Abstract
Human Wiskott-Aldrich syndrome protein (WASP) is a scaffold linking upstream signals to the actin cytoskeleton. In response to intersectin ITSN1 and Rho GTPase Cdc42, WASP activates the Arp2/3 complex to promote actin polymerization. The human pathogen Cryptococcus neoformans contains the ITSN1 homolog Cin1 and the WASP homolog Wsp1, which share more homology with human proteins than those of other fungi. Here we demonstrate that Cin1, Cdc42/Rac1, and Wsp1 function in an effector pathway similar to that of mammalian models. In the cin1 mutant, expression of the autoactivated Wsp1-B-GBD allele partially suppressed the mutant defect in endocytosis, and expression of the constitutively active CDC42Q61L allele restored normal actin cytoskeleton structures. Similar phenotypic suppression can be obtained by the expression of a Cdc42-green fluorescent protein (GFP)-Wsp1 fusion protein. In addition, Rac1, which was found to exhibit a role in early endocytosis, activates Wsp1 to regulate vacuole fusion. Rac1 interacted with Wsp1 and depended on Wsp1 for its vacuolar membrane localization. Expression of the Wsp1-B-GBD allele restored vacuolar membrane fusion in the rac1 mutant. Collectively, our studies suggest novel ways in which this pathogenic fungus has adapted conserved signaling pathways to control vesicle transport and actin organization, likely benefiting survival within infected hosts.
INTRODUCTION
Cryptococcus neoformans is an opportunistic fungal pathogen that infects humans, primarily immunocompromised individuals, causing life-threatening meningitis that is fatal if left untreated (for reviews, see references 18 and 28). In the environment and the infected host, C. neoformans grows primarily as a haploid and unicellular yeast. It has a fully developed actin cytoskeleton network and maintains active actin dynamics during asexual budding, like the budding yeast Saccharomyces cerevisiae (21, 47). During the mating cycle of C. neoformans, the round, yeast-like cells can differentiate into a hyphal form producing sexual fruiting structures and meiotic basidiospores (23). As a human pathogen, C. neoformans employs a multifaceted virulence mechanism, producing an antiphagocytic polysaccharide capsule, the antioxidant melanin pigment, and enzymes such as phospholipase B and urease (for reviews, see references 7, 18, 28, and 34). Since intracellular trafficking and actin organization underlie most of these cellular characteristics, investigators have increasingly studied the areas of polarity establishment, actin organization, vesicle transport, and signaling with regard to roles in fungal pathogenesis. Proteins controlling these pathways in C. neoformans include the small Rho family GTPases Ras, Rac, and Cdc42; secretory proteins Sec4/Sav1, Sec6, and Sec14; and the vacuole protein Vps41 (3, 8, 25, 31, 38, 44, 49, 55). We have recently identified two unique C. neoformans proteins, the intersectin ITSN1 homolog Cin1 and the Wiskott-Aldrich syndrome protein (WASP) homolog Wsp1, that are involved in intracellular trafficking, organization of the actin cytoskeleton, and virulence (38, 39).
WASPs play a general role in the assembly of branched actin filaments by linking small GTPases to the actin cytoskeleton. WASP is an autoinhibitory protein due to the folding of its C-terminal verprolin, cofilin, acidic (VCA) domain to the N-terminal basic domain (B) and GTP-binding domain (GBD). Upon binding by ITSN1 and/or activated Cdc42, the protein becomes unfolded, releasing autoinhibition and allowing its C-terminal A domain to bind and activate downstream targets such as Arp2/3 proteins (22, 36, 45). The cryptococcal WASP homolog Wsp1 contains a GBD not found in other fungi, such as S. cerevisiae or Candida albicans, and it interacts with both Cin1 and Cdc42 in vitro (38, 39).
In S. cerevisiae, budding and the establishment of cell polarity are accomplished through dynamic changes in the actin cytoskeleton, which are regulated by an integrated signaling cascade, including that of the Rho family small GTPases, such as Cdc42, Rac, and Rho (10, 32). Cdc42 is highly conserved among eukaryotic organisms in both sequence and function (19), and it functions through the binding of effector molecules containing the GBD, such as WASPs, to promote actin polymerization (10). So far, two Cdc42 paralogs have been identified in C. neoformans: Cdc42 and Cdc420. Cdc42 plays a more prominent role than Cdc420 in governing phenotypes such as thermal tolerance and septin formation, and the cdc42 mutant strain exhibits a defect in actin repolarization under thermal stress conditions (3).
Mammalian Rac proteins, which share overlapping and complementary functions with Cdc42, regulate mobility and other phenotypes relating to membrane trafficking and the actin cytoskeleton (1, 53). In filamentous fungi, such as Penicillium marneffei, the Rac homolog CflB plays a role in hyphal growth and actin organization (6). In the plant-pathogenic fungus Ustilago maydis, disruption of the RAC1 gene affects cell morphology and hyphal growth in the haploid cell (26). Additionally, U. maydis Rac1 plays a role in vacuole morphology, as evidenced by the facts that the rac1 cells had fragmented vacuoles and the expression of a constitutively active RAC1Q61L allele resulted in growth arrest and in swollen cells containing a single large vacuole (26). Mammalian and plant Rac proteins are thought to function in actin organization through effector WAVE (WASP family verprolin-homologous) proteins, which lack both the WASP homology 1 (WH1) domain and the GBD (9, 33, 52). At least one Rac homolog, Rac1, has been discovered in C. neoformans. In contrast to Cdc42 and Cdc420, Rac1 appears to affect hyphal differentiation downstream of Ras1, with only a modest effect on high-temperature growth (44). No proteins homologous to WAVE proteins have been found in C. neoformans or any other fungi, suggesting a distinct effector(s) for Rac in fungal actin organization.
We have demonstrated previously that the Cin1 and Wsp1 proteins regulate intracellular trafficking and that both proteins interact with Cdc42 through conserved domains (38, 39). Here we present evidence for a Cin1-Cdc42-Wsp1 effector pathway. We also found that Rac1 regulates endocytosis and is required for normal vacuole morphogenesis. A novel Rac1-Wsp1 effector pathway for vacuole morphogenesis is also presented.
MATERIALS AND METHODS
Strains, plasmids, and media.
Cryptococcus neoformans var. neoformans (serotype D) strain JEC21 was used as the parental strain. All other strains used are listed in Table S1 in the supplemental material. Important oligonucleotide primers for PCR amplification and DNA plasmids are listed in Tables S2 and S3 in the supplemental material, respectively. All culture media and reagents were prepared as described previously (38, 39).
Mutant allele and strain construction.
