Abstract
Free-living amoebae are protozoa found in soil and water. Among them, some are pathogenic and many have been described as potential reservoirs of pathogenic bacteria. Their cell cycle is divided into at least two forms, the trophozoite and the cyst, and the differentiation process is named encystment. As cysts are more resistant to disinfection treatments than trophozoites, many studies focused on encystment, but until recently, little was known about cellular, biochemical, and molecular modifications operating during this process. Important signals and signaling pathways at play during encystment, as well as cell responses at the molecular level, have been described. This review summarizes our knowledge and focuses on new findings.
INTRODUCTION
Free-living amoebae (FLA) are protozoa commonly found in soil and water. Some of them are pathogenic (54) and can also harbor pathogenic bacteria (61). Indeed, FLA feed on bacteria by phagocytosis, but some bacteria can resist phagocytosis (23); among them, some, such as Legionella pneumophila, are even able to multiply within FLA (52). Thus, interaction between FLA and L. pneumophila is a major concern for public health (3, 32). FLA have developed a strategy in response to adverse conditions or stresses: they differentiate from trophozoites, the vegetative form, to cyst, the resting form. This differentiation is termed encystment (for a review, see references 44 and 66). Encystment of FLA occurs under various conditions, such as nutrient starvation and osmotic stress, and as a response to bacterial toxins (12, 18, 22, 27, 46). Cysts are particularly resistant to treatments and consequently play a critical role in survival and spreading of FLA and potential intracellular bacteria. Cysts and encystment of FLA have been studied for many years, mainly at the morphological and biochemical levels. More recently, cellular and molecular studies have provided a better understanding of the mechanisms of encystment, but these new findings have never been reviewed. The aim of this paper is to review these new findings.
CYST WALL MORPHOLOGY OF FLA
The differentiation of trophozoites into cysts induces huge morphological changes (Fig. 1 and 2). Cysts are spherical and possess an outer layer, the cyst wall. However, the morphology of cyst walls depends on the genus and species and has been the basis for identification of FLA.
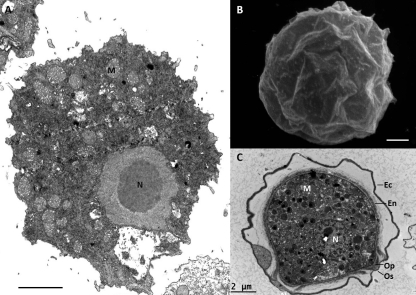
Fig 1.
Electron microscopy of Acanthamoeba sp. (A) Transmission electron microscopy of a trophozoite. (B) Scanning electron microscopy of a cyst. (C) Transmission electron microscopy of a cyst. Ec, ectocyst; En, endocyst; M, mitochondria; N, nucleus; Op, operculum; Os, ostiole. Scale bars = 2 μm.
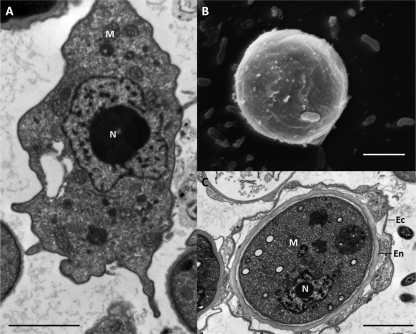
Fig 2.
Electron microscopy of Hartmannella sp. (A) Transmission electron microscopy of a trophozoite. (B) Scanning electron microscopy of a cyst. (C) Transmission electron microscopy of a cyst. Ec, ectocyst; En, endocyst; M, mitochondria; N, nucleus. Scale bars = 2 μm.
The cysts of Acanthamoeba have a double-layered wall. The ectocyst is the external layer, and the endocyst, which is formed after the ectocyst, is the internal fibrillar layer. These layers are composed of at least acid-insoluble proteins (45) and cellulose (62), but the exact composition is not well known. The structure of Acanthamoeba cysts has been well described by electron microscopy (5, 9). One to several holes named ostioles are present at the surface of the cyst wall. It has been proposed that excystment occurs through these ostioles after digestion of the opercula covering the ostioles (9).
