Abstract
During the period 2008 to 2010, we identified 11,386 serum samples with increased (positive) levels of antibodies recognizing Bordetella pertussis antigens. We sought to characterize the distribution of positive antibody result patterns in relation to patient age. IgG and IgA antibodies recognizing pertussis toxin (PT) and filamentous hemagglutinin (FHA) were quantified using a multianalyte immunodetection assay. Four mutually exclusive positive result patterns were observed: increased FHA antibodies only, increased PT IgA but not IgG, increased PT IgG but not IgA, and increased PT IgG and IgA. In patients <21 years old, the predominant pattern was increased PT IgG but not IgA, whereas in patients ≥21 years old, it was increased FHA antibodies only. The proportion of positive serum samples exhibiting increased PT IgA but not IgG was <20% in all age categories but showed a stepwise rise with age. The proportions of positive serum samples exhibiting increased PT IgG and IgA were similar (26 to 32%) in the age categories spanning 11 to 60 years of age but lower in the <11- and >60-year-old groups. In 3 of 5 age categories, a significant rise in the proportion of positive serum samples exhibiting increased FHA antibodies only occurred in 2010. Patterns of positive B. pertussis antibody results varied with age. The predominance of increased FHA antibodies only in patients >20 years old suggests that many adults thought to have B. pertussis infections actually have other infections that induce FHA-reactive antibodies. Similarly, the 2010 rise in the frequency of increased FHA antibodies only in some age groups suggests an increase in non-B. pertussis infections.
INTRODUCTION
Cough illness (pertussis) due to infection with Bordetella pertussis remains a persistent problem in the United States, with epidemic cycles every 2 to 5 years (2, 3, 14). Many cases are not diagnosed because the disease is often not considered, particularly among adolescents and adults, and confirmation of the diagnosis poses challenges. Culture using special media is highly specific, but cultures are often negative by the third week of illness, when the diagnosis may first be considered (1). PCR is being increasingly used for diagnosis and is more sensitive than culture, but sensitivity declines over time (1, 5). Measurement of antibodies can play an important role in the diagnosis of pertussis illness (1, 6–8, 13–17, 20, 22–26, 28), particularly after the second week of illness. Detection of an increased level of IgG or IgA antibody to pertussis toxin (PT) in a patient with prolonged cough illness provides strong support for a diagnosis of recent B. pertussis infection. An increased level of IgG or IgA antibody to filamentous hemagglutinin (FHA) also occurs in B. pertussis infection but is a less specific marker of B. pertussis infection because other Bordetella species express FHA and cross-reacting antibodies are also induced by other bacteria (14, 17, 26). The interpretation of increased antibody positive result patterns can be a challenge due to a variety of factors, including differing assay sensitivities and specificities and the complex relationship between patient age and the B. pertussis antibody isotypes produced following vaccination (25).
Serum samples submitted to the Focus Diagnostics reference laboratory for the B. pertussis antibody panel (PT IgG, PT IgA, FHA IgG, FHA IgA) arrive from all geographic areas of the United States but are not accompanied by information indicating the reason for testing. The panel was originally developed for the serologic diagnosis of pertussis, and conversations with physicians indicate that, in most situations, it is used for this purpose. However, the panel is, on occasion, used by clinicians to assess the response to pertussis vaccination in children or to determine immunity or susceptibility to B. pertussis infection. The panel's use for the latter two reasons is problematic. Specifically, the pertussis vaccines in current use (DTaP and Tdap) also contain other antigens (i.e., pertactin and fimbriae), and antibodies to these antigens are more likely to be serologic correlates of protection (4, 24). As part of our overall goal of providing the most accurate panel interpretations possible for patients of all ages, we sought to characterize the frequencies at which various positive (increased) antibody detection patterns occur and assess the relationship of a given pattern to patient age.