Gene knockout alleles were generated by the split-marker method, and the resulting mutant strains were obtained as described previously (38, 39). Auxotrophic and dominant selection marker genes, including URA5 (encoding orotidine monophosphate pyrophosphorylase), HPT (hygromycin phosphate transferase), NEO (neomycin phosphotransferase), and NAT (nourseothricin N-acetyltransferase) were all used in this study. To obtain the rac1 mutant, the 5′ and 3′ fragments were first amplified with primers PW1197 and PW1200 and with primers PW1198 and PW1201, respectively. The two fragments were digested with BamHI and HindIII and were joined with either the NEO or the HPT gene cassette. The two fragments, amplified with primers PW1196 and PW638 and with primers PW812 and PW1199, respectively, were used to generate the rac1 mutant. The same split-marker approach was also employed to create wsp1 cdc42 and wsp1 rac1 strains in the cdc42 and rac1 mutants by use of alternate drug resistance markers. A cdc42 rac1 mutant strain was also produced by transforming the rac1 mutant with the cdc42::NAT allele. The WSP1-GBD allele linked to the NAT marker was constructed by PCR overlap (11). The first fragment was amplified with primers PW1265 and PW1188, with pGS778 as the template, and the second fragment was amplified with primers PW1189 and PW1399, with the same plasmid as the template. WSP1-GBD was then amplified with primers PW1266 and PW968 by using the two fragments mentioned above as the template and was inserted into the TOPO TA vector (Invitrogen), resulting in pGS783. pGS783 was then digested with BamHI and XbaI, and the insert was cloned into plasmid pGS324 to generate pGS794.
A similar approach was used to generate a WSP1-B-GBD allele linked to the NEO marker. Fragment 1 was amplified with the M13 forward primer and primer PW1455, with pGS462 as the template. With pGS783 as the template, and with primers PW1266 and PW1167, the target fragment was obtained and was inserted into the TA vector (pGS865), which was then digested with BamHI and XbaI and inserted into pGS277, resulting in pGS1228.
The PCR overlap extension technique was also utilized to generate a CDC42Q61L allele. The first fragment was amplified with primer PW1499 and reverse primer M13, and the second fragment was amplified with primer PW1498 and forward primer M13 by using pGS950 as the template. The CDC42Q61L allele was amplified with primers PW1495 and PW1496, cloned into the TOPO TA vector (Invitrogen), and digested with BamHI and BglII to release the CDC42Q61L allele, which was then inserted into plasmid pGS277, generating pGS1219. To link the pleckstrin homology (PH) domain to CDC42Q61L, the PH domain was amplified from pGS931 (containing partial CIN1 cDNA [38]) with primers PW1551 and PW1553. The fragment was TA cloned (pGS1261), released by BamHI and XbaI restriction, and inserted into pGS325 to yield pGS1414. The CDC42Q61L allele with a native CDC42 terminator was released from pGS1403 by BamHI and SpeI restriction and was inserted into pGS1414, yielding pGS1443. pGS690, containing a PGPD1-Dbl homology (DH) fusion gene, was transformed into the wsp1 mutant (GYS237), resulting in strain GYS566 (see Table S1 in the supplemental material). The rac1 mutants expressing the WSP1-GBD and WSP1-B-GBD alleles were obtained by transforming the mutant with pGS794 and pGS875, respectively. The CDC42-GFP-WSP1 fusion gene was generated by PCR linking CDC42 to GFP-WSP1 by use of plasmids pGS1186, pGS1217, and pGS1236.
The promoter sequence from C. neoformans glyceraldehyde phosphate dehydrogenase was used in most gene fusion or truncation constructs unless stated otherwise.
Fluorescence-tagged protein construction.
The green fluorescent protein (GFP)-Cin1 fusion protein was constructed as described previously (38). To produce a GFP-Wsp1 fusion protein, the GFP gene was digested from pGS720 with BamHI and BglII and was fused with the WSP1 gene, which was digested from plasmid pGS869 with BamHI and NotI. The GFP-WSP1 fusion gene was inserted into plasmid pGS324, resulting in pGS1251. The DsRED-WSP1 fusion gene linked to the hygromycin resistance marker gene was constructed similarly, by use of plasmids pGS810, pGS821, and pGS1481. For construction of the DsRED-CDC42 fusion gene, CDC42 was restricted from pGS950 with BamHI and SpeI and was inserted into pGS988, resulting in pGS1005, which contains the NAT marker. The DsRED-RAC1 fusion gene was constructed in a similar manner by use of pGS1011, pGS1035, and pGS1058. Fusion gene constructs were transformed into the respective strains through biolistic transformation, and expression was examined by the ability of each fusion gene to suppress the corresponding mutant's phenotype.
Yeast two-hybrid interaction.
An interaction between Cin1 and Wsp1 was previously established using a yeast two-hybrid system (Invitrogen) for Cin1 and Wsp1 (38, 39). RAC1 cDNA was digested with EcoRI and BglII from pGS1011 and was then inserted into pGADT7. The gene sequence was verified by DNA sequencing. A yeast strain (Gold) from Invitrogen was used as the host strain, and gene transformation was carried out according to the protocol provided by the manufacturer.
Vacuolar fusion following hypotonic stress.
To induce vacuole fusion in C. neoformans, we adapted the hypotonic stress method used for S. cerevisiae (20, 48). Cells grown in yeast extract-peptone-dextrose (YPD) were stained with FM4-64 either directly or after 20 min of hypotonic treatment (10-fold dilution of YPD in water) and were examined microscopically at the time points indicated in the figure legends.
Microscopy.
Cryptococcal cells were prepared and stained with rhodamine-conjugated phalloidin as described previously (38, 39). For FM4-64 staining, cells grown in liquid YPD for 14 to 16 h (mid- to late-logarithmic phase) were harvested by centrifugation at 700 × g for 1 min and were resuspended in ice-cold Hanks' buffered salt solution (HBSS; Invitrogen). FM4-64 was added to the cell suspension at a final concentration of 5 μg/ml.
To observe fluorescent fusion proteins, cells grown for 12 to 14 h in YPD were harvested and were washed twice with sterile distilled water. Cells were also resuspended in water for subsequent microscopic observations.
Optical and fluorescence microscopic observations were carried out on a Zeiss fluorescence microscope (Axio Imager 2) with a 63× (numerical aperture, 1.4) Plan-Apochromat oil immersion lens (Zeiss). Images were captured with an AxioCam camera (Zeiss) and were adjusted for brightness and contrast only by using Adobe Photoshop CS4.
RESULTS
Wsp1 coresides with and functions downstream of Cin1 in endocytosis.