Chávez-Munguía et al. suggest that cellulose is present in both layers of the cyst wall (9), while previous studies proposed that cellulose is present only in the endocyst (30, 66). Recently, it has been shown that the ectocyst presented an irregular surface and that vesicles were found within its wall. Using quick-freeze fracture/deep etching with electron microscopy, it has been shown that the endocyst is thinner and more fibrillar than the ectocyst and resembles the cellulose structure in plant cell walls (29). Encystment has been demonstrated to correlate with development of biocide resistance, with increasing content in alkali-insoluble residues (cellulose) being associated with increasing resistance to most chemical agents (63). Recent studies demonstrated considerable variations in resistance to biocides of closely related Acanthamoeba isolates, suggesting that important variations exist between cyst wall compositions of these isolates (13).
Regarding Naegleria spp., there are reports with discrepant observations concerning the cyst wall structure, likely partly due to the fact that different strains and species were used. Electron microscopy studies reported that the cyst wall of N. gruberi consists of a double layer, with the ectocyst being irregular and approximately 25 nm thick and the endocyst appearing layered and 200 to 450 nm thick. They both join at ostioles that are closed by opercula as described for Acanthamoeba spp. (53). It has also been reported that the outer layer present in the cysts of N. gruberi is absent in N. fowleri and N. jadini (34). A recent analysis suggests that the Naegleria cyst wall is formed only as a single thick fibrillar layer with detached irregular thin loops that might have been confounded with a real ectocyst (8). The detailed composition of Naegleria sp. cyst walls remains unknown, but they are thought to contain chitin, since they are not stained with a cellulose-specific marker (30) but can be stained with calcofluor white M2R, indicating the presence of another kind of β-1-4-linked polysaccharide (8). It should be underlined that chitin is found in Entamoeba cysts (2).
To our knowledge, there is only one publication describing the composition of Hartmannella cyst walls. It seems that the major components of the cyst wall are proteins with a glucose polymer present in low quantity (64). Such as for Naegleria, the cysts of Hartmannella spp. are not stained with a cellulose-specific marker, suggesting that there is no cellulose in the walls (30). A morphological study reported that the cyst wall of Hartmannella vermiformis is composed of a 50-nm-thick endocyst and a 110- to 140-nm-thick ectocyst consisting of multilayered filamentous material (58).
In Balamuthia spp., a third layer, named the mesocyst, has been described (35).
SIGNALS AND SIGNALING PATHWAYS
The signals inducing encystment of FLA are various. Starvation is a major signal for encystment in FLA (46, 50), and it also induces encystment or differentiation in other amoebae, such as Entamoeba and Dictyostelium spp. Osmotic stress also clearly induces encystment of Acanthamoeba, and this is true for osmotic stress induced by various compounds like salts and glucose (6, 12, 18).
Also, the presence of intracellular bacteria could interfere with encystment (14). Francisella tularensis type A strains cause the rapid encystment of Acanthamoeba castellanii (21). Toxin-producing bacteria, such as Pseudomonas aeruginosa, can induce encystment in Naegleria fowleri and Acanthamoeba castellanii (22, 27).
Encystment is efficiently induced by magnesium ions and taurine in a nonnutrient medium (50). Magnesium and taurine induce cyclic AMP (cAMP) synthesis, with cAMP itself being able to induce encystment (49). cAMP induction could be due to activation of adenylate cyclase or inhibition of phosphodiesterase. An inhibitor of phosphodiesterase caused induction of encystment (49).
Encystment of Acanthamoeba is also induced by catecholamines (e.g., epinephrine and norepinephrine) and taurine (60, 65). The encystment induced by epinephrine is likely mediated by a receptor that activates cAMP synthesis (43). Again, there is a similar mechanism in Entamoeba, where a catecholamine-like molecule is produced in response to interaction with galactose-terminated ligands, like mucin. This catecholamine binds to an adrenergic-like receptor and induces cAMP synthesis and encystment of this amoeba (11, 20).
Recently, Siddiqui et al. have shown that galactose enhances Balamuthia encystment (57). These authors suggested that galactose might mediate its response via a galactose binding protein receptor, which has been previously described for Balamuthia (36).