MATERIALS AND METHODS
Serum samples submitted to Focus Diagnostics for pertussis antibody testing were evaluated using a validated tetraplex microsphere-based multianalyte immunodetection assay as previously described (21); the four analytes measured were PT IgG, PT IgA, FHA IgG, and FHA IgA. Results were expressed quantitatively as IU/ml based on interpolation from a secondary standard with assigned values traceable to the World Health Organization international B. pertussis antibody standard (29). Reference ranges, based on the 95th percentile values obtained for a group of 200 blood donor serum samples collected in 2005, were as follows: PT IgG, <45 IU/ml; PT IgA, <10 IU/ml; FHA IgG, <90 IU/ml; FHA IgA, <50 IU/ml. Values within the reference range for a given analyte were considered normal, whereas values above the reference range were considered increased (positive).
Differences among proportions were evaluated by chi-square analysis (MedCalc Software, Mariakerke, Belgium). P values were corrected for multiple comparisons using the Bonferroni adjustment; corrected P values of <0.05 were considered significant.
RESULTS
During the time period 2008 to 2010, we identified 11,386 serum samples with increased levels of PT IgG, PT IgA, FHA IgG, and/or FHA IgA. As shown in Table 1, the patient age distribution observed for serum samples with an increased level of one or more pertussis antibodies was comparable to that observed for serum samples without increased levels of pertussis antibodies. In 2010, the number of specimens submitted for pertussis antibody testing increased by 26% over the 2009 number and the number of specimens positive for any pertussis antibody increased by 20%.
Table 1.
Distribution of samples submitted for B. pertussis antibody testing across age groups in relation to detection of increased levels of any pertussis antibody
| Age group (yr) | % of samples with: |
|
|---|---|---|
| No pertussis antibody increase | Any pertussis antibody increase | |
| <11 | 14 | 15 |
| 11-20 | 9 | 13 |
| 21-40 | 22 | 16 |
| 41-60 | 32 | 27 |
| >60 | 17 | 23 |
| Unknown | 5 | 6 |
The 11,386 serum samples with increased levels of one or more pertussis antibodies were grouped into 4 mutually exclusive result sets: (i) increased FHA IgG and/or IgA only (also referred to as the increased FHA antibody only group), (ii) increased PT IgA but not PT IgG, (iii) increased PT IgG but not PT IgA, and (iv) increased PT IgG and PT IgA. For the latter 3 groups defined by increased levels of one or both PT antibody isotypes, FHA antibody levels were not used as classification criteria; thus, some patients in these groups had increased levels of FHA IgG and/or IgA, whereas others did not. The proportional distribution of the 11,386 serum samples among the 4 antibody detection patterns was as follows: 39% increased FHA antibody only, 8% increased PT IgA but not PT IgG, 29% increased PT IgG but not PT IgA, and 24% increased PT IgG and PT IgA. Table 2 presents the number of serum samples within each positive antibody result set by age category for each of the 3 years (2008, 2009, and 2010) and all 3 years combined. These results were used to calculate the proportions shown in Fig. 1 to 4.
Table 2.
Age distribution of patients with positive B. pertussis antibody result patterns over a 3-year period
| Yr and antibody reactivity increase pattern | No. of samples in each age category |
|||||
|---|---|---|---|---|---|---|
| <11 yr | 11-20 yr | 21-40 yr | 41-60 yr | >60 yr | Unknown | |
| 2008 | ||||||
| FHA IgG and/or IgA only | 53 | 97 | 155 | 307 | 358 | 89 |
| PT IgA, not PT IgG | 6 | 13 | 21 | 74 | 132 | 24 |
| PT IgG, not PT IgA | 285 | 200 | 155 | 184 | 82 | 168 |
| PT IgG + PT IgA | 103 | 134 | 120 | 217 | 116 | 109 |
| 2009 | ||||||
| FHA IgG and/or IgA only | 86 | 136 | 266 | 426 | 411 | 67 |
| PT IgA, not PT IgG | 13 | 10 | 41 | 86 | 131 | 4 |
| PT IgG, not PT IgA | 356 | 234 | 155 | 220 | 85 | 74 |
| PT IgG + PT IgA | 127 | 174 | 155 | 280 | 125 | 52 |
| 2010 | ||||||
| FHA IgG and/or IgA only | 167 | 145 | 339 | 669 | 688 | 21 |
| PT IgA, not PT IgG | 7 | 9 | 39 | 101 | 191 | 5 |
| PT IgG, not PT IgA | 320 | 204 | 176 | 240 | 112 | 13 |
| PT IgG + PT IgA | 161 | 176 | 188 | 316 | 166 | 17 |
| All 3 yr combined | ||||||
| FHA IgG and/or IgA only | 306 | 378 | 760 | 1,402 | 1,457 | 177 |
| PT IgA, not PT IgG | 26 | 32 | 101 | 261 | 454 | 33 |
| PT IgG, not PT IgA | 961 | 638 | 486 | 644 | 279 | 255 |
| PT IgG + PT IgA | 391 | 484 | 463 | 813 | 407 | 178 |
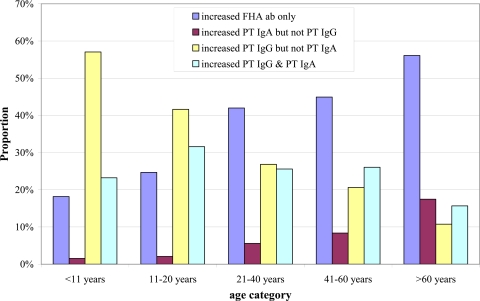
Fig 1.