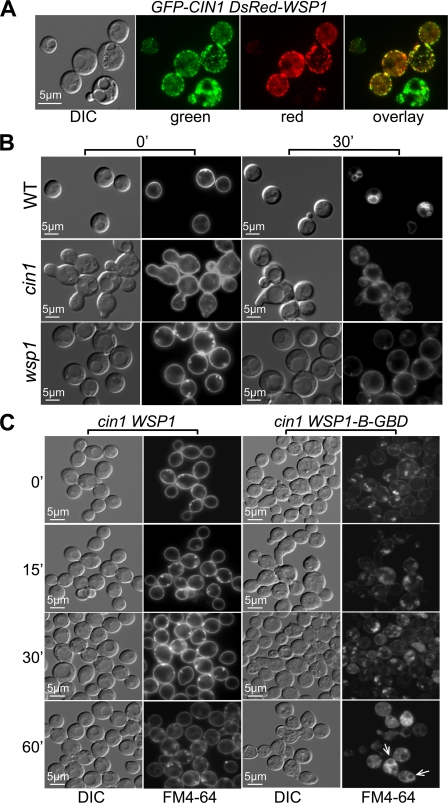
We have shown previously that both Cin1 and Wsp1 regulate intracellular trafficking and the actin cytoskeleton, which underlie the growth, differentiation, and virulence of C. neoformans (38, 39). Cin1 bears a resemblance to the human intersectin protein ITSN, which functions in conjunction with WASP and Rho GTPase Cdc42 protein to regulate intracellular trafficking and maintain the actin cytoskeleton (30). To test the hypothesis that the Cin1, Wsp1, and Cdc42 proteins may function analogously, we examined epistatic relationships among the three proteins. We first validated the previously established interaction between Cin1 and Wsp1 by in vivo colocalization of fluorescence-labeled GFP-Cin1 and DsRed-Wsp1 fusion proteins. Cin1 fluorescence appeared mostly as punctae near the plasma membrane (Fig. 1A, green), and DsRed-Wsp1 was similarly present as punctae near the plasma membrane. Minimal red and green fluorescence was also observed in the cytosol (Fig. 1A, green and red). Overlay of the two images revealed colocalization of the red and green fluorescent signals, suggesting that Cin1 and Wsp1 occupy similar subcellular domains and could interact with each other in vivo (Fig. 1A, overlay).
Fig 1.
C. neoformans Wsp1 is a conserved effector of Cin1 in actin organization. (A) In vivo localization of fluorescence-labeled fusion proteins. The GFP-CIN1 and DsRed-WSP1 fusion genes were expressed in the cin1 mutant strain. GFP-Cin1 is enriched in the plasma membrane as punctae, and DsRed-Wsp1 exhibits similar localization. DIC, differential interference contrast. (B) Endocytic uptake of FM4-64 in the wild-type (WT), cin1, and wsp1 strains. The cin1 and wsp1 mutants were defective in endocytosis, in agreement with previous studies (38, 39). (C) FM4-64 uptake by the cin1 mutant expressing either the wild-type WSP1 gene or the WSP1-B-GBD allele driven by the constitutively active GPD promoter. Wsp1-B-GBD expression results in internalization of FM4-64 as random patches and partial restoration of endocytosis in 60 min (indicated by arrows), in contrast to the pattern observed with the wild-type Wsp1 protein. Cells were prepared for fluorescence microscopy as described in the text.
In wild-type C. neoformans strains, endocytic uptake of FM4-64 occurs immediately upon exposure to the lipophilic dye under ambient temperatures. FM4-64 is concentrated mostly at the plasma membranes of cells kept on ice (Fig. 1B). Incorporation of this dye into internal ring-like structures, which are early endosomes and endosomes, became apparent within 30 min at room temperature (Fig. 1B). In agreement with previous findings, FM4-64 remained in the plasma membrane and was not internalized at 30 min in either the cin1 or the wsp1 mutant strain (Fig. 1B, upper row), indicating that Cin1 and Wsp1 are similarly required for endocytosis (38, 39).
To examine the genetic relationship between Cin1 and Wsp1, we utilized a Wsp1 mutant allele lacking the B domain and the GBD (WSP1-B-GBD). Previous studies of mammalian WASPs indicated that deletion of the B domain and the GBD relieves autoinhibition and results in constitutive WASP activation. The WSP1-B-GBD allele was introduced into the cin1 mutant, with the wild-type WSP1 gene as a control. The cin1 strain overexpressing wild-type WSP1 demonstrated no change in the FM4-64 endocytosis defect (Fig. 1C). In contrast, the cin1 mutant expressing Wsp1-B-GBD showed an FM4-64 staining pattern distinct from that of the cin1 mutant expressing the wild-type Wsp1 allele. FM4-64 was observed both in the plasma membrane and inside the cells as random aggregates at time zero and at 15 and 30 min (Fig. 1). Staining of the aggregates became progressively intensified as the exposure time increased, and at 60 min, ring-like structures resembling endosomes were observed, indicating partial suppression of the endocytosis defect by autoactivated Wsp1. The incomplete suppression of the endocytosis defect and the failed suppression of the cin1 morphology defect indicated partial phenotypic complementation of the cin1 mutant by the WSP1-B-GBD allele (Fig. 1C).
Cdc42 is an effector of Cin1 in actin cytoskeleton organization.
The actin cytoskeleton consists mainly of short-lived actin cables and actin patches. In S. cerevisiae, the actin cable consists of filamentous actin (F-actin), whereas the actin patch is a cortical membrane zone composed of F-actin and actin-binding/regulatory proteins, such as capping proteins (Cap1 and Cap2), actin nucleation proteins (Arp2/3), and regulators (Las17/Bee1) (15, 29, 54). The actin patch is also enriched at the sites of active polarized cell surface growth, such as the bud, the site of cell-cell separation, and the mating projection (35, 40).
Previous studies suggested that C. neoformans maintains actin dynamics similar to those of model yeasts (21, 47). In wild-type strains, F-actin is present in polarized patches enriched in the early, emerging daughter cell and at the contractile ring (Fig. 2A). Consistent with their morphology and cell separation defects, the cin1 and wsp1 mutants display a less defined pattern of F-actin localization (Fig. 2A). In contrast, the cdc42 mutant has intact actin patches at permissive growth temperatures (30°C), although actin filaments are less apparent in this strain than in the wild type (Fig. 2A).
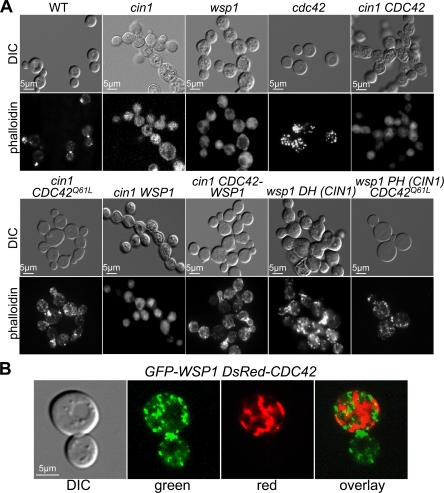
Fig 2.