Also, Akins and Byers reported earlier that a factor, named encystment-enhancing activity (EEA), secreted by Acanthamoeba is able to stimulate encystment (1). The nature of this factor is not described, but the authors suggest that it may be a modified nucleotide, because its activity was sensitive to snake venom phosphodiesterase. However, this factor should be different from cAMP, as cAMP phosphodiesterase was not active against EEA. It is interesting that EEA was produced in high-density cultures; thus, the authors speculated that this factor could act similarly to a quorum-sensing signaling molecule. As well, Eichinger hypothesized that catecholamines might be quorum-sensing molecules in Entamoeba (20), and actually, catecholamines are agonists of autoinducer-3, a quorum-sensing molecule described in some bacteria (59). It suggests that catecholamines might be involved in prokaryote-eukaryote communication. Our recent studies show that higher cell density led to higher rates of encystment in Acanthamoeba, suggesting that this process could be controlled by quorum sensing (E. Fouque, unpublished data).
Few signaling pathways have been proposed to be involved in encystment. In Acanthamoeba, farnesyl protein transferase (FPT III), an inhibitor of Ras farnesylation, reduced encystment (17). Ras is a small GTPase that could activate the mitogen-activated protein kinase (MAPK) pathway. However, other inhibitors of the MAPK pathway (the p38 MAPK inhibitor SB203580 and the MEK inhibitor PD98059) were tested, but they did not show any effects on encystment. Together, these results suggest that the MAPK pathway might be involved in encystment but that the components of this pathway remain to be described. Also, the receptors mediating this pathway remain to be characterized. As cAMP has a direct impact on encystment, cAMP receptors are likely involved, but Ras might also be coupled to the tyrosine kinase family (RTK). In Balamuthia, tyrosine- and phosphatidylinositide 3-kinase (PI3K)-mediated pathways are likely involved, since inhibitors reduce encystment (57). Deciphering the signaling pathways is of primary importance to understanding the mechanism of encystment in FLA, and one should keep in mind that different pathways could be triggered by different signals.
THE CYTOSKELETON
As encystment is associated with morphological changes, it is obvious that cytoskeletal rearrangements might occur. An early publication described the control of actin synthesis during encystment (25). Transcription of actin genes was slightly repressed during early stage of encystment, but protein synthesis was highly reduced, suggesting a translational regulation. A recent study described that actin is partially degraded during the early stage of encystment (28).
An inhibitor of actin polymerization, cytochalasin D, and an inhibitor of Rho kinase, Y27632, repressed encystment of Acanthamoeba. Rho kinase is a small GTPase involved in regulation of actin polymerization (17). These results confirmed that cytoskeleton rearrangement is crucial for encystment. Lately, similar results have been obtained for Balamuthia (57), suggesting that actin control is likely important for encystment of various FLA.
At the protein level, the gelation factor was induced in cysts (4). This factor belongs to the filamin family and is involved in actin cross-linking to maintain cell shape. Conversely, actophorin, a protein from the ADP/cofilin family involved in the actin dynamic, is repressed in cysts (4). These results both suggest that actin turnover is reduced. Recently, three genes encoding proteins of the ADP/cofilin family of Entamoeba invadens have been studied. One of them was repressed during encystment, while the two others were induced during excystment (33).
PROTEASES
Different proteases are likely involved in encystment and/or excystment of Acanthamoeba. One of them, a subtilisin-like serine protease, is clearly associated to encystment. The mRNA corresponding to this protein is induced during encystment (39, 40). The protein is also specifically detected in cysts, as shown by two-dimensional (2D) gel electrophoresis (4, 47). In addition, small interfering RNA (siRNA) experiments targeting the catalytic domain of serine proteases, as well as chemical inhibitors of serine proteases, efficiently reduced encystment in Acanthamoeba (16, 41). Altogether, these various works demonstrate that serine proteases, at least the subtilisin-like serine protease, are crucial for the differentiation into cysts. During differentiation, there is a need for protein turnover, which is completed via lysosomes or ubiquitin-proteasome systems. It is proposed that these proteases may mediate autophagic functions, as the protein associates with autophagosomes during encystment (16, 41). Reinforcing this hypothesis, a recent study showed that an autophagy protein was also involved in encystment. Indeed, this protein was induced in cysts, and repression of its synthesis by siRNA decreased encystment (42). Also, the presence of autolysosomes during encystment was visualized by electron microscopy (5).