Distribution of the four positive B. pertussis antibody (ab) result patterns within different age categories for the entire 3-year study period.
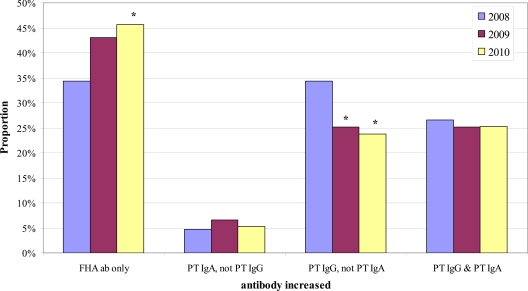
Fig 4.
Yearly proportions of the 4 positive B. pertussis antibody (ab) result patterns of patients in the 41- to 60-year-old age category. The double asterisks indicate that the proportion was significantly different from both the 2008 and 2009 proportions.
Figure 1 presents the distribution of the 4 positive antibody result patterns within a given age category for the entire 3-year study period. The proportion of positive results due to increased PT IgG but not PT IgA gradually decreased with age, whereas the proportion due to increased FHA antibodies only and the proportion due to increased PT IgA but not PT IgG increased with age. No clear-cut relationship between age and the proportion of positive results due to increased PT IgG and PT IgA was observed; however, the lowest percentage was observed in the >60-year-old age category.
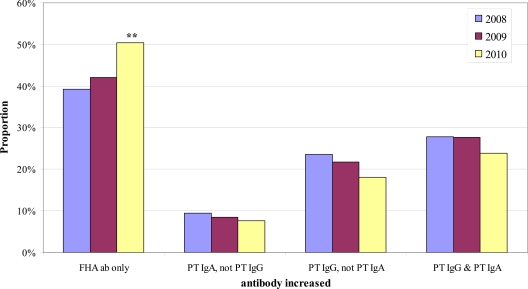
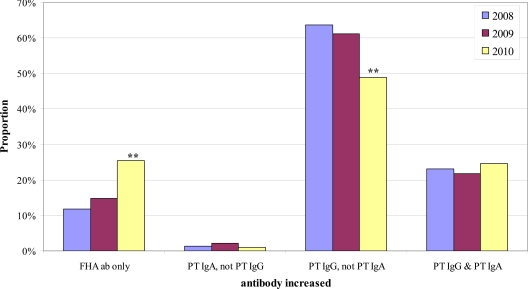
We next sought to determine if the distribution of the 4 positive antibody result patterns within a given age category changed over time. Chi-square analysis identified significant yearly differences in the distribution of positive result patterns in 3 of the 5 age categories: <11 years old, 21 to 40 years old, and 41 to 60 years old. For these 3 age groups, we then performed pairwise comparisons by year of the proportion of samples exhibiting a given positive result pattern. Figure 2 shows the results for the <11-year-old age category; the proportion of positive results due to increased FHA antibodies only was significantly higher in 2010 than in both 2008 and 2009, with a corresponding significant decrease in the proportion of positive results due to increased PT IgG but not PT IgA. Figure 3 shows the results for the 21- to 40-year-old age category. The proportion of positive results due to increased FHA antibodies only was significantly greater in 2010 than in 2008 but not greater than that in 2009. However, the proportion of positive results due to increased PT IgG but not PT IgA in the 21- to 40-year-old age category was significantly lower in both 2009 and 2010 than in 2008 (Fig. 3). Results for the 41- to 60-year-old age category are shown in Fig. 4; the proportion of positive results due to increased FHA antibodies only was significantly higher in 2010 than in each of the previous years.