Cin1, Cdc42, and Wsp1 function collectively to regulate the actin cytoskeleton. (A) F-actin was visualized using rhodamine-conjugated phalloidin staining of the indicated strains. All strains were incubated to the mid- and late-logarithmic phases in YPD prior to incubation in HBSS. WT, wild type. In the cin1 mutant background, the following alleles were expressed using the GPD promoter: the wild-type CDC42 gene, the dominant active CDC42Q61L allele, the wild-type WSP1 gene, and the CDC42-WSP1 fusion allele. In the wsp1 background, the following genes/domains were overexpressed: the DH domain of the CIN1 gene and the PH domain of CIN1 coexpressed with the CDC42Q61L allele. DIC, differential interference contrast. (B) GFP-Wsp1 and DsRed-Cdc42 were coexpressed in the wsp1 cdc42 mutant strain. An overlay of the two images suggests that Wsp1 and Cdc42 occupy shared subcellular domains in vivo.
To investigate whether Cdc42 plays a conserved role in actin organization by functioning downstream of Cin1 but upstream of Wsp1, we performed the following analysis. A constitutively active Cdc42 allele, CDC42Q61L, was generated and transformed into the cin1 mutant strain, with the wild type CDC42 allele as a control. The expression of CDC42Q61L resulted in the formation of abundant actin cables and some actin patches in the cin1 mutant (Fig. 2A). In contrast, the cin1 mutant expressing the wild type CDC42 allele remained defective in actin cable and patch formation (Fig. 2A). This finding is consistent with the hypothesis that Cdc42 is an effector of Cin1 in actin organization, particularly actin cable formation.
To examine the relationship between Cdc42 and Wsp1, we expressed the DH (Rho-guanine nucleotide exchange factor [Rho-GEF]) domain of Cin1, which has been shown to have a GEF function in vitro similar to that of Cdc24 in the wsp1 mutant (38). Expression of the Cin1-DH domain restored near-normal actin cable and patch formation to the wsp1 mutant (Fig. 2A). Interestingly, the constitutively active Cdc42Q61L protein linked to the PH domain of Cin1, which is thought to promote membrane tethering and Cdc42 activity (16, 37), also restored normal actin cable formation and partial actin patch formation to the wsp1 mutant (Fig. 2A). These findings indicated that Cin1 and Cdc42 do not act exclusively on Wsp1 and that additional effectors of C. neoformans Cdc42 might be present.
Wsp1 interacts with Cdc42 to regulate the actin cytoskeleton downstream of Cin1.
Cdc42 was previously shown to interact with Wsp1 in a yeast two-hybrid assay (39). To explore this interaction, DsRed-Cdc42 and GFP-Wsp1 fusion proteins were expressed in a wsp1 cdc42 double mutant, and the patterns of fluorescence were observed. GFP-Wsp1 was localized primarily in punctae or peripheral patches adjacent to the plasma membrane, with some cytoplasmic signals (Fig. 2B). In contrast, DsRed-Cdc42 had more continuous distribution in the plasma membrane and the cytosol (Fig. 2B). Overlay of the two images suggested that the two proteins share certain overlapping distributions (shown as yellow fluorescence) within live cells (Fig. 2B).
Moreover, to further examine the interaction between Wsp1 and Cdc42 and the relevance of that interaction to Cin1, we fused Cdc42 with Wsp1 through a GFP linker. generating a Cdc42-GFP-Wsp1 protein. Overexpression of this fusion protein partially restored the growth phenotype and actin cables and patches of the cin1 mutant (Fig. 2A). Neither Cdc42 nor Wsp1 alone could suppress the phenotype of the cin1 mutant (Fig. 2A). This resulted in an intriguing proposition: either the Cdc42-GFP-Wsp1construct altered the autoinhibitory conformation of Wsp1, resulting in its activation, or Cdc42 could activate Wsp1 through direct association. Regardless, the finding is consistent with established models in which Wsp1 is a direct downstream effector of Cdc42 in actin organization.
Wsp1 is required for the localization of Rac1 at the vacuole membrane.
Taken together, the cumulative data provide evidence that Wsp1 is a conserved effector of Cdc42. However, the wsp1 mutant lacks both actin patches and actin cables, while the cdc42 mutant appears to have normal actin patches but not actin cables (Fig. 3A), suggesting that the role of Wsp1 in actin patches could be activated by other regulators, such as Rac proteins of the Rho GTPase family. The cryptococcal Rac homolog Rac1 was previously shown to exhibit similar and distinct functions with Cdc42 in the regulation of filamentation and thermal resistance; its other roles were unknown (44). To determine whether Rac1 has any roles in vesicle transport and the actin cytoskeleton, we characterized the function of Rac1 in endocytosis, observed the localization of the DsRed-Rac1 fusion protein, and examined the interaction between Rac1 and Wsp1.
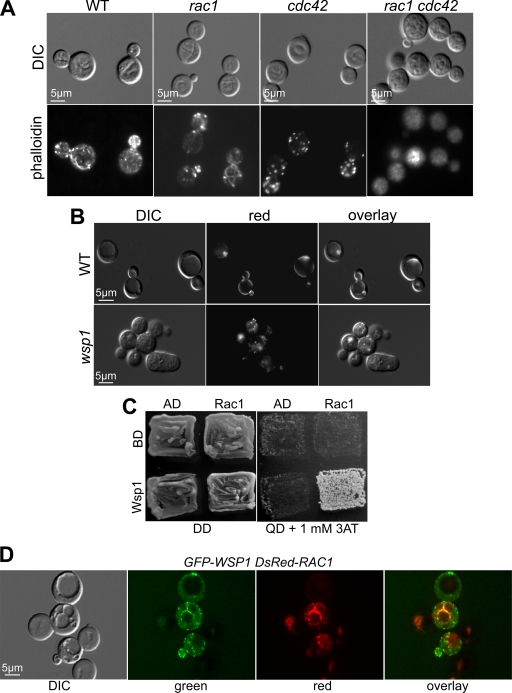
Fig 3.