Recently, another family of proteases, the cysteine proteases, was also reported to be involved in encystment. A study carefully followed by 2D gel electrophoresis the expression of proteins during encystment (28). The authors concluded that most of the changes occurred during the early stage of encystment. They also showed that specific inhibitors of cysteine proteases partially reduce encystment. Cysteine proteases are also induced at the RNA level during encystment (38). In Entamoeba, a cysteine protease, induced during encystment, did not colocalize with autophagosomes, suggesting that it is not involved in autophagy (19).
THE CYST-SPECIFIC PROTEIN CSP21
The first described molecular change in FLA during encystment is the expression of CSP21 (cyst-specific protein of 21 kDa). This protein has been identified by SDS-PAGE analysis. CSP21 is hydrophilic and produced during early stages of encystment (24). Despite the fact that the precise cellular localization is not known, immunodetection results suggest that this protein is associated with cyst walls (24). Studies at the mRNA level have confirmed its early expression during the encystment process (10) and that this expression is specific for cysts (39). However, the role of this protein is not established yet.
CELL WALL SYNTHESIS
Pioneering works have demonstrated that during encystment, Acanthamoeba induces the incorporation of glucose into cellulose (50, 51) and that β-glucan synthetase activity is induced (48). The digestion of cyst walls could be achieved by proteases, cellulase, and chitinase. These enzymes are secreted by Acanthamoeba during excystment (26). Galactose and glucose were present in high levels in the cyst walls (17). During encystment, isocitrate dehydrogenase and isocitrate lyase were regulated, suggesting that lipids were degraded and that acetyl coenzyme A (acetyl-CoA) might be used for cellulose synthesis (37).
Cysts of Acanthamoeba are more resistant against treatments than trophozoites, likely because of their specific wall. As the walls are partly made of cellulose, it has been speculated that inhibition of cellulose synthesis might induce sensitivity. One study has tested a cellulose synthesis inhibitor (2,6-dichlorobenzonitrile) and showed that this inhibitor blocks encystment and favors subsequent treatment (15). The authors logically claimed that the cellulose pathway is a novel target to improve Acanthamoeba treatment.
Another recent study has also targeted cellulose metabolism by inhibiting the glycogen phosphorylase (31). This enzyme is usually involved in glycogen hydrolysis to glucose, which may then serve in cellulose synthesis. The expression of glycogen phosphorylase was inhibited by siRNA methods, leading to a defect in encystment. The treated cells were able to synthesize only their ectocyst layer and not their endocyst layer. Northern blot analysis showed that the expression of glycogen phosphorylase occurred between 8 and 24 h after the induction of encystment.
Also, the expression of two enzymes involved in the glycolytic pathway (enolase and fructose bisphosphate aldolase) was modulated during encystment in different genera. Enolase is expressed during cyst formation in Naegleria fowleri (7) and in Acanthamoeba (4). It is likely that these enzymes might be related to cellulose synthesis (4). The induction of enolase in cysts confirmed a previous study conducted at the mRNA level in Acanthamoeba (39). Finally, enolase is also involved in differentiation of Entamoeba histolytica and was localized at the cyst wall, suggesting a role different from the one in the glycolytic pathway (55, 56).