Fig 2.
Yearly proportions of the 4 positive B. pertussis antibody (ab) result patterns of patients in the <11-year-old age category. Double asterisks indicate that the proportion was significantly different from both the 2008 and 2009 proportions.
Fig 3.
Yearly proportions of the 4 positive B. pertussis antibody (ab) result patterns of patients in the 21- to 40-year-old age category. Asterisks indicate that the proportion was significantly different from the 2008 proportion.
DISCUSSION
A variety of factors complicates the interpretation of B. pertussis antibody detection patterns. One such factor is assay sensitivity; the vast majority of B. pertussis-infected patients exhibit increased levels of IgG recognizing PT and FHA, but only about 60% of these patients also show increased levels of IgA recognizing these antigens (8, 16, 19). An IgG-positive but IgA-negative pattern in a child with a cough can be particularly difficult to interpret, since this same reactivity pattern is found following vaccination (9). Another factor is assay specificity. Because PT is produced only by B. pertussis, detection of antibodies to PT is considered specific for B. pertussis infection or vaccination. FHA, in contrast, is found in other Bordetella species, including B. parapertussis, and patients infected with these organisms exhibit increased levels of FHA antibodies (14, 23, 25). Similarly, proteins closely related to FHA are found in other microorganisms, and infection with these microbes can induce cross-reactive antibodies (14, 26). A third factor is the relationship between patient age and B. pertussis antibody isotypes produced following vaccination. Individuals whose only exposure to B. pertussis antigens occurs via vaccination produce only IgG to PT and FHA (9, 23), whereas vaccination of individuals who have previously experienced B. pertussis infection produces IgA, as well as IgG, antibodies (12). Most young children fall into the first category and thus produce only IgG antibodies following vaccination. However, unrecognized B. pertussis infections are common in children so that some young vaccinated children will have both IgA and IgG responses to immunization (7). Most adolescents and adults, even though having been vaccinated in childhood, have experienced a prior (often unrecognized) B. pertussis infection and exhibit increased levels of IgG and IgA antibodies following vaccination (12). Thus, the expected B. pertussis antibody reactivity pattern of vaccinated adolescents/adults may be indistinguishable from that characterizing acute infection.
Our findings demonstrate that the distribution of 4 mutually exclusive positive B. pertussis antibody result patterns differs markedly depending on patient age. The proportion of positive results due to increased PT IgG and PT IgA, the pattern most strongly associated with recent B. pertussis infection (7, 8, 10, 14, 17, 22, 23, 25), was similar in all ≤60-year-old patient age categories. Increased PT IgG but not PT IgA was the predominant positive result pattern in patients <21 years old, whereas increased FHA antibodies only was the predominant pattern in patients ≥21 years old. Although the proportion of positive results due to increased PT IgA without increased PT IgG was low in all age groups, there was a clear increase in this proportion with age.
Children and teenagers with increased PT IgG but not PT IgA may represent either individuals being evaluated for an appropriate B. pertussis vaccine response or patients with acute B. pertussis infection exhibiting increased PT IgG without increased PT IgA. Unfortunately, none of the samples were accompanied by information we could use to distinguish vaccine response versus infection.