Wsp1 is an effector of Rac1 in actin organization. (A) F-actin was visualized using rhodamine-conjugated phalloidin staining of the indicated strains. WT, wild type. DIC, differential interference contrast. (B) Rac1 is enriched in the vacuolar membrane. The DsRed-Rac1 fusion protein was expressed in the wild-type and wsp1 mutant strains. Rac1 localization near the vacuolar membrane in the wild-type strain was disrupted in the wsp1 mutant. (C) Rac1 interacts with Wsp1 in a yeast two-hybrid assay. The two-hybrid yeast strain was incubated for 3 days on either DD (double-dropout medium [SD-Leu-Trp]), indicating the presence of both vectors, or QD (quadruple-dropout medium [SD-Leu-Trp-His-Ade]), suggesting interaction. AD, empty activation domain vector; BD, empty binding domain vector; Rac1, C. neoformans RAC1 allele in BD; Wsp1, C. neoformans WSP1 allele in AD. (D) GFP-Wsp1 colocalizes with DsRed-Rac1. The GFP-Wsp1 and DsRed-Cdc42 fusion proteins were coexpressed in the wsp1 rac1 double mutant strain. Colocalization of the fluorescent signals around the vacuole was visualized in the overlay image.
In most cells, the rac1 mutant strain exhibited a normal distribution of actin patches and cables, in contrast to the cdc42 mutant strain, which had normal actin patches but not cables, while the wild-type strain had normal actin patches and cables (Fig. 3A). Intriguingly, no definitive actin patches or cables were observed in the rac1 cdc42 mutant strain, indicating that Rac1 and Cdc42 collectively maintain the actin cytoskeleton (Fig. 3A).
The DsRed-Rac1 fusion protein was enriched in the vacuole membrane: fluorescence occurring as near-continuous rings surrounding the vacuoles was very apparent (Fig. 3B, top). In addition, this distribution was dependent on Wsp1, as evidenced by the fact that in the wsp1 mutant, the DsRed-Rac1 fusion protein no longer appeared in the vacuole membrane, occurring instead as small cytoplasmic punctae (Fig. 3B, bottom). The wsp1 mutant showed a defect in vacuole formation, indicating that Wsp1 is essential for vacuolar biogenesis.
Consistent with these findings, Rac1 interacted with Wsp1 in a yeast two-hybrid screen, as evidenced by the fact that robust cell growth occurred on a stringent selective medium (synthetic dropout medium [SD]-Leu-Trp-His-Ade plus 1 mM 3-aminotriazole [3-AT]) in the yeast strain cotransformed with the Rac1- and Wsp1-containing vectors (Fig. 3C). Moreover, the distribution of a GFP-Wsp1 fusion protein partially overlapped that of DsRed-Rac1 along the vacuolar membrane in the rac1 wsp1 strain background, indicating possible colocalization of these two proteins in vivo (Fig. 3D).
Wsp1 is an effector of Rac1 in vacuolar morphogenesis.
To examine the role of Rac1 in intracellular transport, we examined the rac1 mutant for possible defects in endocytosis and/or vacuole morphology. In comparison to the wild-type strain, which displayed normal endocytosis, and the wsp1 mutant strain, with a severe defect in endocytosis, the rac1 mutant strain showed an intermediate phenotype. Immediately after exposure of the rac1 mutant to FM4-64, the dye was concentrated mostly in the plasma membrane, as in the wild-type and wsp1 strains (Fig. 4A). In addition, bright particulate staining occurred inside the periphery of the plasma membrane in the rac1 and wsp1 mutants. After 15 min of exposure, small, ring-like endosomal structures located near the plasma membrane were observed in some of the rac1 mutant cells. Some of the rings were not complete, indicating that the endosomes had not yet completed invagination or scission from the plasma membrane (Fig. 4A, arrows). At 30 min, some of the FM4-64-stained endosomes/vesicles were internalized, but others remained near the plasma membrane (Fig. 4A, arrows). In contrast, the wild-type strain displayed more-complete internalization of the fluorescent dye at 15 and 30 min. As expected, similar endosomes were not found in the wsp1 strain (Fig. 4A) (39). This finding suggests that Rac1 plays an important role in early steps of endocytosis.
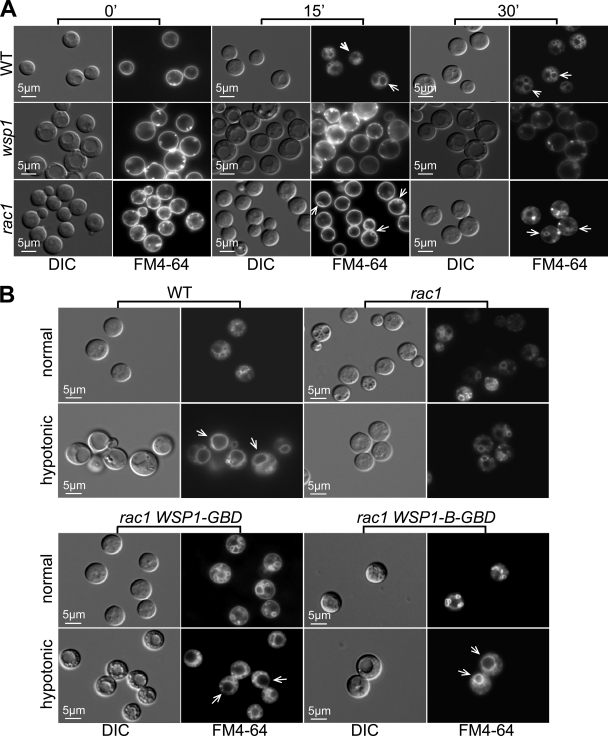
Fig 4.
Rac1 has a role in endocytosis and vacuole morphology mediated by Wsp1. (A) Endocytosis of FM4-64 was assessed in the wild-type (WT), wsp1, and rac1 strains at 0, 15, and 30 min. In contrast to the wild-type strain, which showed early-appearing endosomes, and the wsp1 mutant, which exhibited none, the rac1 strain showed partial endosome formation at 15 min and more mature endosome formation 30 min after exposure to FM4-64. The numbers of endosomes in the rac1 mutant (indicated by arrows) were lower, and the sizes were smaller, than those in the wild type. DIC, differential interference contrast. (B) Rac1 affected Wsp1-mediated vacuole morphology. Hypotonic stress-induced vacuole fusion was observed in the wild-type strain (arrows) but not in the rac1 mutant. Expression of the autoactivated Wsp1-GBD and Wsp1-B-GBD proteins restored at least partial vacuole fusion in the rac1 mutant (arrows). Hypotonic stress was induced by switching cells to diluted YPD medium (10-fold dilution with sterile distilled water) for 20 min. Cells were photographed 5 min after exposure to FM4-64.