CONCLUSION
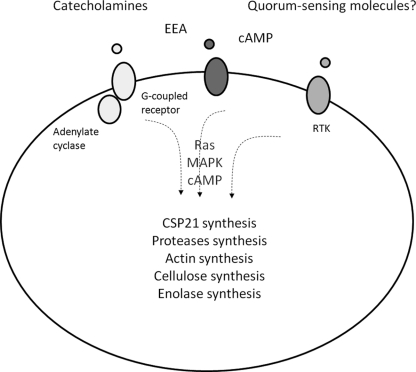
Cysts of FLA are much more resistant to treatment than trophozoites and can survive for a long time in harsh conditions. They are particularly important for persistence and spreading of FLA, leading to potential health problems. Therefore, it is important to study cyst formation in order to find new treatments to inhibit this process. In this paper, we reviewed major biochemical, molecular, and cellular modifications at work during encystment of FLA, but most of the studies have been dealing with Acanthamoeba as summarized in Fig. 3. However, the information is scarce, and we need to focus on several interesting points. First, the signals inducing encystment of FLA are globally well known (starvation and osmotic stress, etc.) even if some of them likely remain to be discovered. For example, we need to clarify if catecholamine is a global signal in every genus of FLA and if catecholamine could be produced by FLA, as it is the case for Entamoeba invadens. Second, there is still a huge lack of information regarding the signaling pathways involved in encystment. Little is known about the receptors inducing encystment, and although Ras, MAPK, and PI3K pathways were recently linked to encystment, there is no clear link between signals, these pathways, and the cellular response. For example, it is unclear which pathway is induced by cAMP, and no pathway has been completely described. Also, we still do not know if these signals use the same or different pathways to induce encystment. If there are independent pathways, we would need to block all of them to inhibit encystment. Third, we speculate that quorum-sensing molecules might be involved in encystment, as suggested by several hints. However, it remains to be confirmed and tested in various FLA. If this is true, inhibition of encystment by disturbing the quorum-sensing communication could be envisioned. Fourth, focusing on cellular response, actin, proteases, and cell wall synthesis are clearly regulated, but we can speculate that other molecules yet to be found are likely involved. Fifth, a growing interest deals with the role of intracellular bacteria that could interfere with the encystment process. Sixth, it is essential to study excystment, as this process has been studied less than encystment. Seventh, as most of the studies have been conducted on Acanthamoeba, there is a need to study more thoroughly other main genera of FLA, such as Naegleria, Balamuthia, and Hartmannella, because not all these genera are phylogenetically related.
Fig 3.
Signals, signaling pathways, and responses involved in the encystment of Acanthamoeba.
With the aim of responding to these questions, modern tools should be used or developed. Future research should be focused on genomic and transcriptomic studies on the main genera to decipher in detail mechanisms of encystment. In particular, there is an urgent need of genome sequences in all major FLA genera, and most of all, we are convinced that transcriptomic studies, via RNA-Seq, should be the priority to get a whole picture of gene regulation during encystment. In order to manipulate gene expression, siRNA has been recently used, but there is a need to develop other tools for mutagenesis and stable transfection.
Eventually, these efforts should help to improve disinfection efficacy and limit health problems due to FLA themselves and/or intracellular pathogenic bacteria.
Footnotes
Published ahead of print 24 February 2012
REFERENCES
- 1. Akins RA, Byers TJ. 1980. Differentiation promoting factors induced in Acanthamoeba by inhibitors of mitochondrial macromolecule synthesis. Dev. Biol. 78:126–140 [DOI] [PubMed] [Google Scholar]
- 2. Arroyo-Begovich A, Carabez-Trejo A. 1982. Location on chitin in the cyst wall of Entamoeba invadens with colloidal gold tracers. J. Parasitol. 68:253–258 [PubMed] [Google Scholar]