The observation that an increase in FHA antibodies only (without an increase in PT antibodies) was the predominant abnormal antibody result pattern among adults suggests that many adults suspected of having pertussis actually have a different infection that induces FHA-reactive antibodies (14, 17, 26). Such infections include Chlamydophila pneumoniae and Mycoplasma pneumoniae infections, both of which have been associated with prolonged cough illness, although the cough duration tends to be shorter than it is with pertussis (18, 27). B. parapertussis, B. bronchiseptica, B. holmesii, and nonencapsulated Haemophilus influenzae also induce FHA-reactive antibodies (14, 26); there may also be other, as-yet-unknown, microbes that trigger the production of such antibodies. Specifically, in a study of army personnel with prolonged cough illness in Korea, it was found that a number had high FHA antibody titers without high PT or pertactin antibody titers and no evidence of infection with either M. pneumoniae or C. pneumoniae (26). Our observation that 56% of patients >60 years old with an abnormal result had increased FHA antibody titers only is consistent with the results of Hodder et al. (11), who found that 39% of patients >65 years old with a ≥2-fold increase in antibodies to a pertussis antigen showed increased FHA antibodies only. A pattern of increased FHA antibodies alone in a patient with a cough illness should suggest the possibility of infection with an organism other than B. pertussis.
To our knowledge, our finding of a direct association between age and the proportion of positive results due to increased PT IgA but not PT IgG has not been documented previously. This association may be due to the cumulative effect of specific exposures to B. pertussis; however, repeated exposures would be expected to increase levels of IgG, as well as IgA, antibodies to PT. Perhaps our cutoff point for PT IgA-positive values (<10 IU/ml) is disproportionally low or our cutoff point for PT IgG (<45 IU/ml) is disproportionally high.
Our interest in comparing positive B. pertussis antibody result patterns over the 3-year study period was sparked, in part, by the 2010 pertussis outbreak in several states, particularly California. Cherry and Seaton (5) documented an increase in specimens submitted to Focus Diagnostics for B. pertussis/parapertussis PCR testing and an increase in the proportion of specimens positive for B. parapertussis (from 7.9% in 2009 to 16.5% in 2010). Nearly all of the samples positive for B. parapertussis in 2010 were from children <11 years old. Similarly, our analysis identified a significant increase in the proportion of positive antibody results due to increased FHA antibodies only, with the most notable increase (from 15% in 2009 to 25% in 2010) occurring among children <11 years old. These findings directly support the PCR data, since patients with B. parapertussis infection would be expected to exhibit increased levels of FHA antibodies but not PT antibodies. We also observed a significant 2010 increase in the proportion of positive results due to increased FHA antibodies only among adults 21 to 60 years old. It is unclear, however, if this increase reflected infection with B. parapertussis (the PCR data did not indicate an increase in B. parapertussis infections in this age group) or that greater testing of adults with a cough illness identified infections with other microorganisms that induce FHA-reactive antibodies (26).
Footnotes
Published ahead of print 22 February 2012
REFERENCES
- 1. CDC 14 February 2011, posting date Pertussis (whooping cough) diagnosis confirmation. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/pertussis/clinical/diagnostic-testing/diagnosis-confirmation.html [Google Scholar]
- 2.Cherry JD. 2005. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics 115: 1422– 1427 [DOI] [PubMed] [Google Scholar]
- 3.Cherry JD. 2010. The present and future control of pertussis. Clin. Infect. Dis. 51: 663– 667 [DOI] [PubMed] [Google Scholar]
- 4.Cherry JD, Gornbein J, Heininger U, Stehr K. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16: 1901– 1906 [DOI] [PubMed] [Google Scholar]
- 5.Cherry JD, Seaton BL. 2012. Patterns of Bordetella parapertussis respiratory illnesses: 2008-2010. Clin. Infect. Dis. 54: 534– 537 [DOI] [PubMed] [Google Scholar]