To study the functional consequence of the interaction between Rac1 and Wsp1, we observed vacuole morphology by staining cells of various genetic backgrounds with FM4-64 following hypotonic stress, which was created by switching cells from rich YPD to hypotonic conditions (YPD diluted 10-fold with sterile distilled water). The wild-type strain exhibited a pattern of normal staining by FM4-64: multiple endosomes/vesicles were seen following 30 min of exposure (Fig. 4B). After 20 min of hypotonic stress followed by 5 min of exposure to FM4-64, most of the wild-type cells showed a large, predominant vesicle (vacuole), likely due to membrane fusion of smaller vacuoles (Fig. 4B, WT; fused vacuoles indicated by arrows). The late-appearing and small vacuole structures of the rac1 mutant exhibited no such transition after hypotonic stress (Fig. 4B, rac1). However, expression of the autoactivated Wsp1 (Wsp1-GBD and Wsp1-B-GBD) alleles resulted in suppression of the rac1 defect in vacuole fusion to a large extent. As a result, a single large vacuole occurred in cells after hypotonic stress (Fig. 4B, rac1 WSP1-GBD and rac1 WSP1-B-GBD, arrows). In addition, the effect of the WSP1-B-GBD allele appeared to be greater than that of the WSP1-GBD allele, since the strain expressing Wsp1-B-GBD more closely resembled the wild-type strain (Fig. 4B). This evidence supports a model in which Wsp1 is a novel effector of Rac1 in vacuole morphology and fusion.
DISCUSSION
Intracellular trafficking is an essential cellular process linked to diverse downstream effects. In mammalian cells, these effects include cell surface protrusions and the migration, uptake, and propulsion of macromolecules (12). In S. cerevisiae, C. albicans, and C. neoformans, intracellular transport underlies cellular growth, polarity establishment, and/or virulence (reviewed in reference 47). There is evidence indicating the presence of distinct endocytic machinery in C. neoformans and likely in other basidiomycetous fungi (38, 39). Given that intracellular trafficking is a conserved process, studies focused on interactions among the Cin1, Wsp1, Cdc42, and Rac1 proteins should reveal the distinction of the Cin1-mediated pathway(s).
Wsp1 as a conserved target of Cin1 in actin organization.
Intracellular trafficking in S. cerevisiae is a highly organized event involving orchestrated functions of many proteins, including the Pan1 endocytic protein, WASP-like Las17/Bee1, Cdc42, and other proteins that link the endocytic cycle to the actin cytoskeleton (14, 43, 51). Pan1 regulates endocytosis and actin organization through its two N-terminal EH (Eps15 homology) domains and one C-terminal A domain. The EH domains bind the SlaI, End3, Ent1/2, and Yap1801/2 proteins (5, 43, 50), while the A domain interacts with the Arp2/3 complex. Pan1 does not interact with Las17/Bee1, which lacks the GBD, but interacts through the inhibitory protein SlaI to activate the Arp2/3 complex (13, 27, 42, 50).
We have proposed that C. neoformans evolves an endocytic pathway more similar to the human ITSN1 pathway than that of S. cerevisiae Pan1. In addition to Cin1, which contains one N-terminal EH domain and the coiled-coil region, C. neoformans also contains a WASP homology 2 (WH2) domain, two SRC homology 3 (SH3) domains, and a C-terminal DH-PH domain (38). C. neoformans also contains a WASP homolog, Wsp1, which has a GBD found only in mammalian WASPs. Given that mammalian WASP is a conserved functional partner of ITSN1 in actin regulation, similar domains in Cin1 and Wsp1 might suggest that certain signaling mechanisms are analogous between this fungal pathogen and mammals.
Here we performed protein localization studies and genetic epistasis analysis that support functional interactions between C. neoformans Cin1 and Wasp1 in endocytic trafficking and morphogenesis. Cin1 and Wsp1 likely operate in a common signaling pathway, given shared phenotypic defects in wsp1 and cin1 mutant strains (39). Partial suppression of the cin1 defect in endocytosis by the autoactivated Wsp1 allele further supports a genetic relationship between the two proteins. The partial suppression also suggests that additional activation mechanisms for Wsp1 and/or additional targets of Cin1 in endocytosis might be present.
Wsp1 as one of the conserved effectors of Cdc42 in actin organization.
The wsp1 mutant strain failed to display normal actin patches and cables, suggesting a role in the regulation of the actin cytoskeleton. The finding that the autoactivated Wsp1-B-GBD allele partially suppressed the cin1 defect in endocytosis further indicates that Wsp1 functions in a manner similar to that of mammalian WASP and that the GBD indeed contributes to the autoinhibition of Wsp1. Wsp1 interacts with Cdc42 in vitro, and this interaction was corroborated through partial colocalization of the fluorescence-labeled Wsp1 and Cdc42 fusion proteins. Importantly, Wsp1 is required for Cdc42 distribution, indicating that a feedback mechanism likely involving Cin1 is present. In a mechanism still not fully understood, Cdc42-GFP-Wsp1 partially suppressed the cin1 defect in the actin cytoskeleton. Such findings are consistent with a proposed role for Wsp1 as an effector of Cdc42 and Cin1. This is an unprecedented finding, since other known fungal WASP homolog proteins, including S. cerevisiae Las17 and C. albicans Wal1, lack the GBD (24, 46) and thus are unlikely to mediate Cdc42 function through similar mechanisms.
Like mammalian Cdc42, which regulates many effects relating to cytoskeleton organization and morphogenesis, fungal Cdc42 has a wide range of functions, ranging from polarity establishment, hyphal differentiation, and cytokinesis to virulence (10, 17, 32). Distinctively, Cdc42 regulates not only polarity establishment but also thermal tolerance, affecting virulence in C. neoformans. While Cdc42 regulates thermal tolerance downstream of Ras1, its role in polarity establishment might be related to the fact that the cdc42 mutant lacks actin cables. Expression of the constitutively activated Cdc42Q61l protein was able to restore both actin patches and cables to the wsp1 strain, suggesting that additional effectors of Cdc42 in actin organization might exist, such as formins, which are also involved in the polymerization of actin.
Wsp1 as a novel effector of Rac1 in vacuole morphology.
Wsp1 is one of the conserved effectors of Cdc42 in the actin cytoskeleton. The wsp1 mutant exhibits a more severe defect in actin organization than the cdc42 mutant, suggesting that additional regulators of Wsp1 might be present. To identify such regulators, we focused on Rac1, which shows similarity to Cdc42 in its general structure. Rac1 was previously determined to function in hyphal differentiation and high-temperature growth downstream of Ras1 (44). Rac proteins exhibit a wide range of functions in fungi, with the exception of S. cerevisiae, which does not have Rac homologs. The Penicillium marneffei Rac homolog CflB plays a role in hyphal growth and actin organization (6). C. albicans Rac1 has an important role in filamentous growth in cells inside a matrix (4). U. maydis rac1 mutant cells have a defect in vacuole morphology, and expression of a constitutively active RAC1Q61L allele caused enlarged cells with a single large vacuole (26). Our results corroborated the role of Rac1 in vacuolar morphogenesis. Moreover, we provided the first evidence that Rac1 regulates early endocytosis as well as vacuolar membrane fusion. Earlier studies with mammals have indicated that Rac proteins can bind to and activate WASP (2, 41). Through several lines of genetic evidence, we similarly concluded that C. neoformans Rac1 is a novel regulator of Wsp1. First, we demonstrated that Rac1 interacts with Wsp1 in vitro and in vivo. Second, Rac1 and Cdc42 are both required for normal actin cytoskeleton structures. Third, Rac1 enrichment in the vacuole membrane requires a functional Wsp1. Lastly, autoactivated Wsp1 suppresses the defect of the rac1 mutant in vacuole morphology upon induction of vacuolar membrane fusion.