- 3. Borella P, Guerrieri E, Marchesi I, Bondi M, Messi P. 2005. Water ecology of Legionella and protozoan: environmental and public health perspectives. Biotechnol. Annu. Rev. 11:355–380 [DOI] [PubMed] [Google Scholar]
- 4. Bouyer S, Rodier MH, Guillot A, Hechard Y. 2009. Acanthamoeba castellanii: proteins involved in actin dynamics, glycolysis, and proteolysis are regulated during encystation. Exp. Parasitol. 123:90–94 [DOI] [PubMed] [Google Scholar]
- 5. Bowers B, Korn ED. 1969. The fine structure of Acanthamoeba castellanii (Neff strain). II. Encystment. J. Cell Biol. 41:786–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byers TJ. 1979. Growth, reproduction, and differentiation in Acanthamoeba. Int. Rev. Cytol. 61:283–338 [DOI] [PubMed] [Google Scholar]
- 7. Chávez-Munguía B, et al. 2011. Naegleria fowleri: enolase is expressed during cyst differentiation. J. Eukaryot. Microbiol. 58:463–468 [DOI] [PubMed] [Google Scholar]
- 8. Chávez-Munguía B, et al. 2009. Ultrastructural study of the encystation and excystation processes in Naegleria sp. J. Eukaryot. Microbiol. 56:66–72 [DOI] [PubMed] [Google Scholar]
- 9. Chávez-Munguía B, et al. 2005. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J. Eukaryot. Microbiol. 52:153–158 [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Orfeo T, Gilmartin G, Bateman E. 2004. Mechanism of cyst specific protein 21 mRNA induction during Acanthamoeba differentiation. Biochim. Biophys. Acta 1691:23–31 [DOI] [PubMed] [Google Scholar]
- 11. Coppi A, Merali S, Eichinger D. 2002. The enteric parasite Entamoeba uses an autocrine catecholamine system during differentiation into the infectious cyst stage. J. Biol. Chem. 277:8083–8090 [DOI] [PubMed] [Google Scholar]
- 12. Cordingley JS, Wills RA, Villemez CL. 1996. Osmolarity is an independent trigger of Acanthamoeba castellanii differentiation. J. Cell Biochem. 61:167–171 [DOI] [PubMed] [Google Scholar]
- 13. Coulon C, Collignon A, McDonnell G, Thomas V. 2010. Resistance of Acanthamoeba cysts to disinfection treatments used in health care settings. J. Clin. Microbiol. 48:2689–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Moraes J, Alfieri SC. 2008. Growth, encystment, and survival, of Acanthamoeba castellanii grazing on different bacteria. FEMS Microbiol. Ecol. 66:221–229 [DOI] [PubMed] [Google Scholar]
- 15. Dudley R, Alsam S, Khan NA. 2007. Cellulose biosynthesis pathway is a potential target in the improved treatment of Acanthamoeba keratitis. Appl. Microbiol. Biotechnol. 75:133–140 [DOI] [PubMed] [Google Scholar]
- 16. Dudley R, Alsam S, Khan NA. 2008. The role of proteases in the differentiation of Acanthamoeba castellanii. FEMS Microbiol. Lett. 286:9–15 [DOI] [PubMed] [Google Scholar]
- 17. Dudley R, Jarroll EL, Khan NA. 2009. Carbohydrate analysis of Acanthamoeba castellanii. Exp. Parasitol. 122:338–343 [DOI] [PubMed] [Google Scholar]
- 18. Dudley R, et al. 2005. Acanthamoeba isolates belonging to T1, T2, T3, T4 but not T7 encyst in response to increased osmolarity and cysts do not bind to human corneal epithelial cells. Acta Trop. 95:100–108 [DOI] [PubMed] [Google Scholar]
- 19. Ebert F, et al. 2008. An Entamoeba cysteine peptidase specifically expressed during encystation. Parasitol. Int. 57:521–524 [DOI] [PubMed] [Google Scholar]
- 20. Eichinger D. 2001. A role for a galactose lectin and its ligands during encystment of Entamoeba. J. Eukaryot. Microbiol. 48:17–21 [DOI] [PubMed] [Google Scholar]
- 21. El-Etr SH, et al. 2009. Francisella tularensis type A strains cause the rapid encystment of Acanthamoeba castellanii and survive in amoebal cysts for three weeks postinfection. Appl. Environ. Microbiol. 75:7488–7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fritzinger AE, Marciano-Cabral F. 2004. Modulation of a “CD59-like” protein in Naegleria fowleri amebae by bacteria. J. Eukaryot. Microbiol. 51:522–528 [DOI] [PubMed] [Google Scholar]