- 6.Deen JL, et al. 1995. Household contact study of Bordetella pertussis infections. Clin. Infect. Dis. 21: 1211– 1219 [DOI] [PubMed] [Google Scholar]
- 7.de Melker HE, et al. 2000. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 38: 800– 806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallander HO. 1999. Microbiological and serological diagnosis of pertussis. Clin. Infect. Dis. 28 (Suppl 2): S99– S106 [DOI] [PubMed] [Google Scholar]
- 9.Heininger U, et al. 1994. Comparative study of Lederle/Takeda acellular and Lederle whole-cell pertussis-component diphtheria-tetanus-pertussis vaccines in infants in Germany. Vaccine 12: 81– 86 [DOI] [PubMed] [Google Scholar]
- 10.Heininger U, Cherry JD, Stehr K. 2004. Serologic response and antibody-titer decay in adults with pertussis. Clin. Infect. Dis. 38: 591– 594 [DOI] [PubMed] [Google Scholar]
- 11.Hodder SL, et al. 2000. Antibody responses to Bordetella pertussis antigens and clinical correlations in elderly community residents. Clin. Infect. Dis. 31: 7– 14 [DOI] [PubMed] [Google Scholar]
- 12.Le T, et al. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT Study. J. Infect. Dis. 190: 535– 544 [DOI] [PubMed] [Google Scholar]
- 13.Marchant CD, et al. 1994. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis, and vaccine effectiveness. J. Infect. Dis. 169: 1297– 1305 [DOI] [PubMed] [Google Scholar]
- 14.Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18: 326– 382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May ML, Doi SA, King D, Evans J, Robson JM. 2012. Prospective evaluation of an Australian pertussis toxin IgG and IgA enzyme immunoassay. Clin. Vaccine Immunol. 19: 190– 197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meade BD, Mink CM, Manclark CR. 1990. Serodiagnosis of pertussis, p 322– 329 Proceedings of the Sixth International Symposium on Pertussis National Institutes of Health, Bethesda, MD [Google Scholar]
- 17.Mink CM, et al. 1992. A search for Bordetella pertussis infection in university students. Clin. Infect. Dis. 14: 464– 471 [DOI] [PubMed] [Google Scholar]
- 18.Miyashita N, Fukano H, Yoshida K, Niki Y, Matsushima T. 2003. Chlamydia pneumoniae infection in adult patients with persistent cough. J. Med. Microbiol. 52: 265– 269 [DOI] [PubMed] [Google Scholar]
- 19.Müller FM, Hoppe JE, Wirsing von König CH. 1997. Laboratory diagnosis of pertussis: state of the art in 1997. J. Clin. Microbiol. 35: 2435– 2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nennig ME, Shinefield HR, Edwards KM, Black SB, Fireman BH. 1996. Prevalence and incidence of adult pertussis in an urban population. JAMA 275: 1672– 1674 [PubMed] [Google Scholar]
- 21.Prince HE, Lape-Nixon M, Matud J. 2006. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin. Vaccine Immunol. 13: 266– 270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt-Grohé S, et al. 1995. Pertussis in German adults. Clin. Infect. Dis. 21: 860– 866 [DOI] [PubMed] [Google Scholar]
- 23.Stehr K, et al. 1998. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics 101: 1– 11 [DOI] [PubMed] [Google Scholar]
- 24.Storsaeter J, Hallander HO, Gustafsson L, Olin P. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16: 1907– 1916 [DOI] [PubMed] [Google Scholar]
- 25.Tondella ML, et al. 2009. International Bordetella pertussis assay standardization and harmonization meeting report. Centers for Disease Control and Prevention, Atlanta, Georgia, United States, 19-20 July 2007. Vaccine 27: 803– 814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent JM, et al. 2000. Prolonged afebrile nonproductive cough illnesses in American soldiers in Korea: a serological search for causation. Clin. Infect. Dis. 30: 534– 539 [DOI] [PubMed] [Google Scholar]
- 27.Wang K, et al. 2011. Mycoplasma pneumoniae and respiratory virus infections in children with persistent cough in England: a retrospective analysis. Pediatr. Infect. Dis. J. 30: 1047– 1051 [DOI] [PubMed] [Google Scholar]
- 28.Wright SW, Edwards KM, Decker MD, Zeldin MH. 1995. Pertussis infection in adults with persistent cough. JAMA 273: 1044– 1046 [PubMed] [Google Scholar]
- 29.Xing D, et al. 2009. Characterization of reference materials for human antiserum to pertussis antigens by an international collaborative study. Clin. Vaccine Immunol. 16: 303– 311 [DOI] [PMC free article] [PubMed] [Google Scholar]