Suppression of the vacuole fusion defect in the rac1 mutant by the Wsp1-GBD and Wsp1-B-GBD alleles was only partial, like the suppression of the cin1 defect by the Wsp1-B-GBD allele. This finding could suggest that additional effectors of Rac1, such as Cin1, might exist and that multiple proteins participate in the regulation of vesicle transport and the actin cytoskeleton. Finally, since Wsp1 is the effector of both Cdc42 and Rac1 in vesicle transport and the actin cytoskeleton, loss of Wsp1 function also negatively affects the distribution, and hence the function, of Cdc42 and Rac1. This may suggest that feedback mechanisms are present, such as that involving Cin1 and Cdc42-Wsp1. No evidence has yet been found to indicate whether the DH domain acts as a GEF for Rac1, but there are Rac-specific GEF homologs, such as DOCK1 (GenBank accession no. XP_573028), in the C. neoformans genome. These proteins remain uncharacterized.
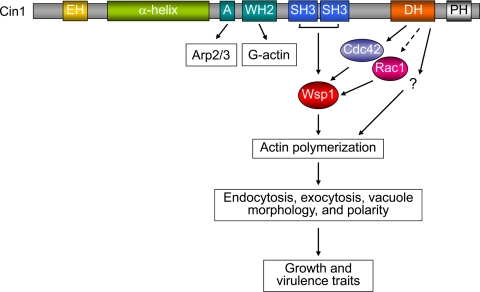
On the basis of these findings, we propose a working model highlighting the interactions between Cin1, Wsp1, Cdc42, and Rac1 and the possible functional consequences of these interactions (summarized in Fig. 5). In this model, Cin1 activates Cdc42 through the DH (Rho-GEF) domain, which, in turn, activates Wsp1 to promote actin polymerization, both of which are required for endocytosis, exocytosis, and other intracellular transport events. In addition to Cdc42, Wsp1 can also be activated by Rac1 for endocytosis and vacuole morphology. The possible function of Cin1-DH with respect to Rac1 has not been substantiated. Cin1 could also modulate Wsp1 through direct binding between the SH3 domains and the proline-rich (PR) (Wsp1) domain. Moreover, Cin1 may promote the extension of branched actin filaments by direct binding of Arp2/3 homologs through the A domain and of monomeric actin (G-actin) through the WH2 domain, respectively.
Fig 5.
Proposed model for the Cin1-mediated endocytic pathway, which regulates actin organization through the collective functions of Cin1, Cdc42, Rac1, and Wsp1. Cin1 is a multimodular adaptor protein proposed to regulate endocytosis, exocytosis, and signaling through interactions with several other proteins (47). Cin1 activates Cdc42 through the DH (Rho-GEF) domain; Cdc42, in turn, activates Wsp1 to promote Arp2/3-mediated actin polymerization. In addition to Cdc42, Wsp1 can also be an effector of Rac1 to regulate endocytosis and vacuole morphology. A possible effect of Cin1-DH on Rac1 has not been substantiated. Cin1 also activates Wsp1 through direct binding between the SRC homology 3 (SH3) (Cin1) and proline-rich (PR) (Wsp1) domains. Moreover, Cin1 may promote the extension of the branched actin filaments by binding directly to Arp2/3 homologs through the A domain and to monomeric actin (G-actin) through the WASP homology 2 (WH2) domain.
Taken together, the multifunctional roles of Cin1, Wsp1, Cdc42, and Rac1 underscore the importance of intracellular trafficking, actin cytoskeleton organization, and signaling in the physiology and virulence of C. neoformans. Further studies of these proteins and their functions will further facilitate the understanding of intracellular transport in microbial pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Amy Whittington for comments and Naeem Uddin and Michelle Nguyen for technical assistance.
This research was supported in part by PHS grants AI063242 and AI050128 (to J.A.A.) and AI054958 and AI074001 (to P.W.).
Footnotes
Published ahead of print 10 February 2012
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Allen WE, Jones GE, Pollard JW, Ridley AJ. 1997. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 110(Pt 6):707–720 [DOI] [PubMed] [Google Scholar]
- 2. Aspenstrom P, Lindberg U, Hall A. 1996. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr. Biol. 6:70–75 [DOI] [PubMed] [Google Scholar]
- 3. Ballou ER, Nichols CB, Miglia KJ, Kozubowski L, Alspaugh JA. 2010. Two CDC42 paralogues modulate Cryptococcus neoformans thermotolerance and morphogenesis under host physiological conditions. Mol. Microbiol. 75:763–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bassilana M, Arkowitz RA. 2006. Rac1 and Cdc42 have different roles in Candida albicans development. Eukaryot. Cell 5:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benedetti H, Raths S, Crausaz F, Riezman H. 1994. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell 5:1023–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyce KJ, Hynes MJ, Andrianopoulos A. 2003. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 116:1249–1260 [DOI] [PubMed] [Google Scholar]
- 7. Buchanan KL, Murphy JW. 1998. What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 4:71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chayakulkeeree M, et al. 2011. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol. Microbiol. 80:1088–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Z, et al. 2010. Structure and control of the actin regulatory WAVE complex. Nature 468:533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cotteret S, Chernoff J. 2002. The evolutionary history of effectors downstream of Cdc42 and Rac. Genome Biol. 3:REVIEWS0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davidson RC, et al. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607–2615 [DOI] [PubMed] [Google Scholar]
- 12. Donaldson J, Segev N. 2009. Regulation and coordination of intracellular trafficking: an overview, p 329–341 In Segev N. (ed), Trafficking inside cells: pathways, mechanisms and regulation. Landes Bioscience, Austin, TX [Google Scholar]
- 13. Duncan MC, Cope MJ, Goode BL, Wendland B, Drubin DG. 2001. Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat. Cell Biol. 3:687–690 [DOI] [PubMed] [Google Scholar]
- 14. Galletta BJ, Chuang DY, Cooper JA. 2008. Distinct roles for Arp2/3 regulators in actin assembly and endocytosis. PLoS Biol. 6:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goley ED, Welch MD. 2006. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7:713–726 [DOI] [PubMed] [Google Scholar]
- 16. Harlan JE, Hajduk PJ, Yoon HS, Fesik SW. 1994. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature 371:168–170 [DOI] [PubMed] [Google Scholar]
- 17. Harris SD. 2011. Cdc42/Rho GTPases in fungi: variations on a common theme. Mol. Microbiol. 79:1123–1127 [DOI] [PubMed] [Google Scholar]
- 18. Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A. 2011. Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC [Google Scholar]