- 23. Greub G, Raoult D. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirukawa Y, Nakato H, Izumi S, Tsuruhara T, Tomino S. 1998. Structure and expression of a cyst specific protein of Acanthamoeba castellanii. Biochim. Biophys. Acta 1398:47–56 [DOI] [PubMed] [Google Scholar]
- 25. Jantzen H. 1981. Control of actin synthesis during the development of Acanthamoeba castellanii. Dev. Biol. 82:113–126 [DOI] [PubMed] [Google Scholar]
- 26. Kaushal DC, Shukla OP. 1976. Release of certain extracellular enzymes during excystment of axenically produced cysts of Hartmannella culbertsoni. Indian J. Exp. Biol. 14:498–499 [PubMed] [Google Scholar]
- 27. Lee X, Reimmann C, Greub G, Sufrin J, Croxatto A. 2012. The Pseudomonas aeruginosa toxin L-2-amino-4-methoxy-trans-3-butenoic acid inhibits growth and induces encystment in Acanthamoeba castellanii. Microbes Infect. 14:268–272 [DOI] [PubMed] [Google Scholar]
- 28. Leitsch D, et al. 2010. Major role for cysteine proteases during the early phase of Acanthamoeba castellanii encystment. Eukaryot. Cell 9:611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lemgruber L, Lupetti P, De Souza W, Vommaro RC, da Rocha-Azevedo B. 2010. The fine structure of the Acanthamoeba polyphaga cyst wall. FEMS Microbiol. Lett. 305:170–176 [DOI] [PubMed] [Google Scholar]
- 30. Linder M, Winiecka-Krusnell J, Linder E. 2002. Use of recombinant cellulose-binding domains of Trichoderma reesei cellulase as a selective immunocytochemical marker for cellulose in protozoa. Appl. Environ. Microbiol. 68:2503–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lorenzo-Morales J, et al. 2008. Glycogen phosphorylase in Acanthamoeba spp.: determining the role of the enzyme during the encystment process using RNA interference. Eukaryot. Cell 7:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loret JF, Greub G. 2010. Free-living amoebae: biological by-passes in water treatment. Int. J. Hyg. Environ. Health 213:167–175 [DOI] [PubMed] [Google Scholar]
- 33. Makioka A, Kumagai M, Hiranuka K, Kobayashi S, Takeuchi T. 2011. Entamoeba invadens: identification of ADF/cofilin and their expression analysis in relation to encystation and excystation. Exp. Parasitol. 127:195–201 [DOI] [PubMed] [Google Scholar]
- 34. Marciano-Cabral F. 1988. Biology of Naegleria spp. Microbiol. Rev. 52:114–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martínez AJ, Schuster FL, Visvesvara GS. 2001. Balamuthia mandrillaris: its pathogenic potential. J. Eukaryot. Microbiol. 2001(Suppl):6S–9S [DOI] [PubMed] [Google Scholar]
- 36. Matin A, Jeong SR, Stins M, Khan NA. 2007. Effects of human serum on Balamuthia mandrillaris interactions with human brain microvascular endothelial cells. J. Med. Microbiol. 56:30–35 [DOI] [PubMed] [Google Scholar]
- 37. Mehdi H, Garg NK. 1987. Changes in the lipid composition and activities of isocitrate dehydrogenase and isocitrate lyase during encystation of Acanthamoeba culbertsoni strain A-1. Trans. R. Soc. Trop. Med. Hyg. 81:633–636 [DOI] [PubMed] [Google Scholar]
- 38. Moon EK, Chung DI, Hong Y, Kong HH. 2011. Expression levels of encystation mediating factors in fresh strain of Acanthamoeba castellanii cyst ESTs. Exp. Parasitol. 127:811–816 [DOI] [PubMed] [Google Scholar]
- 39. Moon EK, Chung DI, Hong YC, Ahn TI, Kong HH. 2008. Acanthamoeba castellanii: gene profile of encystation by ESTs analysis and KOG assignment. Exp. Parasitol. 119:111–116 [DOI] [PubMed] [Google Scholar]
- 40. Moon EK, Chung DI, Hong YC, Kong HH. 2007. Differentially expressed genes of Acanthamoeba castellanii during encystation. Korean J. Parasitol. 45:283–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moon EK, Chung DI, Hong YC, Kong HH. 2008. Characterization of a serine proteinase mediating encystation of Acanthamoeba. Eukaryot. Cell 7:1513–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moon EK, Chung DI, Hong YC, Kong HH. 2009. Autophagy protein 8 mediating autophagosome in encysting Acanthamoeba. Mol. Biochem. Parasitol. 168:43–48 [DOI] [PubMed] [Google Scholar]
