Abstract
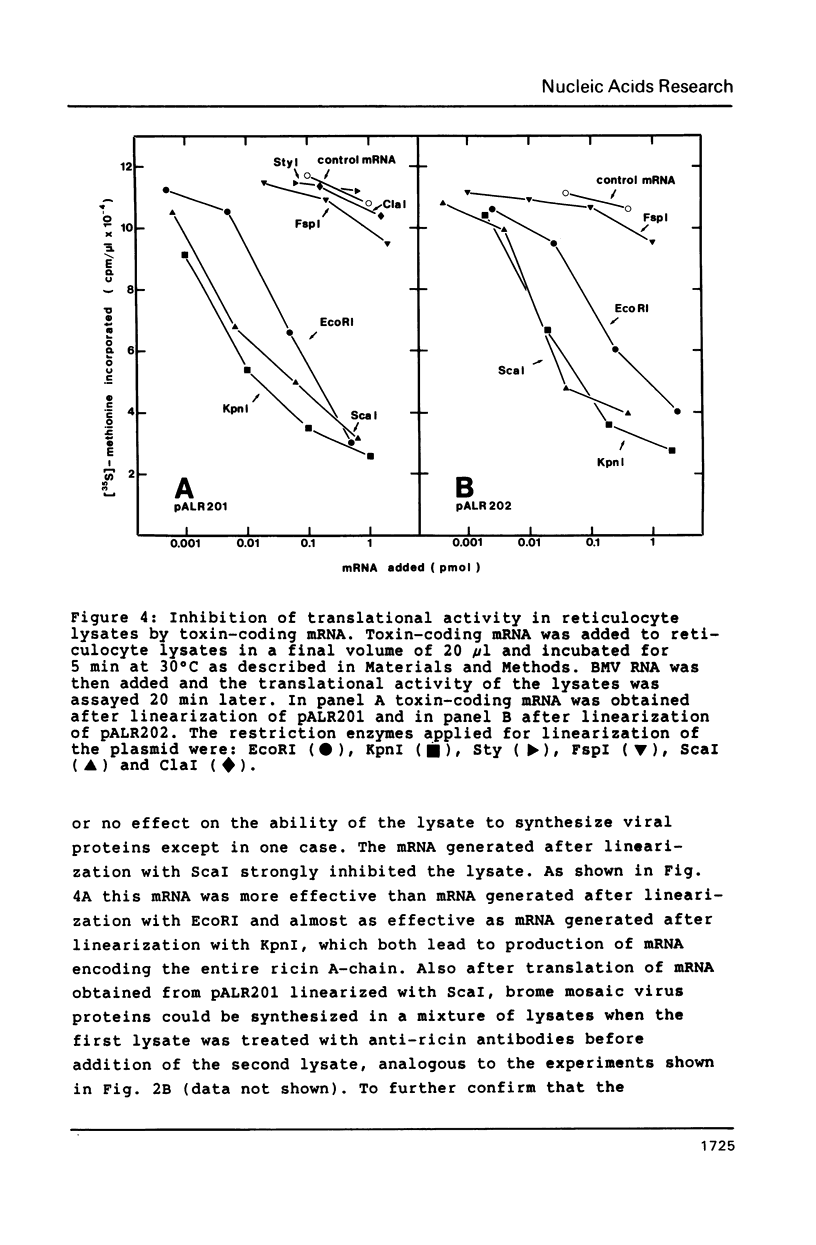
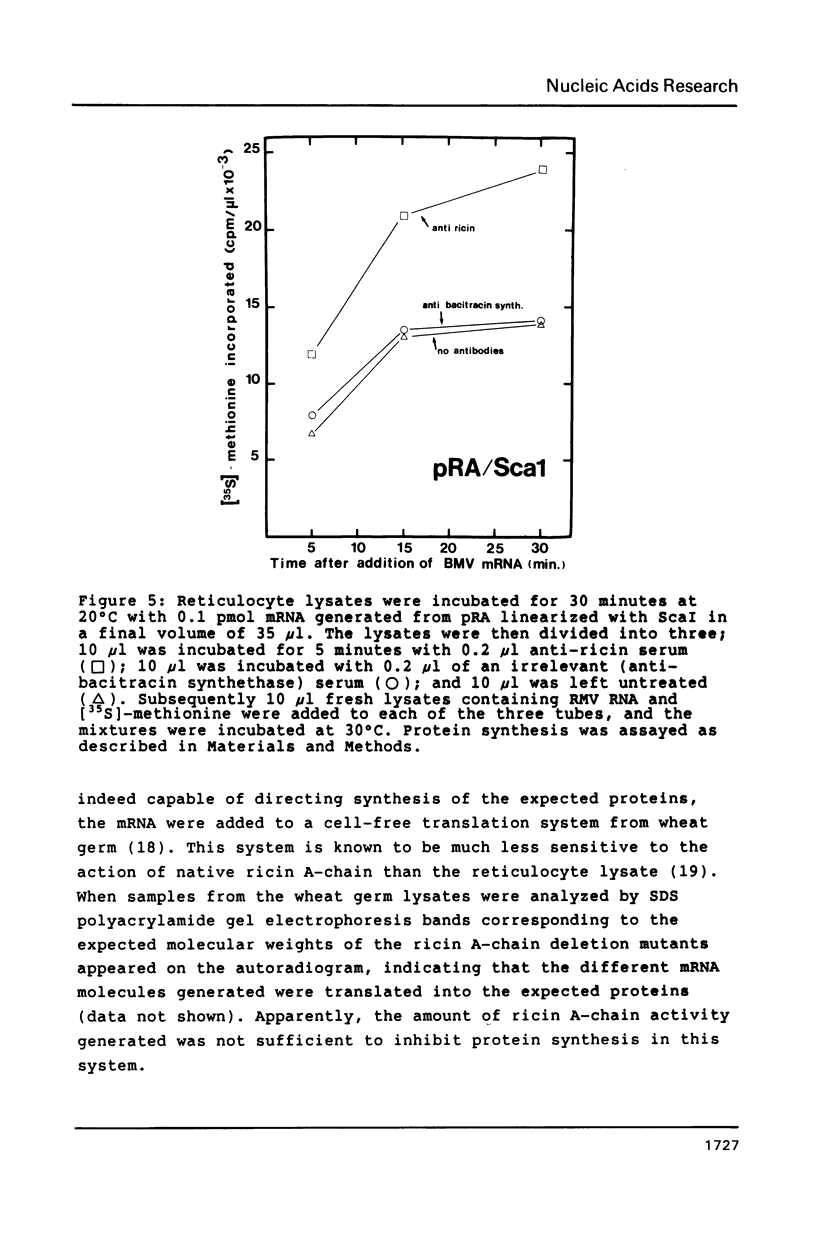
The gene encoding the ricin A-chain was isolated and subcloned into an in vitro expression vector downstream from the SP6 promotor. mRNA encoding the A-chain strongly inhibited the translational activity of reticulocyte lysates. The inhibition correlated with glycosylase activity on rRNA, and could be abolished by addition of antibodies specific for ricin. mRNA generated after linearization of the vector at unique restriction sites within the A-chain coding sequence did not inhibit, except after linearization with ScaI. Also mutants lacking the 28 N-terminal amino acids of native A-chain strongly inhibited the lysates. However, in both cases no glycosylase activity could be observed. We also show that the lack of a stop codon in mRNA does not affect the level of expression as assayed here.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Cawley D. B., Hedblom M. L., Hoffman E. J., Houston L. L. Differential ricin sensitivity of rat liver and wheat germ ribosomes in polyuridylic acid translation. Arch Biochem Biophys. 1977 Aug;182(2):690–695. doi: 10.1016/0003-9861(77)90550-1. [DOI] [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987 Apr 25;262(12):5908–5912. [PubMed] [Google Scholar]