- 19. Johnson DI. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones L, Tedrick K, Baier A, Logan MR, Eitzen G. 2010. Cdc42p is activated during vacuole membrane fusion in a sterol-dependent subreaction of priming. J. Biol. Chem. 285:4298–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kopecka M, et al. 2001. Microtubules and actin cytoskeleton in Cryptococcus neoformans compared with ascomycetous budding and fission yeasts. Eur. J. Cell Biol. 80:303–311 [DOI] [PubMed] [Google Scholar]
- 22. Kurisu S, Takenawa T. 2009. The WASP and WAVE family proteins. Genome Biol. 10:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwon-Chung KJ, Edman JC, Wickes BL. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60:602–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li R. 1997. Bee1, a yeast protein with homology to Wiscott-Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J. Cell Biol. 136:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu X, Hu G, Panepinto J, Williamson PR. 2006. Role of a VPS41 homologue in starvation response, intracellular survival and virulence of Cryptococcus neoformans. Mol. Microbiol. 61:1132–1146 [DOI] [PubMed] [Google Scholar]
- 26. Mahlert M, Leveleki L, Hlubek A, Sandrock B, Bolker M. 2006. Rac1 and Cdc42 regulate hyphal growth and cytokinesis in the dimorphic fungus Ustilago maydis. Mol. Microbiol. 59:567–568 [DOI] [PubMed] [Google Scholar]
- 27. Miliaras NB, Park JH, Wendland B. 2004. The function of the endocytic scaffold protein Pan1p depends on multiple domains. Traffic 5:963–978 [DOI] [PubMed] [Google Scholar]
- 28. Mitchell TG, Perfect JR. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moseley JB, Goode BL. 2006. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 70:605–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Bryan JP. 2010. Intersecting pathways in cell biology. Sci. Signal. 3:re10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panepinto J, et al. 2009. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol. 71:1165–1176 [DOI] [PubMed] [Google Scholar]
- 32. Park HO, Bi E. 2007. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 71:48–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel FB, et al. 2008. The WAVE/SCAR complex promotes polarized cell movements and actin enrichment in epithelia during C. elegans embryogenesis. Dev. Biol. 324:297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perfect JR. 2005. Cryptococcus neoformans: a sugar-coated killer with designer genes. FEMS Immunol. Med. Microbiol. 45:395–404 [DOI] [PubMed] [Google Scholar]
- 35. Pruyne D, Bretscher A. 2000. Polarization of cell growth in yeast. J. Cell Sci. 113:571–585 [DOI] [PubMed] [Google Scholar]
- 36. Ramesh N, Geha R. 2009. Recent advances in the biology of WASP and WIP. Immunol. Res. 44:99–111 [DOI] [PubMed] [Google Scholar]
- 37. Shaw G. 1996. The pleckstrin homology domain: an intriguing multifunctional protein module. Bioessays 18:35–46 [DOI] [PubMed] [Google Scholar]
- 38. Shen G, Whittington A, Song K, Wang P. 2010. Pleiotropic function of intersectin homologue Cin1 in Cryptococcus neoformans. Mol. Microbiol. 76:662–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen G, Whittington A, Wang P. 2011. Wsp1, a GBD/CRIB domain-containing WASP homolog, is required for growth, morphogenesis, and virulence of Cryptococcus neoformans. Eukaryot. Cell 10:521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith MG, Swamy SR, Pon LA. 2001. The life cycle of actin patches in mating yeast. J. Cell Sci. 114:1505–1513 [DOI] [PubMed] [Google Scholar]
- 41. Symons M. 1996. Rho family GTPases: the cytoskeleton and beyond. Trends Biochem. Sci. 21:178–181 [PubMed] [Google Scholar]
- 42. Tang HY, Munn A, Cai M. 1997. EH domain proteins Pan1p and End3p are components of a complex that plays a dual role in organization of the cortical actin cytoskeleton and endocytosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:4294–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang HY, Xu J, Cai M. 2000. Pan1p, End3p, and S1a1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell. Biol. 20:12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vallim MA, Nichols CB, Fernandes L, Cramer KL, Alspaugh JA. 2005. A Rac homolog functions downstream of Ras1 to control hyphal differentiation and high-temperature growth in the pathogenic fungus Cryptococcus neoformans. Eukaryot. Cell 4:1066–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Veltman DM, Insall RH. 2010. WASP family proteins: their evolution and its physiological implications. Mol. Biol. Cell 21:2880–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walther A, Wendland J. 2004. Polarized hyphal growth in Candida albicans requires the Wiskott-Aldrich syndrome protein homolog Wal1p. Eukaryot. Cell 3:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang P, Shen G. 2011. The endocytic adaptor proteins of pathogenic fungi: charting new and familiar pathways. Med. Mycol. 49:449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang YX, Kauffman EJ, Duex JE, Weisman LS. 2001. Fusion of docked membranes requires the armadillo repeat protein Vac8p. J. Biol. Chem. 276:35133–35140 [DOI] [PubMed] [Google Scholar]
- 49. Waugh MS, et al. 2002. Ras1 and Ras2 contribute shared and unique roles in physiology and virulence of Cryptococcus neoformans. Microbiology 148:191–201 [DOI] [PubMed] [Google Scholar]
- 50. Wendland B, Emr SD. 1998. Pan1p, yeast eps15, functions as a multivalent adaptor that coordinates protein-protein interactions essential for endocytosis. J. Cell Biol. 141:71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wendland B, Emr SD, Riezman H. 1998. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol. 10:513–522 [DOI] [PubMed] [Google Scholar]
- 52. Westerberg L, Greicius G, Snapper SB, Aspenstrom P, Severinson E. 2001. Cdc42, Rac1, and the Wiskott-Aldrich syndrome protein are involved in the cytoskeletal regulation of B lymphocytes. Blood 98:1086–1094 [DOI] [PubMed] [Google Scholar]
- 53. Yang FC, et al. 2001. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc. Natl. Acad. Sci. U. S. A. 98:5614–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang HC, Pon LA. 2002. Actin cable dynamics in budding yeast. Proc. Natl. Acad. Sci. U. S. A. 99:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoneda A, Doering TL. 2006. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol. Biol. Cell 17:5131–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.