- 43. Murti CR. 1975. Molecular biology of amoebic encystment. Indian J. Med. Res. 63:757–767 [PubMed] [Google Scholar]
- 44. Murti CR, Shukla OP. 1984. Differentiation of pathogenic amoebae: encystation and excystation of Acanthamoeba culbertsoni—a model. J. Biosci. 6:475–489 [Google Scholar]
- 45. Neff RJ, Neff RH. 1969. The biochemistry of amoebic encystment. Symp. Soc. Exp. Biol. 23:51–81 [PubMed] [Google Scholar]
- 46. Neff RJ, Ray SA, Benton WF, Wilborn M. 1964. Induction of synchronous encystement (differenciation) in Acanthamoeba sp. Methods Cell Physiol. 1:55–83 [Google Scholar]
- 47. Park J, Jeong Y, Ahn T. 2002. Changes in profiles of major proteins in encysting Acanthamoeba castellanii. Korean J. Biol. Sci. 6:341–347 [Google Scholar]
- 48. Potter JL, Weisman RA. 1971. Differentiation in Acanthamoeba: beta-glucan synthesis during encystment. Biochim. Biophys. Acta 237:65–74 [DOI] [PubMed] [Google Scholar]
- 49. Raizada MK, Krishna Murti CR. 1972. Transformation of trophic Hartmannella culbertsoni into viable cysts of cyclic 3′,5′-adenosine monophosphate. J. Cell Biol. 52:743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raizada MK, Murti CR. 1971. Changes in the activity of certain enzymes of Hartmannella (Culbertson strain A-1) during encystment. J. Protozool. 18:115–119 [DOI] [PubMed] [Google Scholar]
- 51. Raizada MK, Murti CR. 1972. Synthesis of RNA, protein, cellulose, and mucopolysaccharide and changes in the chemical composition of Hartmannella culbertsoni during encystment under axenic conditions. J. Protozool. 19:691–695 [DOI] [PubMed] [Google Scholar]
- 52. Rowbotham TJ. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schuster F. 1963. An electron microscope study of the amoebo-flagellate, Naegleria gruberi (Schardinger). II. The cyst stage. J. Protozool. 10:313–320 [DOI] [PubMed] [Google Scholar]
- 54. Schuster FL, Visvesvara GS. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 34:1001–1027 [DOI] [PubMed] [Google Scholar]
- 55. Segovia-Gamboa NC, et al. 2010. Entamoeba invadens, encystation process and enolase. Exp. Parasitol. 125:63–69 [DOI] [PubMed] [Google Scholar]
- 56. Segovia-Gamboa NC, et al. 2011. Differentiation of Entamoeba histolytica: a possible role for enolase. Exp. Parasitol. 129:65–71 [DOI] [PubMed] [Google Scholar]
- 57. Siddiqui R, Jarroll EL, Khan NA. 2010. Balamuthia mandrillaris: role of galactose in encystment and identification of potential inhibitory targets. Exp. Parasitol. 126:22–27 [DOI] [PubMed] [Google Scholar]
- 58. Smirnov AV, Michel R. 1999. New data on the cyst structure of Hartmannella vermiformis Page, 1967 (Lobosea, Gymnamoebia). Protistology 1:82–85 [Google Scholar]
- 59. Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. U. S. A. 100:8951–8956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Srivastava DK, Shukla OP. 1983. Encystment of Acanthamoeba culbertsoni by organic effectors. Indian J. Exp. Biol. 21:444–447 [PubMed] [Google Scholar]
- 61. Thomas V, McDonnell G, Denyer SP, Maillard JY. 2010. Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol. Rev. 34:231–259 [DOI] [PubMed] [Google Scholar]
- 62. Tomlinson G, Jones EA. 1962. Isolation of cellulose from the cyst wall of a soil amoeba. Biochim. Biophys. Acta 63:194–200 [DOI] [PubMed] [Google Scholar]
- 63. Turner NA, Russell AD, Furr JR, Lloyd D. 2000. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J. Antimicrob. Chemother. 46:27–34 [DOI] [PubMed] [Google Scholar]
- 64. Upadhyay JM, Crow S, Cox A. 1984. The cyst wall composition of Hartmannella glebae. Proc. Soc. Exp. Biol. Med. 175:424–428 [DOI] [PubMed] [Google Scholar]
- 65. Verma AK, Raizada MK, Murti CK. 1974. Effect of bioamines on the cellular differentiation of Hartmannella culbertsoni. Biochem. Pharmacol. 23:57–63 [DOI] [PubMed] [Google Scholar]
- 66. Weisman RA. 1976. Differentiation in Acanthamoeba castellanii. Annu. Rev. Microbiol. 30:189–219 [DOI] [PubMed] [Google Scholar]