- Halling K. C., Halling A. C., Murray E. E., Ladin B. F., Houston L. L., Weaver R. F. Genomic cloning and characterization of a ricin gene from Ricinus communis. Nucleic Acids Res. 1985 Nov 25;13(22):8019–8033. doi: 10.1093/nar/13.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Montfort W., Villafranca J. E., Monzingo A. F., Ernst S. R., Katzin B., Rutenber E., Xuong N. H., Hamlin R., Robertus J. D. The three-dimensional structure of ricin at 2.8 A. J Biol Chem. 1987 Apr 15;262(11):5398–5403. [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perara E., Rothman R. E., Lingappa V. R. Uncoupling translocation from translation: implications for transport of proteins across membranes. Science. 1986 Apr 18;232(4748):348–352. doi: 10.1126/science.3961485. [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Lamb F. I., Pappin D. J., Lord J. M. The primary sequence of Ricinus communis agglutinin. Comparison with ricin. J Biol Chem. 1985 Dec 15;260(29):15682–15686. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundan A., Olsnes S., Sandvig K., Pihl A. Preparation and properties of chimeric toxins prepared from the constituent polypeptides of diphtheria toxin and ricin. Evidence for entry of ricin A-chain via the diphtheria toxin pathway. J Biol Chem. 1982 Aug 25;257(16):9733–9739. [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]