [PubMed] [Google Scholar]
- 27.Swidsinski A, et al. 2002. Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44–54 [DOI] [PubMed] [Google Scholar]
- 28.Tamboli CP, Neut C, Desreumaux P, Colombel JF. 2004. Dysbiosis as a prerequisite for IBD. Gut 53:1057. [PMC free article] [PubMed] [Google Scholar]
- 29.Thjodleifsson B, et al. 2003. Subclinical intestinal inflammation: an inherited abnormality in Crohn's disease relatives? Gastroenterology 124:1728–1737 [DOI] [PubMed] [Google Scholar]
- 30.van der Waaij LA, et al. 2005. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm. Bowel Dis. 11:865–871 [DOI] [PubMed] [Google Scholar]
- 31.van der Waaij LA, et al. 2004. Immunoglobulin coating of faecal bacteria in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 16:669–674 [DOI] [PubMed] [Google Scholar]
- 32.Waar K, Degener JE, van Luyn MJ, Harmsen HJ. 2005. Fluorescent in situ hybridization with specific DNA probes offers adequate detection of Enterococcus faecalis and Enterococcus faecium in clinical samples. J. Med. Microbiol. 54:937–944 [DOI] [PubMed] [Google Scholar]
- 33.Walker A, et al. 2011. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wapenaar MC, et al. 2008. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis: an unusual case of ascites. Gut 57:463–467 [DOI] [PubMed] [Google Scholar]
- 35.Willing B, et al. 2009. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm. Bowel Dis. 15:653–660 [DOI] [PubMed] [Google Scholar]
- 36.Xavier RJ, Podolsky DK. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434 [DOI] [PubMed] [Google Scholar]